Abstract
Patient: Male, 57-year-old
Final Diagnosis: Leptomeningeal carcinomatosis
Symptoms: Headache • visual acuity loss
Medication: —
Clinical Procedure: Venous sinus stenting
Specialty: Oncology
Objective:
Unusual or unexpected effect of treatment
Background:
Cerebral venous sinus obstruction associated with leptomeningeal carcinomatosis is an extremely rare complication of advanced non-small-cell lung cancer. There is little information available on the efficacy of therapeutic options because of its rarity and extremely poor prognosis.
Case Report:
A 57-year-old man presented with severe headache, vomiting, and visual loss for 1 month. Head magnetic resonance venography (MRV) showed occlusion of the left transverse sinus. Gd-enhanced MRI showed no abnormal enhancement. Lumbar puncture intracranial pressure was higher than 40 cmH2O. Positive cerebrospinal fluid tumor cytology confirmed the diagnosis of leptomeningeal carcinomatosis (LC). The headache was relieved by repeated lumbar punctures, and ventriculo-peritoneal shunt was performed. Cerebral angiography showed severe stenosis of the left transverse sinus without thrombosis, and significant delay of cerebral circulation. The transverse sinus stenosis was judged to be contributing to raised intracranial pressure, and the patient underwent left transverse sinus stent placement. After the procedure, his visual acuity improved, the visual field was enlarged, and his headache could be controlled by medication. Follow-up Gd-enhanced MRI showed dural enhancement and spinal dissemination. Because molecular biology of the surgical specimen showed epidermal growth factor receptor (EGFR)-activating mutations, he was treated with osimertinib for 2 months. He survived for 8 months following the diagnosis of LC and left transverse sinus stenosis.
Conclusions:
Venous sinus stenting can offer an effective palliative interventional option for symptom relief of severe headache and visual symptoms, even in the end stage of malignancy.
MeSH Keywords: Cranial Sinuses, Lung Neoplasms, Meningeal Carcinomatosis
Bachground
Venous sinus stenting has been accepted as the established treatment for idiopathic intracranial hypertension [1,2]. Characteristic symptoms of intracranial hypertension are intractable headaches, papilledema, and visual symptoms. The incidence of idiopathic intracranial hypertension is reported to be roughly 1–2 per million population. However, use of venous sinus stenting for cerebral sinus stenosis associated with LC has not been well-studied because of the extreme rarity and dismal prognosis of LC [3,4]. The reported median survival of patients with LC in historical data was only 1–3 months. Here, we report a case of venous sinus stenting for transverse sinus stenosis associated with LC in a patient with lung cancer who presented with severe headache and visual symptoms. His symptoms were remarkably relieved after stenting and he survived for 8 months after the diagnosis of LC.
Case Report
Institutional Review Board approval was obtained for this case report. A 57-year-old man who had received chemotherapy using cisplatin and vinorelbine for lung adenocarcinoma in the previous year presented with severe headache and vomiting. He subsequently became almost blind bilaterally 3 months after onset.
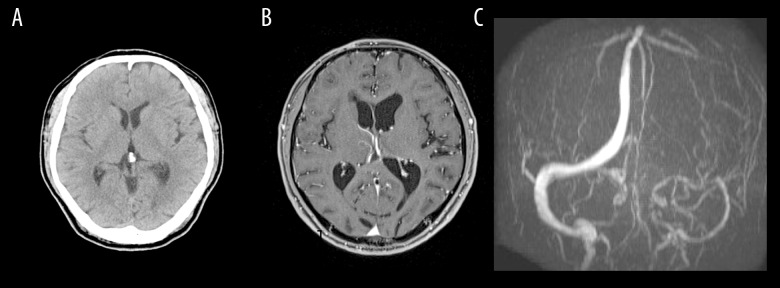
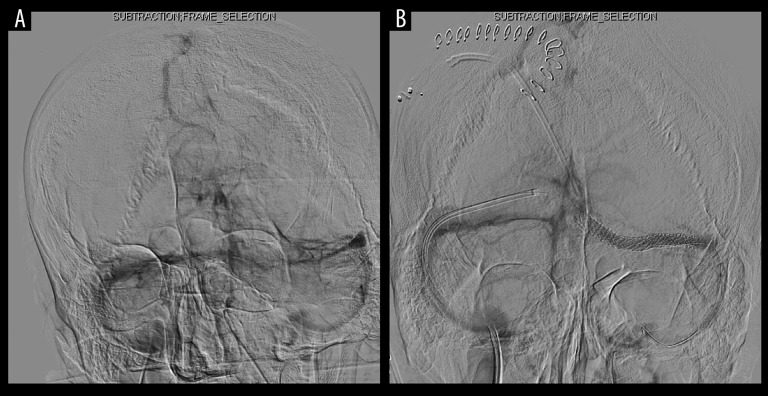
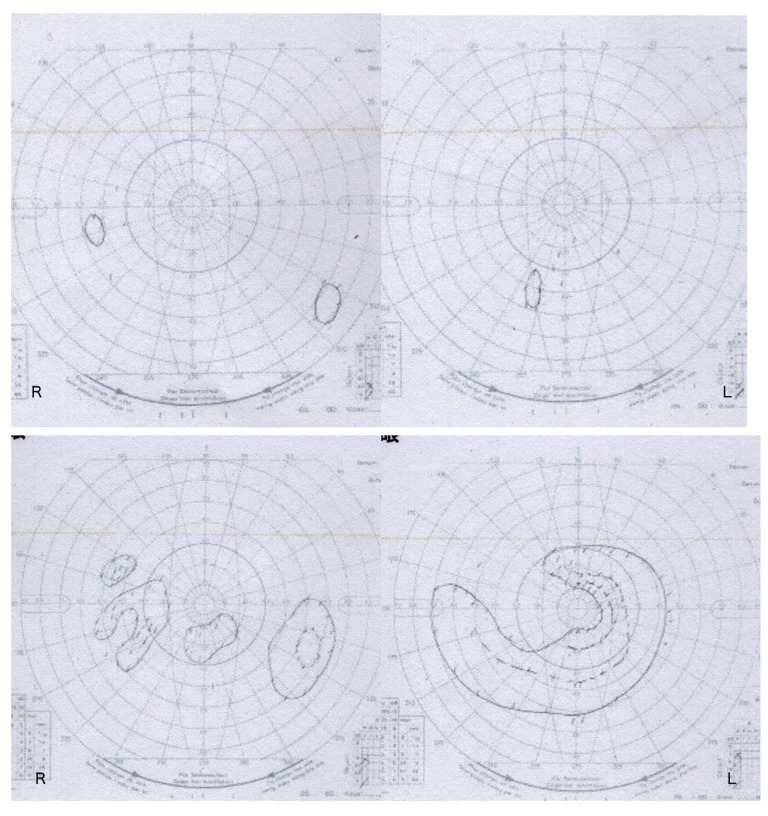
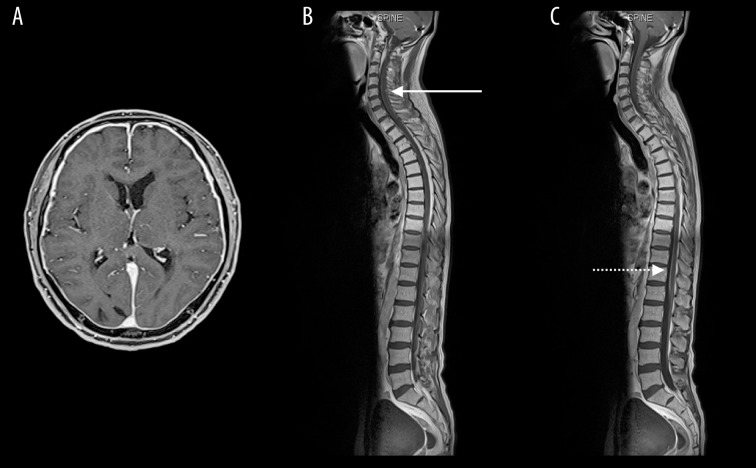
Head CT revealed no significant findings including hydrocephalus, but head MRV showed occlusion of the left transverse sinus (Figure 1). Gd-enhanced MRI showed no abnormal enhancement. Hematological, coagulation, biochemical, and serological findings were normal. Lumbar puncture intracranial pressure was higher than 40 cmH2O. Cerebrospinal fluid cell count, protein, and glucose were 28 cells/μL, 36.1 mg/dL, and 42 mg/dL, respectively. LC was highly suspected by positive cerebrospinal fluid tumor cytology. Medication was not effective in treating the headache, but it was relieved by repeated lumbar punctures. Thus, intracranial hypertension due to left transverse sinus occlusion associated with LC was diagnosed, and we prescribed 250 mg of acetazolamide/day to relieve it. However, he suddenly lost consciousness when the diagnostic catheter angiography was planned. His consciousness was returned to normal by lumbar puncture, and a ventriculo-peritoneal shunt using a programmable valve system of CERTAS® (Codman, Raynham, MA) was performed urgently on the next day. Cerebral angiography showed severe stenosis of the left transverse sinus and delay of cerebral circulation (Figure 2A), as well as significant delay of cerebral circulation. The transverse sinus stenosis was judged to be contributing to raised intracranial pressure, and he underwent left transverse sinus stent placement using Palmaz® Genesis (Cordis, Miami, FL) 1 week after the shunt was placed (Figure 2B). Dual antiplatelet drugs using acetylsalicylic acid 100 mg and clopidogrel 75 mg were administered for 4 days prior to the procedure, as described in the literature [1]. After the procedure, a flicker test showed that his visual acuity had improved, Goldmann perimeter testing showed that the visual field was enlarged (Figure 3), and his headache was controlled by medication. However, a follow-up Gd-enhanced MRI showed dural enhancement and spinal dissemination (Figure 4). Because molecular biology of the surgical specimen obtained from lung cancer the year before showed epidermal growth factor receptor (EGFR)-activating mutations [5], he was treated with osimertinib for 2 months until best supportive care was chosen after progression of disease. The patient survived for 8 months following the diagnosis of LC and left transverse sinus stenosis.
Figure 1.
Head CT scan showed no parenchymal lesions and no hydrocephalus (A). Gd-enhanced MRI showed no enhanced lesions (B). Magnetic resonance venography showed near occlusion of the left transverse sinus (C).
Figure 2.
Cerebral angiography showed severe stenosis of the left transverse sinus (A). After stenting, stenotic lesions of the left transverse sinus were dilated (B).
Figure 3.
Preoperative Goldmann perimeter showed large visual field defects (upper). Postoperative Goldmann perimeter showed remarkable improvement of visual field defects (lower).
Figure 4.
Follow-up Gd-enhanced head MRI showed leptomeningeal enhancement (A). Gd-enhanced spine MRI showed dural enhancement of cervical (solid line) (B) and lumbar (dashed line) (C) spine.
Discussion
LC is caused by the spread of cancer cells to the leptomeninges and by their dissemination within the cerebrospinal fluid [4]; most arises from lung, breast cancer, and melanoma [6]. LC occurs in 3–5% of patients with advanced non-small-cell lung cancer [4]. Reported analysis of LC outcomes in non-small-cell lung cancer indicated a poor median survival of only 3 months and no difference in survival in patients who received whole-brain radiotherapy [7]. The most frequent symptom at onset is intracranial hypertension, and high lumbar puncture intracranial pressure was reported to occur in 57% of cases [3,8]. Because the sensitivity of non-contrast MRI scan is not high, cerebrospinal fluid cytological examination still remains the criterion standard for diagnosis of LC [4].
A retrospective study showed a higher incidence of LC in patients with EGFR mutations than in those with wild-type EGFR [9]. However, after substantial efforts in the characterization of genetic profiles and the development of novel agents with greater central nervous system permeability, promising advances have been made in the management of LC from NSCLC, especially in this subtype [4,10]. The prognosis of patients with LC has dramatically improved from a historical median survival of 1–3 months to 3–11 months [11–14]. Osimertinib is a third-generation EGFR tyrosine kinase inhibitor with impressive efficacy in controlling both systemic and central nervous system disease. Because it has a higher CNS/blood penetration ratio, it is being investigated as a therapy for LC [4,9].
Cerebral venous sinus obstruction is a very rare complication of LC. Direct pressure on the sinus venosus by the tumor (dural/cranial metastasis) and direct tumor extension into the sinus venosus were suggested to be the underlying mechanisms [15]. Few case reports have been published [15–18]. The reported incidence of venous sinus obstruction associated with LC in cancer patients who underwent neurological consultation was 0.02% [19]. However, it may be overlooked because of its non-specific clinical symptoms for LC patients, extremely poor prognosis of LC, and prior requirement for angiographic diagnosis. Thus, data on the efficacy of venous sinus stenting for cerebral venous sinus stenting are quite scarce.
Based on a review of the literature, this appears to be the first case report of venous sinus stenting for venous sinus stenosis associated with LC in the era of molecular targeted therapy [14]. Severe headache and visual symptoms associated with cerebral venous sinus stenosis caused reduced quality of life in our patient. His symptoms were remarkably relieved after venous sinus stenting, and he was discharged home. He died 8 months after diagnosis of LC, and venous sinus stenting offered an effective palliative interventional option for symptom relief of severe headache and visual symptoms, even in the end stage of malignancy.
Conclusions
Venous sinus stenting can offer an effective palliative interventional option for symptom relief of severe headache and visual symptoms, even in the end stage of malignancy with leptomeningeal carcinomatosis in the era of molecular targeted therapy.
Footnotes
Conflicts of interest
None.
References:
- 1.Fargen KM, Liu K, Garner RM, et al. Recommendations for the selection and treatment of patients with idiopathic intracranial hypertension for venous sinus stenting. J Neurointerv Surg. 2018;10:1203–8. doi: 10.1136/neurintsurg-2018-014042. [DOI] [PubMed] [Google Scholar]
- 2.Puffer RC, Mustafa W, Lanzino G. Venous sinus stenting for idiopathic intracranial hypertension: A review of the literature. J Neurointerv Surg. 2013;5:483–86. doi: 10.1136/neurintsurg-2012-010468. [DOI] [PubMed] [Google Scholar]
- 3.Gleissner B, Chamberlain MC. Neoplastic meningitis. Lancet Neurol. 2006;5:443–52. doi: 10.1016/S1474-4422(06)70443-4. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19:e43–55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 5.Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol. 2018;36:911–19. doi: 10.1200/JCO.2017.76.7293. [DOI] [PubMed] [Google Scholar]
- 6.Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology. 2010;74:1449–54. doi: 10.1212/WNL.0b013e3181dc1a69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: Survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–85. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 8.Chu F-N, Lang Y, Sun X-M, Cui L. Meningeal carcinomatosis: A retrospective analysis of seventy-seven cases. Neuroimmunol Neuroinflammation. 2017;4:1–5. [Google Scholar]
- 9.Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22:5130–40. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 10.Pakkala S, Ramalingam SS. Personalized therapy for lung cancer: Striking a moving target. JCI Insight. 2018;3(15):120858. doi: 10.1172/jci.insight.120858. pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Rhun E, Galanis E. Leptomeningeal metastases of solid cancer. Curr Opin Neurol. 2016;29:797–805. doi: 10.1097/WCO.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 12.Liao BC, Lee JH, Lin CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10:1754–61. doi: 10.1097/JTO.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 13.Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11:1962–69. doi: 10.1016/j.jtho.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–37. doi: 10.1016/j.ctrv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Oda N, Sakugawa M, Bessho A, et al. Cerebral venous sinus thrombosis concomitant with leptomeningeal carcinomatosis, in a patient with epidermal growth factor receptor-mutated lung cancer. Oncol Lett. 2014;8:2489–92. doi: 10.3892/ol.2014.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed RM, Halmagyi GM. Malignant meningitis presenting as pseudotumor cerebri. J Neurol Sci. 2013;329:62–65. doi: 10.1016/j.jns.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Brown MT, Friedman HS, Oakes WJ, et al. Sagittal sinus thrombosis and leptomeningeal medulloblastoma. Neurology. 1991;41:455–56. doi: 10.1212/wnl.41.3.455. [DOI] [PubMed] [Google Scholar]
- 18.Li HK, Harding V, Williamson R, et al. Cerebral sinus thrombosis and leptomeningeal carcinomatosis in a patient with ovarian cancer. J Clin Oncol. 2012;30:e19–20. doi: 10.1200/JCO.2011.38.1426. [DOI] [PubMed] [Google Scholar]
- 19.Raizer JJ, DeAngelis LM. Cerebral sinus thrombosis diagnosed by MRI and MR venography in cancer patients. Neurology. 2000;54:1222–26. doi: 10.1212/wnl.54.6.1222. [DOI] [PubMed] [Google Scholar]