Abstract
In the last decade, researchers have searched for predictive surface markers of multipotent mesenchymal stromal/stem cells (MSCs) for ensuring improved therapeutic outcomes following cartilage damage in humans. However, we have achieved only limited progress because of the challenge presented by conflicting data. This commentary provides some evidence to prove a lack of success with current efforts, including an inconsistency in accepted surface markers and chondrogenic potential of MSCs as well as the tissue source–dependent MSC surface markers that correlate with chondrogenic potential. A brief discussion on these disputed topics and perspective about functionally predictive surface markers and standardization of analytic procedures are also highlighted.
Keywords: mesenchymal stromal/stem cell, surface marker, proliferation, chondrogenic differentiation, cartilage
Introduction
As a leading cause of disability among adults, osteoarthritis often results from a biochemical breakdown of articular cartilage in joints 1. Articular cartilage has a poor intrinsic healing capacity because of its avascular structure, immobility of chondrocytes, and low mitotic activity. Compared with conventional surgical methods, autologous cell therapy, growth factor therapy, and biomaterials provide more promising approaches for clinical treatment 2. Human multipotent mesenchymal stromal/stem cell (MSC)-based cell therapy is expected to deliver a promising treatment for cartilage repair because of easy isolation of cells from mesenchymal tissues with higher proliferative and chondrogenic potential 3, 4. Given that MSCs exist in a number of tissues and organs, such as bone marrow, synovial membrane, and adipose tissue 5– 7, to compare research outcomes and promote the development of MSC-based therapy, the International Society for Cellular Therapy defined human MSCs in 2006 8. First, in vitro culture of MSCs must have the ability to adhere to plastic substrates; second, MSCs should express cluster of differentiation 73 (CD73), CD90, and CD105 (>95%), which are measured by flow cytometry. Meanwhile, CD14, CD19, CD34, CD45, and HLA class II should be negative (≤2% positive). Third, MSCs must have osteogenic, chondrogenic, and adipogenic capacities in vitro.
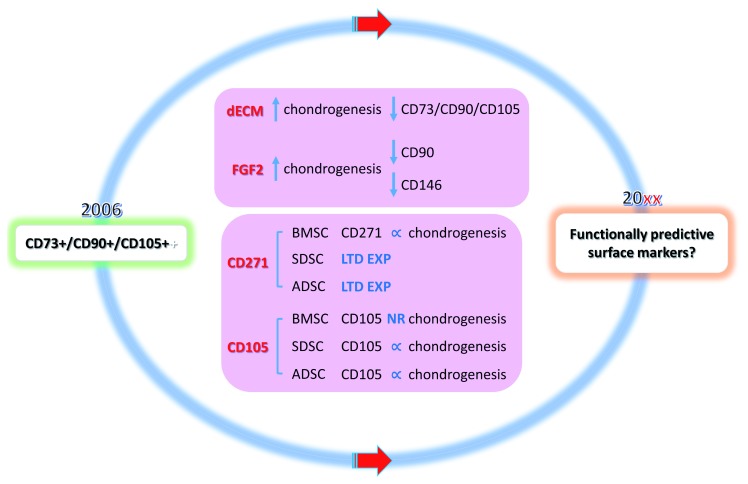
Increasing evidence has shown that human MSC subpopulations which were sorted by some surface markers had better chondrogenic potential for cartilage regeneration. Researchers are trying to find predictive MSC surface markers for ensuring improved therapeutic outcomes. Although some promising MSC surface markers have been comprehensively reviewed 9– 11, Alegre-Aguarón et al. questioned the correlation between stem cell surface markers and chondrogenic potential 12. This commentary provides some evidence to prove a lack of success with current efforts, including an inconsistency in accepted surface markers and chondrogenic potential of MSCs as well as the tissue source–dependent MSC surface markers that correlate with chondrogenic potential. A brief discussion on these disputed topics and perspective about functionally predictive surface markers and standardization of analytic procedures are also highlighted ( Figure 1).
Figure 1. Schematic diagram of our Commentary on ‘Surface markers associated with chondrogenic potential of human mesenchymal stromal/stem cells’.
Human mesenchymal stromal/stem cells (MSCs) were defined in 2006 by the International Society for Cellular Therapy. Unfortunately, currently accepted predictive surface markers do not seem to be ideal candidates to predict MSCs with chondrogenic potential in terms of inconsistency of currently accepted surface markers and chondrogenic potential of MSCs and tissue source–dependent MSC surface markers that correlate with chondrogenic potential. Are functionally predictive surface markers the next target for sorting MSCs in cartilage engineering and regeneration? ∝, positive correlation; ADSC, adipose-derived stromal/stem cell; BMSC, bone marrow-derived stromal/stem cell; dECM, decellularized extracellular matrix; FGF2, fibroblast growth factor 2; LTD EXP, limited expression; NR, not related; SDSC, synovium-derived stromal/stem cell.
Inconsistency of currently accepted surface markers and chondrogenic potential of MSCs
Increasing evidence suggests that environmental preconditioning revitalizes the proliferation and chondrogenic capacity of adult stromal/stem cells 13. Given two good examples that decellularized extracellular matrix (dECM) expansion or fibroblast growth factor 2 (FGF2) pretreatment can promote human MSC proliferation and chondrogenic potential, there is an inconsistency in accepted surface markers and chondrogenic potential of MSCs.
dECM expansion
Recent reports demonstrated that dECM deposited by synovium-derived stromal/stem cells (SDSCs) provided an in vitro microenvironment for SDSC expansion, which dramatically improved proliferation and enhanced chondrogenic potential 14– 16. The flow cytometry data reported by Li et al. showed that, compared with surface markers of human SDSCs grown on tissue culture plastic (TCP), the percentage of CD29, CD90, and CD105 expression of SDSCs grown on dECM decreased slightly but the median fluorescence intensity (MFI) declined dramatically; interestingly, both the percentage and MFI of stage-specific embryonic antigen 4 (SSEA4) increased 17. Zhang et al. also found that, despite nearly 100% expression in SDSCs after expansion on either TCP or dECM substrates, CD29, CD90, and CD105 declined dramatically at the MFI in dECM-expanded SDSCs; the MFI of SSEA4 in dECM-expanded cells increased slightly while the percentage doubled 18. Interestingly, real-time quantitative polymerase chain reaction results showed that SRY-Box9 ( SOX9), type II collagen ( COL2A1), and aggrecan ( ACAN) were significantly upregulated during chondrogenic induction in SDSCs from the dECM group 18. In those two studies, SSEA4 was found to be the only surface marker under evaluation that increased when dECM improved the chondrogenic potential of human SDSCs. However, Li et al. showed that SSEA4(+) expression did not favor human SDSC chondrogenesis because enhanced chondrogenesis occurred in the SSEA4(−) population of cells 19.
FGF2 pretreatment
In 2011, Kim et al. found that the expression percentage of surface marker CD49a in human SDSCs decreased with pretreatment using FGF2 and that FGF2 exerted no effect on the expression levels in CD29, CD44, CD73, CD105, and CD166 20. In that study, the size, weight, and glycosaminoglycan (GAG) accumulation of pellets increased following FGF2 supplementation during cell expansion. In another study, after seven days of monolayer expansion in the presence of FGF2, human SDSCs became significantly smaller and showed a fibroblast-like appearance as well as a decrease in MFI for CD29, CD90, and CD10; however, FGF2-pretreated SDSCs showed significantly increased chondrogenic potential 21. Hagmann et al. reported that FGF2 pretreatment suppressed CD146 expression in human bone marrow–derived stromal/stem cells (BMSCs) and promoted chondrogenic differentiation 22. These studies demonstrated that, during ex vivo expansion, FGF2 is an effective agent to promote human MSC proliferation and chondrogenic potential via upregulation of SOX9 23. However, measured surface markers did not show a positive correlation with the proliferative and chondrogenic potential of MSCs.
Tissue source–dependent MSC surface markers that correlate chondrogenic potential
Some surface markers associated with chondrogenic potential are not equally expressed in all tissue-specific stromal/stem cells. The evidence shown in this section supports the conclusion that the predictive capacity of CD271 and CD105 for MSC chondrogenic potential is tissue source–dependent in terms of MSCs from synovium, bone marrow, and adipose.
CD271
CD271, a low-affinity nerve growth factor receptor, is considered to be a highly selective surface marker for BMSCs 24. The reports from Mifune et al. 25 and Calabrese et al. 26 showed that CD271(+) BMSCs from freshly isolated cells had higher chondrogenic potential as evidenced by increased expression of chondrogenic genes in pellet culture with induction medium compared with CD271(−) BMSCs. Petters et al. demonstrated that, without ex vivo expansion, human bone marrow–derived CD271(+) mononuclear cells could generate sufficient articular cartilage constructs exhibiting high cell viability and remarkable chondrogenic matrix deposition in a type I collagen hydrogel 27.
Given that CD271 plays an important predictive role in chondrogenic potential of human BMSCs, there is still controversy over whether human SDSCs express CD271 28, 29. Some studies reported that CD271 was expressed only in the synovial membrane of patients with osteoarthritis 29, 30. Interestingly, increasing evidence indicates that SDSCs are tissue-specific stromal/stem cells for chondrogenesis 31 and present superior chondrogenic potential and less hypertrophy compared with BMSCs 32.
Despite the low abundance of CD271(+) subpopulation within stromal vascular fraction cells, Quirici et al. found that the CD271(+) subpopulation of adipose-derived stromal/stem cells (ADSCs) had high increments in cell proliferation when compared with unsorted ADSCs 33. Research by Kohli et al. showed that CD271(+) ADSCs from ex vivo expansion had a superior ability to promote cartilage repair compared with unsorted ADSCs 34. However, the study by Beckenkamp et al. showed that, in freshly isolated cells, CD34(+)CD271(+) ADSCs displayed similar in vitro chondrogenic potential at passage 3 compared with CD34(+)CD271(−) ADSCs 35.
CD105
CD105 (endoglin) is a transmembrane protein that regulates cellular proliferation, differentiation, and migration 36. Cleary et al. found that the percentage of CD105 in human BMSCs was not related to subsequent chondrogenic potential since CD105 expression did not change during cell expansion when chondrogenic potential decreased 37. Interestingly, this outcome seems to be inconsistent with the role of CD105 in SDSCs and ADSCs. Arufe et al. in 2009 38 and Chang et al. in 2013 39 demonstrated that the cellular subset of CD105(+) SDSCs from ex vivo expansion possessed greater chondrogenic capacity than the CD105(−) SDSC subset. Jiang et al. found that CD105(+) ADSC subpopulation in in vitro culture had a much stronger chondrogenic potential than CD105(−) subpopulation and had more intensive immunostaining of type II collagen and higher gene expression of COL2A1 and ACAN following chondrogenic induction 40. There is also a report that myrtucommulone-A treatment reduced CD105 expression in expanded human ADSCs along with reduced chondrogenic potential 41.
Discussions and perspectives
In this commentary, we discussed that the same surface markers might perform differently in predicting the chondrogenic potential of MSCs isolated from different tissues. We also highlighted the inconsistency in currently accepted surface markers and chondrogenic potential of MSCs, which brings up the challenge to find more reliable surface markers to meet the demands of future regenerative medicine. A 2017 report from Dickinson et al. 42 raised the concept of “functionally predictive surface markers”, which may convey a promising method to address this issue. In the article, the authors used a genomic profiling strategy to find a functional MSC surface marker that can predict enhanced chondrogenic potential. They found that receptor tyrosine kinase-like orphan receptor 2 (ROR2), the Wnt5a receptor, was upregulated in highly chondrogenic clones and used ROR2 to sort the MSC subpopulation which can produce enhanced cartilage constructs with superior efficacy in an animal cartilage repair model. As a functionally predictive surface marker, ROR2 is believed to be important for chondrogenesis, including initial morphology of the cartilage anlagen and subsequent tuning of mature cartilage 43, as well as mediating Wnt5a signaling in enhancing chondrogenesis by activation of SOX9 44. Intriguingly, a recent report from Stüdle et al. did not find human BMSCs to express ROR2 since the percentage of ROR2(+) cells was lower than 0.1% 45. The authors also found that high variability both across the donors and across clonally derived strains in BMSCs challenged chondrogenic differentiation outcomes. These results indicate that there is a long way to go to find functionally predictive surface markers for stromal/stem cell–based cartilage engineering and regeneration.
Although many studies have focused on the correlation between human MSC surface markers and chondrogenic potential, there is a lack of standard procedures to quantify surface marker expression. Some procedures might influence the outcome, such as enzyme-dependent cell-detaching methods. A 2017 report compared the effect of cell-detaching methods on the positive proportion of surface markers of cultured SDSCs 46. They found that trypsin (catalog number 25200072; Thermo Fisher Scientific, Waltham, WA, USA) obviously reduced the percentage and MFI of CD73(+) cells and CD105(+) cells but had little effect on the percentage of CD90 expression. They also found that TrypLE (catalog number 12563011; Thermo Fisher Scientific) had no influence on the positive proportion of tested surface markers at 30 minutes of digestion but dramatically reduced the CD44(+), CD49c(+), CD73(+), CD140a(+), and CD140b(+) cell populations at 60 minutes of digestion. Collagenase (catalog number C9263; MilliporeSigma, Burlington, MA, USA) was found to reduce the CD58(+), CD105(+), and CD140b(+) cell populations at 120 minutes of digestion. By using flow cytometric analysis, most researchers tend to measure the percentage rather than the MFI of surface markers to assess the influence on MSC chondrogenic potential. However, the percentage analysis method is easily affected by outliers and disregards fluorescent intensity shifts that may show how proliferation and differentiation are progressing by a change in the level of surface marker expression. To overcome the limitations of percentage, Chan et al. proposed an analysis method based on MFI because of its robustness against outliers and increased accuracy 47. Therefore, the relationship between surface markers and chondrogenic ability should be further studied by a method including the MFI of MSCs.
Acknowledgments
We thank Suzanne Danley from the Department of Orthopaedics, West Virginia University, for editing the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Andre J. van Wijnen, Department of Orthopedic Surgery and Biochemistry and Molecular Biology, Mayo Clinic, Rochester, Minnesota, USA
Andrea Barbero, Department of Biomedicine, University Hospital Basel, Basel, Switzerland
Gerjo J. V. M. van Osch, Department of Orthopaedics and Department of Otorhinolaryngology, Erasmus MC, University Medical Center, Rotterdam, The Netherlands
Funding Statement
This work was supported by research grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award number AR067747-01A1) and the Musculoskeletal Transplant Foundation.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Centers for Disease Control and Prevention (CDC): Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50(7):120–5. [PubMed] [Google Scholar]
- 2. Falah M, Nierenberg G, Soudry M, et al. : Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34(5):621–30. 10.1007/s00264-010-0959-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koga H, Engebretsen L, Brinchmann JE, et al. : Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc. 2009;17(11):1289–97. 10.1007/s00167-009-0782-4 [DOI] [PubMed] [Google Scholar]
- 4. Pittenger MF, Mackay AM, Beck SC, et al. : Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. 10.1126/science.284.5411.143 [DOI] [PubMed] [Google Scholar]
- 5. Prockop DJ: Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. 10.1126/science.276.5309.71 [DOI] [PubMed] [Google Scholar]
- 6. De Bari C, Dell'Accio F, Tylzanowski P, et al. : Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–42. [DOI] [PubMed] [Google Scholar]
- 7. Zuk PA, Zhu M, Ashjian P, et al. : Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. 10.1091/mbc.e02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dominici M, Le Blanc K, Mueller I, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Campbell DD, Pei M, et al. : Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells. 2012;1(4):1107–20. 10.3390/cells1041107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lv FJ, Tuan RS, Cheung KM, et al. : Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19. 10.1002/stem.1681 [DOI] [PubMed] [Google Scholar]
- 11. Pérez-Silos V, Camacho-Morales A, Fuentes-Mera L: Mesenchymal Stem Cells Subpopulations: Application for Orthopedic Regenerative Medicine. Stem Cells Int. 2016;2016: 3187491. 10.1155/2016/3187491 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Alegre-Aguarón E, Desportes P, García-Álvarez F, et al. : Differences in surface marker expression and chondrogenic potential among various tissue-derived mesenchymal cells from elderly patients with osteoarthritis. Cells Tissues Organs. 2012;196(3):231–40. 10.1159/000334400 [DOI] [PubMed] [Google Scholar]
- 13. Pei M: Environmental preconditioning rejuvenates adult stem cells' proliferation and chondrogenic potential. Biomaterials. 2017;117:10–23. 10.1016/j.biomaterials.2016.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Pei M: Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A. 2011;17(5–6):703–12. 10.1089/ten.TEA.2010.0339 [DOI] [PubMed] [Google Scholar]
- 15. Pei M, He F, Wei L: Three-Dimensional Cell Expansion Substrate for Cartilage Tissue Engineering and Regeneration: A Comparison in Decellularized Matrix Deposited by Synovium-Derived Stem Cells and Chondrocytes. J Tissue Sci Eng. 2011;2:104 10.4172/2157-7552.1000104 [DOI] [Google Scholar]
- 16. Pei M, Li JT, Shoukry M, et al. : A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater. 2011;22:333–43; discussion 343. 10.22203/ecm.v022a25 [DOI] [PubMed] [Google Scholar]
- 17. Li J, Hansen KC, Zhang Y, et al. : Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35(2):642–53. 10.1016/j.biomaterials.2013.09.099 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Zhang Y, Li J, Davis ME, et al. : Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater. 2015;20:39–50. 10.1016/j.actbio.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Campbell DD, Bal GK, et al. : Can arthroscopically harvested synovial stem cells be preferentially sorted using stage-specific embryonic antigen 4 antibody for cartilage, bone, and adipose regeneration? Arthroscopy. 2014;30(3):352–61. 10.1016/j.arthro.2013.12.009 [DOI] [PubMed] [Google Scholar]
- 20. Kim JH, Lee MC, Seong SC, et al. : Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue Eng Part A. 2011;17(7–8):991–1002. 10.1089/ten.TEA.2010.0277 [DOI] [PubMed] [Google Scholar]
- 21. Pizzute T, Li J, Zhang Y, et al. : Fibroblast Growth Factor Ligand Dependent Proliferation and Chondrogenic Differentiation of Synovium-Derived Stem Cells and Concomitant Adaptation of Wnt/Mitogen-Activated Protein Kinase Signals. Tissue Eng Part A. 2016;22(15–16):1036–46. 10.1089/ten.TEA.2016.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hagmann S, Moradi B, Frank S, et al. : FGF-2 addition during expansion of human bone marrow-derived stromal cells alters MSC surface marker distribution and chondrogenic differentiation potential. Cell Prolif. 2013;46(4):396–407. 10.1111/cpr.12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Correa D, Somoza RA, Lin P, et al. : Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthritis Cartilage. 2015;23(3):443–53. 10.1016/j.joca.2014.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Álvarez-Viej M, Menéndez-Menéndez Y, Otero-Hernández J: CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J Stem Cells. 2015;7(2):470–6. 10.4252/wjsc.v7.i2.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mifune Y, Matsumoto T, Murasawa S, et al. : Therapeutic superiority for cartilage repair by CD271-positive marrow stromal cell transplantation. Cell Transplant. 2013;22(7):1201–11. 10.3727/096368912X657378 [DOI] [PubMed] [Google Scholar]
- 26. Calabrese G, Giuffrida R, Lo Furno D, et al. : Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int J Mol Sci. 2015;16(7):15609–24. 10.3390/ijms160715609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petters O, Schmidt C, Henkelmann R, et al. : Single-Stage Preparation of Human Cartilage Grafts Generated from Bone Marrow-Derived CD271 + Mononuclear Cells. Stem Cells Dev. 2018;27(8):545–55. 10.1089/scd.2017.0218 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Arufe MC, De la Fuente A, Fuentes I, et al. : Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111(4):834–45. 10.1002/jcb.22768 [DOI] [PubMed] [Google Scholar]
- 29. Karystinou A, Dell'Accio F, Kurth TB, et al. : Distinct mesenchymal progenitor cell subsets in the adult human synovium. Rheumatology (Oxford). 2009;48(9):1057–64. 10.1093/rheumatology/kep192 [DOI] [PubMed] [Google Scholar]
- 30. Del Rey MJ, Faré R, Usategui A, et al. : CD271 + stromal cells expand in arthritic synovium and exhibit a proinflammatory phenotype. Arthritis Res Ther. 2016;18:66. 10.1186/s13075-016-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Jones BA, Pei M: Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):301–11. 10.1089/ten.TEB.2012.0002 [DOI] [PubMed] [Google Scholar]
- 32. Pizzute T, Lynch K, Pei M: Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev Rep. 2015;11(1):119–32. 10.1007/s12015-014-9546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quirici N, Scavullo C, de Girolamo L, et al. : Anti-L-NGFR and -CD34 monoclonal antibodies identify multipotent mesenchymal stem cells in human adipose tissue. Stem Cells Dev. 2010;19(6):915–25. 10.1089/scd.2009.0408 [DOI] [PubMed] [Google Scholar]
- 34. Kohli N, Al-Delfi IRT, Snow M, et al. : CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells. Sci Rep. 2019;9(1):3194. 10.1038/s41598-019-39715-z [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Beckenkamp LR, Souza LEB, Melo FUF, et al. : Comparative characterization of CD271 + and CD271 - subpopulations of CD34 + human adipose-derived stromal cells. J Cell Biochem. 2018;119(5):3873–84. 10.1002/jcb.26496 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Gonzalez-Garza MT: Adult stem cell membrane markers: their importance and critical role in their proliferation and differentiation potentials.In: Valarmathi MT (Eds.). Stromal cells-structure, function, and therapeutic implications IntechOpen Limited, London, UK.2018. 10.5772/intechopen.76869 [DOI] [Google Scholar]
- 37. Cleary MA, Narcisi R, Focke K, et al. : Expression of CD105 on expanded mesenchymal stem cells does not predict their chondrogenic potential. Osteoarthritis Cartilage. 2016;24(5):868–72. 10.1016/j.joca.2015.11.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Arufe MC, De la Fuente A, Fuentes-Boquete I, et al. : Differentiation of synovial CD-105 + human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108(1):145–55. 10.1002/jcb.22238 [DOI] [PubMed] [Google Scholar]
- 39. Chang CB, Han SA, Kim EM, et al. : Chondrogenic potentials of human synovium-derived cells sorted by specific surface markers. Osteoarthritis Cartilage. 2013;21(1):190–9. 10.1016/j.joca.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 40. Jiang T, Liu W, Lv X, et al. : Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31(13):3564–71. 10.1016/j.biomaterials.2010.01.050 [DOI] [PubMed] [Google Scholar]
- 41. Izgi K, Sonmez MF, Canatan H, et al. : Long Term Exposure to Myrtucommulone-A Changes CD105 Expression and Differentiation Potential of Mesenchymal Stem Cells. Tissue Eng Regen Med. 2017;14(2):113–21. 10.1007/s13770-016-0020-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Dickinson SC, Sutton CA, Brady K, et al. : The Wnt5a Receptor, Receptor Tyrosine Kinase-Like Orphan Receptor 2, Is a Predictive Cell Surface Marker of Human Mesenchymal Stem Cells with an Enhanced Capacity for Chondrogenic Differentiation. Stem Cells. 2017;35(11):2280–91. 10.1002/stem.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. DeChiara TM, Kimble RB, Poueymirou WT, et al. : Ror2, encoding a receptor-like tyrosine kinase, is required for cartilage and growth plate development. Nat Genet. 2000;24(3):271–4. 10.1038/73488 [DOI] [PubMed] [Google Scholar]
- 44. Topol L, Jiang X, Choi H, et al. : Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162(5):899–908. 10.1083/jcb.200303158 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Stüdle C, Occhetta P, Geier F, et al. : Challenges Toward the Identification of Predictive Markers for Human Mesenchymal Stromal Cells Chondrogenic Potential. Stem Cells Transl Med. 2019;8(2):194–204. 10.1002/sctm.18-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Tsuji K, Ojima M, Otabe K, et al. : Effects of Different Cell-Detaching Methods on the Viability and Cell Surface Antigen Expression of Synovial Mesenchymal Stem Cells. Cell Transplant. 2017;26(6):1089–102. 10.3727/096368917X694831 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Chan LY, Yim EK, Choo AB: Normalized median fluorescence: an alternative flow cytometry analysis method for tracking human embryonic stem cell states during differentiation. Tissue Eng Part C Methods. 2013;19(2):156–65. 10.1089/ten.TEC.2012.0150 [DOI] [PubMed] [Google Scholar]