Abstract
The aim of the study was to examine the influence of single and consortia treatments of drought tolerant rhizobacteria producing ACC deaminase together with additional plant growth promoting (PGP) characteristics on finger millet growth, antioxidant and nutrient concentration under water-stressed and irrigated (no stress) conditions. These rhizobacteria belong to the Variovorax sp. Achromobacter spp. Pseudomonas spp. and Ochrobactrum sp. The single inoculant of RAA3 (Variovorax paradoxus) and a consortium inoculant of four bacteria, i.e., DPC9 (Ochrobactrum anthropi), DPB13 (Pseudomonas palleroniana), DPB15 (Pseudomonas fluorescens) and DPB16 (Pseudomonas palleroniana), significantly boosted the overall growth parameters and nutrient concentrations in leaves of finger millet. Moreover, elevated levels of the reactive oxygen species scavenging enzymes–superoxide dismutase (17.3%, 11.6%), guaiacol peroxidase (38.7%, 22.2%), catalase (33.7%, 21.3%) and ascorbate peroxidase (18.2%, 10.0%); cellular osmolytes–proline (41.5%, 25.0%), phenol (44.5%, 37.5%); higher leaf chlorophyll (64.4%, 30.8%) and a reduced level of hydrogen peroxide (50.7%, 59.5%) and malondialdehyde (48.4%,72.5%) were noted, respectively, after single inoculation of RAA3 and a consortium treatment by strains DPC9 + DPB13 + DPB15 + DPB16, in contrast with non-treated plants mainly under water-stressed conditions. This finding clearly illustrates that PGPB that express ACC deaminase along with additional PGP traits could be an efficient approach for improving plant health in environments, where agricultural practices are reliant on rain for water.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-2046-4) contains supplementary material, which is available to authorized users.
Keywords: Finger millet, Drought stress, 1-Aminocyclopropane-1-carboxylate (ACC) deaminase, Sustainability, Tolerance
Introduction
In the twenty-first century, climate change is a major menace to the agricultural sustainability (Papworth et al. 2015) and immensely influences the worldwide reduction in crop productivity (Ali et al. 2017; Saikia et al. 2018; Danish and Zafar-ul-Hye 2019). For a growing world population the food security relies on the continuing yield increases of key cereal crops; however, the annual crop production is not maintaining pace with the expected future need for food (Grassini et al. 2013). Drought is the major reason of preventive crop production over vast regions of the world. In fact, the vagaries in precipitation patterns in drought affected areas are anticipated to increase in the coming years due to climate change, posing adverse impacts on productivity and wide-ranging losses to agriculture production (Zhu 2016; Ghorchiani et al. 2018; Saleem et al. 2018). Agriculture production mainly depends on rainfall during the farming season, so droughts can be potentially ruinous and can have erratic influences on the productivity of crops (Zhou et al. 2018). Approximately 90% of the world’s agricultural lands are beset with a surfeit of abiotic and biotic stresses. Furthermore, drought is the prevalent abiotic stresses that cause a significant reduction of biological functioning in plants (Wang et al. 2005; Delshadi et al. 2017; Pandey and Gupta 2019; Shirinbayan et al. 2019).
Drought tolerance is a multifaceted phenomenon comprising collections of gene networks. At present, various strategies are being utilized by the researchers to increase the ability of crops to endure drought stress, unluckily, these techniques are highly technical and labour-intensive, and are often hard to use in practice (Gepstein and Glick 2013; Niu et al. 2018). Therefore, another eco-friendly approach may need to be employed at this time. One such scheme could be the application of stress-tolerant rhizobacteria producing ACC deaminase play important roles in improving plant growth and productivity particularly under drought conditions (Forni et al. 2017). Bacteria producing ACC deaminase can diminish many of the negative consequences of ethylene stress imposed upon plants and improve plant growth characteristics by regulating ethylene synthesis (Glick et al. 1998; Glick 2014). A number of studies demonstrated the inoculation of ACC deaminase positive bacterial strains in improving plant fitness during droughts (Mayak et al. 2004; Sarma and Saikia 2014; Chandra et al. 2018a, b, 2019a, b), flooding stress (Grichko and Glick 2001; Farwell et al. 2007), excessive salinity (Kausar and Shahzad 2006; Nadeem et al. 2007), heavy metal stress (Stearns et al. 2005), etc.
Rhizobacteria can significantly ease plant growth inhibition of many agriculture crops including cereals (Santoyo et al. 2016; Numan et al. 2018). Changes in root characteristics are the important traits conferring adaptation in plants during drought stress. However, the application of rhizobacteria alters the root morphology and helps in the acquisition of nutrients and water (Vacheron et al. 2013). Cellular osmotic adjustment by elevated concentration of cellular osmotica (e.g., proline) is an additional significant adaptation in plants during drought. PGPB treatments lead to a boost in plant cellular osmolytes and supports plants during periods of stress (Kohler et al. 2008; Saravanakumar et al. 2011). The accumulation of free radical leads to injury of cell membranes and other cellular machinery in severely drought-stressed plants (Munns 2002). Antioxidant enzymes have the capacity to eliminate free radicals and prevent cell membranes and DNA content from further damage (Chandra et al. 2018b; Saikia et al. 2018). Certain PGPB (e.g., Paenibacillus spp. and Pseudomonas spp.) can raise the antioxidant potential in plants such as common bean, green gram, lettuce and sunflower which contributes to enhancing the tolerance to drought (Figueiredo et al. 2008; Kohler et al. 2008; Sandhya et al. 2009; Sarma and Saikia 2014). The application of PGPB, an approach to raise plant fitness, was selected as it is an inexpensive and relatively simple technique that is well suited to use in developing countries. Therefore, the objectives of the work presented herein were to determine the influence of single and consortia inoculation of PGPB producing ACC deaminase together with other PGP characteristics on (i) growth, (ii) antioxidant potential, and (iii) nutrient concentration (N, P, K, Ca and Na) of finger millet plants under water-stressed and irrigated conditions in two glasshouse experiments. The probable intrinsic mechanisms of drought tolerance were examined by quantifying the reactive oxygen species (ROS) scavenging enzymes and osmolytes in the bacterial single and consortium-treated plants in both the experiments.
Materials and methods
Bacterial strain
In present study, 8 bacterial strains were taken that were previously isolated from rainfed agriculture field of Central Himalaya (Kumaun region), Uttarakhand, India and screened for the production of ACC deaminase together with other PGP characteristics such as phosphate solubilization, indole 3-acetic acid (IAA) production, N2 fixation, ammonia production and siderophore production are given in SUPPLEMENTARY TABLE SI. In addition, two strains, Pseudomonas synxantha R81 (Mathimaran et al. 2012) and Pseudomonas sp. UW4 (Duan et al. 2013) were taken as reference strains for the study. These 10 bacterial strains were investigated for their drought tolerance in nutrient broth medium supplemented with PEG 8000 is given in SUPPLEMENTARY FIG. SI.
Experiment I: influence of rhizobacteria producing ACC deaminase on finger millet plant growth under irrigated and water-stressed conditions
Soil source and properties of potting mixture
The potting mixture sand and soil (3:1) used in the pot experiment was taken from the river bed of Golapar, Haldwani and GBPUA&T Pantnagar, Uttarakhand India, respectively, and determined to have the following physicochemical properties: pH 6.98, organic carbon (7.40 g kg−1), total N (1.90 g kg−1), available P (11.0 mg/kg) and extractable K (139 mg/kg). Soil physicochemical characteristics were analyzed using established methods, i.e., organic carbon (Walkley and Black 1934), available soil P (Olsen 1954), K content by Flame photometry and available N by Kjeldahl digestion.
Plant material
For the growth promoting experiment, the seeds of finger millet (var. VL-149) were procured from VPKAS, Almora, Uttarakhand, India and they are sterilized by gently shaking them in 3% (v/v) NaClO for 3 min followed by 70% ethanol for 1 min and finally with rinsed with sterile deionized water (5–6 times) and pre-germinated on sterile filter paper in Petri plates. After germination, the uniform sizes of seedlings were used for pot experiments.
Inoculation method
The selected bacterial inoculants were grown in 50 ml of 2.5% nutrient broth (Himedia) and incubated in a shaker incubator at 200 rpm and 28 °C. The bacterial culture was standardized to 107–108 cfu/ml, 0.6 of OD600. For experimental set up, seeds were treated with 1 ml bacterial suspension of their respective treatments before planting. Equal amounts of nutrient broth were applied to control plants.
Experimental details
The plants were grown for 40 days in pots (10 cm length and 10 cm internal diameter) containing a 3:1 mixture of sterile soil and sand under glasshouse conditions at a temperature of 28 ± 2 °C, 60% relative humidity, a photoperiod of 16/8 h day/night cycle and a light intensity 400 Em−2 s−1 (400–700 nm). A completely randomized design was used and the experiments were performed with four replicates. In Experiment I and II, a total of 11 and 8 treatments were taken, respectively, and are enumerated in Table 1. The moisture level of potting mixture was retained at 90% of WHC (i.e., 23.50% w/w) during the experiments. The layout of the experiments as-two sets (one for irrigated and another for water-stressed) of pot trials were set up for both the experiments. After 5 weeks of plant growth, one set of pot trials was exposed to water stress by stopping water for 5 days, while alternative set of pots continued to be watered. After 5 days of water-stress (referred to here as drought), the moisture level in the pots was 35% of WHC. The following morphological parameters were noted for both irrigated and water-stressed conditions after 40 days of plant growth, i.e., shoot/root length, root/shoot dry weight and root/shoot (R/S) ratio.
Table 1.
List of bacterial treatments used in the Experiments I and II
| Experiment I | Experiment II | ||
|---|---|---|---|
| Designation | Bacterial isolates/strains | Designation | Combination of bacterial strains |
| Control | Uninoculated | ||
| RAA3 | Variovorax paradoxus | T1 (control) | Uninoculated |
| DPC12 | Pseudomonas sp. | T2 (DPC9 + DPC12) | O. anthropi DPC9 + Pseudomonas sp. DPC12 |
| DPB16 | Pseudomonas palleroniana | T3 (DPB13 + PSA7) | P. palleroniana DPB13 + A. marplatensis PSA7 |
| DPB15 | Pseudomonas fluorescens | T4 (DPB15 + PSB8) | P. fluorescens DPB15 + A. sp. PSB8 |
| PSA7 | Achromobacter marplatensis | T5 (DPB16 + RAA3) | P. palleroniana DPB16 + V. paradoxus RAA3 |
| PSB8 | Achromobacter sp. | T6 (R81 + UW4) | P. synxantha R81 + P. sp. UW4 |
| R81 | Pseudomonas synxantha | T7 (DPC9 + DPB13 + DPB15 + DPB16) | O. anthropi DPC9 + P. palleroniana DPB13 + P. fluorescens DPB15 + P. palleroniana DPB16 |
| DPB13 | Pseudomonas palleroniana | T8 (DPC12 + PSA7 + PSB8 + RAA3) | Pseudomonas sp. DPC12 + A. marplatensis PSA7 + A. sp. PSB8 + V. paradoxus RAA3 |
| UW4 | Pseudomonas sp. | ||
| DPC9 | Ochrobactrum anthropi | ||
Harvesting and plant sample analysis
At the time of harvesting, plants were dug out from the pots and the roots were washed with tap water. For enzymatic analysis the subsamples of finger millet leaves from each treatment of both experiments were stored at − 80 °C. The morphological parameters were noted after initial air drying and samples of root and shoot dried at 65 °C in an electric oven until sample weight remained constant. The dry mass of roots and shoots was then measured.
Experiment II: influence of bacterial consortium producing ACC deaminase on finger millet plant growth under irrigated and water-stressed conditions
Cross-streak test
The selected bacterial strains (10) were assessed for their compatibility among each other on nutrient agar medium in such a way that actively grown culture of one bacterium (100 µl) was spread on the nutrient agar plate and a sterilized paper disc was dipped into actively grown culture of a second bacterium placed on the nutrient agar above the spread bacteria. The plates were incubated at 28 °C for 72 h and observed for the inhibition zone. The lack of inhibition zone shows the compatibility with corresponding bacterial strains and the presence of inhibition zone shown the incompatibility. All the bacterial strains used in the study were found to be compatible with each other. On the basis of compatibility total seven combinations were made (Table 1) and evaluated for their growth promoting effect on finger millet.
Seed bacterization, pot assay and sample analyses
Similar to glass house experiment I, the method of seed treatment, pot trial setup and analyses were employed for the experiment II. The germinated seeds were treated with 1 ml suspensions of bacterial strains and the strains were mixed in equal ratio to attain the desired absorbance and for the control treatment 2.5% sterile nutrient broth was used (Himedia).
Methods used for plant biochemical analysis (experiments I and II)
The biochemical analysis of finger millet leaves was quantified by standard procedures as follows: Chlorophyll (Arnon 1949), total proline (Bates et al. 1973), lipid peroxidation (Heath and Packer 1968), H2O2 (Alexieva et al. 2001), total phenol (Zieslin and Ben-Zaken 1993), guaiacol peroxidase (GPX) (Urbanek et al. 1991), ascorbate peroxidase (APX) (Nakano and Asada 1981), superoxide dismutase (SOD) (Beauchamp and Fridovich 1971) and catalase (CAT) (Beers and Sizer 1952).
Nutrient concentration analysis
The dried leaf samples of finger millet plants were used for N, P, K, Ca and Na estimation. The P content of the leaf sample was assessed according to methods described by Jackson (1973). The estimation K+, Ca2+ and Na+ concentrations was done with the help of Flame photometry and the N content was measured by Kjeldahl digestion.
Statistical analysis
The data were subjected to ANOVA (IBM SPSS statistics 20) and tested for significance using DMRT with a significant level p < 0.05.
Results
Experiment I: influence of rhizobacteria producing ACC deaminase on finger millet plant growth under irrigated and water-stressed conditions
Plant growth characteristics
The application of bacterial inoculants had a noteworthy positive effect on the growth parameters of finger millet (i.e., shoot length, root elongation, root and shoot dry weight) as compared to untreated seedlings both under water-stressed and irrigated conditions (Table 2). The treatment RAA3 stimulated the maximum increase in shoot length (27.8%), followed by strains DPC12 (25.2%), and R81 (23.3%) over untreated water-stressed plants. Likewise, under irrigated conditions, strain RAA3, DPC12 and R81 facilitated the maximum enhancement of shoot length 31.4%, 29.3% and 25.5%, respectively, over controls. Under water-stressed conditions, a maximum of 26.8% increase of root length was noted with RAA3-treated plants followed by DPC12 (26.5%) and DPB13 (24.6%), respectively, as compared to non-treated controls, whereas under irrigated conditions the RAA3 treated plants showed a maximum 40.0% increase in root length followed by DPC12 (33.9%) and R81 (33.7%) as compared to the irrigated control plants. Moreover, it has also been noticed that all the treatments shown a substantial impact on the shoot dry weight which ranged from 1.30 to 2.80-fold and root dry weight which ranged from 1.38 to 2.79-fold under both water-stressed and irrigated conditions as compared to their respective controls. The impact of bacterial inoculants on R/S ratio were found non-significant both under water-stressed and irrigated conditions.
Table 2.
Effect of PGPB inoculation containing ACC deaminase activity on growth attributes of finger millet plants under irrigated and drought conditions (Experiment I)
| Treatments | (I) Shoot length (cm) | (II) Root length (cm) | (III) Shoot dry weight (g) | (IV) Root dry weight (g) | (V) R/S ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | |
| Control | 38.11a | 36.73A | 13.70a | 13.50A | 0.14a | 0.09A | 0.13a | 0.13A | 0.96a | 1.64B |
| RAA3 | 55.54c | 50.88D | 22.83d | 18.44C | 0.39e | 0.24D | 0.36e | 0.26C | 0.93a | 1.09A |
| DPC12 | 53.93c | 49.13D | 20.72c | 18.38C | 0.34d | 0.23D | 0.31d | 0.24C | 0.91a | 1.09A |
| DPB16 | 50.43bc | 46.69C | 19.88bc | 16.75BC | 0.33d | 0.18C | 0.28cd | 0.19B | 0.87a | 1.09A |
| DPB15 | 49.06bc | 45.09BC | 20.69c | 16.55BC | 0.30cd | 0.17BC | 0.24bc | 0.20B | 0.79a | 1.26AB |
| PSA7 | 47.80bc | 44.5BC | 18.85bc | 17.38BC | 0.23bc | 0.15BC | 0.22bc | 0.18B | 0.95a | 1.25AB |
| PSB8 | 45.78b | 44.25B | 18.75bc | 16.13B | 0.21b | 0.14B | 0.22bc | 0.18B | 1.03a | 1.38AB |
| R81 | 51.18c | 47.88CD | 20.65c | 17.25BC | 0.32d | 0.17BC | 0.28cd | 0.20B | 0.91a | 1.20AB |
| DPB13 | 46.65bc | 44.31BC | 19.50bc | 17.91C | 0.27c | 0.16BC | 0.26c | 0.19B | 0.99a | 1.33AB |
| UW4 | 48.51bc | 44.84BC | 19.94bc | 16.88BC | 0.29cd | 0.11AB | 0.25c | 0.19B | 0.89a | 1.75B |
| DPC9 | 47.33bc | 46.50C | 18.43b | 17.75BC | 0.25bc | 0.15BC | 0.21bc | 0.17B | 0.86a | 1.14AB |
(I) shoot length, (II) root length, (III) shoot dry weight, (IV) root dry weight and (V) R/S ratio of root to shoot dry weight. Values are SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
The results of two factor analysis was revealed that the water-stress condition significantly decreased the total chlorophyll content by 35.2%, shoot dry weight by 42.1%, shoot length by 6.3% and root dry weight by 22.5% as compared to the no-stress conditions, while irrespective of the watering conditions, the bacterial inoculants RAA3 significantly increased the total chlorophyll content by 60.7%, shoot dry weight by 65.2%, shoot length by 29.7%, and root dry weight by 58.2% as compared to non-treated control plants. In addition, a significant interaction effect of bacterial treatments and watering conditions was found for shoot/root dry weight, R/S ratio and total chlorophyll given in SUPPLEMENTARY TABLE SII.
Biochemical responses of finger millet to individual inoculants
Plants that suffered from water-stress condition had significantly lower chlorophyll contents (1.82-fold) as compared to the non-treated irrigated control plants (Table 3). However, the various inoculants caused a noteworthy increase in total chlorophyll (1.31–2.81-fold) content under water-stressed conditions, as compared to non-inoculated plants. Under irrigated conditions, the bacterial treatments increased the total chlorophyll content (1.47–2.41-fold) as compared to non-treated control. Plants suffering from water stress exhibited a 1.30-fold increase of proline content in leaf tissues of non-treated plants and up to 1.80-fold for treated plants. There was no marked change between non-treated and treated plants for the proline content in irrigated conditions (Fig. 1I). In addition, bacterial treatment had a significant effect on total phenolic content (3.2–44.5%) under water-stressed compared to irrigated conditions (7.1–29.1%) over respective non-treated plants (Table 3).
Table 3.
Effect of PGPB inoculation containing ACC deaminase activity on total chlorophyll, total phenol, malondialdehyde (MDA), Catalase (CAT) and ascorbate peroxidase (APX) content of finger millet under irrigated and drought conditions (Experiment I)
| Treatments | Total chlorophyll (mg/g Fresh weight) | Total phenol (μg/mg) GAE | MDA (µg/g Fresh weight) | CAT (µmol/min/mg protein) | APX (nmol/min/mg protein) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | |
| Control | 0.85a | 0.47A | 28.13a | 46.33A | 15.48b | 18.49C | 115.46a | 135.44A | 739.55ab | 1245.73A |
| RAA3 | 2.05d | 1.31C | 39.68b | 83.50E | 10.90a | 12.46A | 122.92ab | 204.38C | 746.15b | 1523.09C |
| DPC12 | 1.47bc | 1.03BC | 31.69ab | 68.22CD | 13.05ab | 15.05B | 127.59ab | 199.10C | 749.83b | 1547.89C |
| DPB16 | 1.65c | 1.19C | 37.63b | 74.68D | 11.04a | 13.76AB | 126.27ab | 220.66D | 735.27ab | 1729.83E |
| DPB15 | 1.27b | 0.88B | 31.44a | 66.93C | 11.61ab | 15.20B | 135.88b | 188.96BC | 744.10b | 1734.75E |
| PSA7 | 1.29b | 0.61AB | 31.15a | 56.97B | 13.48ab | 15.60B | 129.88ab | 180.46BC | 734.95ab | 1581.37CD |
| PSB8 | 1.25b | 0.72AB | 30.27a | 47.88A | 13.91b | 15.20B | 122.44ab | 179.07B | 721.88a | 1437.81B |
| R81 | 1.35b | 0.94BC | 38.56b | 72.07CD | 12.33ab | 13.68AB | 138.05b | 176.22B | 757.74 b | 1699.11DE |
| DPB13 | 1.31b | 0.76B | 36.97b | 68.22CD | 11.76ab | 14.62B | 130.25b | 193.51C | 730.29ab | 1673.51DE |
| UW4 | 1.37b | 0.94BC | 31.81ab | 63.38BC | 12.90ab | 14.91B | 129.15ab | 193.80C | 746.95b | 1642.65D |
| DPC9 | 1.36b | 1.01BC | 32.66ab | 63.69BC | 13.19ab | 15.44B | 122.04ab | 188.60BC | 749.51b | 1650.28D |
Values are SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
GAE gallic acid equivalent
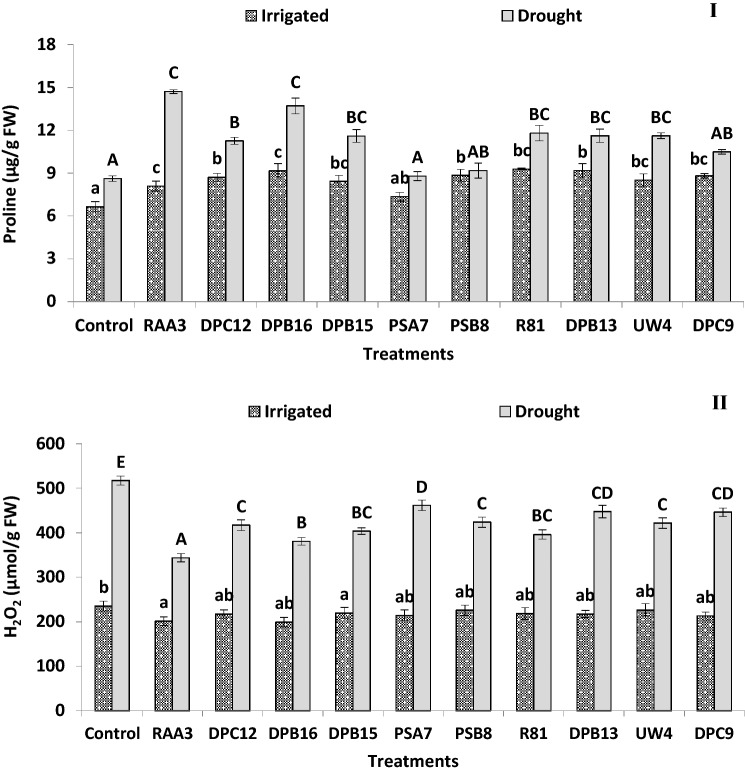
Fig. 1.
Effect of irrigated and drought conditions on (I) proline and (II) hydrogen peroxide (H2O2) content of PGPB producing ACC deaminase inoculated finger millet plants (Experiment I). Error bars indicate SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
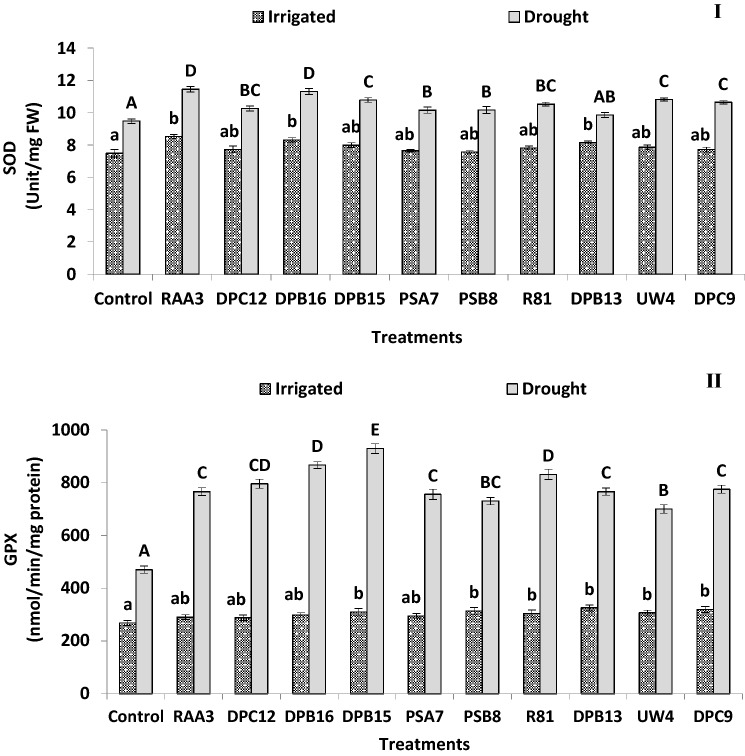
Bacterially treated plants exhibited lower H2O2 content (1.12–1.51-fold) under water-stressed conditions compared to non-treated control plants. However, there was no obvious difference between bacterially treated and non-treated plants regarding their H2O2 content (Fig. 1II) in irrigated conditions. Under water-stressed conditions, application of strain RAA3, R81 and DPB16 diminished the MDA levels by 48.4%, 35.2% and 34.3%, respectively, over control plants, suggesting that these strains have shown a vital interplay in reduction of oxidative damage of lipids under water-stressed conditions (Table 3). However, under irrigated conditions, the MDA content and the activity of ROS scavenging enzymes was not different between non-treated and bacterially treated plants, while there were significant increases in the activity of SOD (1.04–1.21-fold) (Fig. 2I), GPX (1.49–1.98-fold) (Fig. 2 II), and CAT (1.30–1.63-fold), and APX (1.15–1.39-fold) in inoculated plants in the presence of water-stress compared to untreated controls (Table 3).
Fig. 2.
Effect of irrigated and drought conditions on (I) superoxide dismutase (SOD) and (II) guiacol peroxidase (GPX) content of PGPB producing ACC deaminase inoculated finger millet plants (Experiment I). Error bars indicate SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
Nutrient concentration of finger millet
It has been found that drought-stressed condition significantly decreased the nutrient concentrations of finger millet plants. However, rhizobacteria possessing ACC deaminase increases the N (6.2–35.7%), P (18.8–38.0%), K+ (9.7–24.0%), Ca2+ (9.9–22.8%) and Na+ (9.0–36.3%) contents of finger millet under water-stressed conditions. In addition, under irrigated conditions, the bacterial treatments significantly increased the N (17.7–42.1%), P (15.9–36.2%), K+ (7.6–19.7%), Ca2+ (7.8–36.5%) and Na+ (7.6–27.5%) content as compared to non-treated control plants (Fig. 3).
Fig. 3.
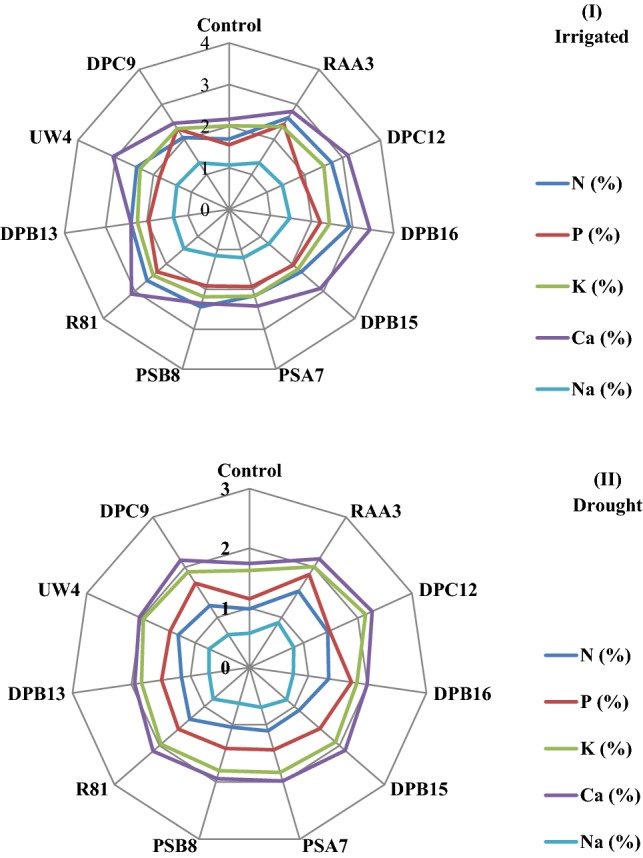
Effect of (I) irrigated and (II) drought conditions on foliar nutrient contents of PGPB producing ACC deaminase inoculated finger millet plants (Experiment I)
Experiment II: influence of bacterial consortium producing ACC deaminase on finger millet plant growth under irrigated and water-stressed conditions
Plant growth characteristics
Inoculation of finger millet plant with consortia of rhizobacteria producing ACC deaminase had a significant effect on shoot/root dry weight, root elongation, shoot length and R/S ratio (Table 4). The consortia of four (T7) and two (T5) inoculants exhibited a maximum enhancement in shoot length of at least 21.9% and 21.4%, respectively, as compared to non-treated water-stressed plants. The consortia T5 and T8 treated plants also exhibited a greater root length in both under water-stressed and irrigated conditions. Moreover, it has also been noticed that the consortia-treated plants revealed a substantial impact from 23.4 to 73.9% on the shoot and root dry weight in both water-stressed and no-stress conditions as compared to their non-treated plants. However, the impact of bacterial consortia on root development was greater with a noticeable upsurge in the R/S ratio under water-stressed conditions, whereas under irrigated conditions no substantial difference was observed among the bacterially treated and non-treated plants.
Table 4.
Effect of PGPB consortium inoculation containing ACC deaminase on growth attributes of finger millet plants under irrigated and drought conditions (Experiment II)
| Treatments | (I) Shoot length (cm) | (II) Root length (cm) | (III) Shoot dry weight (g) | (IV) Root dry weight (g) | (V) R/S ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | |
| T1 (control) | 37.00a | 35.68A | 12.07a | 10.17A | 0.16a | 0.13A | 0.16a | 0.05A | 0.97b | 0.43A |
| T2 | 45.93b | 43.25BC | 16.84b | 14.33BC | 0.31bc | 0.25C | 0.24bc | 0.15B | 0.78ab | 0.61B |
| T3 | 46.73bc | 44.68BC | 16.63b | 13.42BC | 0.27b | 0.21B | 0.21b | 0.15B | 0.77ab | 0.71B |
| T4 | 46.33b | 41.15B | 15.88b | 12.96B | 0.32bc | 0.23BC | 0.26c | 0.14B | 0.82ab | 0.62B |
| T5 | 48.53bc | 45.42C | 19.13bc | 15.67C | 0.34c | 0.27CD | 0.27c | 0.19C | 0.79ab | 0.73B |
| T6 | 47.40bc | 41.67B | 17.17b | 13.67BC | 0.35c | 0.28CD | 0.29cd | 0.15B | 0.84b | 0.56AB |
| T7 | 52.53c | 45.67C | 19.38bc | 14.75BC | 0.44d | 0.30D | 0.32d | 0.16BC | 0.73ab | 0.55AB |
| T8 | 50.73c | 43.83BC | 21.23c | 15.92C | 0.36c | 0.29CD | 0.33d | 0.21C | 0.91b | 0.74B |
(I) shoot length, (II) root length, (III) shoot dry weight, (IV) root dry weight and (V) R/S ratio of root to shoot dry weight. Values are SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
Likewise experiment I, a two way ANOVA was executed for some of the morphological parameters and it was found that the water-stress condition significantly decreased the shoot dry weight by 24.0%, root dry weight by 41.1%, shoot length by 9.0% and total chlorophyll content by 33.8% as compared to the irrigated condition. On contrary, T7 treated plants irrespective of watering conditions significantly increased the shoot dry weight by 59.4% and shoot length by 26.0%, whereas treatment T8 significantly increased the total chlorophyll content by 41.1% and root dry weight by 59.1% as compared to non-treated plants. It was also observed that there was no substantial difference in the R/S ratio of two watering conditions, whereas many of the treatments exerted a significant effect on the R/S ratio as compared to non-inoculated plants. A significant interaction effect of bacterial treatments and watering conditions was found for the shoot/root dry weight, R/S ratio and total chlorophyll content given in SUPPLEMENTARY TABLE SIII.
Biochemical responses of finger millet to consortia inoculants
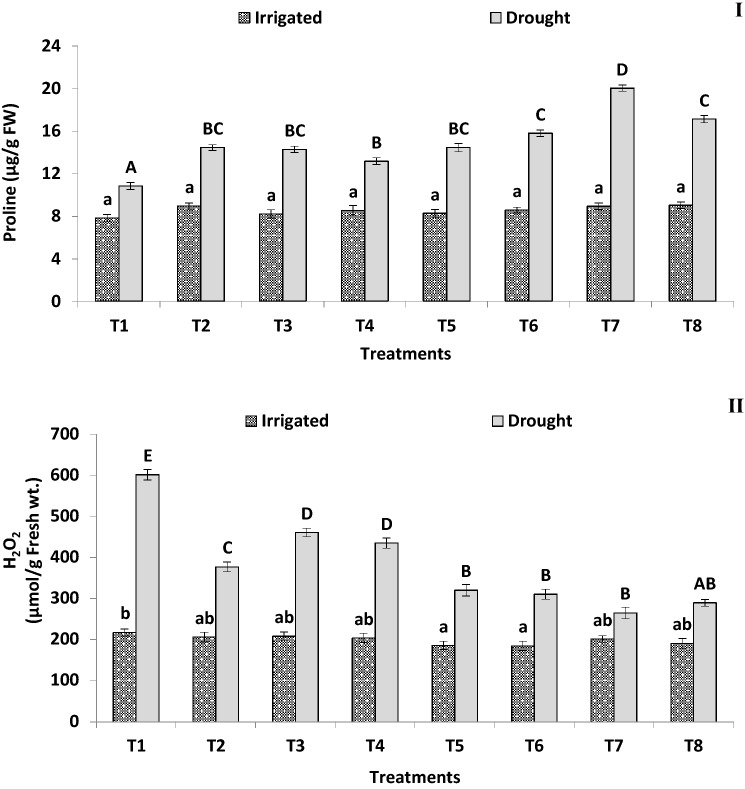
Water-stressed conditions negatively affect the leaf chlorophyll content in finger millet. However, plants treated with bacterial consortia producing ACC deaminase shown a substantial increase in chlorophyll content in both under irrigated (1.23–1.79-fold) and drought (1.32–1.65-fold) conditions, as compared to their corresponding non-treated control plants (Table 5). In addition, consortia inoculants revealed positive effects on several biochemical parameters under water-stressed conditions, whereas the effects under irrigated conditions were smaller. Under irrigated conditions, no significant difference was recorded between non-treated and bacterially treated plants for total proline and phenol contents, whereas an increase of 1.21–1.85-fold in leaf proline concentrations was noted in the consortia-treated plants (Fig. 4I) and 1.29–2.04-fold in total phenol content (Table 5) under water-stressed conditions.
Table 5.
Effect of PGPB consortium inoculation containing ACC deaminase on total chlorophyll, total phenol, malondialdehyde (MDA), catalase (CAT) and ascorbate peroxidase (APX) content of finger millet under irrigated and drought conditions (Experiment II)
| Treatments | Total chlorophyll (mg/g Fresh weight) | Total phenol (μg/mg) GAE | MDA (µg/g Fresh weight) | CAT (µmol/min/mg protein) | APX (nmol/min/mg protein) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | Irrigated | Drought | |
| T1 (control) | 1.03 | 0.74 | 31.74 | 52.15 | 17.20 | 22.51 | 81.21 | 90.19 | 473.37 | 819.36 |
| T2 | 1.62 | 1.06 | 34.05 | 83.37 | 15.63 | 13.05 | 82.06 | 114.61 | 485.19 | 910.30 |
| T3 | 1.57 | 0.99 | 35.83 | 67.20 | 14.62 | 12.19 | 84.85 | 104.95 | 505.96 | 866.39 |
| T4 | 1.27 | 0.97 | 35.17 | 77.41 | 16.20 | 14.05 | 87.87 | 122.84 | 489.31 | 945.90 |
| T5 | 1.73 | 1.02 | 37.58 | 84.93 | 16.77 | 12.04 | 87.61 | 124.17 | 517.41 | 986.07 |
| T6 | 1.65 | 1.09 | 37.87 | 88.07 | 15.34 | 12.23 | 90.95 | 128.82 | 505.30 | 997.74 |
| T7 | 1.77 | 1.22 | 35.49 | 106.64 | 14.91 | 9.00 | 89.58 | 139.00 | 503.63 | 1085.69 |
| T8 | 1.85 | 1.18 | 37.09 | 99.21 | 15.77 | 11.33 | 90.33 | 121.67 | 518.03 | 1027.34 |
Values are SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
GAE gallic acid equivalent
Fig. 4.
Effect of irrigated and drought conditions on (I) proline and (II) hydrogen peroxide (H2O2) content of PGPB consortium producing ACC deaminase inoculated finger millet plants (Experiment II). Error bars indicate SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
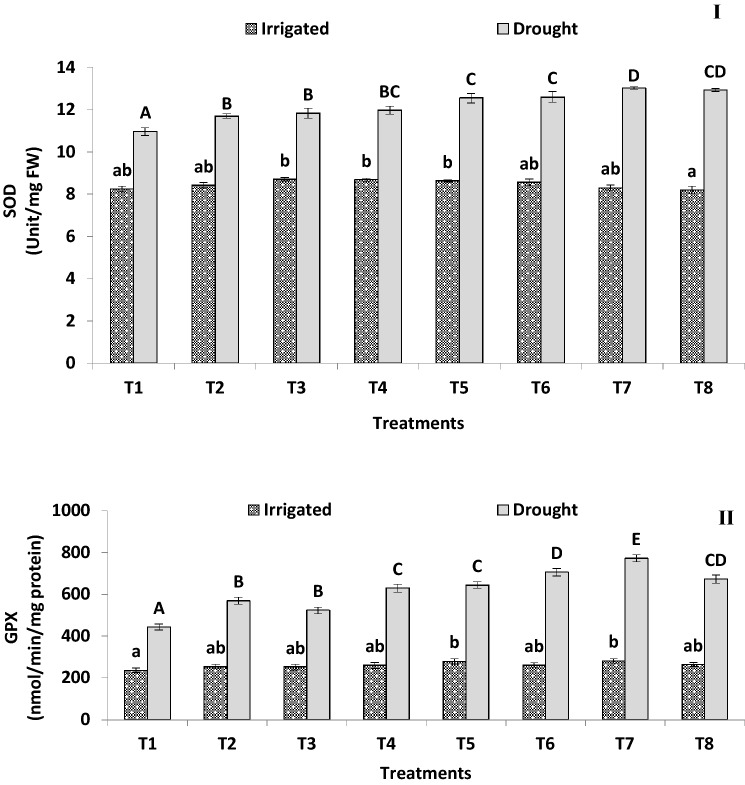
A decrease of leaf MDA content 98.7% and 86.9% was noted with treatment T7 and T6 consortia-treated plants, respectively, as compared to non-treated control plants under water-stressed conditions (Table 5). Likewise, under water-stressed conditions bio-consortia-treated plants reduced the H2O2 content from 30.5 to 126.6% compared to the non-treated control (Fig. 4II). However, there was a general increase in the activity of SOD (1.13–1.26-fold) (Fig. 5I), GPX (1.18–1.74-fold) (Fig. 5II), CAT (1.16–1.54-fold) and APX (1.06–1.25-fold) (Table 5) in inoculated finger millet plants in the presence of water-stressed conditions compared to the non-treated control plants. In general, there was no evident difference in MDA, H2O2 and SOD concentrations with bacterial treatment under irrigated conditions.
Fig. 5.
Effect of irrigated and drought conditions on (I) superoxide dismutase (SOD) and (II) guiacol peroxidase (GPX) content of PGPB consortium producing ACC deaminase inoculated finger millet plants (Experiment II). Error bars indicate SEM of three replicates. Treatment means within columns with the different letters indicate statistically significant differences (p < 0.05) after DMRT
Nutrient concentration of finger millet
Drought conditions caused a noteworthy reduction in the nutrient concentration, i.e., N (41.5%), P (34.0%), K+ (40.9%), Ca2+ (37.0%) and Na+ (31.8%) of finger millet as compared to irrigated non-treated control plants. The bacterial consortia significantly increased the N (33.2–56.3%), P (43.4–57.9%), K+ (19.7–41.9%), Ca2+ (16.8–38.3%), and Na+ (20.0–35.5%) concentrations in finger millet under water-stressed conditions. Similarly, the inoculation of finger millet with bacterial consortia significantly increased the N (33.9–54.4%), P (35.1–54.5%), K+ (1.2–28.1%), Ca2+ (13.9–44.5%) and Na+ (17.1–39.4%) concentrations as compared to untreated plants under no-stress conditions (Fig. 6).
Fig. 6.
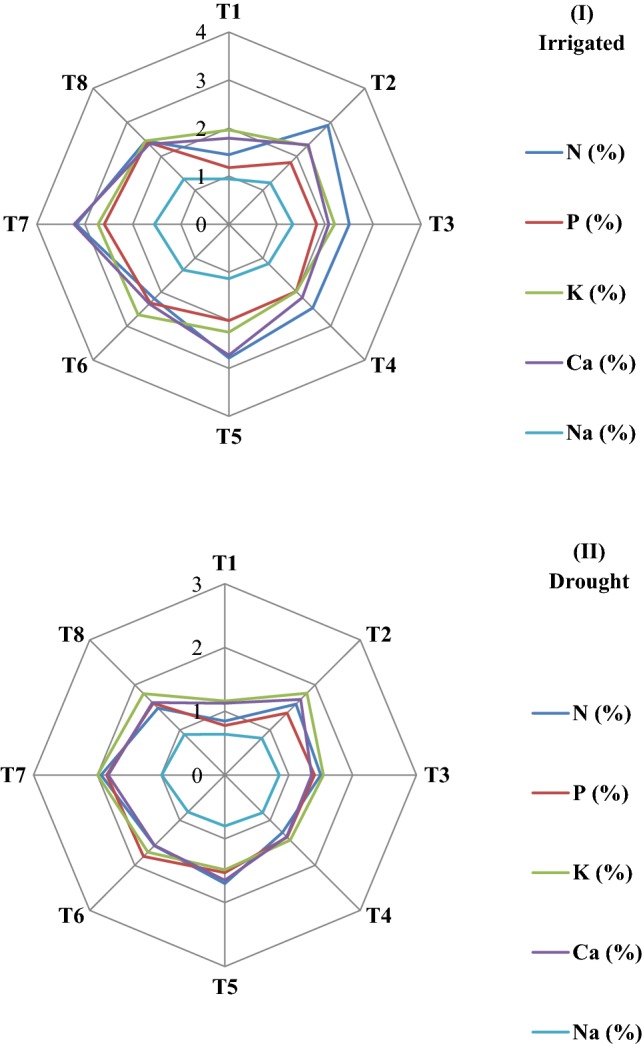
Effect of (I) irrigated and (II) drought conditions on foliar nutrient contents of PGPB consortium producing ACC deaminase inoculated finger millet plants (Experiment II)
Discussion
Water stress is a key concern of worldwide agriculture that significantly restricts crop yields of cultivable land (Kang et al. 2009; Saikia et al. 2018). Therefore, attaining improved crop health and productivity under drought stress conditions is a vital threat for sustainable agriculture. Numerous PGPB have the intrinsic capacity of enhancing the growth and providing drought tolerance to inoculated plants. PGPB that have ACC deaminase producing activity may be particularly helpful to plants under stress conditions by lowering the levels of stress hormone ethylene (Kruasuwan and Thamchaipenet 2018; Govindasamy et al. 2020). In this study, the potential of bacterial ACC deaminase along with other PGP traits was demonstrated to improve the growth of finger millet under water-stressed and no-stress conditions in two glasshouse experiments (i.e., individual and consortium). The observations that strain RAA3 (V. paradoxus) and a combination of DPC9 (Ochrobactrum anthropi), DPB13 (Pseudomonas palleroniana), DPB15 (Pseudomonas fluorescens) and DPB16 (Pseudomonas palleroniana) promoted the shoot and root growth of finger millet under drought stress agreed with previous finding in which improved root and shoot growth was noticed upon treating the plants with ACC deaminase producing bacterial inoculants (Ashraf and Akram 2009; Saikia et al. 2018). These findings also agreed with previous studies of Chandra et al. (2019a) and Yasmin et al. (2013) who revealed that bacterial inoculants application exhibited an increase in the R/S ratio as compared to non-treated plants under drought-stressed conditions.
Better root growth facilitates enhance water intake from the soil. In lack of soil moisture condition, plants release ACC, abscisic acid and ethylene which act as stress cues in plant tissues through the transpiration stream (Mayak et al. 2004). Although PGPB do not directly boost the nutrient and water accessibility to crops, they effectively do so by augmenting root development during drought (Fleury et al. 2010). In this study, maximum shoot and root length and shoot/root dry biomass was noted following individual inoculation of plants by strain RAA3. In addition, higher plant height and shoot dry biomass was reported following combined inoculation of plants with treatment T7 and root elongation and root dry biomass was facilitated following inoculation with multi-strain combinations of treatment T8 as compared to non-treated control when plants were subjected to water-stressed conditions. Earlier reports of Kohler et al. (2008) presented that bacterial strains (e.g., Pseudomonas mendocina) that had the capacity to secrete IAA significantly enhanced plant growth characteristics under drought-stressed conditions. These results clearly indicate that rhizobacteria isolated from rainfed agriculture field could be highly efficient in promoting plant health under water-stressed conditions. Our finding also supports the results obtained by Castillo et al. (2013) and Kumar et al. (2016) have revealed that individual and consortia inoculants significantly increased the growth parameters of sunflower and chickpea under both non-stressed and drought-stressed conditions. These results are attributed to the capability of PGPB to increase plant growth and yield by synthesizing IAA, increasing nutrient and water acquisition and antagonizing phytopathogens, all of which helps plants to endure water stress (Kaushal and Wani 2016; Shirinbayan et al. 2019).
In plants, the prompt response to drought stress is a decrease of photosynthetic efficiency, which, as a result, lowers energy production and metabolite accumulation. Treatment of finger millet with single inoculants (e.g., RAA3) or consortium (e.g., treatment T7) inoculation partially eliminated the drought induced damage on growth by sustaining the chlorophyll content. An earlier report demonstrated that bacterial consortium BBS, i.e., AR156 (Bacillus cereus) + SM21 (Bacillus subtilis) + XY21 (Serratia sp.) significantly increased the leaf chlorophyll content by 27.4% in comparison to non-inoculated control plants which suggested that consortium application facilitated chlorophyll content retention in cucumber leaves following water stress (Wang et al. 2012). Another study demonstrated that application of BB (i.e., Bacillus amyloliquefaciens Bk7 and Brevibacillus laterosporus B4) significantly improved the photosynthetic activity in rice when challenged with different environmental stresses (Kakar et al. 2016). These findings indicate a higher photosynthetic efficacy of inoculated plants than uninoculated water-stressed control plants.
Moreover, bacterial application shows a direct effect on the accumulation of phenolic and proline content. Plant cellular osmolytes are important determinants of plant response to stresses (Ashraf and Harris 2004). In our study, single inoculation with strain RAA3 (1.80-fold) and consortia-treated plants (1.85-fold) displayed higher proline accumulation as counterpart to non-treated plants when exposed to water-stressed conditions. This elevated level of proline contributes to osmotic adjustment and protects the structure of macromolecules and cell membranes (Valentovic et al. 2006; Harb et al. 2010; Ghorbanpour et al. 2013; Chandra et al. 2019b). Compatible solutes including glycine betaine, sugars and proline were concentrated in plant parts under environmental stressed conditions and supports plants’ ability to sustain cytosolic pH, boost the level of various enzymes, act as molecular chaperones and control the intercellular redox reaction (Sandhya et al. 2010; Carlson et al. 2020). Our study exhibited a direct correlation between bacterially treated plants and osmolyte accumulation, which could also be an imperative bacterial cause for water-stress lessening in finger millet plants. Increased total phenol content (TPC) was noticed in the treated plants under water-stressed conditions. This could be a useful marker for the strategies of drought tolerance in inoculated plants. In fact, the increased accumulation of phenolics in leaves may be considered as a strategy to alleviate the impact of drought induced damage in plants (Chandra et al. 2019a; Chiappero et al. 2019).
Previous studies also indicated that imposition of drought to plants leads to higher lipid peroxidation (Trovato et al. 2008). In the present study, it was noticed that water stress substantially increased MDA content in non-inoculated plants as compared to inoculated ones. Our results showed that treatment of finger millet plants with strain RAA3 and with consortium T7 significantly decreased the MDA content by 48.4% and 98.7%, respectively, under drought stress over non-treated plants. The decline in MDA content indicates the capacity of treatment T7 to reduce the extent of lipid peroxidation of the plasma lemma to defend leaf cell membrane from the drought induced damage. These finding are similar with the results obtained by Khan et al. (2019) who found that bacterial application significantly diminished the MDA level in plants subjected to water stress.
Previous finding of Bharti et al. (2016) and Chandra et al. (2018a, b) revealed that bacterial application amended the ROS machinery in stress-imposed plants in contrast to the non-treated drought-stressed plants. The augmentation of different ROS scavengers in finger millet plants is recognized as key parameter for water-stress mitigation by ACC deaminase producing bacteria. The level of POD and CAT increased significantly in consortium-treated stressed plants in contrast to the control plants. The enhanced level of ROS scavenging enzymes provided a protective mechanism in water-stressed finger millet plants by detoxifying the reactive H2O2, ·OH and 1O2. The single and consortia application of bacteria significantly boosted the level of CAT, APX, SOD, and GPX indicating that drought tolerance may be contributed through the increased activity of these antioxidant enzymes. Our study is strongly supported by the results of Kakar et al. (2016) who revealed that a mixture of Bacillus amyloliquefaciens Bk7 and Brevibacillus laterosporus B4 treated plants revealed a higher degree of enzymatic antioxidants, conferred IST (induced systematic tolerance) and improved rice plant fitness when subject to water stress. In fact, numerous researchers have described that activities of CAT, GPX, APX and SOD are increased under drought stress (Wu et al. 2006; Patel and Hemantaranjan 2012; Goswami and Deka 2020).
NPK are the crucial macronutrients for growth and development of the plants. The results of the present study indicate that bacterial application of consortium T7 has the capability to produce siderophores, indole acetic acid, and solubilize inorganic phosphate together with ACC deaminase activity are supposed to be helpful for better nutrient content and plant growth stimulation under irrigated and water-stressed conditions. The results of previous studies suggest that microbes enhance the phosphorus content in crops by mineralizing organic P in soil and also solubilizing various inorganic phosphates, which is a beneficial attribute for the augmentation of plant growth (Chen et al. 2006; Kumar et al. 2016).
Conclusions
The present work aimed to study the role of drought tolerant ACC deaminase plant growth promoting bacteria bacterium to evaluate the plant growth under drought and no-stress conditions. Besides ACC deaminase activity, the tested bacterial strain possesses other plant growth promoting properties including IAA production, siderophores production, nitrogen fixation and phosphate solubilization ability that could mitigate drought stress-induced damages and establish induced systemic tolerance to the finger millet plants. Finding of the experiments presented here indicate that single inoculation of RAA3 or a consortia inoculation of treatment T7, i.e., DPC9 + DPB13 + DPB15 + DPB16 improve the overall health and growth of the finger millet plants under no-stress and water-stressed conditions. In addition, inoculation with these treatments also improved the compatible solutes, enzymatic and non-enzymatic antioxidants to counteract the drought stress in finger millet. In conclusion, this work opens up the possibility to evaluate the potential of these PGPB in enhancing crop growth and productivity under field conditions as biofertilizer in alleviating drought stress faced by the plants. These results suggested that RAA3 and treatment T7 could be used as a bioinoculant to improve the productivity of plants growing under drought stress.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financed by AISRF supported by the Governments of India and Australia. This study was also partially supported by an Indo-Philippines collaboration project.
Author contributions
DC and AKS were involved in the designing and execution of the work. DC majorly conducted all the experiments, lab work, analyzed data, and prepared the manuscript draft. AKS, BG and RS corrected the manuscript and gave the final approval for the version to be published.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Dinesh Chandra, Email: dchandra.009@gmail.com.
Rashmi Srivastava, Email: rsri_10@yahoo.co.in.
Bernard R. Glick, Email: glick@uwaterloo.ca
Anil Kumar Sharma, Email: anilksharma_99@yahoo.com.
References
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24:1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- Ali F, Bano A, Fazal A. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017;82:363–375. doi: 10.1007/s10725-017-0267-2. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol Adv. 2009;27:744–752. doi: 10.1016/j.biotechadv.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Ashraf MPJC, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–16. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- Bharti N, Pandey SS, Barnawal D, Patel VK, Kalra A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci Rep. 2016;6:34768. doi: 10.1038/srep34768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R, Tugizimana F, Steenkamp PA, Dubery IA, Hassen AI, Labuschagne N. Rhizobacteria-induced systemic tolerance against drought stress in Sorghum bicolor (L.) Moench. Microbiol Res. 2020;30:126388. doi: 10.1016/j.micres.2019.126388. [DOI] [PubMed] [Google Scholar]
- Castillo P, Escalante M, Gallardo M, Alemano S, Abdala G. Effects of bacterial single inoculation and co-inoculation on growth and phytohormone production of sunflower seedlings under water stress. Acta Physiol Plant. 2013;35:2299–2309. doi: 10.1007/s11738-013-1267-0. [DOI] [Google Scholar]
- Chandra D, Srivastava R, Glick BR, Sharma AK. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere. 2018;28:227–240. doi: 10.1016/S1002-0160(18)60013-X. [DOI] [Google Scholar]
- Chandra D, Srivastava R, Sharma AK. Influence of IAA and ACC deaminase producing fluorescent pseudomonads in alleviating drought stress in wheat (Triticum aestivum) Agric Res. 2018;7:290–299. doi: 10.1007/s40003-018-0305-y. [DOI] [Google Scholar]
- Chandra D, Srivastava R, Gupta VV, Franco CM, Paasricha N, Saifi SK, Tuteja N, Sharma AK. Field performance of bacterial inoculants to alleviate water stress effects in wheat (Triticum aestivum L.) Plant Soil. 2019;441:261–281. doi: 10.1007/s11104-019-04115-9. [DOI] [Google Scholar]
- Chandra D, Srivastava R, Sharma AK. Evaluation of ACC deaminase producing rhizobacteria to alleviate water stress impacts in wheat (Triticum aestivum L.) plants. Can J Microbiol. 2019 doi: 10.1139/cjm-2018-0636. [DOI] [PubMed] [Google Scholar]
- Chen YP, Rekha PD, Arunshen AB, Lai WA, Young CC. Phosphate solubilizing bacteria from subtropical soil and their tri-calcium phosphate solubilizing abilities. Appl Soil Ecol. 2006;34:33–41. doi: 10.1016/j.apsoil.2005.12.002. [DOI] [Google Scholar]
- Chiappero J, del Rosario Cappellari L, Alderete LG, Palermo TB, Banchio E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind Crops Prod. 2019;139:111553. doi: 10.1016/j.indcrop.2019.111553. [DOI] [Google Scholar]
- Danish S, Zafar-ul-Hye M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci Rep. 2019;9:5999. doi: 10.1038/s41598-019-42374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delshadi S, Ebrahimi M, Shirmohammadi E. Effectiveness of plant growth promoting rhizobacteria on Bromus tomentellus Boiss seed germination, growth and nutrients uptake under drought stress. S Afr J Bot. 2017;113:11–18. doi: 10.1016/j.sajb.2017.07.006. [DOI] [Google Scholar]
- Duan J, Jiang W, Cheng Z, Heikkila JJ, Glick BR. The complete genome sequence of the plant growth-promoting bacterium Pseudomonas putida UW4. PLoS ONE. 2013;8:e58640. doi: 10.1371/journal.pone.0058640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell AJ, Vesely S, Nero V, McCormack K, Rodriguez H, Shah S, Dixon DG, Glick BR. Tolerance of transgenic canola (Brassica napus) amended with ACC deaminase-containing plant growth-promoting bacteria to flooding stress at a metal-contaminated field site. Environ Pollut. 2007;147:540–545. doi: 10.1016/j.envpol.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Figueiredo MV, Burity HA, Martínez CR, Chanway CP. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol. 2008;40:182–188. doi: 10.1016/j.apsoil.2008.04.005. [DOI] [Google Scholar]
- Fleury D, Jefferies S, Kuchel H, Langridge P. Genetic and genomic tools to improve drought tolerance in wheat. J Exp Bot. 2010;61:3211–3222. doi: 10.1093/jxb/erq152. [DOI] [PubMed] [Google Scholar]
- Forni C, Duca D, Glick BR. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil. 2017;410:335–356. doi: 10.1007/s11104-016-3007-x. [DOI] [Google Scholar]
- Gepstein S, Glick BR. Strategies to ameliorate abiotic stress-induced plant senescence. Plant Mol Biol. 2013;82:623–633. doi: 10.1007/s11103-013-0038-z. [DOI] [PubMed] [Google Scholar]
- Ghorbanpour M, Hatami M, Khavazi K. Role of plant growth promoting rhizobacteria on antioxidant enzyme activities and tropane alkaloid production of Hyoscyamus niger under water deficit stress. Turk J Biol. 2013;37:350–360. doi: 10.3906/biy-1209-12. [DOI] [Google Scholar]
- Ghorchiani M, Etesami H, Alikhani HA. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric Ecosyst Environ. 2018;258:59–70. doi: 10.1016/j.agee.2018.02.016. [DOI] [Google Scholar]
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Glick BR, Penrose DM, Li J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- Goswami M, Deka S. Plant growth-promoting rhizobacteria—alleviators of abiotic stresses in soil: a review. Pedosphere. 2020;30:40–61. doi: 10.1016/S1002-0160(19)60839-8. [DOI] [Google Scholar]
- Govindasamy V, George P, Kumar M, Aher L, Raina SK, Rane J, Annapurna K, Minhas PS. Multi-trait PGP rhizobacterial endophytes alleviate drought stress in a senescent genotype of sorghum [Sorghum bicolor (L.) Moench] 3 Biotech. 2020;10:13. doi: 10.1007/s13205-019-2001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassini P, Eskridge KM, Cassman KG. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat Commun. 2013;4:2918. doi: 10.1038/ncomms3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichko VP, Glick BR. Amelioration of flooding stress by ACC deaminase-containing plant growth-promoting bacteria. Plant Physiol Biochem. 2001;39:11–17. doi: 10.1016/S0981-9428(00)01212-2. [DOI] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MM, Pereira A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010;154:1254–1271. doi: 10.1104/pp.110.161752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jackson MC. Soil chemist analysis. New Delhi: Prentice Hall Pvt. Ltd.; 1973. [Google Scholar]
- Kakar KU, Ren XL, Nawaz Z, Cui ZQ, Li B, Xie GL, Hassan MA, Ali E, Sun GC. A consortium of rhizobacterial strains and biochemical growth elicitors improve cold and drought stress tolerance in rice (Oryza sativa L.) Plant Biol. 2016;18:471–483. doi: 10.1111/plb.12427. [DOI] [PubMed] [Google Scholar]
- Kang Y, Khan S, Ma X. Climate change impacts on crop yield, crop water productivity and food security—a review. Prog Nat Sci. 2009;19:1665–1674. doi: 10.1016/j.pnsc.2009.08.001. [DOI] [Google Scholar]
- Kausar R, Shahzad SM. Effect of ACC-deaminase containing rhizobacteria on growth promotion of maize under salinity stress. J Agric Soc Sci. 2006;2:216–218. doi: 10.1139/W09-092. [DOI] [Google Scholar]
- Kaushal M, Wani SP. Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol. 2016;66:35–42. doi: 10.1007/s13213-015-1112-3. [DOI] [Google Scholar]
- Khan N, Bano A, Rahman MA, Guo J, Kang Z, Babar MA. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci Rep. 2019;9:2097. doi: 10.1038/s41598-019-38702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler J, Hernández JA, Caravaca F, Roldán A. Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol. 2008;35:141–151. doi: 10.1071/FP07218. [DOI] [PubMed] [Google Scholar]
- Kruasuwan W, Thamchaipenet A. 1-Aminocyclopropane-1-carboxylate (ACC) deaminase-producing endophytic diazotrophic Enterobacter sp. EN-21 modulates salt-stress response in sugarcane. Plant Growth Regul. 2018;37:849–858. doi: 10.1007/s00344-018-9780-4. [DOI] [Google Scholar]
- Kumar M, Mishra S, Dixit V, Kumar M, Agarwal L, Chauhan PS, Nautiyal CS. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.) Plant Signal Behav. 2016;11:e1071004. doi: 10.1080/15592324.2015.1071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathimaran N, Srivastava R, Wiemken A, Sharma AK, Boller T. Genome sequences of two plant growth-promoting fluorescent Pseudomonas strains, R62 and R81. J Bacteriol. 2012;194:3272–3273. doi: 10.1128/JB.00349-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166:525–530. doi: 10.1016/j.plantsci.2003.10.025. [DOI] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol. 2007;53:1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Niu X, Song L, Xiao Y, Ge W. Drought-Tolerant plant growth-promoting rhizobacteria associated with foxtail millet in a semi-arid agroecosystem and their potential in alleviating drought stress. Front Microbiol. 2018;8:2580. doi: 10.3389/fmicb.2017.02580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Bashir S, Khan Y, Mumtaz R, Shinwari ZK, Khan AL, Khan A, Ahmed AH. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: a review. Microbiol Res. 2018;209:21–32. doi: 10.1016/j.micres.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Olsen SR. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington: USDA; 1954. [Google Scholar]
- Pandey S, Gupta S. ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Microbiol. 2019;10:1506. doi: 10.3389/fmicb.2019.01506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papworth A, Maslin M, Randalls S. Is climate change the greatest threat to global health? Geogr J. 2015;181:413–422. doi: 10.1111/geoj.12127. [DOI] [Google Scholar]
- Patel PK, Hemantaranjan A. Antioxidant defense system in chickpea (Cicer arietinum L.): influence by drought stress implemented at pre- and post- anthesis stage. Am J Plant Physiol. 2012;7:164–173. doi: 10.3923/ajpp.2012.164.173. [DOI] [Google Scholar]
- Saikia J, Sarma RK, Dhandia R, Yadav A, Bharali R, Gupta VK, Saikia R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci Rep. 2018;8:3560. doi: 10.1038/s41598-018-21921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AR, Brunetti C, Khalid A, Della Rocca G, Raio A, Emiliani G, De Carlo A, Mahmood T, Centritto M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE. 2018;13:e0191218. doi: 10.1371/journal.pone.0191218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya VZAS, Grover M, Reddy G, Venkateswarlu B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol Fert Soils. 2009;46:17–26. doi: 10.1007/s00374-009-0401-z. [DOI] [Google Scholar]
- Sandhya VSKZ, Ali SZ, Grover M, Reddy G, Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. doi: 10.1007/s10725-010-9479-4. [DOI] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Saravanakumar D, Kavino M, Raguchander T, Subbian P, Samiyappan R. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol Planta. 2011;33:203–209. doi: 10.1007/s11738-010-0539-1. [DOI] [Google Scholar]
- Sarma RK, Saikia R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014;377:111–126. doi: 10.1007/s11104-013-1981-9. [DOI] [Google Scholar]
- Shirinbayan S, Khosravi H, Malakouti MJ. Alleviation of drought stress in maize (Zea mays) by inoculation with Azotobacter strains isolated from semi-arid regions. Appl Soil Ecol. 2019;133:138–145. doi: 10.1016/j.apsoil.2018.09.015. [DOI] [Google Scholar]
- Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR. Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem. 2005;43:701–708. doi: 10.1016/j.plaphy.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Trovato M, Mattioli R, Costantino P. Multiple roles of proline in plant stress tolerance and development. Rend Lincei. 2008;19:325–346. doi: 10.1007/s12210-008-0022-8. [DOI] [Google Scholar]
- Urbanek H, Kuzniak-Gebarowska E, Herka K. Elicitation of defence responses in bean leaves by Botrytis cinerea polygalacturonase. Acta Physiol Plant. 1991;13:43–50. [Google Scholar]
- Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Moënne-Loccoz Y, Muller D, Legendre L, Wisniewski-Dyé F, Prigent-Combaret C. Plant growth-promoting rhizobacteria and root system functioning. Front Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentovic P, Luxova M, Kolarovic L, Gasparikova O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006;52:186–191. doi: 10.17221/3364-PSE. [DOI] [Google Scholar]
- Walkley A, Black CA. An examination of different methods for determining soil organic matter and proposed modifications of the chromic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA. Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol. 2005;162:465–472. doi: 10.1016/j.jplph.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Yang W, Wang C, Gu C, Niu DD, Liu HX, Wang YP, Guo JH. Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS ONE. 2012;7:e52565. doi: 10.1371/journal.pone.0052565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QS, Xia RX, Zou YN. Reactive oxygen metabolism in mycorrhizal and nonmycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol. 2006;163:1101–1110. doi: 10.1016/j.jplph.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Yasmin H, Bano A, Samiullah Screening of PGPR isolates from semi-arid region and their implication to alleviate drought stress. Pak J Bot. 2013;45:51–58. [Google Scholar]
- Zhou Y, Liu Y, Peng C, Li X, Zhang M, Tian X, Li J, Li Z, Duan L. Coronatine enhances drought tolerance in winter wheat by maintaining high photosynthetic performance. J Plant Physiol. 2018;228:59–65. doi: 10.1016/j.jplph.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Zhu JK. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieslin N, Ben-Zaken R. Peroxidase activity and presence of phenolic substances in peduncle of rose flower. Plant Physiol Biochem. 1993;31:333–339. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.