Abstract
Objective:
To test the association between statin use and prostate volume (PV) change over time using data from REDUCE, a 4-year randomized controlled trial testing dutasteride for prostate cancer chemoprevention.
Subjects/patients and methods:
We identified men with a baseline negative prostate biopsy from REDUCE who did not undergo prostate surgery or develop prostate cancer over the trial period. Men reported statin use at baseline. PV was determined from transrectal ultrasound performed to guide prostate biopsy at baseline, 2- and 4-years post-randomization. Multivariable generalized estimating equations tested differences in PV change over time by statin use, overall and stratified by treatment arm. We tested for interactions between statins and time in association with PV using the Wald test.
Results:
Of 4,106 men, 17% used statins at baseline. Baseline PV did not differ by statin use. Relative to non-users, statin users had decreasing PVs over the trial period (p=0.027). Similar patterns were seen in dutasteride and placebo arms, though neither reached statistical significance. Mean estimated PV was modestly but significantly lower in statin users relative to non-users in the dutasteride arm at 2-years (4.5%, p=0.032) and 4-years (4.0%, p=0.033), with similar (3–3.3%) but non-significant effects in the placebo arm.
Conclusion:
If confirmed, our findings support a role for statins in modestly attenuating PV growth, with a magnitude of effect in line with previously-reported PSA-lowering effects of statins (~4%). Future studies are needed to assess whether this putative role for statins in PV growth could impact lower urinary tract symptom development or progression.
Keywords: 5-alpha Reductase Inhibitors/therapeutic use, Middle Aged, Prostate, Body Mass Index, Obesity
INTRODUCTION
Benign prostatic hyperplasia (BPH) is common among older men.(1) The condition is associated with a constellation of lower urinary tract symptoms (LUTS) that can impair quality of life.(2–4) While the underlying mechanisms associated with its occurrence have not yet been established, chronic inflammation of prostatic tissue has been hypothesized to be a critical etiologic component.(1, 5, 6) Importantly, incidence of BPH and its associated comorbidities is projected to increase as the US population ages and as the prevalence of conditions that promote systemic inflammation, such as obesity, continue to increase.(7) Hence, effective treatment and, ideally, preventive strategies are urgently needed to limit the morbidities associated with this growing public health problem.
Some recent studies suggest that statins may reduce prostate volume (PV) growth and LUTS,(8–10) but results have not been consistent.(11) Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are a class of drugs widely prescribed to treat hypercholesterolemia. The inhibition of HMG-CoA reductase leads to improvement of serum lipid parameters by limiting conversion of HMG-CoA to mevalonate, a key intermediate in cholesterol biosynthesis. The resulting reduction in serum cholesterol, known to be beneficial for reducing the incidence of cardiovascular disease, may also benefit prostatic tissue. Through reduction of intracellular mevalonate levels, isoprenoid synthesis is limited which inhibits cellular proliferation and differentiation.(12) In addition, statins have anti-inflammatory properties,(13) along with other effects including increased apoptosis and autophagy.(14) While these cellular mechanisms provide rationale linking statins to altered metabolism in prostatic tissue,(15, 16) the association between statins and benign prostate conditions remains unclear.
The REduction by DUtasteride of prostate Cancer Events (REDUCE) chemoprevention trial randomized men with a negative prostate biopsy to dutasteride or placebo and performed repeat transrectal ultrasound (TRUS)-guided biopsies at 2- and 4-years (17), providing an opportunity to examine the effect of statin use on TRUS-measured PV growth over the 4-year trial period. We previously showed that statin use was associated with reduced chronic histologic inflammation of benign prostate tissue in REDUCE.(18) In light of our previous work and the known systemic anti-inflammatory properties and apoptotic properties of statins, we hypothesized that statin use may lead to an attenuation of PV growth over time.
SUBJECTS/PATIENTS AND METHODS
Study Population
REDUCE was a 4-year double-blind, randomized placebo-controlled trial testing the effect of dutasteride (0.5 mg/day) versus placebo on the incidence of prostate cancer. The methods and trial results have been previously published.(17) Briefly, the 4-year multinational trial randomized men between 50 and 75 years of age meeting the following criteria: serum prostate-specific antigen (PSA) levels between 2.5 to 10 ng/mL, having had a single negative prostate biopsy (6 – 12 cores) 6 months prior to enrollment, presenting with an International Prostate Symptom Score (IPSS) <25, or <20 while receiving α-blockers for the treatment of BPH. Baseline biopsies were centrally reviewed to confirm a negative prostate cancer diagnosis. Men were excluded if they had a history of prostate cancer, high-grade intraepithelial neoplasia, or atypical small acinar proliferation. Of 8,321 men randomized to the trial, 199 (1.3%) were excluded because they did not receive the drug, had a positive baseline biopsy or the biopsy was not reviewed, leaving 8,122 in the ‘efficacy’ study. Of the 8,122 men, we excluded men from our analysis who had baseline TRUS-measured PV >80 cc (as per REDUCE inclusion criteria, n=163), those who had prostate surgery during the trial period (n=741), those diagnosed with prostate cancer during trial period (n=976) as prostate cancer presence may affect PV, men with missing PV values (n=1,567) and men using other lipid-lowering agents alone or in combination with statins [fenofibrate (n=34), ezetimibe (n=16), gemfibrozil (n=25)]. Men with missing data for body mass index (BMI; n=58), diabetes status (n=1), BPH status (n=2), dihydrotestosterone (DHT) level (n=55), testosterone level (n=10), smoking status (n=2) and alcohol intake (n=15) were also excluded, leaving 4,457 participants.
Exposure and covariate assessment
A detailed medical history was obtained at baseline for medical comorbidities, smoking history, medication and alcohol use. Participants reported all medications taken at baseline, however, statin type, dose and duration were unavailable; hence, statin use was dichotomized to users and non-users at baseline. The presence of BPH at baseline was defined according to the discretion of the treating physician.
Outcome assessment
As per trial protocol subjects underwent TRUS to guide prostate biopsy at years 2 and 4. TRUS was also performed at baseline for screening prior to randomization, unless already performed within the preceding 6 months. PV was calculated using measurements obtained by TRUS and the prostate ellipsoid formula (the length of anteroposterior, cephalocaudal, and transverse axes multiplied by π/6). We identified 351 men with PV values at follow-up years 2 or 4 that met our criteria for outliers defined as the top or bottom 2.5% of the ratio of PV difference to the baseline value [(follow-up PV – baseline PV)/ baseline PV × 100)] across all men for that specific time point. We assumed that these extreme values arose from errors in measurement or data transcribing, and therefore we excluded those 351 men from our analysis for a final sample size of 4,106.
Statistical analysis
Baseline characteristics by statin use were examined using the Wilcoxon rank sum test and χ2 tests for continuous and categorical variables, respectively.
Generalized estimating equations (GEE) models were used to examine the association between statin use (vs. non-use) and changes in PV over time, overall and stratified by treatment arm. A first-order autoregressive correlation structure for within-subject PV was assumed. Models were adjusted for characteristics at randomization: age (continuous), race (white, black, other), BMI (continuous, log-transformed), BPH (yes vs. no), diabetes (yes vs. no), DHT (continuous, log-transformed), serum testosterone (continuous, log-transformed), geographic region (North America, Europe, other), smoking (never, former, current), alcohol use (0, 1–7, >7 drinks/week), and treatment arm (dutasteride vs. placebo; for overall analyses only). We also included a time indicator (categorical: baseline, 2-year visit, 4-year visit) and interaction terms for time × statin and time × dutasteride (for overall analyses only) to account for changes in associations with PV over time. Linear combinations of estimates from the GEE models were used to display the differences in PV (ΔPV) between statin users vs. non-statin users at baseline, 2-year, and 4-year visits. We tested for interactions between statin use and PV change over time, and between treatment arm and PV change over time using the Wald test. We also reported mean estimated PV and 95% confidence intervals (CIs) from the multivariable models at each time point overall and by statin use, overall and stratified by treatment arm (dutasteride vs. placebo). These mean estimated PV values were also displayed graphically.
Statistical analyses were performed using Stata, version 13.1 (Stata Corp., College Station, TX).
RESULTS
Characteristics of statin users and non-users
Of 4,106 men, 692 (17%) were statin users at baseline (Table 1). Relative to non-users, statin users were slightly older and more likely to live in North America than in Europe or elsewhere. Statin users also had a slightly higher BMI, were more likely to have been diagnosed with diabetes, less likely to have BPH, and had lower DHT and testosterone levels. Statin users were also more likely to be former smokers and less likely be never smokers. There were no differences between statin users and non-users with respect to alcohol consumption, IPSS or baseline PV.
Table 1:
Baseline characteristics by statin use in REDUCE
| Statin non-user (N=3,414) | Statin user (N=692) | p value | |
|---|---|---|---|
| Age | 0.0121 | ||
| Median (IQR) | 62 (58, 67) | 63 (58, 67) | |
| Race | 0.0722 | ||
| White | 3124 (92%) | 649 (94%) | |
| Black | 57 (2%) | 12 (2%) | |
| Other | 233 (7%) | 31 (4%) | |
| Geographic region | <0.0012 | ||
| North America | 737 (22%) | 329 (48%) | |
| Europe | 2120 (62%) | 318 (46%) | |
| Other | 557 (16%) | 45 (7%) | |
| BMI (kg/m2) | <0.0011 | ||
| Median (IQR) | 26.7 (24.7, 29.1) | 27.2 (25.2, 29.8) | |
| Treatment arm | 0.6942 | ||
| Placebo | 1656 (49%) | 330 (48%) | |
| Dutasteride | 1758 (51%) | 362 (52%) | |
| BPH | 0.0012 | ||
| No | 1108 (32%) | 268 (39%) | |
| Yes | 2306 (68%) | 424 (61%) | |
| IPSS | |||
| Median (IQR) | 7 (4, 12) | 7 (4, 12) | 0.6081 |
| Diabetes | <0.0012 | ||
| No | 3197 (94%) | 595 (86%) | |
| Yes | 217 (6%) | 97 (14%) | |
| DHT (nmol/L) | <0.0011 | ||
| Median (IQR) | 1.2 (0.9, 1.7) | 1.1 (0.8, 1.5) | |
| Testosterone (nmol/L) | <0.0011 | ||
| Median (IQR) | 15.0 (11.2, 19.5) | 13.5 (10.5, 17.3) | |
| Smoker | 0.0042 | ||
| Never | 1578 (46%) | 283 (41%) | |
| Former | 1328 (39%) | 316 (46%) | |
| Current | 508 (15%) | 93 (13%) | |
| Alcohol use | 0.4042 | ||
| 0 drinks/week | 835 (24%) | 186 (27%) | |
| 1–6 drinks/week | 1426 (42%) | 281 (41%) | |
| ≥7 drinks/week | 1153 (34%) | 225 (33%) | |
| Prostate volume (cc) | 0.6311 | ||
| Median (IQR) | 43.9 (34.1, 56.3) | 44.7 (35.0, 56.1) |
Wilcoxon
Chi-Square
Associations between statin use, dutasteride, and PV change over time
On multivariable analysis among all patients, PV was similar between statin users vs. non-users (p=0.52) and between treatment arms at baseline (p=0.96, Table 2). However, both statin use and dutasteride use were associated with a reduction in PV growth over the trial period (p-interactions=0.021 and <0.001, respectively). Specifically, relative to non-use, statin use was associated with decreased PV at 2-years (ΔPV = −1.75, 95% CI [−3.16, −0.33], p=0.015) and at 4-years (ΔPV = −1.63, 95% CI [−3.10, −0.16], p=0.030). The effect of dutasteride on lowering PV was roughly 10-fold greater than the statin-associated magnitude of effect at both 2-years (ΔPV = −12.3, 95% CI [−13.3, −11.2], p<0.001) and 4-years (ΔPV = −18.1, 95% CI [−19.2, −17.1], p<0.001). When stratified by treatment arm, there were similar magnitudes of association between statin use and PV change over time as observed in the study population as a whole, though no significant interactions between statin use and time were seen in either the placebo arm (p-interaction=0.26) or dutasteride arm (p=0.055). Considering each time point separately, though there was an inverse direction of effect, statin use was not significantly associated with lower PV in the placebo arm at 2-years (p=0.18) or at 4-years (p=0.32). However, in the dutasteride arm, statin use was associated with significantly lower PV at 2-years (ΔPV = −1.94, 95%CI [−3.71, −0.16], p=0.032) and at 4-years (ΔPV = −2.00, 95%CI [−3.84, −0.16], p=0.033). Sensitivity analysis stratified by geographic region showed similar patterns of statin-associated PV changes in North America, Europe and other regions (data not shown).
Table 2:
Linear combinations for differences in prostate volume (ΔPV) change over time by statin and dutasteride use in the 4-year REDUCE study, calculated from multivariable1 generalized estimating equations
| Baseline | 2-year visit | 4-year visit | p-interaction exposure × visit |
||||
|---|---|---|---|---|---|---|---|
| ΔPV (95% CI) | p-value | ΔPV (95% CI) | p-value | ΔPV (95% CI) | p-value | ||
| Use | 0.03 (−1.00, 1.05) | 0.96 | −12.3 (−13.3, −11.2) | <0.001 | −18.1 (−19.2, −17.1) | <0.001 | |
| Use | −0.30 (−2.53, 1.92) | 0.79 | −1.52 (−3.75, 0.71) | 0.18 | −1.18 (−3.51, 1.14) | 0.32 | |
| Use | 0.59 (−2.36, 1.18) | 0.52 | −1.94 (−3.71, −0.16) | 0.032 | −2.00 (−3.84, −0.16) | 0.033 | |
Multivariable models contain visit, statin use, treatment arm, age, race, BMI, BPH, diabetes, DHT, testosterone, geographic region, smoking, alcohol consumption, and interaction terms: statin × visit and dutasteride × visit
Mean estimated PV as a function of statin use
To facilitate clinical interpretation of our results, we calculated mean estimated PVs from the multivariable-adjusted GEE models presented above. Overall mean estimated PV at baseline in REDUCE was 45.6 cc (Table 3). As anticipated given established effects of aging on prostate growth, PV increased over time in the placebo arm of the trial, from 45.6 cc at baseline, to 51.9 cc at 2-years and 58.0 cc at 4-years, corresponding to a mean increase in PV of 12.4 cc or 27% over the 4-year trial period. In the dutasteride arm, mean estimated PV decreased from 45.7 cc at baseline to 39.6 cc (13% reduction) at 2-years, remaining stable at 4-years (39.8 cc; Table 3).
Table 3:
Estimated mean prostate volume1 over the REDUCE trial period according to statin use, overall and by treatment arm
| Baseline | Year 2 | Year 4 | |
|---|---|---|---|
| Statin user | 45.3 (44.0–46.5) | 44.0 (42.8–45.3) | 47.2 (45.9–48.6) |
| Statin user | 45.2 (43.8–46.6) | 50.4 (49.0–51.8) | 56.6 (55.2–58.1) |
| Statin user | 45.3 (43.9–46.6) | 38.1 (36.8–39.5) | 38.5 (37.1–39.9) |
measured in cc and represented as mean (95% CI); prostate volume estimates were derived from the GEE model presented in Table 2
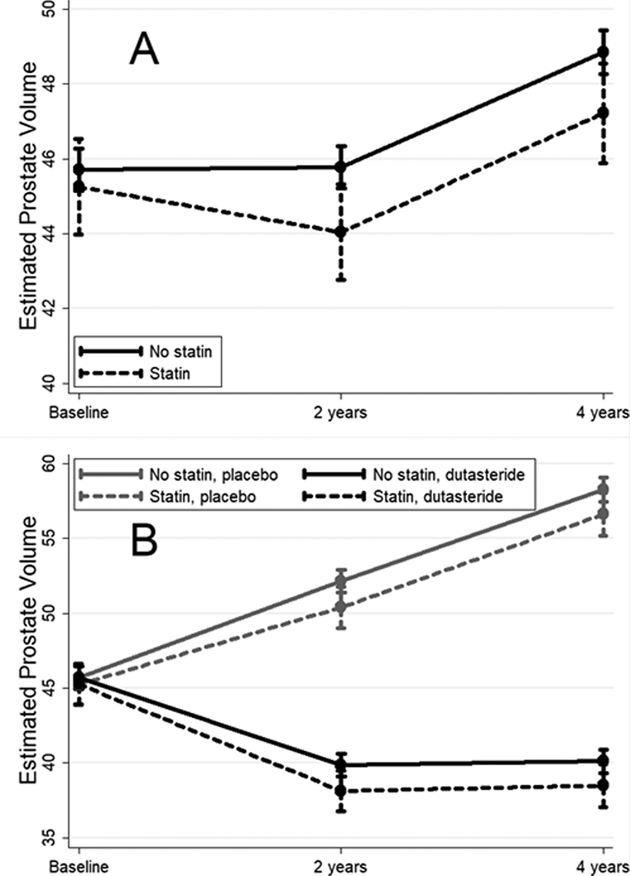
Mean estimated PVs at baseline were similar between statin users and non-users, both overall and in the dutasteride and placebo arms (all p≥0.52; Table 3, illustrated in Figure 1). However, statin users had slightly but significantly smaller mean estimated PVs than non-users in the dutasteride arm at 2-years (4.5% smaller; 38.1 vs. 39.9 cc; p=0.032) and 4-years (4.0% smaller; 38.5 vs. 40.1 cc; p=0.033). Similar magnitudes of differences in mean estimated PVs between statin users and non-users were seen in the placebo arm at 2- and 4-years (3–3.3% smaller), though these differences were not statistically significant (p≥0.18).
Figure 1:
Mean estimated prostate volume with 95% CIs (measured in cc; derived from the multivariable GEE model presented in Table 2) over the REDUCE trial period according to statin use. Panel A shows results for all men regardless of treatment arm while panel B shows results stratified by treatment arm
DISCUSSION
BPH is a progressive disease commonly affecting older men and known to have wide-ranging negative effects on quality of life manifesting as LUTS. The prevalence of BPH-associated LUTS in the US ranges from 50 – 75% among men aged 50 years of age and older, with approximately 80% of men affected by 70 years of age.(19) Identification of factors contributing to BPH development and progression is important to inform prevention and treatment efforts. In this secondary analysis of data from the REDUCE chemoprevention trial, we report that statin use was associated with a modest but significant ~4% attenuation of PV growth over the 4-year trial period. If confirmed, our results point to a potential role for statins in modestly attenuating age-related increases in PV. Whether this degree of PV growth attenuation could affect the development or progression of BPH-associated LUTS requires further study.
In addition to age, certain lifestyle factors have been associated with BPH/LUTS in observational studies, including obesity and poor metabolic health.(20) In addition to the established role of dyslipidemia in cardiac ischemia, high cholesterol has been linked to prostate ischemia in animal models, characterized by reduced prostatic blood flow resulting in hypoxia and elevated levels of hypoxia-induced growth factors leading to prostate hyperplasia.(21, 22) In line with this mechanism, human studies have shown that abnormal vasculature may contribute to hyperplastic growth in BPH.(23) While these studies point to a role for high cholesterol in prostate ischemia, whether statin use could improve prostate vascular health has not been tested to our knowledge. Previously in REDUCE, we reported that high total serum cholesterol and low high-density lipoprotein (HDL) were associated with increased incidence of LUTS.(24) A large retrospective study from Taiwan reported a higher prevalence of BPH among men with metabolic syndrome, particularly among those with low HDL levels.(25) These findings were in keeping with those from a prospective community-based cohort which reported higher incidence of BPH among diabetic men with elevated low-density lipoprotein (LDL) levels.(26) While these studies support an effect of dysregulated serum lipid levels on BPH, evidence for a role of statins is less clear. An epidemiological study of older male health professionals found no evidence to support a role for statins in LUTS prevention.(27) However, a study of men from the Boston area found a significant inverse association between statin use and LUTS in older (60+ years of age) but not younger (<60 years) men.(10) Another US-based study reported a significant inverse association between statin use and new-onset BPH and LUTS, in men 40–79 years of age.(9) Finally, a Korean study found that one year of statin use was associated with reduced PSA and PV among men receiving α-blockers for the treatment of BPH.(28) Consistent with some of the results from these observational studies, we observed a lower prevalence of BPH at baseline among statin users in REDUCE, despite statin users having a higher prevalence of BPH risk factors including older age and higher BMI.
Given somewhat promising findings from observational studies, a number of randomized trials (8, 11, 29, 30) have tested the potential of statins to reduce BPH-associated symptoms. A Phase II trial testing atorvastatin alone for reducing prostate size and improving existing LUTS in 319 men >50 years of age with LDL levels between 100–190 mg/dl reported that despite a significant reduction in LDL levels over the 6-month trial period, the effect on PV was modest (4% reduction) and not statistically significant.(11) Another trial that assigned men with BPH to finasteride alone (if serum cholesterol levels were normal) or to finasteride + lovastatin (if serum cholesterol levels were elevated) for 4 months, found that statin use did not significantly alter IPSS, PV or PSA levels.(29) However, a 6-month intervention in men with BPH and metabolic syndrome reported greater improvements in IPSS when α-blockers were combined with atorvastatin, compared to men taking α-blockers alone.(30) Finally, a study that assigned men over 60 years of age with BPH to statins vs. placebo for 12 months showed that statin treatment lowered systemic inflammation, IPSS and PV in these older men, with more pronounced effects among obese men and among those with high cholesterol.(8) The mean reduction in PV after 12 months of simvastatin use in this study was 11 ml, corresponding to almost a 25% reduction in PV, well in excess of findings from other studies. However, the mean reduction in PV after 12 months of atorvastatin use was 3.5 ml, corresponding to a 7% reduction in PV, closer in line with the Mills et al. 6-month atorvastatin trial (reporting a 2 ml or 4% mean reduction in PV), (11) as well as with our observational findings (~1.5 cc or ~4% lower PVs in statin users vs. non-users). Collectively, the results from these trials as well as from our observational study suggest a modest but consistent effect of statins to attenuate PV growth.
Both laboratory and epidemiological studies support an effect of statins on prostate biology, with current evidence pointing to a role for statins in reducing prostate inflammation and proliferation.(16) Two studies reported that statin treatment inhibited BPH in rat models,(31, 32) through mechanisms including reducing expression of prostate oxidative stress markers (32) and lowering serum levels of pro-inflammatory interleukin-6 and pro-growth insulin-like growth factor (IGF)-1 (31). Another study showed that in vitro lovastatin treatment caused apoptosis of human prostate stromal cells derived from patients undergoing surgery for BPH.(33) Using data from REDUCE, our group previously demonstrated that statin use was associated with lower rates of histological inflammation in both benign prostate (18) and prostate tumor tissue.(34) Others have shown that statins lower PSA levels, a marker closely linked to PV, in prostate cancer-free men (35–37) thereby lending further support for anti-proliferative effects of statins on benign prostate tissue. Indeed, these prior studies showed that statin users had ~4% lower PSA levels relative to non-users. In keeping with these data, we and others (11) found that statins reduced PV by ~4%, suggesting that statin-related reductions in PSA could be due to PV changes. These consistent results across studies for both PSA and PV reductions in association with statin use lend credence both to our results and to the hypothesis that statins significantly, albeit modestly, impact prostate biology. As such, multiple lines of evidence from both laboratory and epidemiological studies support a biological effect of statins on prostate inflammation and growth.
Our results should be considered in the context of study limitations. First, we lacked data regarding type, dose and duration of statin use. Therefore, we do not know how long men were using statins prior to trial entry. Without these data it is difficult to explore why statin use was not associated with baseline PV despite many men likely using statins before trial entry, though one possible explanation could be the restricted range of baseline PV imposed by REDUCE eligibility criteria.(4) Moreover, we lacked adherence data and therefore we do not know if men continued their use of statins during the follow-up period though we assumed they did for the purposes of this analysis. Adherence rates to statin therapy range from 80–90%, based on findings from previous statin trials of roughly similar durations as REDUCE.(38) Any discontinuation of statin use among users (or indeed initiation of statin use among non-users) during the REDUCE trial period would be expected to bias our results towards the null. As such the effect of statins on PV change may be even more pronounced than that observed in this analysis. In addition, this study was limited to men with a restricted range of IPSS values and PV that rendered them eligible for the REDUCE trial. As such, this may limit the generalizability of our results. Specifically, eligibility criteria requiring PSA levels between 2.5–10 ng/ml enriched the REDUCE trial for men with larger prostates (median baseline PV was 44.0 cc, IQR 34.2, 56.3; although none had prostates >80 cc as per REDUCE eligibility criteria) and with relatively pronounced continued prostate enlargement (27% increase in estimated PV in the placebo arm over the 4-year trial period). This is in contrast to a previous study examining the association between statin use and risk of benign prostatic enlargement defined as prostate volume ≥30 ml.(9) While we did observe a lower prevalence of BPH among statin users in REDUCE at baseline, our analysis did not specifically address a potential role for statins in primary prevention of BPH, but rather examined their potential to attenuate PV growth over time in a patient population requiring urological care. We lacked data regarding median lobe enlargement, previously observed to be more common in larger prostates,(39) and therefore could not examine whether there was any effect of its presence or absence on PV trajectories in association with statin use. We also lacked dietary and physical activity data and therefore were unable to include these as covariates in our models which could give rise to residual confounding. We did not include data on serum cholesterol levels and therefore could not explore cholesterol-mediated versus direct effects of statin use on PV change. Limited numbers of non-white men prevented race-stratified analyses. Finally, PV estimation using TRUS is subject to measurement error. The magnitude and direction of the measurement error has been shown to vary by prostate size, and is more pronounced for smaller (<30 cc) prostates, which tend to be underestimated, compared to larger (>30 cc) prostates, which tend to be overestimated.(39) Given that REDUCE is enriched for men with larger prostates, this provides some reassurance that TRUS-estimated PV is relatively accurate in this group. Study strengths include the use of data from a multinational trial that measured PV at regular pre-specified intervals. Also, all men underwent multiple prostate biopsies allowing us to eliminate men with underlying prostate cancer from our analysis. Study-mandated biopsy and TRUS measurements at 2-year intervals limited possible screening biases associated with statin use.
In conclusion, we report that statin use was associated with a modest attenuation of prostate enlargement over time in the REDUCE trial. The suggestion that PV changes were slightly more pronounced when statins were combined with dutasteride (4–4.5% vs. 3–3.3% in the placebo arm) may point to potential added benefit if confirmed by future studies. Given that prostate enlargement and BPH are prevalent conditions in Western society conferring a reduced quality of life, it is possible that even modest attenuation of prostate enlargement could have a clinical and public health benefit. Future studies are needed to validate our findings, and to examine the association between statin use and risk of BPH and associated LUTS.
Funding:
This study was supported by the Irish Cancer Society John Fitzpatrick Fellowship (E.H. Allott), and NIH 1K24CA160653 (S.J. Freedland).
Abbreviations
- BMI
Body Mass Index
- BPH
benign prostatic hyperplasia
- LUTS
lower urinary tract symptoms
- PV
prostate volume
- REDUCE
REduction of DUtasteride in Cancer Events
Footnotes
Conflicts of interest statement: None of the authors declare any conflicts of interest
REFERENCES
- 1.Donnell RF. Benign prostate hyperplasia: a review of the year’s progress from bench to clinic. Current Opinion in Urology. 2011;21(1):22–6. [DOI] [PubMed] [Google Scholar]
- 2.Saigal CS, Joyce G. Economic costs of benign prostatic hyperplasia in the private sector. The Journal of urology. 2005;173(4):1309–13. [DOI] [PubMed] [Google Scholar]
- 3.Garraway WM, Kirby RS. Benign prostatic hyperplasia: effects on quality of life and impact on treatment decisions. Urology. 1994;44(5):629–36. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG. Definition of at-risk patients: baseline variables. BJU international. 2006;97 Suppl 2:7–11; discussion 21–2. [DOI] [PubMed] [Google Scholar]
- 5.Schenk JM, Kristal AR, Neuhouser ML, Tangen CM, White E, Lin DW, et al. Biomarkers of Systemic Inflammation and Risk of Incident, Symptomatic Benign Prostatic Hyperplasia: Results From the Prostate Cancer Prevention Trial. American Journal of Epidemiology. 2010;171(5):571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Q, Wang Z, Liu G, Daneshgari F, MacLennan GT, Gupta S. Metabolic Syndrome, Inflammation and Lower Urinary Tract Symptoms – Possible Translational Links. Prostate cancer and prostatic diseases. 2016;19(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozumdar A, Liguori G. Persistent Increase of Prevalence of Metabolic Syndrome Among U.S. Adults: NHANES III to NHANES 1999–2006. Diabetes Care. 2011;34(1):216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Zeng X, Dong L, Zhao X, Qu X. The effects of statins on benign prostatic hyperplasia in elderly patients with metabolic syndrome. World journal of urology. 2015;33(12):2071–7. [DOI] [PubMed] [Google Scholar]
- 9.St Sauver JL, Jacobsen SJ, Jacobson DJ, McGree ME, Girman CJ, Nehra A, et al. Statin use and decreased risk of benign prostatic enlargement and lower urinary tract symptoms. BJU international. 2011;107(3):443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall SA, Chiu GR, Link CL, Steers WD, Kupelian V, McKinlay JB. Are statin medications associated with lower urinary tract symptoms in men and women? Results from the Boston Area Community Health (BACH) Survey. Annals of epidemiology. 2011;21(3):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills IW, Crossland A, Patel A, Ramonas H. Atorvastatin treatment for men with lower urinary tract symptoms and benign prostatic enlargement. European urology. 2007;52(2):503–9. [DOI] [PubMed] [Google Scholar]
- 12.Babcook MA, Joshi A, Montellano JA, Shankar E, Gupta S. Statin Use in Prostate Cancer: An Update. Nutrition and Metabolic Insights. 2016;9:NMI.S38362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78. [DOI] [PubMed] [Google Scholar]
- 14.He Z, Mangala LS, Theriot CA, Rohde LH, Wu H, Zhang Y. Cell killing and radiosensitizing effects of atorvastatin in PC3 prostate cancer cells. J Radiat Res. 2012;53(2):225–33. [DOI] [PubMed] [Google Scholar]
- 15.Hoque A, Chen H, Xu X-c. Statin Induces Apoptosis and Cell Growth Arrest in Prostate Cancer Cells. Cancer Epidemiology Biomarkers & Prevention. 2008;17(1):88–94. [DOI] [PubMed] [Google Scholar]
- 16.Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ. The current evidence on statin use and prostate cancer prevention: are we there yet? Nature reviews Urology. 2017;14(2):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–202. [DOI] [PubMed] [Google Scholar]
- 18.Allott EH, Howard LE, Vidal AC, Moreira DM, Castro-Santamaria R, Andriole GL, et al. Statin Use, Serum Lipids, and Prostate Inflammation in Men with a Negative Prostate Biopsy: Results from the REDUCE Trial. Cancer Prev Res (Phila). 2017;10(6):319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am. 2016;43(3):289–97. [DOI] [PubMed] [Google Scholar]
- 20.Parsons JK. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Current opinion in urology. 2011;21(1):1–4. [DOI] [PubMed] [Google Scholar]
- 21.Kozlowski R, Kershen RT, Siroky MB, Krane RJ, Azadzoi KM. Chronic ischemia alters prostate structure and reactivity in rabbits. The Journal of urology. 2001;165(3):1019–26. [PubMed] [Google Scholar]
- 22.Saito M, Tsounapi P, Oikawa R, Shimizu S, Honda M, Sejima T, et al. Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci Rep. 2014;4:3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berger AP, Horninger W, Bektic J, Pelzer A, Spranger R, Bartsch G, et al. Vascular resistance in the prostate evaluated by colour Doppler ultrasonography: is benign prostatic hyperplasia a vascular disease? BJU international. 2006;98(3):587–90. [DOI] [PubMed] [Google Scholar]
- 24.Feng T, Howard LE, Vidal AC, Moreira DM, Castro-Santamaria R, Andriole GL, et al. Serum cholesterol and risk of lower urinary tract symptoms progression: Results from the Reduction by Dutasteride of Prostate Cancer Events study. International journal of urology : official journal of the Japanese Urological Association. 2017;24(2):151–6. [DOI] [PubMed] [Google Scholar]
- 25.Yoo S, Oh S, Park J, Cho SY, Cho MC, Jeong H, et al. The impacts of metabolic syndrome and lifestyle on the prevalence of benign prostatic hyperplasia requiring treatment: historical cohort study of 130 454 men. BJU international. 2019;123(1):140–8. [DOI] [PubMed] [Google Scholar]
- 26.Parsons JK, Bergstrom J, Barrett-Connor E. Lipids, lipoproteins and the risk of benign prostatic hyperplasia in community-dwelling men. BJU international. 2008;101(3):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondul AM, Giovannucci E, Platz EA. A prospective study of statin drug use and lower urinary tract symptoms in older men. American journal of epidemiology. 2013;178(5):797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SH, Park TJ, Bae MH, Choi SH, Cho YS, Joo KJ, et al. Impact of treatment with statins on prostate-specific antigen and prostate volume in patients with benign prostatic hyperplasia. Korean J Urol. 2013;54(11):750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatiou KN, Zaglavira P, Skolarikos A, Sofras F. The effects of lovastatin on conventional medical treatment of lower urinary tract symptoms with finasteride. Int Braz J Urol. 2008;34(5):555–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 30.Cakir SS, Ozcan L, Polat EC, Besiroglu H, Kocaaslan R, Otunctemur A, et al. Statins are effective in the treatment of benign prostatic hyperplasia with metabolic syndrome. Aging Male. 2018:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Shen F, Dong L, Zhao X, Qu X. Influence and pathophysiological mechanisms of simvastatin on prostatic hyperplasia in spontaneously hypertensive rats. Urologia internationalis. 2013;91(4):467–73. [DOI] [PubMed] [Google Scholar]
- 32.Ishola IO, Tijani HK, Dosumu OO, Anunobi CC, Oshodi TO. Atorvastatin attenuates testosterone-induced benign prostatic hyperplasia in rats: role of peroxisome proliferator-activated receptor-gamma and cyclo-oxygenase-2. Fundam Clin Pharmacol. 2017;31(6):652–62. [DOI] [PubMed] [Google Scholar]
- 33.Padayatty SJ, Marcelli M, Shao TC, Cunningham GR. Lovastatin-induced apoptosis in prostate stromal cells. J Clin Endocrinol Metab. 1997;82(5):1434–9. [DOI] [PubMed] [Google Scholar]
- 34.Banez LL, Klink JC, Jayachandran J, Lark AL, Gerber L, Hamilton RJ, et al. Association between statins and prostate tumor inflammatory infiltrate in men undergoing radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010;19(3):722–8. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton RJ, Goldberg KC, Platz EA, Freedland SJ. The influence of statin medications on prostate-specific antigen levels. Journal of the National Cancer Institute. 2008;100(21):1511–8. [DOI] [PubMed] [Google Scholar]
- 36.Mondul AM, Selvin E, De Marzo AM, Freedland SJ, Platz EA. Statin drugs, serum cholesterol, and prostate-specific antigen in the National Health and Nutrition Examination Survey 2001–2004. Cancer causes & control : CCC. 2010;21(5):671–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang SL, Harshman LC, Presti JC Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28(25):3951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonbank A, Drexel H, Agewall S, Lewis BS, Dopheide JF, Kjeldsen K, et al. Reasons for disparity in statin adherence rates between clinical trials and real-world observations: a review. Eur Heart J Cardiovasc Pharmacother. 2018;4(4):230–6. [DOI] [PubMed] [Google Scholar]
- 39.Bienz M, Hueber PA, Al-Hathal N, McCormack M, Bhojani N, Trinh QD, et al. Accuracy of transrectal ultrasonography to evaluate pathologic prostate weight: correlation with various prostate size groups. Urology. 2014;84(1):169–74. [DOI] [PubMed] [Google Scholar]