Abstract
Background:
Very preterm (VPT; <30 weeks gestation) children are a heterogeneous group, yet the co-occurrence of psychiatric and neurodevelopmental impairments remains unclear. Moreover, the clinical and socio-environmental factors that promote resilient developmental outcomes among VPT children are poorly understood.
Methods:
125 children (85 VPT and 40 full-term) underwent neurodevelopmental evaluation at age 5-years. Parents and teachers completed measures of internalizing, externalizing, attention-deficit/hyperactivity (ADHD), and autism symptoms. Psychiatric and neurodevelopmental measures were analyzed using Latent Profile Analysis. Multinomial regression examined the extent that infant, sociodemographic, and family factors, collected prospectively from birth to follow-up, independently differentiated resilient and impaired children.
Results:
Four latent profiles were identified, including a Typically-Developing Group which represented 27.1% of the VPT group and 65.0% of the full-term group, an At-Risk Group with mild psychiatric and neurodevelopmental problems (VPT 44.7%, full-term 22.5%), a Psychiatric Group with moderate-to-severe psychiatric ratings (VPT 12.9%, full-term 10.0%), and a school-based Inattentive/Hyperactive Group (VPT 15.3%, full-term 2.5%). Clinical diagnoses were highest among the Psychiatric Group (80%). Factors that differentiated resilient and impaired subgroups of VPT children included prolonged exposure to maternal psychosocial distress (p≤.04), current family dysfunction (p≤.05), and maternal ADHD symptoms (p≤.02), whereas social risk index scores differentiated resilient and impaired full-term children (p<.03).
Conclusion:
Lower levels of maternal distress, family dysfunction, and maternal ADHD symptoms were associated with resilience among VPT children. Maternal distress and family dysfunction are modifiable factors to be targeted as part of psychiatric interventions embedded in the long-term care of VPT children.
Keywords: Prematurity, Psychiatric impairments, Neurodevelopment, Latent profile analysis
Very preterm (VPT; <30 weeks gestational age, GA) children are at increased risk of attention deficit hyperactivity (ADHD), autism spectrum (ASD) and anxiety disorders, along with neurodevelopmental impairments, compared to full-term (FT) children (Johnson et al., 2010; Woodward et al., 2009). However, preterm children are a heterogeneous group and while many demonstrate impairments, others resemble typically-developing FT children (Johnson et al., 2018). The factors that promote resilient developmental outcomes despite being born preterm remain poorly understood.
Few follow-up studies have examined developmental heterogeneity among VPT children using cluster-based approaches, which assume that a population is comprised of distinct subgroups. Using parent-report measures of cognitive, language, behavioral, socio-emotional, and autistic problems in moderate-to-late preterm (MLPT; 32–36 weeks GA) infants, Johnson et al. (2018) identified three subgroups spanning an unimpaired group of resilient infants, at-risk infants, and infants who exemplified the preterm phenotype with cognitive, socio-emotional, and ASD impairments. As VPT infants are born earlier and experience greater clinical complications than MLPT infants (Bastek et al., 2008), VPT infants are at increased risk of cognitive, motor, and behavioral impairments in childhood (Allotey et al., 2018). VPT children may, therefore, demonstrate different or more severe profiles of impairment than MLPT children. Moreover, few cluster-based analyses of preterm children have included standardized neurodevelopmental tasks beyond infancy when impairments may be more readily discernable (Krasner et al., 2015; Poehlmann-Tynan et al., 2015). Given the lack of trans-diagnostic studies that include both psychiatric and neurodevelopmental measures, it remains unclear how comorbid psychiatric impairments co-occur with neurodevelopmental impairments. Prior studies have also relied upon parent-report (Johnson et al., 2018; Krasner et al., 2015; Poehlmann-Tynan et al., 2015) without complementary teacher-reports which may identify subgroups of VPT children with situational or pervasive difficulties (Bora, Pritchard, Moor, Austin & Woodward, 2011).
Infant clinical factors, socio-demographic adversity, maternal depression, and negative parenting differentiate resilient and impaired subgroups of VPT children (Johnson et al., 2018; Poehlmann-Tynan et al., 2015). Although mothers of VPT children report greater parenting stress (Treyvaud, Lee, Doyle & Anderson, 2014), reduced involvement in children’s cognitive stimulation (Lean, Paul, Smyser & Rogers, 2018), and more family dysfunction (Treyvaud et al., 2014), these proximal socio-environmental factors have not been examined in relation to developmental heterogeneity among VPT children. Proximal factors directly shape the home environment and likely explain a greater proportion of variance in outcome than distal factors (Molfese, DiLalla & Bunce, 1997). The investigation of proximal socio-environmental factors in relation to heterogeneity in outcomes may elucidate potentially modifiable family factors to be targeted in the individualized follow-up care of VPT infants.
In addition to proximal socio-environmental factors, the extent that heritable factors are associated with developmental heterogeneity among VPT children is unknown. ADHD (Thapar & Stergiakouli, 2009), ASD (Lyall et al., 2014), and intellectual ability (Kirkpatrick, McGue, Iacono, Miller, & Basu 2014) are highly heritable. However, links between heritable family factors and heterogeneity among VPT children remain unknown. Consideration of heritable family background factors may inform individualized interventions that enhance parent-child functioning for VPT children at greatest risk of impairment. The aims of this study were to: 1) examine profiles of risk and resilience in VPT and FT children at age 5-years using dimensional multi-informant measures of psychopathology and standardized measures of neurodevelopment, and 2) identify the clinical, social, and family factors associated with risk and resilience in VPT children.
METHODS
Sample
This study consisted of 104 VPT (≤ 30 weeks GA) infants, born 2007–2010, who were recruited from a Level-III Neonatal Intensive Care Unit. VPT children underwent assessments at ages 2- (87/104, 84%) and 5-years (85/104, 82%). VPT children lost to follow-up were born to young mothers (≤18-years) (p=.02) and had public health insurance (p=.002), but there were no differences in infant clinical characteristics (p>.05). The FT comparison group (37–41 weeks GA) was recruited through two methods. Thirty demographically-similar FT children were recruited from the local communities of VPT children at age 5-years. Ten additional FT children were recruited as infants from an adjoining hospital’s obstetric service and assessed at age 5-years. Characteristics of the sample are shown in Table 1. Exclusion criteria included parent unable to give informed consent, infant chromosomal/congenital abnormality, or suspected/proven congenital infection. Additional exclusion criteria for FT infants included acidosis on cord blood gases and maternal positive urine drug screen. Written informed consent was obtained from all caregivers. Study procedures were approved by the Institutional Review Board.
Table 1.
Infant Clinical and Social Background Characteristics
| Very Preterm (n=85) |
Full Term (n=40) |
p | |
|---|---|---|---|
| Clinical Factors | |||
| Gestational age (weeks) | 26.51 (1.8) | 39.50 (0.8) | <.001 |
| Birthweight (grams) | 932.14 (254.1) | 3420.43 (516.5) | <.001 |
| Male, %(n) | 43.5 (37) | 42.5 (17) | .91 |
| Multiple birth, %(n) | 35.3 (30) | ||
| Antenatal steroids not administered, %(n) | 8.2 (7) | ||
| Postnatal dexamethasone administered, %(n) | 10.6 (9) | ||
| Confirmed Sepsis, %(n) | 23.9 (28) | ||
| Necrotizing enterocolitis, %(n) | 5.9 (5) | ||
| Patent ductus arteriosus, %(n) | 38.8 (33) | ||
| Prolonged oxygen supplementation, %(n) | 54.1 (46) | ||
| Neontal Brain Abnormalities | |||
| Periventricular leukomalacia grade 3/4, %(n) | 3.5 (3) | ||
| Intraventricular hemorrhage grade 3/4, %(n) | 7.1 (6) | ||
| Moderate/severe white matter abnormality, %(n) | 34.1 (29) | ||
| Maternal Social Background | |||
| Social risk index | 1.49 (1.3) | 1.35 (1.4) | .58 |
| African American, %(n) | 36.5 (31) | 42.5 (17) | .52 |
| ≤18 years at delivery, %(n) | 4.7 (4) | 2.5 (1) | .56 |
| No High School qualification, %(n) | 4.7 (4) | 7.5 (3) | .53 |
| Single parent household, %(n) | 45.9 (39) | 37.5 (15) | .38 |
| Public health insurance, %(n) | 57.6 (49) | 45.0 (18) | .19 |
| Income-to-needs ratio | 1.93 (2.0) | 2.21 (2.2) | .49 |
Note. Means and standard deviations reported unless indicated.
Measures
Psychiatric and Neurodevelopmental Functioning.
Cognitive, language, and motor skills at age 5-years were assessed by testers blinded to group membership using the Wechsler Preschool Primary Scales of Intelligence-III (WPPSI-III; Wechsler, 2004), Clinical Evaluation of Language Fundamentals-Preschool 2 (CELF-P2; Semel, Wiig, & Secord, 2004), and the Movement Assessment Battery for Children-2 (MABC-2; Henderson, Sugden & Barnett, 2007), respectively. Mothers completed the Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P; executive function; Gioia, Espy, & Isquith, 2003), Child Behavior Checklist (CBCL; internalizing and externalizing problems; Achenbach & Rescorla, 2001), Conners’ Rating Scale-Revised (CRS-R; ADHD symptoms; Conners, 2000), and Social Responsiveness Scale-2 (SRS-2; ASD symptoms; Constantino & Gruber, 2012). Teachers completed the Teacher Report Form (TRF; internalizing and externalizing problems; Achenbach & Rescorla, 2001), CRS-R, and SRS-2. Across parent and teacher measures of psychopathology, t-scores below 60 represent the normal range of functioning, t-scores 60–70 represent mild-to-moderate problems, and t-scores >70 represent moderate-to-severe problems. Measures of excutive function were included for supplementary analyses (please see Online Appendix S1). A more detailed description of study measures, as well as a summary in table form (Table S1), is provided in Online Appendix S1.
Clinical Diagnoses.
Internalising and externalizing disorders were obtained from the semi-structured Preschool Age Psychiatric Assessment (PAPA; Egger & Angold, 2006) interview which was administered by a trained clinical coordinator and completed by the child’s parent. Diagnoses included ADHD, conduct disorder, oppositional defiant disorder, anxiety disorders, and specific phobia. We used two methods to obtain information regarding ASD: 1) a diagnosis from the child’s psychiatrist or psychologist as reported by the parent during the scheduling of their follow-up visit, and 2) an observational assessment using the Autism Diagnostic Observation Schedule (ADOS; Lord & Rutter, 2001) which was administered by a trained psychometrician blind to children’s birth group and history of psychopathology. ADHD and anxiety symptom severity was determined from PAPA symptom counts. Ratings from the Childhood Autism Rating Scale-II (CARS-II; Schopler, Reichler, DeVellis, & Daly, 1980) assessed ASD symptom severity.
Social and Family Background.
A composite social risk score was calculated using five maternal factors which were dichotomized (present=1, absent=0) and summed (range: 0–5). Factors included young mother at delivery (age ≤18-years), African-American, no high school qualification, public health insurance, and single-parent household (Lean, Paul, Smyser, Smyser, & Rogers, 2018a). Socioeconomic status was estimated using income-to-needs ratio (United States Census Bureau, 2015).
A maternal psychosocial distress index was created using five key measures which were dichotomized (present=1, absent=0) based upon standardized cut-offs or the most impaired tertile score of the sample and summed (range: 0–5). Measures included the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996), Strait Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), Parenting Stress Index (PSI; Abidin, 1990), Social Support Questionnaire (SSQ; Sarason, Levine, Basham, & Sarason, 1983), and PSI Adverse Life Events scale (Abidin, 1990). The General Dysfunction subscale of the McMaster Family Assessment Device (FAD; Epstein, Baldwin, & Bishop, 1983) evaluated family dysfunction. The StimQ-Preschool (StimQ-P; Dreyer, Mendelsohn, & Tamis-LeMonda, 1996) provided a measure of cognitive stimulation in the home. Maternal ADHD and ASD symptoms were evaluated with self-report versions of the CRS-R and SRS-2. Mothers also completed observer-report versions to obtain ADHD and ASD symtoms on the child’s biological father. Maternal intellectual quotient (IQ) was assessed with the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) which is co-normed with the Wechsler Adult Intelligence Scales-III.
Preterm-Only Measures.
Infant clinical risk was determined from clinical factors collected from medical records, which were dichotomized (present=1, absent=0) and summed (range: 0–10): intrauterine growth restriction, prolonged oxygen supplementation, did not receive antenatal steroids, received dexamethasone, necrotizing enterocolitis, confirmed sepsis, patent ductus arteriosus, retinopathy of prematurity, ≥3 SD decrease in weight-for-height/length from birth to term-equivalent age, and >75th percentile for duration of parenteral nutrition (Lean et al., 2018a).
At term-equivalent age, VPT infants underwent an MRI scan. Images were acquired using a Siemens 3T scanner. MRI images were qualitatively scored for the severity of white matter abnormalities (WMA) spanning cystic lesions, focal signal abnormalities, myelination delay, corpus callosum thinning, lateral ventricle dilatation, and cerebral volume reduction (Kidokoro, Neil, & Inder, 2013). Brain tissue volumes (total, cerebral spinal fluid, white matter, cerebellum, cortical and deep nuclear gray matter) were obtained from T2-weighted structural MRI images and processed using automated Morphologically Adaptive Neonatal Tissue Segmentation and Advanced Normalization Tools packages (Beare et al., 2016).
At age 2-years, VPT children’s cognitive, language, and motor development was assessed with the Bayley Scales of Infant Development 3rd Edition (Bayley-III; Bayley, 2005). Mothers completed the Infant-Toddler Social and Emotional Assessment (ITSEA; Carter, Briggs-Gowan, Jones & Little, 2003) to assess externalizing, internalizing, regulatory, and socio-emotional problems. Identical social background questionnaires were administered to obtain longitudinal 2-year measures of social risk, maternal distress, and family dysfunction.
Data Analysis
Latent Profile Analysis (LPA) with bootstrapping was performed on dimensional psychiatric and neurodevelopmental measures for VPT and FT children at age 5-years (n=125) using MPlus (Version 7). LPA performed using bootstrapped ratio likelihood tests with high-quality indicators for >100 subjects has adequate statistical power (~80%) to detect at least three latent groups (a=.05) (Dziak, Lanza & Tan, 2014; Wurpts & Geiser, 2014). LPA model fit was evaluated via lower bootstrapped Akaike Information Criterion (AIC) and Adjusted Bayesian Information Criterion (ABIC) values, bootstrapped Lo-Mendell-Rubin Adjusted Likelihood Ratio Test p-value ≤0.05, and interpretability of the profiles. Entropy values closer to 1.00 indicated greater certainty in latent profile assignment. Subsequent LPA was also performed first including all singletons and the first sibling from preterm multiples, and then performed including all singletons and the second sibling from preterm multiples. As the exclusion of preterm siblings did not alter the results, data for all subjects are presented.
Analysis of variance and chi-square tests were used to compare latent profiles across continuous and categorical clinical and socio-environmental variables. Pair-wise comparisons with Tukey HSD adjustment for multiple comparisons are reported. Missing data (range 5.6–12.0%) were imputed in SPSS (Version 24). Paternal ADHD and ASD symptoms were not presumed missing at random due to absentee fathers and therefore not imputed. Data were then re-analyzed in the VPT cohort separately.
Multinomial logistic regression was used to examine the independent predictors of latent profile assignment among VPT children. Independent variables included infant clinical, social risk, maternal distress, and family dysfunction scores from ages 2- and 5-years. Models were extended to include maternal IQ and ADHD symptoms. Due to smaller sample size in the FT group (n=40), a reduced multinomial regression analysis was performed in the FT cohort including social risk, maternal distress, and family dysfunction scores as independent variables.
RESULTS
Latent Profile Analysis
The LPA (Figure 1) supported a four profile solution that had lower AIC (4743.11) and ABIC (4949.57) values, and the highest entropy (0.96) (Table S1, Online Appendix S2). The average posterior probability ratings of final latent profile assignment were excellent (>.96). LPA performed with VPT and FT children separately produced similar results (Figure S1, Online Appendix S2).
Figure 1.
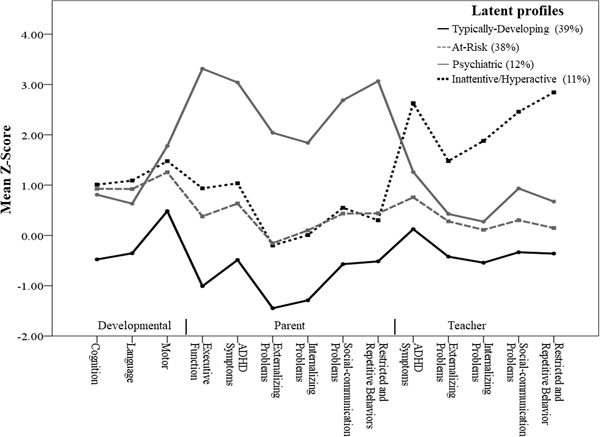
Figure 1 shows four profiles spanning a Typically-Developing Group (39%), an At-Risk Group (38%), a Psychiatric Group (12%) and a school-based Inattentive/Hyperactive Group (11%). Note: standardized scores for developmental measures are reverse coded to correspond with parent- and teacher-reports of psychopathology.
The Typically-Developing Group (39.2% of the total sample, Profile 1) was comprised of children who had neurodevelopmental and psychiatric functioning within the normal range based on published norms (Table 2). The At-Risk Group (37.6%, Profile 2) was comprised of children who obtained lower neurodevelopmental scores and although this profile was rated higher by parents and teachers across psychiatric domains compared to the Typically-Developing Group, psychiatric ratings were in the normal range. Two additional profiles with prominent psychiatric impairments were identified. The Psychiatric Group (12.0%, Profile 3) was characterized by mild-to-moderate internalizing problems and moderate-to-severe problems in executive function, ADHD symptoms, externalizing and problems, and ASD symptoms on parent-report measures (Table 2). All children in the Psychiatric Group had parent-ratings above the normal range (i.e., t-score >60), and teachers also rated 53% of children in this group above the normal range. The Inattentive/Hyperactive Group (11.2%, Profile 4) performed the least well on cognitive and language measures. This teacher-identifed group had mild-to-moderate across internalizing and externalizing domains, and moderate-to-severe ratings for ADHD and ASD symptoms. For this profile, both teachers (mean=76.29, SD=10.9) and parents (mean=60.36, SD=13.6) reported increased ADHD symptoms compared to the other psychiatric domains (Table 2). Teachers rated all of the children in this group above the normal range and parents rated 64% of children in this group above the normal range. A similar pattern of latent profile differences across neurodevelopmental and psychiatric measures was found when the analysis was performed among the VPT group seperately (see Table S2, Appendix S2). See Online Appendix S2 for latent profile differences on additional exeutive function tasks (Table S3).
Table 2.
Neurodevelopmental and Psychiatric Features of Latent Profiles at Age 5-Years
| Typically- Developing (n=49) |
At-Risk (n=47) | Psychiatric (n=15) |
Inattentive/ Hyperactive (n=14) |
p | |
|---|---|---|---|---|---|
| Neurodevelopment | |||||
| Cognition | 107.16 (10.60)a,b,c | 86.11 (12.0)a | 87.87 (13.1)b | 84.86 (12.0)c | <.001 |
| Language | 105.34 (15.5)a,b,c | 86.17 (14.5)a | 90.53 (13.6)b | 83.64 (17.6)c | <.001 |
| Motor | 8.58 (3.3)a,b,c | 6.14 (3.2)a | 3.62 (2.1)b | 5.57 (3.4)c | <.001 |
| Parent-report Symptoms | |||||
| Executive function | 39.92 (6.5)a,b,c | 53.79 (8.7)a,d | 83.13 (12.9)b,d,f | 59.36 (14.6)c,f | <.001 |
| ADHD | 45.12 (5.5)a,b,c | 56.34 (9.4)a,d | 80.40 (8.0)b,d,f | 60.36 (13.6)c,f | <.001 |
| Externalizing | 35.53 (7.7)a,b,c | 48.45 (8.2)a,d | 70.40 (8.7)b,d,f | 48.00 (11.1)c,f | <.001 |
| Internalizing | 37.10 (6.7)a,b,c | 51.00 (8.7)a,d | 68.40 (9.0)b,d,f | 50.07 (11.1)c,f | <.001 |
| Social-Communication | 44.29 (4.4)a,b,c | 54.34 (6.7)a,d | 76.87 (8.5)b,d,f | 55.50 (7.0)c,f | <.001 |
| Autistic behaviors | 44.84 (3.5)a,b,c | 54.38 (7.9)a,d | 80.67 (9.6)b,d,f | 53.00 (7.5)c,f | <.001 |
| Teacher-report Symptoms | |||||
| ADHD | 51.24 (10.4)a,b,c | 57.57 (9.6)a,e | 62.60 (12.5)b,f | 76.29 (10.9)c,e,f | <.001 |
| Externalizing | 45.78 (8.3)a,b,c | 52.79 (9.7)a,e | 54.27 (9.7)b,f | 64.79 (6.2)c,e,f | <.001 |
| Internalizing | 45.78 (8.5)a,b,c | 51.11 (7.4)a,e | 52.73 (10.4)b,f | 68.79 (11.0)c,e,f | <.001 |
| Social-Communication | 46.65 (7.6)a,b,c | 53.02 (6.5)a,d,e | 59.33 (10.9)b,d,f | 74.57 (8.2)c,e,f | <.001 |
| Restricted and Repetitive Behaviors | 46.39 (5.0)a,b,c | 51.47 (6.1)a,e | 56.73 (12.0)b,f | 78.43 (9.3)c,e,f | <.001 |
Note. Means and standard deviations reported. Pair-wise comparison p<.05 Profile
1 vs. 2
1 vs. 3
1 vs. 4
2 vs. 3
2 vs. 4
3 vs. 4
Latent Profile Differences on Background Factors
Demographics.
The Typically-Developing Group had a higher proportion of children from the FT group (65.0%, n=26) than the VPT group (27.1%, n=23, p<.001). The majority of VPT children (44.7%, n=38) were classified into the At-Risk Group, compared to 22.5% (n=9) of the FT group (p=.02). Regarding the two psychiatric profiles, 12.9% (n=11) of the VPT group was classified in the Psychiatric Group compared to 10% (n=4) of the FT group (p=.77); and 15.3% (n=13) of the VPT group was classified in the Inattentive/Hyperactive Group compared to 2.5% (n=1) of the FT group (p=.04). There were no differences by sex or race (p>.05).
Clinical Diagnoses.
At age 5-years, no clinical diagnoses were observed in the Typically-Developing Group (p<.001, Table S3, Online Appendix S2). The Psychiatric Group had the highest rate of clinical diagnoses (80%) compared to 40% of the At-Risk (p=.01) and 36% of the Inattentive/Hyperactivity (p=.02) Groups. Rates of ADHD were highest among the Psychiatric Group (46.7%), and although this rate was higher than the Inattentive/Hyperactivity Group (21.4%), this difference was not significant (p>.05). The Psychiatric Group also had greater ADHD symptom severity than both the At-Risk (p<.001) and Inattentive/Hyperactivity (p=.008) Groups. Rates of ASD were higher among the Psychiatric Group (33.3%) than the At-Risk Group (10.6%, p=.05), although symptom severity was similar (p=.93). The Psychiatric Group had more severe anxiety symptoms than both the At-Risk (p=.003) and Inattentive/Hyperactivity (p=.004) Groups, but these groups were similar in terms of rates of anxiety disorders (p=.14).
Social and Family Characteristics.
Compared to the Typically-Developing Group, children in the At-Risk and Psychiatric Groups were more likely to be raised in single parent households (p<.001), have public health insurance (p≤.04), and have higher levels of social risk (p≤.01) (Table 3). The At-Risk and Psychiatric Groups also had greater family dysfunction (p≤.05) and maternal distress (p<.001), and reduced cognitive stimulation in the home (p=.004) and maternal IQ (p=.001). Between the impaired profiles, the Psychiatric Group had the highest levels of maternal depression (p≤.03), anxiety (p=.02), parenting stress (p<.001), and ADHD (p<.001) and ASD symptoms (p≤.005). The Psychiatric Group also had fathers with high ADHD (p≤.04) and ASD (p≤.02) ratings.
Table 3.
Home and Family Factors Associated with Latent Profiles at Age 5-Years
| Typically- Developing (n=49) |
At-Risk (n=47) | Psychiatric (n=15) |
Inattentive/ Hyperactive (n=14) |
p | |
|---|---|---|---|---|---|
| Public health insurance, %(n) | 38.8 (19)a,b | 59.6 (28)a | 73.3 (11)b | 64.3 (9) | .05 |
| Single parent household, %(n) | 18.4 (9)a,b | 61.7 (29)a | 66.7 (10)b | 42.9 (6) | <.001 |
| Social risk index | 0.90 (1.2)a,b | 1.83 (1.4)a | 2.07 (1.4)b | 1.43 (1.2) | .003 |
| Income-to-needs ratio | 2.56 (2.3) | 1.76 (1.9) | 1.27 (1.0) | 1.79 (2.2) | .09 |
| Family dysfunction | 1.36 (0.1)a,b | 1.57 (0.1)a | 1.78 (0.1)b | 1.64 (0.1) | .001 |
| Cognitive stimulation | 45.51 (0.7)a | 41.46 (0.7)a | 41.58 (1.3) | 43.65 (1.4) | .004 |
| Maternal IQ | 102.88 (1.7)a | 92.32 (1.9)a | 94.45 (3.3) | 99.14 (3.2) | .001 |
| Maternal distress index | 0.45 (0.2)a,b | 1.10 (0.2)a,d | 2.20 (0.3)b,d,f | 0.90 (0.3)f | <.001 |
| Depression | 2.69 (1.0)a,b | 6.78 (1.0)a,d | 13.32 (1.9)b,d,f | 5.89 (1.9)f | <.001 |
| Anxiety | 29.51 (1.4)a,b | 36.40 (1.5)a | 43.72 (2.6)b,f | 33.40 (3.7)f | <.001 |
| Parenting stress | 19.87 (3.9)a,b | 36.04 (4.0)a,d | 79.54 (7.4)b,d,f | 36.28 (7.1)f | <.001 |
| Stressful life events | 37.70 (4.2)a,b,c | 62.23 (4.3)a | 71.90 (7.8)b | 67.27 (7.8)c | <.001 |
| Social support | 5.39 (0.2) | 4.96 (.20) | 4.95 (0.3) | 5.12 (0.3) | .37 |
| Parental Symptoms | |||||
| Maternal ADHD | 38.95 (1.4)a,b | 45.44 (1.4)a | 52.73 (2.8)b | 45.46 (3.2) | <.001 |
| Paternal ADHD (n=80) | 40.80 (1.6)a,b | 49.77 (1.9)a,d | 59.9 (3.1)b,d | 56.20 (4.4) | <.001 |
| Maternal ASD | 42.32 (1.0)a,b | 49.11 (1.1)a,d | 57.70 (1.9)b,d,f | 48.04 (2.2)f | <.001 |
| Paternal ASD (n=84) | 43.72 (1.5)a,b | 52.04 (1.7)a,d | 61.82 (2.8)b,d, f | 45.00 (3.8)f | <.001 |
Note. Means and standard errors reported unless indicated. Pair-wise comparison p<.05 Profile
1 vs. 2
1 vs. 3
1 vs. 4
2 vs. 3
2 vs. 4
3 vs. 4
Preterm-Only Analysis.
Table S4 (Online Appendix S2) shows that within the VPT cohort, higher rates of ADHD (p=.03) and ASD (p=.003), and more severe anxiety symptoms (p<.001), were present among the Psychiatric Group at age 5-years. As in the whole-group analysis, VPT children in the Psychiatric Group had greater family dysfunction (p=.007), maternal distress (p<.001), and maternal and paternal ADHD (p≤.003) and ASD (p≤.001) symptoms (Table S5, Online Appendix S2).
Infant clinical factors did not differentiate the latent profiles of VPT children (p>.05, Table S6, Online Appendix S2). VPT children in the Psychiatric Group had smaller adjusted cerebellar volumes than the Typically-Developing (p=.01) and At-Risk (p=.02) Groups. At age 2-years, the Hyperactive/Inattentive Group obtained the lowest Bayley-III cognitive (p=.002), language (p<.001), and motor scores (p=.001) (Table S4, Online Appendix S2). The Psychiatric Group had the poorest ITSEA externalizing (p=.006), dysregulation (p=.005), and socio-emotional competency (p=.009) scores.
Multivariate Analysis of Social and Family Factors
Table S7 (Online Appendix S2) summarizes the neonatal, 2-year, and 5-year factors that independently differentiated the impaired groups of VPT children from Typically-Developing VPT children. In the At-Risk Group, neither infant clinical risk factors nor social risk, maternal distress, or family dysfunction assessed at either time-point uniquely differentiated this group from the Typically-Developing Group (p>.05). Greater maternal distress at both the 2-year (p=.02) and 5-year (p=.02) assessments differentiated the Psychiatric Group from the Typically-Developing Group, whereas infant clinical risk, social risk, and family dysfunction did not (p>.05). Greater maternal distress at the 2-year assessment also differentiated Inattentive/Hyperactive VPT children from Typically-Developing VPT children (p=.04). However, at the 5-year follow-up, maternal distress was no longer significant (p=.29) and instead, higher levels of family dysfunction differentiated the profiles (p=.03).
In the extended model, no associations were found for maternal IQ (p>.05). Higher maternal ADHD symptoms independently differentiated the At-Risk Group from the Typically-Developing Group (p=.01). Higher levels of maternal ADHD were associated with the Psychiatric Group (p=.02) and explained a greater proportion of variance than maternal distress, which was attenuated (p=.14). However, the inclusion of maternal ADHD symptoms (p=.07) did not alter associations regarding increased family dysfunction in the Inattentive/Hyperactive Group (p=.05). Additional multivariate analysis suggested that paternal ADHD symptoms was not significant (data not shown).
Excluding the FT child in the Inattentive/Hyperactive Group, the results of the supplementary multivariate analysis performed in the FT cohort indicated that higher levels of social risk independently differentiated the At-Risk (B=1.40, p=.007) and Psychiatric (B=1.52, p=.03) Groups from the Typically-Developing Group. Maternal distress and family dysfunction were not significant (p>.05).
DISUCUSSION
This study identified a Typically-Developing and three adverse profiles of VPT and FT children at age 5-years using multi-informant reports of psychopathology and standardized neurodevelopmental tasks. As some prior studies have not included control groups (Krasner et al., 2015; Poehlmann-Tynan et al., 2015), current findings highlight that approximately one quarter of reslient VPT children resemble the majority of demographically-similar FT children. Although our reported rate of resilient preterm children is lower compared to other MLPT cohorts (Johnson et al., 2018; Poehlmann-Tynan et al., 2015), VPT infants are at higher risk of impairments due to greater extent of prematurity (Allotey et al., 2018). FT children were also included in the LPA and thus VPT children’s membership in the resilient profile was based relative to controls.
The At-Risk Group, comprised of the majority of VPT children, had slightly elevated ratings across psychiatric domains with parents and teachers agreeing on the severity of symptoms. The two smaller Psychiatric and Inattentive/Hyperactive Groups had clear impairments in ADHD, socio-emotional, and ASD domains. Both parents and teachers rated the Psychiatric and Inattentive/Hyperactive Groups higher than the At-Risk Group. However, the Psychiatric Group had higher parent-report ratings whereas the Inattentive/Hyperactive Group had higher teacher-report ratings, indicating that parents and teachers disagreed on the severity of symptoms for approximately a quarter of VPT children, which is similar to previous reports (Bora et al., 2011). As the Psychiatric Group had the highest rate of clinical diagnoses, it is possible that parents were aware of their child’s symptoms whereas teachers may not have had this information (Bora et al., 2011). An alternative interpretation of our results concerns the possibility that mothers with depression and anxiety might be over-reporting symptoms in their children (Gartstein, Bridgett, Dishion & Kaufman, 2009), or that highly dysregulated preterm children may be harder to parent subsequently leading to higher levels of maternal distress (Quist et al., 2019; Treyvaud et al., 2011). As the Inattentive/Hyperactive Group was almost exclusively comprised of VPT children, this group may be representative of the preterm phenotype characterized by problems across ADHD, internalizing, and socio-communication domains (Johnson & Marlow, 2011). However, we interpret this finding with caution given that the specific pattern of symptoms in this profile was not readily identified by parent-report. Teachers may be identifying group of VPT children who demonstrate greater emotional and behavioral problems in structured educational settings relative to same-age peers (Scott et al., 2012), potentially due to increased family dysfunction in the home.
In bivariate analyses, lower levels of socio-demographic disadvantage, reduced family dysfunction, and greater cognitive stimulation in the home at age 5-years were associated with the Typically-Developing Group (see Lean et al., 2018a; Poehlmann-Tynan et al., 2015; Treyvaud et al., 2012). Maternal distress and parental ADHD/ASD symptoms clearly varied across the impaired subgroups of VPT children, with higher maternal distress index scores and parental ADHD symptoms reported for the Psychiatric Group. While our study was not designed to test the genetic effects of heritable factors, maternal psychopathology may demonstrate links with childhood internalizing and externalizing disorders through genetic, neurobiological, and environmental mechanisms. First, along with heritable genetic risks, maternal depression underlies childhood internalizing disorders via in-utero exposure to maternal stress (Martini, Knappe, Beesdo-Baum, Lieb, & Wittchen, 2010) and inflammatory processes (Bilbo & Schwarz, 2012). Second, mothers experiencing high levels of psychosocial distress may face greater challenges in providing supportive home environments (Blair & Raver, 2016). Third, links between maternal psychosocial adversity and child psychopathology are mediated by adverse pre-and post-natal exposures on infant structural and functional brain connectivity (Bernier, Calkins, & Bell, 2016). VPT children in the Psychiatric Group had reduced neonatal cerebellar volumes, which supports known links between cerebellar abnormalities and psychopathology (Parker et al., 2007). While we did not find assocations with infant clinical factors, prior cluster-based studies of preterm children report that gestational age and poorer neonatal health differentiate profiles of impairment (Johnson et al., 2018; Poehlmann-Tynan et al., 2015). Discrepancies between findings may be attributable to prior studies including different clinical factors in composite measures of neonatal health (Poehlmann-Tynan et al., 2015).
In multivariate analyses, higher levels of maternal distress at both the 2- and 5-year assessments independently differentiated the Psychiatric Group from the Typically-Developing Group of VPT children. This association was subsequently accounted for by maternal ADHD symptoms. A large population based study reported that 28–32% of women with ADHD also have co-occurring depression or anxiety (Solberg et al., 2018). Early exposure to maternal distress at age 2-years was also associated with the Hyperactive/Inattentive Group, although by age 5-years, family dysfunction was the key independent factor differentiating this group from the Typically-Developing Group. While bivariate analyses suggested that the At-Risk Group had more single-parent households and greater family dysfunction, maternal ADHD symptoms was the only factor uniquely differentiating the At-Risk Group from the Typically-Developing Group in multivariate analyses. Within the FT cohort, social risk differentiated impaired from Typically-Developing children. Maternal distress and family dysfunction differentiated the typically-developing and impaired profiles of VPT children, but did not differentiate profiles of FT children. The difference in risk factors might support previous reports of increased psychopathology and dysfunctional parenting among mothers of preterm infants compared to mothers of term infants (Martini et al., 2010; Treyvaud et al., 2014).
Study findings highlight differential pathways to psychiatric impairment with VPT children placed at risk due to maternal psychosocial adversity and family dysfunction and/or the direct/indirect effects of maternal ADHD. The lack of findings with regards to infant clinical factors optimistically suggests that proximal socio-environmental influences are critical for promoting resilient outcomes among VPT infants. Early interventions that target maternal psychopathology, parenting stress, and parent-child interactions have been shown to be successful in improving maternal and infant outcomes (Beebe et al., 2018). Our findings emphasize the need to monitor and support longer-term maternal and family functioning alongside the provision of individualized strategies to improve the developmental outcomes of heterogeneous groups of VPT children.
Study strengths included high sample retention, the use of multi-informant dimensional measures of psychopathology alongside standardized neurodevelopmental tasks, and the inclusion of a demographically-similar control group. Study limitations included modest sample size (n=125). As the Psychiatric and Inattentive/Hyperactive Groups were the smallest profiles, non-significant differences between these profiles may be attributable to profile size and small effect sizes. As the FT group was recruited at age 5-years, we were unable to examine longitudinal associations between socio-environmental risk factors and heterogeneity in the FT group. We also acknowledge that as we primarily relied upon parent report of children’s externalizing and internalizing disorders using the PAPA, rates of psychopathology may have been slightly over- or under-reported. However, in the Psychiatric Profile, higher rates of PAPA-identified externalizing and internalizing disorders were consistent with the increased rates of ASD which was assessed using a behavioral observation administered by a blinded psychometrician. Future research should endeavor to link heterogeneity among VPT children to differences in structural and functional brain connectivity.
Conclusions
Despite being born preterm, approximately one quarter of resilient VPT children resemble the majority of their FT peers. VPT children are, however, a heterogeneous group with three quarters of VPT children demonstrating psychiatric symptoms that vary in severity across settings. Although infant clinical factors did not differentiate profiles of psychiatric impairments in VPT children, key family factors included maternal psychosocial distress, family dysfunction, and maternal ADHD symptoms. Study findings highlight not only the differential pathways to risk and resilience for VPT children, but also the potentially modifiable factors to be targeted in early interventions to support maternal functioning and parenting.
Supplementary Material
Key Points:
VPT children are a heterogeneous group. While many VPT children demonstrate psychiatric and neurodevelopmental impairments, others resemble typically-developing term-born children.
The socio-environmental and family background factors that promote resilient outcomes among VPT children are poorly understood.
Maternal psychosocial distress (depression, anxiety, parenting stress, stressful events, dissatisfaction with social support), family dysfunction, and ADHD symptoms differentiated resilient and psychiatrically impaired VPT children, whereas socio-demographic adversity differentiated typically-developing and impaired term-born children.
The lack of findings with regards to infant clinical factors optimistically suggests that socio-environmental influences are critical for promoting resilient outcomes among VPT infants.
Follow-up care should support longer-term maternal and family functioning alongside the provision of individualized strategies to improve the outcomes of heterogeneous groups of VPT children.
Acknowledgements
Funding was provided by the National Institutes of Health (R01-HD057098, R01-MH113570, K02-NS089852, UL1-TR000448, K23-MH105179), Intellectual and Developmental Disabilities Research Center at Washington University (U54-HD087011), Cerebral Palsy International Research Foundation, The Dana Foundation, The Child Neurology Foundation, and The Doris Duke Charitable Foundation. The authors would like to thank the Washington University Neonatal Developmental Research Group and the families involved with the study. The authors have declared that they have no competing or potential conflicts of interest.
Abbreviations:
- FT
full-term
- LPA
latent profile analysis
- MLPT
moderate-to-late preterm
- VPT
very preterm
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Conflict of interest statement: No conflicts declared.
REFERENCES
- Abidin RR (1990). Parenting Stress Index (PSI). Charlottesville, VA: Pediatric Psychology Stress. [Google Scholar]
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E… Thangaratinam, S. (2018). Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG: An International Journal of Obstetrics & Gynaecology, 125(1), 16–25. [DOI] [PubMed] [Google Scholar]
- Bastek JA, Sammel MD, Paré E, Srinivas SK, Posencheg MA, & Elovitz MA (2008). Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. American Journal of Obstetrics and Gynecology, 199(4), 367.e1–367.e8. [DOI] [PubMed] [Google Scholar]
- Bayley N (2005). Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III). San Antonio, TX: Pearson. [Google Scholar]
- Beare RJ, Chen J, Kelly CE, Alexopoulos D, Smyser CD, Rogers CE Thompson DK. (2016). Neonatal Brain Tissue Classification with Morphological Adaptation and Unified Segmentation. Frontiers in Neuroinformatics, 10(12), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Beebe B, Myers MM, Lee SH, Lange A, Ewing J, Rubinchik N… Welch MG. (2018). Family nurture intervention for preterm infants facilitates positive mother–infant face-to-face engagement at 4 months. Developmental Psychology, 54(11), 2016–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A, Calkins SD, & Bell MA (2016). Longitudinal Associations Between the Quality of Mother–Infant Interactions and Brain Development Across Infancy. Child Development, 87(4), 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, & Schwarz JM (2012). The immune system and developmental programming of brain and behavior. Frontiers in Neuroendocrinology, 33(3), 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2016). Poverty, Stress, and Brain Development: New Directions for Prevention and Intervention. Academic Pediatrics, 16(3), S30–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora S, Pritchard VE, Moor S, Austin NC, & Woodward LJ (2011). Emotional and behavioural adjustment of children born very preterm at early school age. Journal of Paediatrics and Child Health, 47(12), 863–869. [DOI] [PubMed] [Google Scholar]
- Carter AS, Briggs-Gowan MJ, Jones SM, & Little TD (2003). The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. Journal of Abnormal Child Psychology, 31(5), 495–514. [DOI] [PubMed] [Google Scholar]
- Conners CK (2000). Conners’ Rating Scales-Revised Technical Manual. New York, USA: Multi-Health Systems Inc. [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social Responsiveness Scale, Second Edition (SRS-2). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Dreyer BP, Mendelsohn AL, & Tamis-LeMonda CS (1996). Assessing the Child’s Cognitive Home Environment Through Parental Report; Reliability and Validity. Early Development and Parenting, 5(4), 271–287. [Google Scholar]
- Dziak JJ, Lanza ST, & Tan X (2014). Effect Size, Statistical Power and Sample Size Requirements for the Bootstrap Likelihood Ratio Test in Latent Class Analysis. Structural Equation Modeling : A Multidisciplinary Journal, 21(4), 534–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, & Angold A (2006). Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. Journal of Child Psychology and Psychiatry, 47(3), 313–337. [DOI] [PubMed] [Google Scholar]
- Epstein NB, Baldwin LM, & Bishop D (1983). The McMaster family assessment device. Journal of Marital and Family Therapy, 9(2), 171–180. [Google Scholar]
- Gartstein MA, Bridgett DJ, Dishion TJ, & Kaufman NK (2009). Depressed Mood and Maternal Report of Child Behavior Problems: Another Look at the Depression–Distortion Hypothesis. Journal of Applied Developmental Psychology, 30(2), 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, & Isquith PK (2003). BRIEF-P: Behavior Rating Inventory of Executive Function–Preschool Version. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Henderson SE, Sugden DA, & Barnett AL (2007). Movement assessment battery for children-2: Movement ABC-2: Examiner’s manual. Pearson; São Paulo. [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, & Marlow N (2010). Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. Journal of the American Academy of Child & Adolescent Psychiatry, 49(5), 453–463. [PubMed] [Google Scholar]
- Johnson S, & Marlow N (2011). Preterm birth and childhood psychiatric disorders. Pediatric Research, 69(5 Pt 2), 11R–8R. 10.1203/PDR.0b013e318212faa0 [DOI] [PubMed] [Google Scholar]
- Johnson S, Waheed G, Manktelow BN, Field DJ, Marlow N, Draper ES, & Boyle EM (2018). Differentiating the Preterm Phenotype: Distinct Profiles of Cognitive and Behavioral Development Following Late and Moderately Preterm Birth. The Journal of Pediatrics, 193, 85–92.e1. [DOI] [PubMed] [Google Scholar]
- Kidokoro H, Neil JJ, & Inder TE (2013). New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR. American Journal of Neuroradiology, 34(11), 2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick RM, McGue M, Iacono WG, Miller MB, & Basu S (2014). Results of a “GWAS plus:” general cognitive ability is substantially heritable and massively polygenic. PloS One, 9(11), e112390. 10.1371/journal.pone.0112390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasner AJ, Turner JB, Feldman JF, Silberman AE, Fisher PW, Workman CC… Whitaker AH. . (2015). ADHD Symptoms in a Non-Referred Low Birthweight/Preterm Cohort: Longitudinal Profiles, Outcomes, and Associated Features. Journal of Attention Disorders. 10.1177/1087054715617532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean RE, Paul RA, Smyser CD, & Rogers CE (2018). Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. Journal of Child Psychology and Psychiatry, 59(2), 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean RE, Paul RA, Smyser TA, Smyser CD, & Rogers CE (2018). Social Adversity and Cognitive, Language, and Motor Development of Very Preterm Children from 2 to 5 Years of Age. The Journal of Pediatrics, 203, 117–184.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, & Rutter M (2001). Autism Diagnostic Observation Schedule, Second Edition Torrance, CA: WPS Publishing, LLC. [Google Scholar]
- Lyall K, Constantino JN, Weisskopf MG, Roberts AL, Ascherio A, & Santangelo SL (2014). Parental Social Responsiveness and Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry, 71(8), 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini J, Knappe S, Beesdo-Baum K, Lieb R, & Wittchen H-U (2010). Anxiety disorders before birth and self-perceived distress during pregnancy: Associations with maternal depression and obstetric, neonatal and early childhood outcomes. Early Human Development, 86(5), 305–310. [DOI] [PubMed] [Google Scholar]
- Molfese VJ, DiLalla LF, & Bunce D (1997). Prediction of the Intelligence Test Scores of 3- to 8-Year-Old Children by Home Environment, Socioeconomic Status, and Biomedical Risks. Merill-Palmer Quarterly, 43(2), 219–234. [Google Scholar]
- Parker J, Mitchell A, Kalpakidou A, Walshe M, Jung H-Y, Nosarti C… Allin M (2007). Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain, 131(5), 1344–1351. [DOI] [PubMed] [Google Scholar]
- Poehlmann-Tynan J, Gerstein E, Burnson C, Weymouth L, Bolt D, Maleck S, & Schwichtenberg A (2015). Risk and Resilience in Preterm Children at Age 6. Development and Psychopathology, 27(3), 843–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist M, Kaciroti N, Poehlmann-Tynan J, Weeks HM, Asta K, Singh P, & Shah PE (2019). Interactive Effects of Infant Gestational Age and Infant Fussiness on the Risk of Maternal Depressive Symptoms in a Nationally Representative Sample. Academic Pediatrics. 10.1016/j.acap.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason IG, Levine HM, Basham RB, & Sarason BR (1983). Assessing social support: The Social Support Questionnaire. Journal of Personality and Social Psychology, 44(1), 127–139. [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, & Daly K (1980). Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) | SpringerLink. Journal of Autism and Developmental Disorders, 10(1), 91–103. [DOI] [PubMed] [Google Scholar]
- Scott MN, Taylor HG, Fristad MA, Klein N, Espy KA, Minich N, & Hack M (2012). Behavior Disorders in Extremely Preterm/Extremely Low Birth Weight Children in Kindergarten. Journal of Developmental and Behavioral Pediatrics, 33(3), 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig EH, & Secord WA (2004). Clinical Evaluation of Language Fundamentals Preschool (2nd Ed). San Antonio, TX: Harcourt Assessment PsyCorp. [Google Scholar]
- Solberg BS, Halmøy A, Engeland A, Igland J, Haavik J, & Klungsøyr K (2018). Gender differences in psychiatric comorbidity: a population-based study of 40 000 adults with attention deficit hyperactivity disorder. Acta Psychiatrica Scandinavica, 137(3), 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, & Jacobs GA (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychology Press. [Google Scholar]
- Thapar A, & Stergiakouli E (2009). An Overview on the Genetics of ADHD: An Overview on the Genetics of ADHD. Acta Psychologica Sinica, 40(10), 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treyvaud K, Doyle LW, Lee KJ, Roberts G, Cheong JLY, Inder TE, & Anderson PJ (2011). Family functioning, burden and parenting stress 2years after very preterm birth. Early Human Development, 87(6), 427–431. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Inder TE, Lee KJ, Northam EA, Doyle LW, & Anderson PJ (2012). Can the home environment promote resilience for children born very preterm in the context of social and medical risk? Journal of Experimental Child Psychology, 112(3), 326–337. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Lee KJ, Doyle LW, & Anderson PJ (2014). Very Preterm Birth Influences Parental Mental Health and Family Outcomes Seven Years after Birth. The Journal of Pediatrics, 164(3), 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. (2015). Poverty Thresholds. Retrieved December 6, 2016, from Poverty website: https://www.census.gov/topics/income-poverty/poverty.html
- Wechsler D (2001). Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wechsler D (2004). WPPSI-III: Administration and scoring manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, & Austin NC (2009). Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Archives of Disease in Childhood. Fetal and Neonatal Edition, 94(5), F339–344. [DOI] [PubMed] [Google Scholar]
- Wurpts IC, & Geiser C (2014). Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Frontiers in Psychology, 5(920). 10.3389/fpsyg.2014.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


