Abstract
Aim
Spontaneous subarachnoid haemorrhage (SAH) caused by ruptured cerebral aneurysm is a severe subtype of haemorrhagic stroke. Although the incidence of SAH is relatively low among all cerebrovascular diseases, the mortality is the highest. The critical management of SAH is challenging. We provide this evidence-based guideline to present current and comprehensive recommendations for the diagnosis and treatment of non-trauma SAH.
Methods
A formal literature search of MEDLINE (1 January 1990–30 June 2019) was performed. Data were synthesised with the use of evidence tables. Writing group members met by teleconference to discuss data-derived recommendations. The Chinese Stroke Association’s levels of evidence grading algorithm was used to grade each recommendation. The guideline draft was reviewed by Chinese Stroke Association’s Stroke Fellow Committees. It is intended that this guideline be fully updated every 3 years.
Results
Evidence-based guidelines are presented for the care of patients presenting with non-trauma SAH. The focus of the guideline was subdivided into transfer and systems of care, diagnosis flowchart, aetiology and differentiation, prevention of rebleeding, surgical and endovascular repair of ruptured aneurysms, management of vasospasm and delayed cerebral ischaemia, management of hydrocephalus, management of seizures and management of medical complications.
Conclusions
The guideline offers a framework for SAH management. Early professional and aggressive care of SAH might help dramatically.
Keywords: subarachnoid, stroke
Spontaneous subarachnoid haemorrhage (SAH) caused by ruptured cerebral aneurysm is a severe subtype of haemorrhagic stroke with an incidence between 1 and 27 per 100 000 persons per year. The incidence of SAH varied by region and ethnicity. It was higher in women and increased with ageing.1 The annual incidence of SAH in the Chinese population also varies among different studies. In northern China, the incidence is generally higher, about 6.2 per 100 000 persons per year in Baotou, Inner Mongolia.2 In central and southern regions of China, the incidence is relatively low, about 2.2 per 100 000 persons per year (95% CI 1.2 to 5.4) in Changsha, 1.1 per 100 000 persons per year in Shanghai,3 1.2 per 100 000 persons per year in Shunde, Guangdong Province,4 and 3.02–4.45 per 100 000 persons per year in Chengdu.5 However, it is as high as 6.25 per 100 000 persons per year in Taiwan6 and as low as 1.6 per 100 000 persons per year (95% CI 0.8 to 4.1) in Beijing.3 There were a few studies on the prevalence of SAH in Asia. Only one national study in South Korea reported that the prevalence of SAH was 19.8~21.0 per 100 000 persons per year.7
Excluding injuries and iatrogenic factors, common causes of spontaneous SAH include aneurysms, cavernous haemangioma, venous thrombosis, arteriovenous malformations and cerebral arteriovenous fistula,8 cerebral amyloid angiopathy,9–11 tumour bleeding, special vasculitis/encephalitis10 and abnormal blood coagulation.12 Rare causes of SAH include postpartum eclampsia and reversible cerebral vasoconstriction syndrome.9 According to the anatomical site that a SAH develops and is seen on head CT scan, SAH is classified into convex SAH and non-convex SAH. The former is more common in venous system embolism, venous sinus thrombosis and cerebral amyloid angiopathy,10 11 while the latter is more common in aneurysmal rupture.
Acute intensive care should be provided to all patients with SAH. Acute-phase monitoring and symptomatic treatment to prevent complications are the priorities. The focus of such management may vary depending on the levels of stroke centres that can provide. For a comprehensive stroke centre, the most sophisticated and modern approach emphasising on interventional, neurosurgical and multidiscipline team approach is recommended.
This current guideline is one of the seven guidelines by the Chinese Stroke Association. The methodology on literature review, assignment of level of evidence and grade of recommendation has been published earlier. This guideline mainly concentrated on the discussion and recommendation related to spontaneous SAH on these four sections: the diagnosis and severity assessment of SAH, the early management of SAH, and the treatment and prevention of complications of SAH. The full text of this guideline is available in the online supplemental material.
svn-2019-000296supp001.pdf (500.4KB, pdf)
Section 1: diagnosis and severity assessment of spontaneous SAH
The diagnostic flowchart is shown in figure 1.
Figure 1.
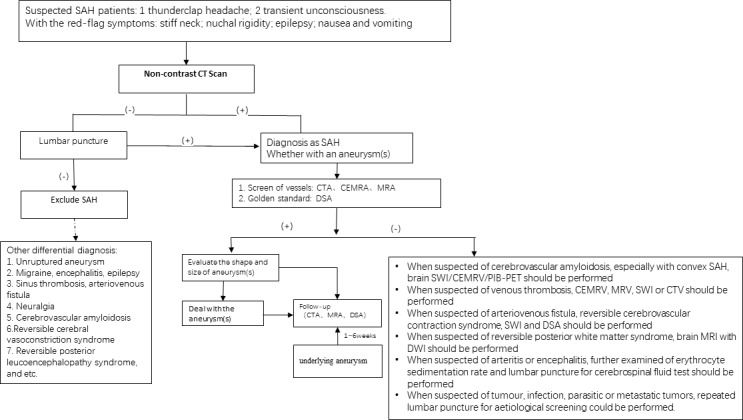
The flowchart of SAH diagnoses. DWI, diffusion-weighted imaging; MRA, magnetic resonance angiography; SAH, subarachnoid haemorrhage; SWI, susceptibility-weighted imaging; CEMRA, contrast enhancement magnetic resonance angiography; CEMRV, contrast enhancement magnetic resonance venogram; CTV, computed tomographic venogram
This section made the recommendations for the areas of SAH care in clinical and imaging diagnoses, aetiology of SAH and SAH severity assessment.
Recommendations
Acute onset of severe headache is one of the initial and major symptoms. The Ottawa headache tool may be useful to differentiate the diagnoses (class I, level of evidence B).
Non-contrast head CT should be the initial imaging study for patients with suspected spontaneous SAH; if non-diagnostic, lumbar puncture should be considered (class Ⅱa, level of evidence B).
CT angiography (CTA) is essential for finding the aetiology of SAH; therefore, each stroke centre can perform CTA 24 hours/7 days (class I, level of evidence B). Contrast magnetic resonance angiography (MRA) and three-dimensional time-of-flight MRA are optional choices for patients who are ineligible to have a CTA. For suspected arteriovenous malformation that causes SAH, susceptibility-weighted imaging (SWI) should be performed (class IIa, level of evidence B).
MRI (with fluid-attenuated inversion recovery, diffusion-weighted imaging (DWI), SWI or T2*) is a reasonable tool to explore the aetiology of SAH. Lumbar puncture should be performed in patients with a negative CT of the head (class IIa, level of evidence C).
DSA can be used as a standard reference for discovering the aetiology of SAH and a tool to further evaluate the treatment, such as interventional therapy or surgery (class IIa, level of evidence B). If the initial DSA is negative, DSA might not be essential to repeat within 1–6 weeks, if CTA is available (class IIb, level of evidence C).
The initial clinical severity and prognosis of SAH should be evaluated by the use of a clinical grading system such as the Hunt and Hess Scale or the World Federation of Neurosurgical Societies (WNFS) scale (class I, level of evidence B).
The risk of delayed cerebral infarction (DCI) and cerebral vasospasm (CVS) in SAH should be evaluated by the use of a relatively simple radiographical grading scale, such as the Fisher Grading Scale or the modified Fisher Grading Scale (class IIb, level of evidence B).
Section 2: SAH early management
Acute intensive care should be offered to all patients with SAH. Acute-phase monitoring and treating symptomatically to avoid complications are the goals. Different levels of stroke centres should focus on various key elements. For a comprehensive stroke centre, emphasising on intervention, neurosurgery and multidiscipline team, and stroke care quality and its improvement process should be emphasised. In general, the flowchart of SAH acute management is shown in figure 2.
Figure 2.
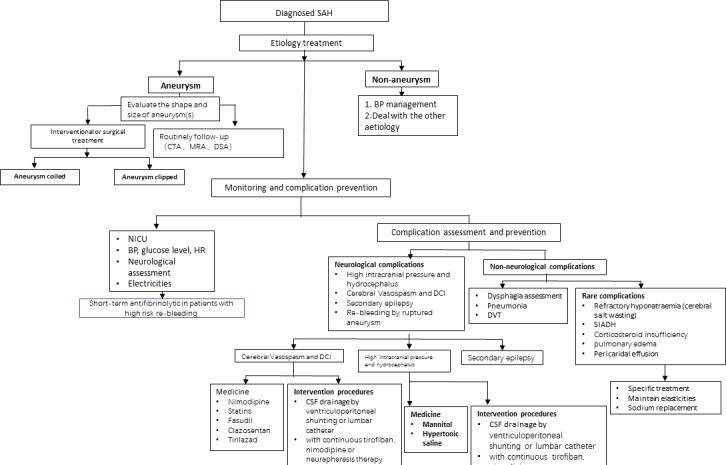
SAH acute management flowchart. BP, blood pressure; CSF, cerebrospinal fluid; DCI, delayed cerebral infarction; DVT, deep vein thrombosis; SAH, subarachnoid haemorrhage; NICU, neurointensive care unit; SIADH, syndrome of inappropriate antidiuretic hormone secretion
General management
Recommendations
-
Patients with severe SAH should be transferred to an experienced comprehensive hospital for further intervention in order to reduce mortality. Severe SAH is defined as having an aneurysm, more than 3 points on the Hess-Hunt Scale or a grade of 4~5 on the WFNS scale (class IIa, level of evidence B).
1.1 All stroke centres should operate a CT scanner 24 hours/7 days. A comprehensive stroke centre should have a team of neurosurgeons, neurointerventionalists and neurointensivists, which is essential to provide high quality of care (class IIa, level of evidence B).
Intensive blood pressure (BP) management in the early phase of SAH is recommended. Systolic blood pressure (SBP) is reasonable to be kept below 160 mm Hg and to reduce variations (class IIa, level of evidence B). However, dropping SBP too low (<130 mm Hg) or having high variability might be harmful (class III, level of evidence B).
A BP-lowering agent should be administrated intravenously during the acute phase. Calcium channel blockers such as nicardipine or beta-blockers such as labetalol are appropriate agents to reach and maintain the target BP range (class I, level of evidence B). However, hypotension can be induced from continuous intra-arterial nimodipine infusion (class IIb, level of evidence B).
Patients with SAH should be kept calm and should avoid nervous conditions or stress. Constipation should be prevented. Bathing alone should be avoided (class I, level of evidence C).
Hyperglycaemia in patients with SAH is associated with poor prognosis and increased mortality (class IIb, level of evidence A). However, aggressive control of hyperglycaemia may not change the clinical outcome. Hypoglycaemia should be avoided (class IIb, level of evidence B).
Treatment should focus on vasospasm and rebleeding prevention. For patients with severe headache, comprehensive management, including headache relief, needs to be considered (class I, level of evidence C).
Rebleeding and haemostasis treatment
Due to the high risk of rebleeding during the first 2 weeks post-SAH, early prevention of rebleeding is important for a better clinical outcome. For patients with SAH, endovascular treatments, such as aneurysm clipping as well as hybrid operation, are preferred since the risk of rebleeding may be reduced. Medical treatment is preferred for patients with severe SAH who do not have an aneurysm or with contraindication to intervention.
Recommendations
For most patients with SAH with a ruptured aneurysm, endovascular or surgical treatment should be considered as soon as possible (less than 72 hours) in order to reduce the risk of rebleeding (class I, level of evidence B).
The interventional or surgical treatment plan should be discussed and determined by the multidisciplinary team, experienced neurosurgeons and neurointerventionalists (class I, level of Evidence C).
For patients with an aneurysm suitable for either interventional or microsurgical treatment, interventional therapy is preferred (class I, level of evidence A). For patients with SAH older than 70 and with a Hunt-Hess Scale score between 4 and 5, endovascular treatment is preferred (class IIa, level of evidence B).
Antifibrinolytic drugs may help reduce the risk of rebleeding after SAH in a short term, but these are unable to improve the long-term clinical outcomes. If the patient still has a significant risk of rebleeding before intervention and there is no absolute contraindication, tranexamic acid can be used for a short term (<72 hours) (class IIb, level of evidence B). However, it should not be used in patients during postsurgical or endovascular therapy (class III, level of evidence C).
Section 3: complications
This section discussed the treatment recommendations of common and rare neurological complications such as high intracranial pressure and hydrocephalus, CVS and DCI, secondary epilepsy, pneumonia, deep vein thrombosis (DVT) prophylaxis, hypotension, refractory hyponatraemia, hypoxaemia, acute pulmonary oedema and acute heart failure.
Neurological complications
Recommendations
Patients with clinical symptoms of increased intracranial pressure (ICP) can be treated with hypertonic saline or mannitol (class IIa, level of evidence B).
For patients with elevated ICP or a massive effect, cerebrospinal fluid (CSF) drainage via ventriculostomy is recommended (class I, level of evidence A). The additional use of intravenous tirofiban and nimodipine therapy in patients with ventricular drainage or neurapheresis therapy might be helpful (class IIb, level of evidence C).
Oral or intravenous nimodipine after SAH onset is recommended (class I, level of evidence A).
Statins have no additional benefit for DCI or functional outcome and should not be used routinely in patients with aneurysmal SAH (aSAH) (class IIb, level of evidence B).
Fasudil may be superior to nimodipine to prevent CVS (class IIa, level of evidence B).
CSF drainage can significantly reduce the incidence of CVS and DCI and improve the overall treatment effect on patients with CVS (class IIa, level of evidence B).
Clazosentan might reduce aSAH vasospasm-related morbidity/all-cause mortality in a dose-dependent manner; however, there might be no significant improvement on outcome. Considering it is associated with complications such as anaemia, pulmonary oedema, low BP and other adverse reactions, it could be selectively used for patients with aSAH (class IIa, level of evidence B).
Tirilazad could be added in patients with SAH at a higher risk of CVS (class IIb, level of evidence B).
Agents such as cilostazol, intravenous edaravone, low-molecular-weight heparin, flunarizine, ozagrel and alprostadil might have the benefit of preventing delayed CVS (class IIb, level of evidence B).
Intravenous magnesium sulfate does not improve clinical outcome after aSAH; therefore, routine administration of magnesium might not be recommended (class IIb, level of evidence A).
Higher haemoglobin levels at baseline indicates better outcome. However, packed red blood cell transfusion is not recommended (class IIb, level of evidence B).
For patients with SAH with severe CVS, endovascular treatment with balloon angioplasty may be considered (class IIb, level of evidence B).
Routine prophylactic antiepileptic drug use is not recommended. Such practice might increase the risk of CVS and DCI (class III, level of evidence A).
For patients with SAH with aneurysms located in the lateral fissure or having a convex SAH, there is a higher risk of seizure. If there is no contraindication, it is reasonable to treat with interventional embolisation instead of surgical clipping (class IIa, level of evidence B).
For treating secondary epilepsy in patients with SAH, long-term use of antiepileptic drugs is recommended (class IIa, level of evidence B).
Non-neurological complications
Recommendations
Patients with SAH developed pneumonia have a poor prognosis and increased mortality (class IIa, level of evidence B).
Patients with SAH who are older, developed status epilepticus, with severe SAH and are ventilator dependent have a higher risk of developing pneumonia (class IIb, level of evidence B). If not contraindicated, prophylactic treatment with antibiotics and early use of tracheostomy might be beneficial (class IIb, level of evidence C).
Asymptomatic lower extremity DVT is not uncommon in patients with SAH and is associated with prolonged hospital stay and poor prognosis (class IIa, level of evidence A).
The risk of DVT is relatively high in male patients postaneurysmal surgery who are bedridden and with severe SAH (class IIa, level of evidence B). Subcutaneous or intravenous heparin may be effective to prevent DVT if not contraindicated (class IIb, level of evidence B).
Routine 3-hour therapy is not recommended in patients with SAH. It may benefit some patients with hypovolaemia (class III, level of evidence B).
For patients with refractory hyponatraemia, syndrome of inappropriate antidiuretic hormone secretion or cerebral salt wasting syndrome, sodium correction with restriction of fluids may be indicated and close monitoring of central venous pressure may be helpful (class IIb, level of evidence C).
Footnotes
Y-XG and QD contributed equally.
Collaborators: Chinese Stroke Association Stroke Council Guideline Writing Committee: Yongjun Wang (yongjunwang@ncrcnd.org.cn, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Jizong Zhao (zhaojz205@163.com / zhaojz@public.bta.net.cn, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Vice-Chairman: Anding Xu (tlil@jnu.edu.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital, Jinan University, Guangzhou, China), Members of Academic Committee: Kangning Chen (ckn_640827@126.com, Department of Neurology, The Southwest Hospital, the First Affiliated Hospital of Third Military Medical University, Chongqing, China), Junbo Ge (ge.junbo@zs-hospital.sh.cn, Shanghai Institute of Cardiovascular Diseases, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China), Li Guo (guoli6@163.com, Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, China), Li He (heli2003new@126.com, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China), Bo Hu (hubo@hust.edu.cn, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (HUST), Wuhan, China), Yong Huo (huoyong@263.net.cn, Department of Cardiology, Peking University First Hospital, Beijing, China), Linong Ji, (jiln@bjmu.edu.cn, Department of Endocrinology and Metabolism, Peking University People's Hospital, Medicine at Peking University, Beijing, China), Xunming Ji (robertjixm@hotmail.com / jixunming@vip.163.com, Department of Neurosurgery, Xuanwu Hospital, Capital University of Medicine, Beijing, China), Tielin Li (ielin2013@126.com / tielin.li@tom.com, Zhujiang Hospital of Southern Medical University, Guangzhou, China), Liping Liu (lipingsister@gmail.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Benyan Luo (luobenyan@zju.edu.cn, Department of Neurology, 1st Affiliated Hospital of Zhejiang University, Hangzhou, China), Zhongrong Miao (zhongrongm@163.com, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Xiaoyuan Niu (niuxiaoyuan1958@163.com, Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, China), Bin Peng (pengbin3@hotmail.com, Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China), Dingfeng Su (dfsu@smmu.edu.cn, Department of Pharmacology, the Second Military Medical University (SMMU), Shanghai, China), Beisha Tang (bstang7398@163.com, Department of Neurology, Xiangya Hospital, Central South University, Changsha, China), Chen Wang (wangchen-tr2002@163.com, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Ning Wang (nwang900@yahoo.com, Department of Neurology and Institute of Neurology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China), Shuo Wang (captain9858@vip.sina.com, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Wei Wang (wwang@vip.126.com / wwang@tjh.tjmu.edu.cn, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China), Xin Wang (wang.xin@zs-hospital.sh.cn, Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China), Yilong Wang, (yilong528@aliyun.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China), Shizheng Wu (wushizheng2005@hotmail.com, Qinghai Province People's Hospital, Xining, China, Peng Xie (xiepeng@cqmu.edu.cn, Chongqing Medical University (CQMU), Chongqing, China), Yuming Xu (13903711125@126.com / xym13903711125@126.com, Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China), Yun Xu (xuyun20042001@aliyun.com, Department of Neurology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China), Yi Yang (doctoryangyi@163.com / doctor_yangyi@hotmail.com, Department of Neurology, the First Hospital of Jilin University, Changchun, China), Jinsheng Zeng (zengjs@pub.guangzhou.gd.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital of Sun Yat-Sen University, Guangdong, China), Chaodong Zhang (scdzhang@163.com, The First affiliated Hospital of China Medical University, Shenyang, China), Tong Zhang (zt61611@sohu.com, Capital Medical University School of Rehabilitation Medicine, China Rehabilitation Research Center, Beijing, China), Zhuo Zhang (zzhuo005@gmail.com, Beijing Anzhen Hospital, Capital Medical University, Beijing, China), Gang Zhao (zhaogang@fmmu.edu.cn, Department of Neurology, Xijing Hospital, The 4th Military Medical University, Xi’an, China), Xingquan Zhao (zxq@vip.163.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China).
Contributors: QD and Y-XG designed the protocol and framework and also participated in revision. YD drafted the sections of diagnosis strategy and general management and revised the whole manuscript. Z-NG drafted the section of epidemiology and initial imaging process. QL drafted the section of medicine treatment. WN drafted the section of surgical treatment. HG reviewed all the studies’ design and interpretation, and confirmed the level of evidence and classification.
Funding: This research received specific funding from Chinese Stroke Association Guidelines Writing Committee.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Chinese Stroke Association Stroke Council Guideline Writing Committee:
Yongjun Wang, Jizong Zhao, Anding Xu, Kangning Chen, Junbo Ge, Li He Li Guo, Bo Hu, Yong Huo, Linong Ji, Xunming Ji, Tielin Li, Liping Liu, Benyan Luo, Zhongrong Miao, Bin Peng XiaoyuanNiu, Beisha Tang DingfengSu, Chen Wang, Ning Wang, Shuo Wang, Wei Wang, Xin Wang, Yilong Wang, Shizheng Wu, Peng Xie, Yuming Xu, Yun Xu, Yi Yang, Jinsheng Zeng, Chaodong Zhang, Tong Zhang, Zhuo Zhang, Gang Zhao, and Xingquan Zhao
References
- 1. de Rooij NK, Linn FHH, van der Plas JA, et al. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. Journal of Neurology, Neurosurgery & Psychiatry 2007;78:1365–72. 10.1136/jnnp.2007.117655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu G, Li Y, Cheng G, et al. Incidence of aneurysmal subarachnoid hemorrhage in Baotou, inner Mongolia. Journal of Brain and Neurological Diseases(Chinese) 2016;11:670–4. [Google Scholar]
- 3. Jiang B, Wang W-zhi, Chen H, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke 2006;37:63–8. 10.1161/01.STR.0000194955.34820.78 [DOI] [PubMed] [Google Scholar]
- 4. Liang W, Huang R, Lee AH, et al. Hospitalizations for incident stroke in Shunde district, Foshan, South China. Neuroepidemiology 2008;30:101–4. 10.1159/000120022 [DOI] [PubMed] [Google Scholar]
- 5. Zhang W. Epidemiological investigation on cerebral apoplexy in community of Chendu City. West China Medical Journal(Chinese) 2011;01:41–3. [Google Scholar]
- 6. Lin H-L, Soo K-M, Chen C-W, et al. Incidence, National trend, and outcome of nontraumatic subarachnoid haemorrhage in Taiwan: initial lower mortality, poor long-term outcome. Biomed Res Int 2014;2014:1–5. 10.1155/2014/274572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon J-W, Lee HJ, Hyun MK, et al. Trends in the incidence of subarachnoid hemorrhage in South Korea from 2006–2009: an ecological study. World Neurosurg 2013;79:499–503. 10.1016/j.wneu.2012.07.032 [DOI] [PubMed] [Google Scholar]
- 8. Gross BA, Frerichs KU, Du R. Sensitivity of CT angiography, T2-weighted MRI, and magnetic resonance angiography in detecting cerebral arteriovenous malformations and associated aneurysms. J Clin Neurosci 2012;19:1093–5. 10.1016/j.jocn.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 9. Khurram A, Kleinig T, Leyden J. Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 2014;45:1151–3. 10.1161/STROKEAHA.113.004298 [DOI] [PubMed] [Google Scholar]
- 10. Theodorou A, Lachanis S, Alexopoulos P, et al. Teaching NeuroImages: acute convexity subarachnoid hemorrhage: an underrecognized presentation of CAA-ri. Neurology 2019;93:e524-e525 10.1212/WNL.0000000000007873 [DOI] [PubMed] [Google Scholar]
- 11. Calviere L, Viguier A, Patsoura S, et al. Risk of intracerebral hemorrhage and mortality after convexity subarachnoid hemorrhage in cerebral amyloid angiopathy. Stroke 2019;50:2562–4. 10.1161/STROKEAHA.119.026244 [DOI] [PubMed] [Google Scholar]
- 12. Vieco PT, Shuman WP, Alsofrom GF, et al. Detection of circle of Willis aneurysms in patients with acute subarachnoid hemorrhage: a comparison of CT angiography and digital subtraction angiography. AJR Am J Roentgenol 1995;165:425–30. 10.2214/ajr.165.2.7618571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
svn-2019-000296supp001.pdf (500.4KB, pdf)


