Summary
Microphthalmia-associated transcription factor (Mitf) is essential for melanocyte development and function and regulates anti-apoptotic Bcl2 expression. We hypothesized that cellular deficiency of Mitf can influence melanocyte survival in response to ultraviolet (UV) radiation. Primary melanocyte cultures were prepared from neonatal wild-type mice and congenic animals heterozygous for Mitf mutations Mitfmi-vga9/+ and MitfMi-wh/+ and exposed to UV irradiation. Wild-type melanocytes were more resistant to UV-induced apoptosis than melanocytes partially deficient in Mitf activity, as determined by relative levels of intracellular melanin and relative activation of Mitf target genes Tyr, Tyrp1, Dct, and Cdk2. Comparative experiments with wild-type cells and congenic albino melanocytes demonstrated that these differences are not due to differences in melanin content, implicating Mitf as a primary determinant of UV-dependent melanocyte survival. Mitf activity correlated directly with resistance to UV-induced apoptosis in melanocytes. Mitf was important not only for regulating the expression of anti-apoptotic Bcl-2 following UV irradiation, but also the expression of the pro-apoptotic BH3-only Bad protein and activation of the extrinsic apoptotic pathway. Hence, Mitf is a multifaceted regulator of UV-induced apoptosis in melanocytes.
Keywords: apoptosis, melanocyte, melanoma, Mitf, ultraviolet, Fas, mice
Introduction
Although skin cancer is the most common malignancy in the United States, understanding its causes, particularly those of melanoma—the most lethal form of skin cancer—is limited. Ultraviolet (UV) radiation (280–400 nm) is the major environmental carcinogen for non-melanoma skin cancer and episodes of sunburn from solar or tanning bed UV radiation during childhood to young adulthood significantly increase the risk of developing melanoma (Lindholm et al., 2004; Young, 2004). As a first line of defense against chemical and physical agents, the skin has evolved special mechanisms to protect against damage by UV radiation. Melanocytes comprise approximately 4% of the cells in human epidermis. They protect the skin from photodamage by producing melanin, a pigment with natural sunscreen and antioxidant properties, and transferring melanin to keratinocytes (De Leeuw et al., 2001; Hoogduijn et al., 2004; Smit et al., 2001; Tadokoro et al., 2003, 2005b). Melanocytes have a low capacity for self-renewal. Therefore mechanisms that regulate melanocyte survival and maintenance of genomic stability are important in preventing cell death and malignant transformation.
The microphthalmia-associated transcription factor (MITF/Mitf) is a master regulator of melanocyte differentiation, development and survival (Goding, 2000; Steingrimsson et al., 2004; Widlund and Fisher, 2003). MITF/Mitf is a basic helix-loop-helix leucine zipper (bHLHzip) transcription factor that is critical in the transcription of pigment pathway genes such as tyrosinase (Tyr), tyrosinase-related protein-1 (Tyrp-1) and dopachrome tautomerase (Dct) (Bentley et al., 1994; Du et al., 2003; Jiao et al., 2004; Ludwig et al., 2004; Schwahn et al., 2005; Tomita et al., 1989; Yasumoto et al., 1997). MITF/Mitf binds to the M-box motif in these promoters to increase their expression and production of melanogenic proteins. In humans, mutations in the MITF gene lead to Waardenburg Syndrome type IIa which is characterized by skin and hair discoloration and deafness due to a lack of melanocytes in skin, hair, and inner ear (Price and Fisher, 2001; Tassabehji et al., 1994).
Microphthalmia-associated transcription factor/Mitf controls the survival of both melanocytes and melanoma cells through its regulation of the anti-apoptotic molecule Bcl-2 (McGill et al., 2002), which is expressed at high levels in the skin (Plettenberg et al., 1995). MITF is a substrate for caspase cleavage in human melanocytes and melanoma cells (Larribere et al., 2005). The C-terminal fragment of MITF protein generated by caspase cleavage has proapoptotic activity that sensitizes melanoma cells to death signals while an uncleavable form of MITF renders them resistant to apoptosis. Thus, the balance between the cleaved and uncleaved form of MITF influences melanocyte survival. Overexpression of MITF enhanced melanocyte survival and contributed to malignant transformation (Garraway et al., 2005). However, overexpression of Bcl-2 in MITF-deficient cells only partially rescued them from apoptosis, suggesting that MITF regulates additional survival genes. For example, Busca et al. (2005) showed that MITF binds to the Hif-1α promoter, strongly stimulating Hif-1α gene expression which in turn, reduced caspase 3 cleavage.
Ultraviolet radiation induces the production of nerve growth factor, stem cell factor, α-MSH and a variety of cytokines by non-melanocytic cells in the skin which can inhibit apoptosis in melanocytes by inducing DNA repair and upregulating Bcl-2 or reducing Bax production (Blume-Jensen et al., 2000; Bohm et al., 2005; Karlsson et al., 2003; Ono and Han, 2000; Rosette and Karin, 1996; Sakata et al., 2003; Zhai et al., 1996). However, the role MITF plays in mediating the effects of UV radiation on melanocyte function and survival is poorly understood. UV radiation can stimulate melanogenesis by directly increasing MITF expression (Kim et al., 2003; Tadokoro et al., 2005a) or MITF promoter activity (Lin et al., 2002), and indirectly by triggering Akt, p38 and cAMP pathways which activate the transcription factors cAMP response element binding protein (CREB), NFkB and MITF (Goding, 2000; Kadekaro et al., 2003; Goding, 2000; Kadekaro et al., 2003). Phosphorylation of p38 activates the Usf-1 transcription factor, which is required for UV activation of the tyrosinase promoter, pro-opiomelanocortin (POMC) and MC1R genes that play a crucial role in regulating melanin synthesis (Corre et al., 2004; Galibert et al., 2001).
Because exposure of melanocytes to UV radiation is an important risk factor in the etiology of melanoma, we investigated whether mutations in Mitf may influence apoptosis in melanocytes after UV exposure. We used two congenic strains of mice, C57BL/6J-MitfMi-wh/+ and C57BL/6J-Mitfmi-vga9/+, that are heterozygous for mutations in the Mitf gene to examine the role of Mitf in regulating mitochondrial and receptor-triggered apoptotic pathways in cultures of UV-irradiated primary melanocytes.
Results
Mice with Mitf mutations and the primary cultures of melanocytes established from them
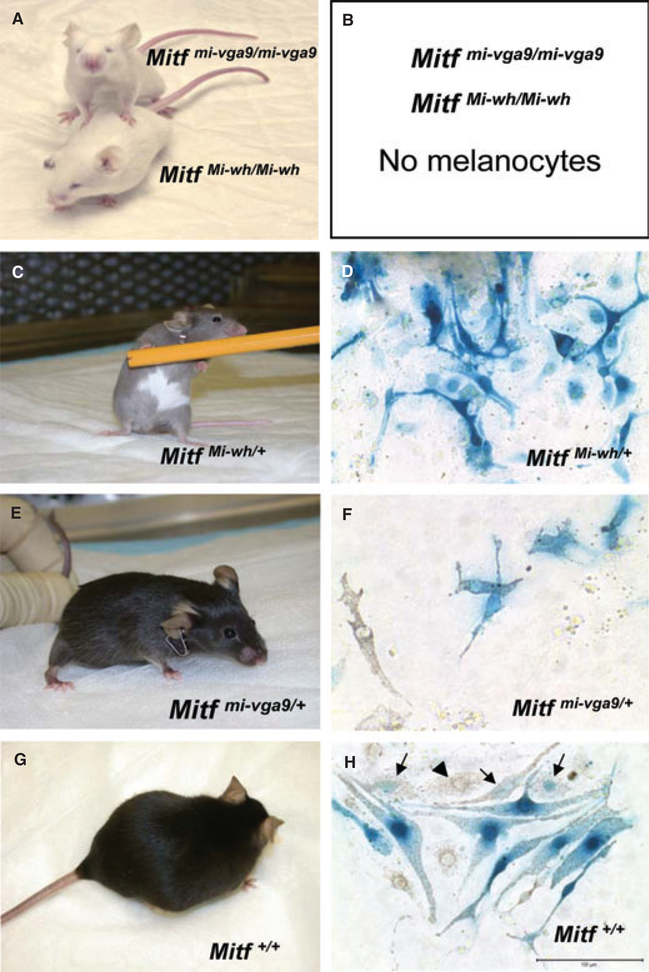
We investigated the role of Mitf in regulating apoptotic pathways using cells derived directly from mice with Mitf mutations with distinct pigmentary and molecular phenotypes. Over two dozen spontaneous and induced mutations in Mitf have been described to date (Steingrimsson et al., 2004). We used mice with two distinct Mitf mutations, Mitf Mi-wh and Mitf mi-vga9, whose mutations can affect coat color either in the homozygous (C57BL/6-Mitf Mi-wh/Mi-wh and C57BL/6-Mitf mi-vga9/mi-vga9 ) or heterozygous (C57BL/6-Mitf Mi-wh/+ only) states (Figure 1). The Mitf Mi-wh mutation is an Ile212Asn substitution that affects the DNA-binding domain of the Mitf protein, resulting in severely defective DNA-binding activity and, in the absence of the alternatively-spliced 6 amino acid exon 6A, a dominant-negative effect (Hemesath et al., 1994; Steingrimsson et al., 2004). Mice homozygous for the Mi-wh mutation (Figure 1A) have white coats, small, slightly pigmented eyes, inner ear defects and reduced fertility. Heterozygotes have a substantial ventral white spot and a gray-brown hypopigmented coat color on the C57BL/6 background (Figure 1C) (Steingrimsson et al., 2004). In contrast, the transgene insertional mutation mi-vga9 severely reduces the expression of the Mitf gene (Hodgkinson et al., 1993; Tachibana et al., 1992). Mice homozygous for this mutation (Figure 1A), like C57BL/6-Mitf Mi-wh/Mi-wh mice, lack melanocytes, resulting in a white coat phenotype with small red eyes and hearing defects (Hodgkinson et al., 1993; Steingrimsson et al., 2004). Heterozygotes have a normal pigmented phenotype (Figure 1E), although, unlike wild-type mice, they can exhibit a visible phenotype in the compound heterozygous state with other Mitf mutant alleles (Bismuth et al., 2008; Goding, 2000). Hence, C57BL/6 mice and congenic C57BL/6-Mitf Mi-wh/+ and C57BL/6-Mitf mi-vga9/+ mice comprise an allelic series of increasing functional severity on both the phenotypic and molecular level (C57BL/6-Mitf Mi-wh/+ > C57BL/6-Mitf mi-vga9/+ > C57BL/6). This provides a convenient framework for investigating the effects of relative Mitf dosage in primary cells derived from the mice. In order to examine the effects of these mutations on melanocytes, congenic mice heterozygous for these mutations were created by crossing male C57BL/6-Mitf Mi-wh/Mi-wh and C57BL/6-Mitf mi-vga9/mi-vga9 mice with C57BL/6J-[Tg]Dct-LacZ females (Hornyak et al., 2001). Intercross with the C57BL/6J-[Tg]Dct-LacZ mice and expression of the Dct-LacZ transgene permitted the identification of cells of melanocytic lineage in cultures from these animals.
Figure 1.
Phenotype of Mitf mutant mice and their melanocytes. (A) C57BL/6J-MitfMi-wh/Mi-wh and C57BL/6J-Mitfmi-vga9/mi-vga9 homozygous mutant mice. The homozygous mutants lack melanocytes, have microphthalmia (small eyes) and are deaf due to defective melanocyte development. (B) No melanocytes can be isolated from (A). (C) C57BL/6J-MitfMi-wh/+ heterozygous mouse. The Mi-wh mutation in Mitf has a dominant negative effect in vitro and a semi-dominant phenotype in vivo, resulting in grey coat color and large belly spot. (D) Cultured C57BL/6J-MitfMi-wh/+ melanocytes expressing [Tg]Dct-lacZ transgene and stained with X-gal. (E) C57BL/6J Mitfmi-vga9/+ heterozygous mouse. (F) Cultured C57BL/6J-Mitfvga–9/+ melanocytes expressing [Tg]Dct-lacZ transgene and stained with X-gal. (G) C57BL/6 mouse with wild type Mitf. (H) Cultured C57BL/6J wild-type melanocytes expressing [Tg]Dct-lacZ transgene and stained with X-gal. Arrows indicate melanin-containing cells that are losing their [Tg]Dct-lacZ expression. Arrowhead indicates a melanin-containing melanocyte no longer expressing [Tg]Dct-lacZ. All melanocyte cultures were stained for β-galactosidase expression with X-gal (blue). Bar = 100 μm.
Primary cultures of dermal cells from newborn heterozygous and wild-type Mitf mice initially contained approximately 2–4% melanoblasts and melanocytes, detected by their expression of the Dct-LacZ reporter transgene (not shown). Following two rounds of geneticin selection removing rapidly dividing fibroblasts (Halaban and Alfano, 1984), 75–90% of the remaining cells were of the melanocytic lineage, positive for LacZ expression. The time from initial isolation of dermal cells from newborn mice to the time of study after two rounds of geneticin treatment was approximately 1 month.
Cultures of dermal cells from C57BL/6-MitfMi-wh/+ [Tg]Dct-LacZ mice generally lacked visible melanin but were identifiable as melanoblasts or melanocytes because of their dendritic morphology and the presence of blue stain for β-galactosidase (Figure 1D). Melanocytes from C57BL/6-Mitfmi-vga9/+ [Tg]Dct-LacZ mice were initially dendritic and unpigmented but began to lose their dendrites and acquired melanin by the third week of culture (Figure 1F). Interestingly, we noted that melanocyte cultures from wild type C57BL/6-Mitf+/+[Tg]Dct-LacZ mice (Figure 1H, arrows and Figure S1) tended to lose dendricity and evidence of Dct:LacZ activity as cytoplasmic melanin granules appeared.
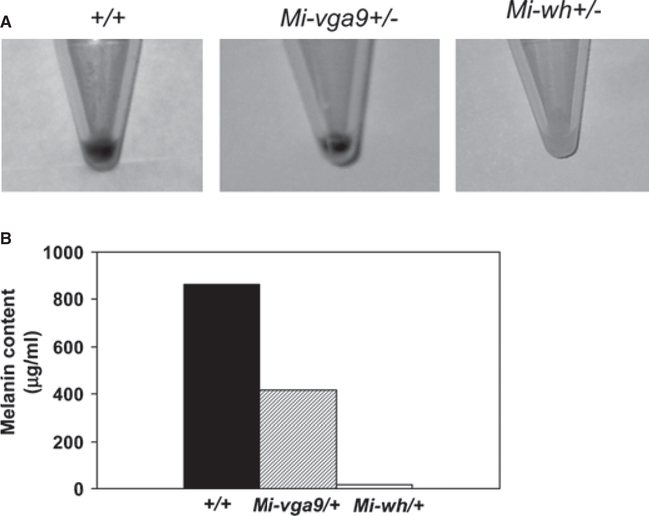
The melanin content of the cultures was determined chemically (Figure 2B). C57BL/6-Mitf+/+[Tg]Dct-LacZ cells with wild-type Mitf produced twice as much melanin as melanocytes from C57BL/6-Mitfmi-vga9/+[Tg] Dct-LacZ mice even though these animals are phenotypically indistinguishable from the congenic C57BL/6-Mitf+/+[Tg]Dct-LacZ controls. Melanocytes from C57BL/6-MitfMi-wh/+[Tg]Dct-LacZ mice contained minimal melanin both visually (Figure 2A right panel) and chemically (Figure 2B).
Figure 2.
Melanin content of primary melanocyte cultures. Equal numbers (1 × 106) of cells from wild-type C57BL/6J, C57BL/6J-Mitfmi-vga9/+, and C57BL/6J-MitfMi-wh/+ mice were pelleted (A) and melanin extracted. The melanin content of the cells is shown in (B).
Mitf gene mutations, melanin, and UV-induced cell survival
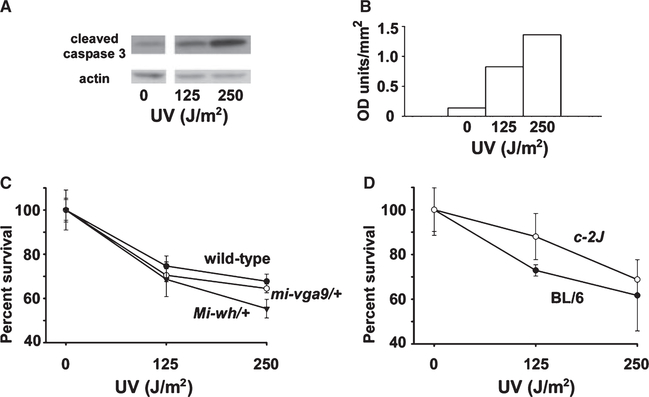
Primary melanoblasts/melanocytes from the three genotypes C57BL/6-Mitf+/+, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ were treated with a range of UV exposures to determine their sensitivity to UV-induced apoptosis. Figure 3C demonstrates that cell survival declined after 24 h with increasing doses of UV radiation. Melanocytes from C57BL/6-MitfMi-wh/+ heterozygotes were most sensitive to UV, with decreasing UV sensitivity observed for C57BL/6-Mitfmi-vga9/+ cells and wild-type cells, respectively (Figure 3C). All three genotypes showed marked toxicity in response to UV exposure. Activation of caspase 3, as evidenced by the appearance of cleaved caspase 3 (Figure 3A), was induced in a dose-dependent fashion in melanocytic cultures over a range of 0–250 J/m2 UV radiation.
Figure 3.
Dose response of melanocytes to UV radiation. Primary cultures of melanocytes and melanoblasts from neonatal mice were exposed in serum-free PBS to 0, 125 or 250 J/m2 UV radiation. After washing, fresh media was added and the culture continued for 24 h at 37°C after which the cells were lysed for determination of protein expression with Western blotting (A, B) or utilized in the MTS assay for determination of relative viable cell number. (A) Expression of cleaved caspase 3 in primary C57BL/6 wild-type melanocytes following exposure to increasing doses of UV. β-actin was used as a loading control. (B) Densitometry analysis of (A), with cleaved caspase 3 normalized to actin. (C) Viability of melanocyte cultures from C57BL/6 and congenic C57BL/6J-MitfMi-wh/+ and C57BL/6J-Mitfmi-vga9/+ neonatal mice following irradiation with increasing doses of UV, measured with the MTS assay. Viability decreases as a function of increased Mitf mutational severity (wild-type >Mi-wh/+ > mi-vga9/+). (D) Viability of melanocyte cultures from C57BL/6 and congenic albino C57BL/6J-Tyrc−2J neonatal mice following irradiation with increasing doses of UV, measured with the MTS assay. No significant difference was observed between the viability of albino and pigmented melanocytes following UV irradiation. Error bars represent standard error of the mean.
The reduced survival of melanocytes heterozygous for mutations in Mitf upon UV irradiation (Figure 3C) implied that the effective intracellular dosage of Mitf might be a primary factor determining melanocyte survival resistance to UV. However, it remained possible that lower amounts of melanin in the irradiated Mitf melanocytes (Figure 2B), rather than Mitf dosage itself, were the primary determinant of survival. To distinguish between these possibilities, we isolated both pigmented and non-pigmented, albino primary murine melanocytes. Primary cultures were developed from C57BL/6 and congenic, albino C57BL/6-Tyrc−2J mice to test directly the effect of melanin upon melanocyte survival following UV irradiation. Because these mice lacked the Dct-lacZ transgene, we utilized immunofluorescence with an antibody targeting the melanocyte marker Tyrp1 to assess enrichment of the cultures for melanocytes.
In contrast to the results obtained with Mitf mutant melanocytes, UV irradiation of wild-type C57BL/6 primary melanocytes and congenic albino C57BL/6-Tyrc−2J melanocytes revealed comparable amounts of cell death with increasing UV doses, with a trend to enhanced survival of albino melanocytes under these conditions (Figure 3D, Figure S3). Use of an anti-Tyrp1 antibody for immunofluorescence identification of melanocytes in these cultures revealed that 70–90% of cells in this experiment exhibited Tyrp-1 immunoreactivity (Figure S2), comparable to the enrichment observed in the previously described experiments. In a separate experiment, using an independently isolated set of melanocytes enriched at a lower level (40–60%), similar results were obtained (Figure S3). These results establish Mitf as a critical determinant of UV-induced melanocyte resistance to death, and also demonstrate that the effect of Mitf is a primary effect of Mitf on intracellular survival mechanisms rather than a secondary effect due to its regulation of the amount of intracellular melanin.
Mitf gene mutations, melanin, and UV-induced apoptosis
The previous experiments established a primary role for Mitf protecting melanocytes from UV-induced death. Mitf directly regulates the anti-apoptotic gene Bcl2 (Mcgill et al., 2002), which is reduced in primary human melanocytes following UV irradiation (Bohm et al., 2005; Kadekaro et al., 2005). Based upon these observations, we proposed that diminished Mitf-dependent regulation of Bcl2 might underlie enhanced cell death in UV-irradiated, Mitf-deficient melanocytes, and examined the effect of UV on key apoptotic pathway components in these cells.
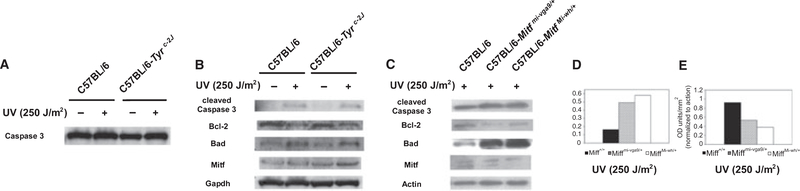
Caspase 3, the effector caspase (Youle and Strasser, 2008), was expressed at comparable levels in pigmented C57BL/6 and albino C57BL/6-Tyrc−2J melanocytes, although following 250 J/m2 UV irradiation, slightly more could be detected in each of these cell types (Figure 4A). Consistent with a role for apoptosis in UV-induced cell death, UV enhanced production of cleaved caspase 3 in both pigmented and albino cells (Figure 4B). UV not only consistently diminished amounts of anti-apoptotic Bcl-2, as in human melanocytes (Bohm et al., 2005; Kadekaro et al., 2005), but also increased its pro-apoptotic, BH3-only interactor Bad in these cells. The similarity between these changes observed in pigmented and albino melanocytes, which also exhibited comparable levels of Mitf that may have been slightly increased in each following UV irradiation (Figure 2B), are consistent with their similar rates of UV-induced cell death (Figure 3D, Figure S3).
Figure 4.
Effect of UV on apoptosis in primary wild-type, albino, and Mitf-deficient melanocytes. Primary cultures of C57BL/6, C57BL/6-Tyrc−2J, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ melanocytes were exposed to 0 or 250 J/m2 UV radiation. Expression of apoptotic and anti-apoptotic proteins was studied using Western blotting from cell lysates collected 24 h later. (A) Expression of caspase 3 by C57BL/6 and C57BL/6-Tyrc−2J melanocytes. (B) Expression of apoptosis-related proteins caspase 3 (cleaved), Bcl-2, and Bad in irradiated and unirradiated pigmented C57BL/6 and albino C57BL/6-Tyrc−2J primary melanocytes. (C) Expression of apoptosis-related proteins in irradiated C57BL/6, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ primary melanocytes. (D) Quantification of cleaved caspase 3 expression in irradiated C57BL/6, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ primary melanocytes in (C). (E) Ratios of quantified Bcl-2 and Bad expression (Bcl-2:Bad ratio) from irradiated C57BL/6, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ primary melanocytes in (C).
In contrast to congenic pigmented and albino melanocytes, UV irradiation induced changes in cleaved caspase 3, Bcl-2, and Bad between pigmented C57BL/6 melanocytes and their congenic, Mitf-deficient counter-parts C57BL/6-MitfMi-wh/+ and C57BL/6-Mitfmi-vga9/+. Increased cleaved caspase 3 was generated in C57BL/6-MitfMi-wh/+ and C57BL/6-Mitfmi-vga9/+ melanocytes following UV (Figure 4C, D). Compared to the slightly lowered level of Bcl-2 observed in normoallelic, or wild-type, Mitf melanocytes following UV (Figure 4B), Bcl-2 in Mitf-deficient melanocytes was decreased even further (Figure 4C). Bad, which was slightly increased in wild-type Mitf melanocytes (Figure 4B), was induced disproportionately in Mitf-deficient cells (Figure 4C). C57BL/6-Mitfmi-vga9/+ and C57BL/6-MitfMi-wh/+ exhibited a profound (43 and 61% respectively) decrease in the ratio of Bcl-2 to Bad when compared to melanocytes with wild-type Mitf following UV exposure (Figure 4E). The magnitude of these changes correlates directly with the propensity of these cells to exhibit enhanced cell death following UV (Figure 3C). These results demonstrate that the intracellular level of Mitf regulates the Bcl-2:Bad ratio in melanocytes following UV irradiation.
UV has been reported to activate directly the extrinsic, or death-receptor (Fas/CD95) apoptotic pathway independent of ligand binding (Aragane et al., 1998), prompting us to examine key elements of this pathway as well in Mitf-deficient melanocytes. UV exposure slightly induced Fas/CD95, and resulted in the generation of significantly more cleaved caspase 8, in Mitf-deficient melanocytes (Figure S4A, B). The expression of pro-apoptotic Bax was unchanged, and the expression of pro-apoptotic Bak varied slightly without a clear trend (Figure S4). These results suggest, first of all, that extrinsic pathway activation may contribute to enhanced apoptotic cell death in UV-irradiated, Mitf-deficient melanocytes. They also suggest that increased induction of pro-apoptotic Bcl-2 homologs is not responsible for enhanced apoptosis in the Mitf mutant cells studied. Instead, this data supports a model whereby inactivation of Bcl-2 by increased expression of Bad, and perhaps other Bcl-2-interacting BH3-only family members (Youle and Strasser, 2008), alone is sufficient to trigger increased apoptosis via the intrinsic pathway in Mitf-deficient cells.
UV irradiation and MITF-dependent gene expression
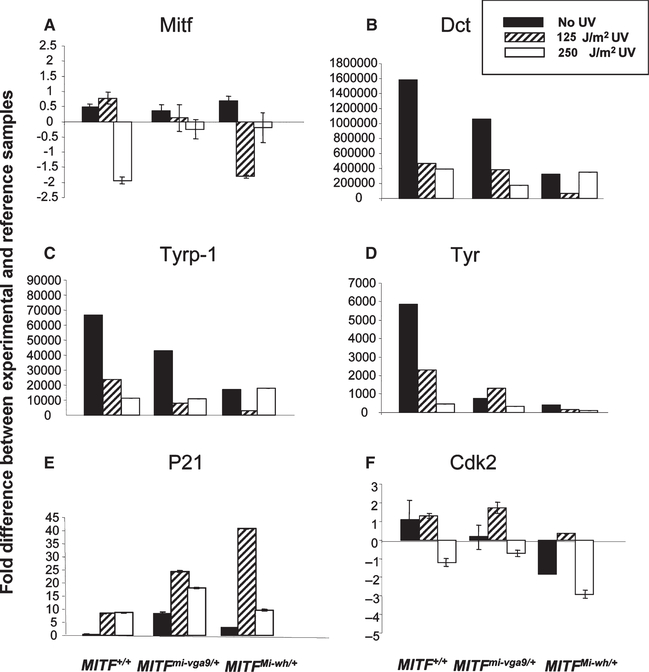
The preceding results demonstrate that the resistance of melanocytes to UV-induced death, substantiated by changes in molecular markers of apoptosis, increases according to the order MitfMi-wh/+ < Mitfmi-vga9/+ < +/+, with the least resistance observed in melanocytes heterozygous for the MitfMi-wh mutation which has the most severe heterozygous phenotype and dominant-negative activity in vitro (Hemesath et al., 1994). The limited numbers of cells we obtained in primary cultures restricted the ability to use reporter gene assays to estimate the activity of Mitf within these cells in order to correlate precisely the resistance to UV-induced apoptosis with Mitf transcriptional activity. However, the melanogenic genes Tyr, Tyrp1, and Dct are known transcriptional targets of Mitf (Bentley et al., 1994; Jiao et al., 2004; Yavuzer and Goding, 1994), as are the cell cycle regulatory genes Cdk2 (Du et al., 2004) and p21 (Carreira et al., 2005). We examined the expression of these Mitf target genes in +/+, Mitfmi-vga9 /+, and MitfMi-wh/+ cells using quantitative RT-PCR to assess their relative levels of Mitf activity.
Wild-type, C57BL/6-Mitfmi-vga9/+, and C57BL/6-Mitf Mi-wh/+ melanocyte cultures were irradiated and RNA extracted 24 h later to measure UV-dependent changes in gene expression. Although the relative level of Mitf gene transcription was similar in unirradiated cells regardless of mutational status, the expression of Mitf diminished with UV irradiation in the mutant cells and at the higher UV dose in wild-type cells (Figure 5A). Despite the comparable levels of Mitf in unirradiated cells, the expression of the Mitf target genes Tyr, Dct/Tyrp2, and Tyrp1 was highest in the cells with wild type Mitf, intermediate in Mitfmi-vga9/+, and lowest in MitfMi-wh/+ mutants (Figure 5B–D). These findings demonstrate a direct correlation between the estimated level of Mitf activity in each of these cell types and the expression of melanogenic genes known to be regulated by Mitf, and also are consistent with the amounts of melanin produced by the cells of each genotype (Figure 2). The expression of Cdk2 varied similarly in unirradiated cells (Figure 5F), whereas the expression of p21 was relatively low in unirradiated cultures and did not follow a similar pattern (Figure 5E).
Figure 5.
Real-time PCR analysis of RNA from melanocyte cultures. Cultures of melanocytes from the genotypes shown in Figure 1 were subjected to 0, 125 J/m2 or 500 J/m2 UV radiation and their RNA harvested 24 h later as described in the Methods. Data are expressed as the fold difference in gene expression between the experimental sample and a reference sample standard, as described in the Methods, using β-actin as an internal control.
As with Mitf, UV radiation decreased transcription of its melanogenic target genes Tyr, Tyrp1, and Dct in all three genotypes (Figure 5B–D), consistent with the reduction in Mitf expression that was observed. In contrast, p21 expression was strongly induced by UV (Figure 5E), similar to the induction observed previously following irradiation of fibroblasts (Reinke and Lozano, 1997) and normal human skin (Murphy et al., 2002).
Discussion
Previous studies showed that Mitf regulates Bcl-2 expression (McGill et al., 2002) and that Bcl-2 activity is crucial for the establishment of the melanocyte stem cell (Nishimura et al., 2002) in the follicular niche (Nishimura et al., 2005). Primary cultures of murine Bcl2−/− melanocytes showed increased cell death following UV irradiation (Gillardon et al., 1999). These observations on the role of Mitf and Bcl-2 in melanocyte survival in one context, the maintenance of the melanocyte stem cell, together with the effect of Bcl-2 on melanocyte survival following UV stimulation, suggested that Mitf might play a role in melanocyte survival following UV stimulation as well.
The present study examined the apoptotic response to UV radiation by primary murine melanocytes and whether mutations in Mitf influence activation of apoptotic pathways. The results show that in melanocytes, UV-induced apoptosis is more complex than previously appreciated and is controlled at multiple points involving both the intrinsic (mitochondrial) and extrinsic (death receptor) pathways. We found that, in the absence of Mitf mutations (i.e., with wild-type Mitf), UV induces cell death in primary murine melanocytes, confirming the results demonstrated previously with human (Bohm et al., 2005; Kadekaro et al., 2005; Zhai et al., 1996) and murine (Gillardon et al., 1999; Hill et al., 1997) melanocytes. UV irradiation decreases the expression of Bcl-2 in primary murine melanocytes, extending results obtained previously with primary human melanocytes (Bohm et al., 2005; Kadekaro et al., 2005). In addition to reducing Bcl-2, UV increases expression of the pro-apoptotic BH3-domain protein Bad, a new finding. An increase in the Bad:Bcl-2 ratio is consistent with an apoptotic mechanism of death for these cells. These changes are present and similar in both congenic pigmented and albino melanocytes, correlating with the equivalent amounts of UV cell death we found in these cell types. A previous study (Hill et al., 1997) found a lower rate of UV-induced cell death from combined UVA and UVB (FS20 lamps) in albino melan-c melanocytes, an immortalized murine cell line derived from albino mice, compared to pigmented melan-a melanocytes, immortalized from C57BL/6-derived melanocytes. Our data (Figure 3D, Figure S3) revealed a trend to increased survival of primary albino C57BL/6-Tyrc−2J melanocytes, a finding consistent with the aforementioned results from immortalized cells, although this difference did not reach levels of statistical significance. It is possible that the increased range of UV irradiation used in the previous experiments, inherent differences between primary and immortalized cells, or differences in the time course and assay type utilized between these two studies account for the differences.
The enhanced levels of cell death observed in Mitf-deficient primary melanocytes (Figure 3C) demonstrate that the amount of functional Mitf present intracellularly is a primary determinant of resistance to UV-induced apoptosis. Decreasing amounts of functional Mitf, due to haploinsufficiency (Mitfmi-vga9/+) or heterozygosity for a dominant-negative mutation (MitfMi-wh/+), translated into reduced melanocyte survival following UV irradiation. We found that in addition to regulating anti-apoptotic Bcl-2, Mitf also regulates levels of pro-apoptotic protein Bad. However, we did not detect a significant increase in levels of the pro-apoptotic Bcl-2 homologs Bak or Bax upon UV irradiation in Mitf-deficient melanocytes. We propose that an increase in the Bad:Bcl-2 ratio, enabling greater engagement of anti-apoptotic Bcl-2 by the pro-apoptotic BH3 ligand Bad, is sufficient to enhance apoptosis in the Mitf-deficient cells independent of increases in Bak or Bax. Such a mechanism is consistent with recent evidence supporting an indirect activation model for BH3-like ligand action (Willis et al., 2007). Consistent with our results, experiments with human melanocytes also found no change at the protein level in the expression of Bax upon UV-irradiation, although Bad was not examined in this report (Bivik et al., 2005).
The preferential activation of caspase 8 in Mitf-deficient melanocytes (Figure S4) also implicates Mitf in the regulation of extrinsic apoptotic pathway activity in UV-induced apoptosis in melanocytes. This was unexpected, because, unlike the interaction that has been described between Mitf and Bcl-2 in the intrinsic pathway, interactions between Mitf and components of the extrinsic apoptotic pathway have not been described. In a previous study, UV irradiation was found not to change the expression of FAS/CD95 in normal human melanocytes (Bohm et al., 2005). Hence, the level of FAS/CD95 expression itself, which may have varied somewhat in our results (Figure S4), is not likely to be relevant to the degree of apoptosis observed. Direct activation of FAS/CD95 in keratinocytes by UV light was observed in a prior study (Aragane et al., 1998), leaving open the possibility that Mitf regulates an intermediate step between UV-induced Fas activation and caspase 8 cleavage in melanocytes as well. Mitf is a substrate for effector caspases (Larribere et al., 2005), but it is unclear how, for example, preferential depletion of Mitf in Mitf-deficient cells could, via a feedback loop, induce preferential activation of caspase 8 upstream (Youle and Strasser, 2008). Interestingly, blockade of Kit signaling with the monoclonal antibody ACK2 results in a decline in Bcl-2 and an increase in caspase 8 cleavage (Kimura et al., 2005), findings similar to ours. Because of the dependence of Mitf activation upon Kit signaling (Hemesath et al., 1998), this may be another clue that there exists a currently undefined interaction between Mitf and the mechanism of caspase 8 activation requiring further study.
Our use of congenic mice heterozygous for distinct mutations in Mitf that affect melanocyte development and pigment production allowed us to evaluate its effects using cells progressively less functional for intact Mitf. Quantitative measurement of melanin showed that primary melanocyte cultures from C57BL/6-Mitfmi-vga9/+ heterozygotes produced only half as much melanin as cells from mice with wild-type Mitf even though their coat colors were phenotypically indistinguishable. These results suggest that coat color is only a rough indicator of melanin content. Melanin has protective effects on melanocytes (Kadekaro et al., 2003, Yamazaki et al., 2004). The level of Mitf activity, inferred both by the relative amount of melanin production in our melanocytes as well as by the relative expression levels of melanogenic enzyme genes driven by Mitf, inversely correlated with susceptibility to UV-induced apoptosis. Thus, melanocytes with wild-type Mitf were least sensitive to killing by UV and had the lowest amounts of cleaved caspase 8 and caspase 3, cells from C57BL/6-Mitf Mi-wh/+ heterozygotes were the most sensitive to UV and had the highest amounts of these fragments, and cells from C57BL/6-Mitfmi-vga9/+ heterozygotes were intermediate in these findings.
Our results demonstrate that Mitf expression in melanocytes inhibits UV-induced apoptosis via a mechanism that is independent of melanin production. They extend the role of Mitf in the prevention of apoptosis beyond its ability to transcriptionally regulate Bcl-2 to a role in shifting the balance between pro-apoptotic and anti-apoptotic molecules, which has been shown to determine a cell’s apoptotic fate (Korsmeyer, 1999; (Willis et al., 2007). In wild-type mice with the highest relative level of Mitf expression, this balance leans toward survival while decreasing the levels of Mitf expression shifts the balance toward apoptosis.
Materials and methods
Mice
C57BL/6J mice were purchased from The Jackson Laboratories (Bar Harbor, ME, USA). C57BL/6J-MitfMi-wh/MitfMi-wh and C57BL/6J-Mitfmi-vga9/Mitfmi-vga9 were a kind gift from Dr. Lynn Lamoreux, College of Veterinary Medicine, Texas A & M University. CD1 outbred mice containing the Dct-LacZ transgene (Hornyak et al., 2001) were backcrossed onto the C57BL/6J genetic background for at least six generations to produce the C57BL/6-[Tg]Dct-LacZ animals used in the experiments. Breeding colonies were maintained in our animal facility in accordance with National Institutes of Health and American Association for Assessment and Accreditation of Laboratory Animal Care International Guidelines. The animals were housed in filter-protected cages and provided with National Institutes of Health open formula mouse chow and water ad libitum. All procedures were approved by the Henry Ford Health System Institutional Animal Care and Use Committee. For experiments comparing the effects of UV radiation on albino and wild-type melanocytes, B6(Cg)-Tyrc−2J/J mice, obtained from The Jackson Laboratories, and C57BL/6 mice, obtained from the NCI-Frederick Animal Production Program (APA), were housed under similar conditions under an approved animal protocol of the Center for Cancer Research, National Cancer Institute.
Cell Cultures
Melanocytes were isolated from the skin using an adaptation of a previously described method (Abdel-Malek et al., 2001). Details are provided in Appendix S1.
UV radiation treatment
Ultraviolet irradiation was administered using either a single FS40 lamp or dual FS20 lamps (National Biological, Twinsburg, OH, USA). FS40 lamps produce 0.5% UVC (260–280 nm), 62% UVB (280–320 nm), and 38% UVA (320–400 nm) with a peak emission at 313 nm. The average irradiance of the source was approximately 1.58 ×·10−4 W/cm2 at 17 cm distance, or 1.99 ×·10−4 W/cm2 at 25 cm distance, as measured by an IL1700 research radiometer (International Light, Newburyport, MA, USA) with a UVB-1 filter (#25628) and SED 240 detector, or a SolaSure Solutell radiometer, respectively. Immediately prior to UV radiation treatment, the media was removed from each culture, the cells washed once with PBS and fresh PBS added. Cultures were exposed to UV radiation (1–2 min depending upon dose and device) under sterile conditions, in the absence of the plastic dish cover. Following UV exposure the PBS was removed and replaced with serum-containing culture medium. Control cultures were identically treated but were not UV irradiated. The effect of UV radiation on cell proliferation and survival was measured by plating equal numbers of transplanted melanocyte cultures from each genotype into 24 flat-bottom well tissue culture plates (Costar, Corning, NY, USA). Two to three days later the culture medium was removed and replaced with PBS and the cells exposed to UV radiation as described above. Control cultures were treated identically but not irradiated. The PBS was replaced with 0.5 ml media per well and the incubation continued. After 24 h, 0.1 ml MTS reagent (Promega Corp., Madison, WI, USA) was added to each well of quadruplicate cultures and the absorbance of the culture supernatants was measured in a Beckman Coulter AD340 ELISA Reader (Beckman Coulter, Fullerton, CA, USA) at 490 nm 1–20 h later, depending upon the experiment and number of plated cells, according to the manufacturer’s instructions.
Melanin measurement
Melanin was measured using the method kindly provided by Dr. Zalfa Abdel-Malek, Department of Dermatology, The University of Cincinnati, Cincinnati, OH. Details are provided in Appendix S1.
Western blotting
Twenty four hours after UV exposure cells were scraped from the dishes, replicate cultures pooled and the cells were pelleted by centrifugation at 500 g for 10 min. The supernatants were discarded and cell pellets lysed using ice-cold RIPA buffer with enzyme inhibitors 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM ethylenediaminetetraacetic acid (EDTA), 5 μg/ml aprotinin, 5 μg/ml leupeptin, 2 mM sodium orthovanadate (Na3VO4), and 1 mM sodium fluoride (NaF). Cell debris was pelleted by centrifugation at 13 400 g for 3 min. The supernatants were collected and protein measured using a Bradford assay (BioRad, Hercules, CA) according to the manufacturer’s instructions. 10–20 μg of protein per sample was loaded in a denaturing 12.5% or 15% SDS-PAGE gel. Following electrophoretic separation, the protein was transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) and blocked with 5% non-fat milk (Carnation, Glendale, CA, USA) in 20 mM Tris-buffered saline containing 0.05% Tween-20 (TBST) buffer overnight at 4°C. The membrane was washed ×3 with TBST and incubated with primary antibody overnight at 4°C. Details of antibodies used and detection are presented in Appendix S1.
Quantitative real-time PCR analysis of gene expression
Melanocyte-containing cultures from newborn wild-type, C57BL/6-MitfMi-wh/+[Tg]Dct-LacZ and C57BL/6-Mitfmi-vga9/+[Tg]Dct-LacZ heterozygous mice were exposed to 0, 125 J/m2, or 250 J/m2 UV radiation as described above. 24 h later the cells were removed from their culture dishes using a cell scraper and the cells counted and sedimented by centrifugation at 500 g for 5 min. The supernatant was discarded and the cell pellet was resuspended in 1 ml ice-cold RLT lysis buffer (Qiagen Sciences, Valencia, CA, USA) and stored at −80°C. RNA was isolated and quantitative real-time PCR performed essentially as previously described (Urzua et al., 2006), but with β-actin as a reference transcript assayed under identical conditions respective to the gene of interest. Additional details of the analysis of the quantitative real-time PCR analysis are provided in Appendix S1.
Beta-galactosidase (β-gal) staining
Cells were fixed in 0.4% paraformaldehyde/PBS for 15 min at room temperature and washed thrice with PBS for 5 min. each. The slides were incubated in a solution of 100 mM Tris pH 7.5 containing 0.1 M phosphate buffer pH 7.3, 2 mM magnesium chloride, 0.1% sodium deoxycholate, 0.02% NP40, 5 mM potassium ferrocyanide, and 5 mM potassium ferricyanide and 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal, Gold BioTechnologies, St. Louis, MO, USA). X-gal was prepared as a 20 mg/ml solution in dimethylformamide and stored at −20°C until use. The tissue was incubated in the staining solution in the dark at room temperature overnight or until a blue signal appeared. Cells were post-fixed for 15 min in a 4% paraformaldehyde/PBS solution and photographed.
Supplementary Material
Figure S1. Melanin content, phenotype and β-galactoside expression in primary melanocyte cultures shown in Figure 1. C57BL/6J-MitfMi-wh/+ [Tg]Dct-LacZ melanocytes shown in Figure 1D; C57BL/6J Mitfmi-vga9/+ [Tg]Dct-LacZ heterozygous melanocytes shown in Figure 1F, and C57BL/6J[Tg]Dct-LacZ melanocytes with wild type Mitf shown in Figure 1H. Arrows indicate melanin-containing cells that are losing their Dct-LacZ expression. Arrowhead indicates a melanin-containing melanocyte with lower [Tg]Dct-LacZ expression. All melanocyte cultures were stained for β-galactosidase (blue). Bar = 100 μm.
Figure S2. Anti-Tyrp1 (Mel-5) immunostaining of pigmented C57BL/6 and albino C57BL/6-Tyrc–2J primary melanocytes. (A) Bright field image of pigmented C57BL/6 primary melanocytes. (B) Anti-Tyrp1 immunofluorescent image of pigmented C57BL/6 primary melanocytes. (C) DAPI staining of pigmented C57BL/6 primary melanocytes. Arrowheads in A, B, and C denote the same cell in respective panels. (D) Bright field image of albino C57BL/6-Tyrc–2J primary melanocytes. (E) Anti-Tyrp1 immunofluorescent image of albino C57BL/6-Tyrc–2J primary melanocytes. (F) DAPI staining of albino C57BL/6-Tyrc–2J primary melanocytes. Arrowheads in E and F denote the same cell in respective panels. (Cell is not visible in D due to lack of pigment).
Figure S3. Viability of melanocyte cultures from C57BL/6 and congenic albino C57BL/6J-Tyrc–2J neonatal mice following irradiation with increasing doses of UV, measured with the MTS assay. In this primary culture, 40–60% of the cells were Tyrp1+ as determined by anti-Tyrp1 immunofluorescence as in Figure S2. No significant difference was observed between the viability of albino and pigmented melanocytes following UV irradiation.
Figure S4. Effects of UV irradiation on extrinsic apoptotic pathway components and pro-apoptotic Bcl-2 family members in wild-type and Mitf-deficient melanocytes. Expression of apoptosis-related proteins was studied using Western blotting from cell lysates collected 24 h later. (A) Expression of extrinsic pathway proteins Fas, caspase 8, cleaved caspase 8, and pro-apoptotic Bcl-2 family members Bak and Bax in C57BL/6, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ primary melanocytes 24 h after irradiation with 250 J/m2 UV. (B) Quantification of Western blotting data from A.
Appendix S1. Supplementary Methods.
Significance.
Ultraviolet (UV)-induced apoptosis is an important mechanism that prevents the accumulation of DNA damage in irradiated skin cells. High intensity UV exposure, for example, leads to the p53-dependent formation of ‘sunburn cells’, or apoptotic keratinocytes, in human skin. The determinants of UV-induced apoptosis in melanocytes are less well understood. Mitf is a transcription factor critical for melanocyte survival at various stages of development at least in part through its regulation of anti-apoptotic Bcl-2. Our finding that Mitf protects melanocytes against UV-induced apoptosis extends these findings to other apoptotic pathways, and reveals a primary role of Mitf in the protection of the melanocyte against UV assault. Modulation of melanocyte apoptosis in response to UV may be an important mode of cutaneous photoprotection.
Acknowledgements
We thank Alice Croxen and Payal Shah for excellent technical assistance. We also thank Kenneth N. Barton for assistance in image analyses. We are grateful to Ronald P. Pelley for critical reading of this manuscript and Dr. Colin Goding for advice and assistance with the manuscript. We also thank Dr. Edward DeFabo for use and calibration of an ultraviolet light box. This work was supported by grants R01-AR47951 (FMS) from the National Institutes, DAMD17-01-1-1709 (FMS) from the Department of Defense, NIH K08 AR01992 (to TJH) and (in part) by the Intramural research program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- Abdel-Malek ZA, Scott MC, Furumura M, Lamoreux ML, Ollmann M, Barsh GS, and Hearing VJ (2001). The melano-cortin 1 receptor is the principal mediator of the effects of agouti signaling protein on mammalian melanocytes. J. Cell Sci 114, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, and Schwarz T (1998). Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell Biol 140, 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley NJ, Eisen T, and Goding CR (1994). Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol. Cell. Biol 14, 7996–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth K, Skuntz S, Hallsson JH, Pak E, Dutra AS, Steingrimsson E, and Arnheiter H (2008). An unstable targeted allele of the mouse Mitf gene with a high somatic and germline reversion rate. Genetics 178, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivik CA, Andersson EB, and Rosdahl IK (2005). Wavelength-specific effects on UVB-induced apoptosis in melanocytes. A study of Bcl-2/Bax expression and keratinocyte rescue effects. Melanoma Res. 15, 7–13. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Jiang G, Hyman R, Lee KF, O’gorman S, and Hunter T (2000). Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet 24, 157–162. [DOI] [PubMed] [Google Scholar]
- Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, and Schwarz A (2005). alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem 280, 5795–5802. [DOI] [PubMed] [Google Scholar]
- Busca R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B, Thyss R, Fitsialos G, Larribere L, Virolle T, Barbry P, Pouyssegur J, Ponzio G, and Ballotti R (2005). Hypoxia-inducible factor 1a is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. J. Cell Biol 170, 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert MD, Denat L, Larue L, and Goding CR (2005). Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433, 764–769. [DOI] [PubMed] [Google Scholar]
- Corre S, Primot A, Sviderskaya E, Bennett DC, Vaulont S, Goding CR, and Galibert MD (2004). UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1). J. Biol. Chem 279, 51226–51233. [DOI] [PubMed] [Google Scholar]
- De Leeuw SM, Smit NP, Van Veldhoven M, Pennings EM, Pavel S, Simons JW, and Schothorst AA (2001). Melanin content of cultured human melanocytes and UV-induced cytotoxicity. J. Photochem. Photobiol. B 61, 106–113. [DOI] [PubMed] [Google Scholar]
- Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, and Fisher DE (2003). MLANA/MART1 and SILV/P-MEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am. J. Pathol 163, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Widlund HR, Horstmann MA, Ramaswamy S, Ross K, Huber WE, Nishimura EK, Golub TR, and Fisher DE (2004). Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565–576. [DOI] [PubMed] [Google Scholar]
- Galibert MD, Carreira S, and Goding CR (2001). The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J. 20, 5022–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Widlund HR, Rubin MA et al. (2005). Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Moll I, Meyer M, and Michaelidis TM (1999). Alterations in cell death and cell cycle progression in the UV-irradiated epidermis of bcl-2-deficient mice. Cell Death Differ. 6, 55–60. [DOI] [PubMed] [Google Scholar]
- Goding CR (2000). Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 14, 1712–1728. [PubMed] [Google Scholar]
- Halaban R, and Alfano FD (1984). Selective elimination of fibroblasts from cultures of normal human melanocytes. In Vitro 20, 447–450. [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Steingrímsson E, Mcgill G, Hansen MJ, Vaught J, Hodgkinson CA, Arnheiter H, Copeland NG, Jenkins NA, and Fisher DE (1994). Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 8, 2770–2780. [DOI] [PubMed] [Google Scholar]
- Hemesath TJ, Price ER, Takemoto C, Badalian T, and Fisher DE (1998). MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 391, 298–301. [DOI] [PubMed] [Google Scholar]
- Hill HZ, Hill GJ, Cieszka K, Plonka PM, Mitchell DL, Meyenhofer MF, Xin P, and Boissy RE (1997). Comparative action spectrum for ultraviolet light killing of mouse melanocytes from different genetic coat color backgrounds. Photochem. Photobiol 65, 983–989. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, and Arnheiter H (1993). Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74, 395–404. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Cemeli E, Ross K, Anderson D, Thody AJ, and Wood JM (2004). Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+. Exp. Cell Res 294, 60–67. [DOI] [PubMed] [Google Scholar]
- Hornyak TJ, Hayes DH, Chiu L-Y, and Ziff EB (2001). Transcription factors in melanocyte development: distinct roles for Pax-3 and Mitf. Mech. Dev 101, 47–59. [DOI] [PubMed] [Google Scholar]
- Jiao Z, Mollaaghababa R, Pavan WJ, Antonellis A, Green ED, and Hornyak TJ (2004). Direct interaction of Sox10 with the promoter of murine Dopachrome Tautomerase (Dct) and synergistic activation of Dct expression with Mitf. Pigment Cell Res. 17, 352–362. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, and Abdel-Malek ZA (2003). Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res. 16, 434–447. [DOI] [PubMed] [Google Scholar]
- Kadekaro AL, Kavanagh R, Kanto H et al. (2005). alpha-Melano-cortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 65, 4292–4299. [DOI] [PubMed] [Google Scholar]
- Karlsson R, Engstrom M, Jonsson M, Karlberg P, Pronk CJ, Richter J, and Jonsson JI (2003). Phosphatidylinositol 3-kinase is essential for kit ligand-mediated survival, whereas interleukin-3 and flt3 ligand induce expression of antiapoptotic Bcl-2 family genes. J. Leukoc. Biol 74, 923–931. [DOI] [PubMed] [Google Scholar]
- Kim TJ, Cho MK, Lee JS, Whang KU, Jin SY, and Hoshino T (2003). The expression of melanogenic proteins in Korean skin after ultraviolet irradiation. J. Dermatol 30, 665–672. [DOI] [PubMed] [Google Scholar]
- Kimura S, Kawakami T, Kawa Y, Soma Y, Kushimoto T, Nakamura M, Watabe H, Ooka S, and Mizoguchi M (2005). Bcl-2 reduced and fas activated by the inhibition of stem cell factor/KIT signaling in murine melanocyte precursors. J. Invest. Dermatol 124, 229–234. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ (1999). BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 59, 1693s–1700s. [PubMed] [Google Scholar]
- Larribere L, Hilmi C, Khaled M, Gaggioli C, Bille K, Auberger P, Ortonne JP, Ballotti R, and Bertolotto C (2005). The cleavage of microphthalmia-associated transcription factor, MITF, by caspases plays an essential role in melanocyte and melanoma cell apoptosis. Genes Dev. 19, 1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CB, Babiarz L, Liebel F, Roydon Price E, Kizoulis M, Gendimenico GJ, Fisher DE, and Seiberg M (2002). Modulation of microphthalmia-associated transcription factor gene expression alters skin pigmentation. J. Invest. Dermatol 119, 1330–1340. [DOI] [PubMed] [Google Scholar]
- Lindholm C, Andersson R, Dufmats M, Hansson J, Ingvar C, Moller T, Sjodin H, Stierner U, and Wagenius G (2004). Invasive cutaneous malignant melanoma in Sweden, 1990–1999. A prospective, population-based study of survival and prognostic factors. Cancer 101, 2067–2078. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Rehberg S, and Wegner M (2004). Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett. 556, 236–244. [DOI] [PubMed] [Google Scholar]
- McGill GG, Horstmann M, Widlund HR et al. (2002). Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109, 707–718. [DOI] [PubMed] [Google Scholar]
- Murphy M, Mabruk MJ, Lenane P, Liew A, Mccann P, Buckley A, Billet P, Leader M, Kay E, and Murphy GM (2002). The expression of p53, p21, Bax and induction of apoptosis in normal volunteers in response to different doses of ultraviolet radiation. Br. J. Dermatol 147, 110–117. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, and Nishikawa S (2002). Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, and Fisher DE (2005). Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724. [DOI] [PubMed] [Google Scholar]
- Ono K, and Han J (2000). The p38 signal transduction pathway: activation and function. Cell. Signal 12, 1–13. [DOI] [PubMed] [Google Scholar]
- Plettenberg A, Ballaun C, Pammer J, Mildner M, Strunk D, Weninger W, and Tschachler E (1995). Human melanocytes and melanoma cells constitutively express the Bcl-2 proto-oncogene in situ and in cell culture. Am. J. Pathol 146, 651–659. [PMC free article] [PubMed] [Google Scholar]
- Price ER, and Fisher DE (2001). Sensorineural deafness and pigmentation genes: melanocytes and the Mitf transcriptional network. Neuron 30, 15–18. [DOI] [PubMed] [Google Scholar]
- Reinke V, and Lozano G (1997). Differential activation of p53 targets in cells treated with ultraviolet radiation that undergo both apoptosis and growth arrest. Radiat. Res 148, 115–122. [PubMed] [Google Scholar]
- Rosette C, and Karin M (1996). Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274, 1194–1197. [DOI] [PubMed] [Google Scholar]
- Sakata S, Sakamaki K, Watanabe K, Nakamura N, Toyokuni S, Nishimune Y, Mori C, and Yonehara S (2003). Involvement of death receptor Fas in germ cell degeneration in gonads of Kit-deficient Wv/Wv mutant mice. Cell Death Differ. 10, 676–686. [DOI] [PubMed] [Google Scholar]
- Schwahn DJ, Timchenko NA, Shibahara S, and Medrano EE (2005). Dynamic regulation of the human dopachrome tautomerase promoter by MITF, ER-alpha and chromatin remodelers during proliferation and senescence of human melanocytes. Pigment Cell Res. 18, 203–213. [DOI] [PubMed] [Google Scholar]
- Smit NP, Vink AA, Kolb RM, Steenwinkel MJ, Van Den Berg PT, Van Nieuwpoort F, Roza L, and Pavel S (2001). Melanin offers protection against induction of cyclobutane pyrimidine dimers and 6–4 photoproducts by UVB in cultured human melanocytes. Photochem. Photobiol. 74, 424–430. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, and Jenkins NA (2004). Melanocytes and the microphthalmia transcription factor network. Annu. Rev. Genet 38, 365–411. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Hara Y, Vyas D, Hodgkinson C, Fex J, Grundfast K, and Arnheiter H (1992). Cochlear disorder associated with melanocyte anomaly in mice with a transgenic insertional mutation. Mol. Cell. Neurosci 3, 433–445. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Kobayashi N, Zmudzka BZ, Ito S, Wakamatsu K, Yamaguchi Y, Korossy KS, Miller SA, Beer JZ, and Hearing VJ (2003). UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin. FASEB J. 17, 1177–1179. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, and Hearing VJ (2005a). Mechanisms of skin tanning in different racial//ethnic groups in response to ultraviolet radiation. J. Investig. Dermatol 124, 1326–1332. [DOI] [PubMed] [Google Scholar]
- Tadokoro T, Yamaguchi Y, Batzer J, Coelho SG, Zmudzka BZ, Miller SA, Wolber R, Beer JZ, and Hearing VJ (2005b). Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Invest. Dermatol 124, 1326–1332. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Newton VE, and Read AP (1994). Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat. Genet 8, 251–255. [DOI] [PubMed] [Google Scholar]
- Tomita Y, Takeda A, Okinaga S, Tagami H, and Shibahara S (1989). Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem. Biophys. Res. Commun 164, 990–996. [DOI] [PubMed] [Google Scholar]
- Urzua U, Roby KF, Gangi LM, Cherry JM, Powell JI, and Munroe DJ (2006). Transcriptomic analysis of an in vitro murine model of ovarian carcinoma: functional similarity to the human disease and identification of prospective tumoral markers and targets. J. Cell. Physiol 206, 594–602. [DOI] [PubMed] [Google Scholar]
- Widlund HR, and Fisher DE (2003). Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22, 3035–3041. [DOI] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T et al. (2007). Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Okamoto H, Miyauchi-Hashimoto H, Matsumura Y, Itoh T, Tanaka K, Kunisada T, and Horio T (2004). XPA gene-deficient, SCF-transgenic mice with epidermal melanin are resistant to UV-induced carcinogenesis. J. Invest. Dermatol 123, 220–228. [DOI] [PubMed] [Google Scholar]
- Yasumoto K-I, Yokoyama K, Takahashi K, Tomita Y, and Shibahara S (1997). Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J. Biol. Chem 272, 503–509. [DOI] [PubMed] [Google Scholar]
- Yavuzer U, and Goding CR (1994). Melanocyte-specific gene expression: role of repression and identification of a melanocyte-specific factor, MSF. Mol. Cell. Biol 14, 3494–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, and Strasser A (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol 9, 47–59. [DOI] [PubMed] [Google Scholar]
- Young AR (2004). Tanning devices–fast track to skin cancer? Pigment Cell Res. 17, 2–9. [DOI] [PubMed] [Google Scholar]
- Zhai S, Yaar M, Doyle SM, and Gilchrest BA (1996). Nerve growth factor rescues pigment cells from ultraviolet-induced apoptosis by upregulating BCL-2 levels. Exp. Cell Res 224, 335–343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Melanin content, phenotype and β-galactoside expression in primary melanocyte cultures shown in Figure 1. C57BL/6J-MitfMi-wh/+ [Tg]Dct-LacZ melanocytes shown in Figure 1D; C57BL/6J Mitfmi-vga9/+ [Tg]Dct-LacZ heterozygous melanocytes shown in Figure 1F, and C57BL/6J[Tg]Dct-LacZ melanocytes with wild type Mitf shown in Figure 1H. Arrows indicate melanin-containing cells that are losing their Dct-LacZ expression. Arrowhead indicates a melanin-containing melanocyte with lower [Tg]Dct-LacZ expression. All melanocyte cultures were stained for β-galactosidase (blue). Bar = 100 μm.
Figure S2. Anti-Tyrp1 (Mel-5) immunostaining of pigmented C57BL/6 and albino C57BL/6-Tyrc–2J primary melanocytes. (A) Bright field image of pigmented C57BL/6 primary melanocytes. (B) Anti-Tyrp1 immunofluorescent image of pigmented C57BL/6 primary melanocytes. (C) DAPI staining of pigmented C57BL/6 primary melanocytes. Arrowheads in A, B, and C denote the same cell in respective panels. (D) Bright field image of albino C57BL/6-Tyrc–2J primary melanocytes. (E) Anti-Tyrp1 immunofluorescent image of albino C57BL/6-Tyrc–2J primary melanocytes. (F) DAPI staining of albino C57BL/6-Tyrc–2J primary melanocytes. Arrowheads in E and F denote the same cell in respective panels. (Cell is not visible in D due to lack of pigment).
Figure S3. Viability of melanocyte cultures from C57BL/6 and congenic albino C57BL/6J-Tyrc–2J neonatal mice following irradiation with increasing doses of UV, measured with the MTS assay. In this primary culture, 40–60% of the cells were Tyrp1+ as determined by anti-Tyrp1 immunofluorescence as in Figure S2. No significant difference was observed between the viability of albino and pigmented melanocytes following UV irradiation.
Figure S4. Effects of UV irradiation on extrinsic apoptotic pathway components and pro-apoptotic Bcl-2 family members in wild-type and Mitf-deficient melanocytes. Expression of apoptosis-related proteins was studied using Western blotting from cell lysates collected 24 h later. (A) Expression of extrinsic pathway proteins Fas, caspase 8, cleaved caspase 8, and pro-apoptotic Bcl-2 family members Bak and Bax in C57BL/6, C57BL/6-Mitfmi-vga9/+, and C57BL/6-MitfMi-wh/+ primary melanocytes 24 h after irradiation with 250 J/m2 UV. (B) Quantification of Western blotting data from A.
Appendix S1. Supplementary Methods.