Abstract
Acute dynamic exercise mobilizes CD34+ hematopoietic stem cells (HSCs) to the bloodstream, potentially serving as an economical adjuvant to boost the collection of HSCs from stem cell transplant donors. The mechanisms responsible for HSC mobilization with exercise are unknown but are likely due to hemodynamic perturbations, endogenous granulocyte-colony stimulating factor (G-CSF), and/or β2-adrenergic receptor (β2-AR) signaling. We characterized the temporal response of HSC mobilization and plasma G-CSF following exercise, and determined the impact of in vivo β-AR blockade on the exercise-induced mobilization of HSCs. Healthy runners (n= 15) completed, in balanced order, two single bouts of steady state treadmill running exercise at moderate (lasting 90-min) or vigorous (lasting 30-min) intensity. A separate cohort of healthy cyclists (n=12) completed three 30-min cycling ergometer trials at vigorous intensity after ingesting: (i) 10mg bisoprolol (β1-AR antagonist); (ii) 80mg nadolol (β1 + β2-AR antagonist); or (iii) placebo, in balanced order with a double-blind design. Blood samples collected before, during (runners only), immediately after, and at several points during exercise recovery were used to determine circulating G-CSF levels (runners only) and enumerate CD34+ HSCs by flow cytometry (runners and cyclists). Steady state vigorous but not moderate intensity exercise mobilized HSCs, increasing the total blood CD34+ count by ~4.15±1.62 Δcells/μl (+202±92%) compared to resting conditions. Plasma G-CSF increased in response to moderate but not vigorous exercise. Relative to placebo, nadolol and bisoprolol lowered exercising heart rate and blood pressure to comparable levels. The number of CD34+ HSCs increased with exercise after the placebo and bisoprolol trials, but not the nadolol trial, suggesting β2-AR signaling mediated the mobilization of CD34+ cells [Placebo: 2.10±1.16 (207±69.2%), Bisoprolol 1.66±0.79 (+163±29%), Nadolol: 0.68±0.54 (+143±36%) Δcells/μL]. We conclude that the mobilization of CD34+ HSCs with exercise is not dependent on circulating G-CSF and is likely due to the combined actions of β2-AR signaling and hemodynamic shear stress.
Keywords: Hematopoietic stem cell transplantation, Exercise immunology, Exercise intensity, CD133, β-AR blockade, Progenitor cells, Nadolol, Bisoprolol, Granulocyte colony stimulating factor (G-CSF)
1. Introduction
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the preferred treatment for many patients with blood cancers, genetic disorders and primary immune deficiency disorders (Copelan, 2006; Holtick et al., 2014; Passweg et al., 2012). Hematopoietic stem cells (HSCs) are preferentially collected from the peripheral blood of healthy donors following a period of granulocyte-colony stimulating factor (G-CSF) therapy designed to proliferate and mobilize CD34+ HSCs from the bone marrow (Barrett and Treleaven, 1998; England et al., 2016). Donors typically undergo apheresis when the blood CD34+ count exceeds 10 cells/μL as this increases the likelihood of collecting 4–4.5 × 106CD34+ cells/kg patient body weight, which is required for a successful transplant (Panch et al., 2017). Donors may receive G-CSF therapy over 4–5 days and undergo apheresis collection to reach HSC collection targets (Bonig and Papayannopoulou, 2012; Grigg et al., 1995; Hosing, 2012). Unfortunately, the time commitment and cumbersome apheresis procedures required for HSC donation, as well as the uncomfortable side effects and known toxicities associated with G-CSF and other HSC mobilizing therapeutics (e.g. the CXCR-4 inhibitor, Plerixafor), have been identified as factors that may deter would be HSC donors (Bonig and Papayannopoulou, 2012; Treleaven and Barrett, 2009). Therefore, there is a need to find new methods that will facilitate HSC collections and help lower donor burden in the alloHSCT setting.
It is well known that a single bout of dynamic aerobic exercise elicits an almost instantaneous increase in the circulating leukocyte count (Simpson et al., 2015). It has also been shown that hematopoietic progenitor cells are mobilized with exercise, presumably to aid in the repair of tissue damaged from the exercise bout (Camargo et al., 2003; Palermo et al., 2005; Wahl et al., 2007). The progenitor cells mobilized by exercise include HSCs and endothelial progenitor cells, both of which may increase 1–2.5-fold above resting values (Bonsignore et al., 2010; Krüger et al., 2014; Mobius-Winkler et al., 2009; Thijssen et al., 2006). While the number of HSCs mobilized by exercise is small in comparison to the major leukocyte subtypes (i.e. granulocytes, lymphocytes and monocytes), the therapeutic potential of HSCs has led to the hypothesis that exercise may serve as a simple and economical adjuvant to increase their isolation from donor blood to facilitate several clinical procedures such as alloHSCT and regenerative medicine (Baker et al., 2017; Emmons et al., 2016; Riddell et al., 2015; Simpson et al., 2017).
It remains to be established if exercise can evoke a mobilization of CD34+ cells comparable to exogenous G-CSF therapy. Reports in the literature are equivocal; most studies indicate that prolonged or exhaustive exercise elicits only modest increases in the CD34+ cell count to the order of 2–12 cells/μL, which would be largely inferior to exogenous G-CSF therapy (Baker et al., 2017; Krüger et al., 2014; Mobius-Winkler et al., 2009). However, (Zaldivar et al., 2007) reported that high intensity exercise in young boys mobilized to the order of 150–175 CD34+cells/μL. Moreover, the optimal mode, duration and intensity of exercise, as well as the underlying mechanisms responsible for HSC mobilization, remain to be determined. It is possible that endogenous G-CSF released during exercise evokes the mobilization of HSCs from the bone marrow to the blood as a means to stimulate the production of mature immune cells or facilitate tissue regeneration (Krüger et al., 2014; Zaldivar et al., 2007). In addition, the binding of catecholamines to β2-adrenergic receptors (β-AR) (Cancelas and Williams, 2006) and/or increases in hemodynamic shear stress that accompany sustained elevations in cardiac output and blood pressure might facilitate the HSC mobilization during exercise (Simpson et al., 2015). To the best of our knowledge, no study has established the effects of exercise intensity on plasma G-CSF levels or attempted to determine the time course relationship between endogenous G-CSF and HSC mobilization in response to acute exercise. Also unknown are the effects of in vivo β-AR blockade and hemodynamic perturbations on the exercise-induced mobilization of HSCs.
The primary aim of this study was to determine the feasibility of using acute dynamic exercise as a surrogate method to G-CSF therapy for mobilizing HSCs in healthy donors. We compared the response of short vigorous intensity exercise with that of prolonged moderate intensity exercise to determine if either protocol would evoke HSC mobilization to levels comparable with those of exogenous G-CSF therapy that have been reported in the literature (Barrett and Treleaven, 1998; Hosing, 2012; Treleaven and Barrett, 2009). We also characterized the temporal response of HSC mobilization and endogenous G-CSF release in response to each exercise bout. Finally, we determined the impact of β-AR blockade in vivo on CD34+ and CD133+ HSC mobilization with exercise, comparing a non-preferential β1 + β2-AR antagonist (nadolol) to a preferential β1-AR antagonist (bisoprolol) to delineate the effects of β2-AR signaling from the alterations in hemodynamic shear stress that accompany β1-AR blockade. We hypothesized that: (i) HSC mobilization would be greatest after vigorous exercise compared to moderate intensity exercise but not large enough to be considered a surrogate for pharmaceutical mobilizing agents, and (ii) the mobilization of HSCs with exercise would be dependent on plasma G-CSF and/or β2-AR signaling.
2. Methods
2.1. Participants
All participants were 18–44 years of age and classified as ‘low risk’ for maximal exercise testing in accordance with the ACSM/AHA criteria (PAR-Q & You, American College of Sports Medicine, 2013). Participants were excluded if they used tobacco products within six months of screening, had a high intake of alcohol (>2 drinks per day), regularly used any medication known to affect the immune system within the last 6-months, were pregnant, or had any autoimmune disease. Participants were asked to abstain from caffeine consumption and vigorous exercise for 24 h prior to each laboratory visit. This study was performed in two parts with separate groups of participants. For Part 1, participants (n=15) were required to regularly engage in 1-3h of vigorous exercise per week, the equivalent of running at least 5–10 miles per week (Jackson et al., 1990). For Part 2, Participants (n=12) were required to be familiar with cycling exercise and screened to ensure they were free of any contraindications to ingesting β-blocker medication. This included an assessment of pulmonary function to exclude the presence of undiagnosed asthma. Both parts of this study were conducted by the same group of investigators in the Laboratory of Integrated Physiology at the University of Houston. All participants provided written informed consent prior to participating and the Committee for the Protection of Human Participants (CPHS) at the University of Houston approved both parts of this study. The physical characteristics of the participants enrolled in Part 1 and Part 2 of this study are shown in Table 1.
Table 1.
Physical characteristics of the participants in Part 1 (n=15) and Part 2 (n= 12). Data are mean±SD. P values for group main effects are reported. ND: not determined.
| Part 1 n=15 |
Part 2 n=12 |
p value |
|
|---|---|---|---|
| % Female | 47% | 17% | 0.202 |
| Age (yrs) | 28.3±7.8 | 30.6±6.4 | 0.525 |
| Height (cm) | 172.7±9.2 | 175.3±6.3 | 0.251 |
| Mass (kg) | 73.1±12.9 | 80.5±13.3 | 0.908 |
| Resting Blood Pressure (mmHg) | |||
| Systolic | 113.8±9.0 | 116.9±8.4 | 0.742 |
| Diastolic | 75.3±5.0 | 76.2±6.6 | 0.506 |
| Resting Glucose (mg/dL) | 88.8±8.6 | 89.8±9.6 | 0.667 |
| Resting Total Cholesterol (mg/dL) | 121.6±17.3 | 139.1±26.4 | 0.190 |
| Relative Body Fat (%) | 25.1±7.0% | ND | ND |
| BMD (g/cm2) | 1.2±0.1 | ND | ND |
| Running velocity at first ventilatory threshold (km/hr) | 11.1±1.7 | ND | ND |
| Cycling power output at blood lactate threshold | ND | 157.5±24.2 | ND |
2.2. Experimental design: Part 1
Part 1 was concerned with comparing the response of short vigorous intensity exercise with that of prolonged moderate intensity exercise. Participants in the Part 1 cohort made three separate visits to the laboratory, with a period of 1–3 weeks between visits. Visit 1 required the participants to undergo preliminary health screening and risk stratification to ensure study inclusion and exclusion criteria were met. Eligible participants then completed a maximal graded exercise test. Visit 2 and Visit 3 required the participants to perform a single bout of treadmill running exercise consisting of either: (i) 30-min of steady state running at +15% of ventilatory threshold (VT) (vigorous); or (ii) 90-min of steady state running at −5% of VT (moderate). Each exercise bout was performed on separate days in random order. The collection of blood samples occurred at the exact same time of day between trials, subjects came in at the same time of day for their pre-exercise samples, although exercise for the vigorous intensity trial began 1h later than the moderate intensity trial so that both exercise bouts finished at the exact same time of day. An assessment of body composition via a whole body DXA (Dual-energy X-ray absorptiometry) scan (Hologic Discovery W DXA, Marlborough, MA, USA) was made during the same visit as the vigorous intensity trial.
2.2.1. Maximal exercise test to determine VO2 peak and ventilatory threshold (VT)
During the first laboratory visit, participants completed a maximal exercise protocol on an indoor motor driven treadmill (Woodway Desmo Treadmill, Waukesha, WI). Participants were fitted with a heart rate monitor (FT4, © Polar Electro, Kempele, Finland) and a facemask that covered their nose and mouth for the continuous measurement of heart rate and respiratory gases, respectively. The facemask was connected to a metabolic cart for real time measurement of respiratory gases (Cosmed CPET, Rome, Italy). The test began with the subject running at a 1% incline at a speed of 6.4km/h. The speed of the treadmill was increased by 0.8km/h every 2min until a respiratory exchange ratio (RER) >1.1 was reached, after which the incline was increased by 2% every minute until volitional exhaustion. VO2peak and maximum heart rate was determined from the test. The ventilatory threshold (VT) was defined as the first ventilatory breakpoint and determined using the V-slope method (Beaver et al., 1986).
2.2.2. Main Exercise Trials
Visits 2 and 3 began between 07:00 and 10:00. Prior to exercise, an intravenous catheter (3/4 in. BD Nexiva™ Closed IV Catheter System, BD vacutainers™, Franklin Lakes, NJ, USA) was inserted to an ante-cubital vein for blood sample collection before, during and after exercise. Blood was collected in a 3mL vacutainer® tube spray-coated with K2EDTA and a 10-mL tube with Silica Clot Activator, Polymer Gel, Silicone-Coated Interior SST™ (BD vacutainer™, Franklin Lakes, NJ, USA) for the separation of blood serum. The IV-catheter was flushed with 5 mL isotonic saline (BD PosiFlush, Franklin Lakes, NJ, USA) after each blood draw, and a 2 mL “waste” tube was drawn immediately prior to collecting the next blood sample to remove residual saline. For the vigorous exercise trial, participants were asked to run at a pace corresponding to +15% of the pre-determined VT velocity for 30 min. Blood samples were collected from the IV catheter at rest, during exercise after 15 min, immediately post exercise, and again at 1, 2, and 3-h post exercise. For the moderate intensity exercise trial, participants were asked to run at a pace corresponding to −5% of the pre-determined VT velocity for 90 min. Blood samples were collected from the IV catheter at rest, during exercise after 15-min and 60-min, immediately post exercise, and again at 1, 2, and 3-h post exercise. The blood sample collected after 15-min of exercise in both the vigorous and moderate intensity trials allowed us to compare the effects of exercise intensity on HSC mobilization at the same duration of exercise.
2.3. Experimental design: Part 2
Part 2 investigated the impact of β-AR blockade in vivo on CD34+ and CD133+ HSC mobilization with exercise. Participants in the Part 2 cohort made five visits to the laboratory, with a period of 1–3 weeks between visits. Visit 1 required the participants to undergo preliminary health screening and risk stratification to ensure study inclusion and exclusion criteria were met. On the second visit, eligible participants completed a discontinuous cycling protocol on an indoor ergometer to determine the individual blood lactate threshold following procedures we have described previously (Bigley et al., 2014). Participants completed the lactate threshold test using their personal road bike mounted to an indoor cycling trainer (Computrainer, RacerMate, USA), or on a fixed laboratory cycling ergometer (Velotron, LAB Model, RacerMate Inc., Seatle WA). The main exercise trials were completed during visits 3–5, which required the participants to cycle for 30-min at a steady state power output corresponding to +10% of the individual blood lactate threshold (considered comparable to the vigorous intensity trial described in Part 1). The main exercise trials commenced 3h after the participant ingested (i) 10mg of bisoprolol (β1AR antagonist), (ii) 80 mg of nadolol (β1+β2-AR antagonist), or (iii) placebo in counter balanced order using a double-blind design.
2.3.1. Main exercise trials
Participants visited the laboratory between 07:00 and 10:00 to provide a resting blood sample, which was collected via standard phlebotomy (BD Vacutainer Safety-Lok™, Franklin Lakes, NJ, USA) into the aforementioned collection tubes either spray-coated with K2EDTA or containing a serum gel separator. Resting blood pressure and heart rate was also determined at this time. Participants then ingested the medication (placebo, bisoprolol or nadolol), which was transferred directly to the participant’s mouth from a pre-dispensed opaque vial. Participants were then asked to sit quietly in the laboratory for 3h, after which another blood sample was a collected and additional resting blood pressure and heart rate measurements were recorded. A capillary blood sample was collected for blood lactate determination (P-GM7 Micro-Stat Analyzer, Analox instruments Ltd., London, UK). Participants were then fitted with the apparatus required to monitor electrocardiogram activity (12-lead) and measure respiratory gas exchange continuously during exercise (Quark CPET, COSMED, Pavona di Albano Laziale, Italy). Following 10-min of easy cycling (<50% of the prescribed exercise intensity), participants were asked to cycle for 30-min at a steady-state power output corresponding to +10% of the pre-determined individual blood lactate threshold on the same cycling apparatus that was used during visit 2. Capillary blood samples, blood pressure and rating of perceived exertion was determined after 10, 20 and 30-min of exercise. Subsequent intravenous blood samples were collected immediately after exercise cessation and at 1h post exercise. The main exercise trials began 3 h after drug ingestion because peak serum concentrations of bisoprolol and nadolol are reached within 2–4 h after oral administration, with a half-life 10–12 h and of 14–24 h respectively (Kostis et al., 1984; Le Coz et al., 1991; Leopold et al., 1986; Schafer-Korting et al., 1984). All trials were completed at the same time of day and a minimum 1-week washout period was interspersed between trials to eliminate any carry over effects of drug ingestion.
2.4. Blood processing and analysis
All blood samples collected from the participants in Part 1 and Part 2 were processed under identical conditions using the same laboratory reagents and apparatus unless otherwise stated. Complete blood counts were determined using an automated clinical grade hematology analyzer (Mindray BC-2800, Shenzhen, China) for the enumeration of total leukocytes, granulocytes, monocytes and lymphocytes. All blood samples were analyzed in duplicate with a co-efficient of variation <5.5%.
2.4.1. Enumeration of CD34+ and CD133+ HSCs in peripheral blood
Whole blood (200μL) was labeled with an FITC conjugated anti-CD34 monoclonal antibody (mAb) (eBioscience, San Diego, CA, USA) and a PE conjugated anti-CD133 (AC133) mAb (Miltenyi Biotec, Bergisch Gladbach, Germany) and allowed to incubate in the dark at room temperature for 30 min. Samples were then mixed with a red blood cell lysing buffer (eBioscience, San Diego, CA, USA) for 20 min, and washed 3X with phosphate-buffered saline (PBS) before being resuspended in 200 μL of PBS for analysis. Immunophenotyping was performed by 2-color flow cytometry (BD Accuri C6, Ann Arbor, MI, USA). Lymphocytes and monocytes were identified by their light scatter properties and electronically gated (CFlow® software v2). We determined CD34+ and CD133+ expression within the electronic gate of total mononuclear cells. Fluorescent signals were collected in logarithmic mode using two separate detector filters for FITC and PE. For each sample, 1 × 105 gated lymphocyte+monocyte events were acquired at a flow rate of 100 μL/minute, allowing for the acquisition of >100 CD34+ and CD133+ events within the total mononuclear cell gate. The percentage of total mononuclear cells expressing CD34 or CD133 was multiplied by the combined lymphocyte and monocyte count from the automated hematology analyzer to determine the number of CD34+ or CD133+ cells/μl of whole blood.
2.4.2. Determination of serum G-CSF, epinephrine, norepinephrine and cortisol levels
Serum was separated from whole blood and stored at −80 °C until analysis. G-CSF was determined in serum for the participants in the Part 1 cohort, and epinephrine, norepinephrine and cortisol was determined in serum for the participants in the Part 2 cohort. All serum samples were analyzed in duplicate using commercially available high sensitivity ELISA kits in accordance with manufacture’s instruction (Human G-CSF Quantikine High Sensitivity ELISA, R&D Systems, Minneapolis, MN, USA; 2-CAT ELISA Fast Track, LDN, Nordhorn, Germany; Cortisol ELISA, LDN, Nordhorn, Germany).
2.5. Statistical analysis
Data is presented as means±standard deviation. All statistical analyses were completed using SPSS (v. 22.0; Chicago, IL). To ensure that subjects had similar physical characteristics and fitness status between Parts 1 and 2, one-way ANOVAs and Fisher’s exact test were used to compare the groups. To determine physiological differences between trials for each study, one-way ANOVAs were used with Bonforroni post hoc to determine differences between placebo and β-blocker trials. Maximum likelihood, linear mixed models (LMM) were used to analyze all immune cell counts. For Part 1, the model included main effects for exercise time (pre-exercise, during-exercise: +15-min (vigorous)/+1 h (moderate), immediately post exercise, recovery: 1 h, 2 h and 3 h post exercise), exercise duration/intensity (moderate and vigorous) and serum G-CSF levels (continuous) for all dependent variables (numbers of immune cells and immune cell subpopulations in whole blood). For Part 2, the model included the main effects for trial (placebo, bisoprolol and nadolol) and exercise time (pre-drug, pre-exercise, post-exercise, and 1h-post exercise). Additionally, we modeled interaction effects between the main effects. Significance was set at p<.05.
3. Results
All participants successfully completed all exercise protocols. No significant differences (p>.05) in physical characteristics were seen between participants recruited for Part 1 or Part 2 (Table 1). For Part 1; 15 subjects were recruited (7 female) (age: 28.3±7.8 yrs, height: 172.7±9.2 cm, body mass: 73.1±12.9 kg). For Part 2; 12 subjects were recruited (2 females) (age: 30.6±6.4 yrs, height: 175.3±6.3 cm, body mass: 80.5±13.3 kg). In the Part 1 cohort, running velocity (km/hr) (12.3±1.9 vs 10.3±1.6), rating of perceived exertion (RPE) (16.1±2.5 vs 14.1±1.5), and average heart rate (bpm) (177.8±7.7 vs 168.0±10.6) was significantly higher in the vigorous compared to the moderate intensity trial (p<.05) (Table 2). In the Part 2 cohort, exercising heart rate (HR) and systolic blood pressure (SBP) was lower in both the nadolol [HR (bpm): 117.7±8.9 vs 158.2±9.2; SBP (mmHg): 135.5±12.6 vs 157.9±11.4] and bisoprolol (HR(bpm): 123.3±7.8 vs 158.2±9.2; SBP (mmHg): 140.8±11.2 vs 157.9±11.4] trials compared to placebo (p<.05), but no significant differences were found for exercising heart rate or systolic blood pressure between the nadolol and bisoprolol trials (p>.05). Borg rating of perceived exertion (RPE) (Borg, 1998) was higher during the final 20 min of exercise in the nadolol (15.8±2.1 vs 14±1.5), but not bisoprolol (15.4±2.6 vs 14±1.5) trials when compared to placebo (p>.05). Blood lactate was higher during the final 20 min of exercise in the bisoprolol (3.8±1.2 vs 3.0±1.0) but not in the nadolol (2.9±0.7 vs 3.0±1.0) trial when compared to placebo (p>.05). Serum levels of cortisol were higher post exercise in the nadolol (25.5±6.4 vs 19.7±5.5) but not bisoprolol (23.4±5.8 vs 19.7±5.5) trial when compared to placebo, but no differences were found for epinephrine or norepinephrine between drug trials (Table 3). For the Part 1 cohort, main effects of sex (male or female) were found for total lymphocytes, monocytes and serum G-CSF levels (p<.05), with females having higher levels than males (data not shown). However, changes in these variables in response to the exercise bouts did not differ between males and females (p>.05; data not shown). We did not determine sex differences in the Part 2 cohort as only 2/12 participants were female.
Table 2.
Exercise measures of the participants in Part 1 (n=15). Data are mean±SD. P values for group main effect are reported.
| (Mean±SD) | Vigorous | Moderate | p value |
|---|---|---|---|
| Velocity (km/h) | 12.3±1.9 | 10.3±1.6 | <0.001 |
| Rating of Perceived Exertion (RPE) | 16.1±2.5 | 14.1±1.5 | 0.016 |
| Average Heart Rate (beats per minute) | 177.8±7.7 | 168.0±10.6 | 0.001 |
| %Heart Rate Max | 92.7±4.7% | 87.5±4.9% | 0.004 |
Table 3.
Exercise measures of the participants in Part 2 (n= 12). All participants completed all 3 trials. Heart rate, blood pressure, RPE and blood lactate were averaged during the last 20-min of exercise. Epinephrine, norepinephrine and cortisol data were obtained from serum samples collected at pre-exercise and immediately post exercise in a subset of participants (n=8). Data are mean±SD. Main effects for trials are reported. Statistical differences from placebo and nadolol are indicated by * and #, respectively, p<.05.
| Exercise Measures | Placebo | Bisoprolol | Nadalol | Trial p value |
|---|---|---|---|---|
| Heart Rate (beats per minute) | 158.2±9.2 | 123.3±7.8* | 117.7±8.9* | <0.001 |
| Cycling Power (Watts) | 157.5±24.2 | 157.5±24.2 | 157.5±24.2 | 0.999 |
| Blood pressure (mmHg) | ||||
| Systolic | 157.9±11.4 | 140.8±11.2* | 135.5±12.6* | <0.001 |
| Diastolic | 76.7±6.2 | 75.5±12.1 | 73.4±5.3 | 0.124 |
| Rating of Perceived Exertion (RPE) | 14±1.5 | 15.4±2.6 | 15.8±2.1* | 0.020 |
| Blood Lactate (mM) | 3.0±1.0 | 3.8±1.2*# | 2.9±0.7 | 0.013 |
| Epinephrine (ng/mL) | ||||
| Pre-Exercise | 0.04±0.03 | 0.06±0.04 | 0.06±0.03 | 0.113 |
| Post Exercise | 0.14±0.13 | 0.16±0.12 | 0.41±0.39 | 0.156 |
| Norepinephrine (ng/mL) | ||||
| Pre-Exercise | 0.8±0.3 | 0.9±0.3 | 0.9±0.3 | 0.475 |
| Post Exercise | 1.6±0.6 | 1.8±0.7 | 1.5±0.6 | 0.567 |
| Cortisol (ng/mL) | ||||
| Pre-Exercise | 13.1±2.3 | 19.0±5.66 | 16.1±5.9 | 0.172 |
| Post Exercise | 19.7±5.5 | 23.4±5.8 | 25.5±6.4* | 0.011 |
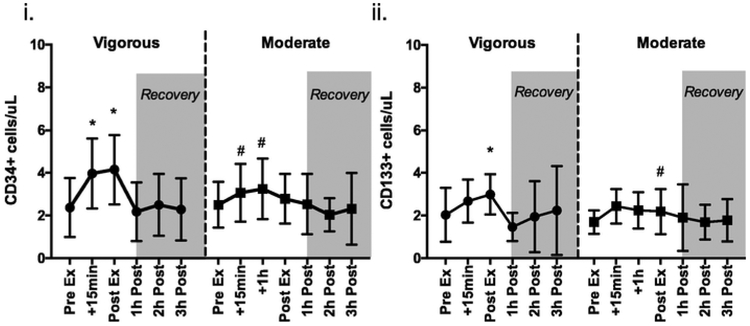
3.1. Short duration vigorous intensity exercise mobilizes greater numbers of CD34+ and CD133+ HSCs than long duration moderate intensity exercise
The effects of vigorous and moderate intensity running exercise on the mobilization of CD34+ and CD133+ HSCs and their kinetic response are depicted in Fig. 1. Changes in circulating leukocyte, lymphocyte and monocyte counts in response to vigorous and moderate intensity exercise are shown in Table 4. There was a significant main effect of exercise time [F (6, 167.029)=11.377, p<.001], exercise trial [F (1,167.029) = 14.120, p<.001] and a significant interaction effect [F (1,167.028)=4.080, p<.001] between exercise time x exercise trial for circulating CD34+ HSCs cells/μL. This was driven by a significant (p<.05) increase in the number of CD34+ HSCs at +15-min of vigorous vs +1h moderate intensity (4.2±1.42 vs 3.25±1.43; p<.001) and immediately post-exercise for vigorous vs moderate intensity (4.40±1.37 vs 2.78±1.15;p<.001). We also compared the effects of exercise intensity independently of exercise duration by comparing the +15-min sample obtained from the vigorous and moderate intensity trials. The number of CD34+ HSCs was a significantly higher after +15-min of vigorous compared to +15-min of moderate intensity exercise (4.20±1.42 vs 3.06±1.35, p<.01).
Fig. 1.
The effects of exercise intensity (Vigorous or Moderate) on (i) CD34+ and (ii) CD133+ HSCs (cells/μL) in the Part 1 cohort. Values are mean±SD. Differences from pre-exercise indicated by *, between trials indicated by #, p<.05.
Table 4.
The total number of leukocytes, lymphocytes, monocytes and HSCs found in peripheral blood before, during and after exercise in the Part 1 cohort. Data are mean±SD. Main effects of time (pre-exercise, during-exercise, post-exercise, 1 h, 2 h and 3 h post-exercise), trial (moderate intensity and vigorous intensity) and time x trial interactions are shown. During-Exercise measures were obtained at +15-min for the vigorous trial and +60-min for the moderate trial. Statistical differences from pre-exercise and between exercise trials are indicated by * and #, respectively, p < .05.
| Pre-Exercise | During-Exercise | Post-Exercise | 1 h Post | 2 h Post | 3 h Post | Time F (p-value) | Trial F (p-value) | Interaction F (p-value) | |
|---|---|---|---|---|---|---|---|---|---|
| Leukocytes (×103 cells/μL) | |||||||||
| Vigorous | 24.4±26.5 | 40.3±41.5 | 42.5±43.5* | 32.7±34.6 | 38.8±44.6* | 37.7±44.5* | 17.739 (<0.001) | 2.346 (0.127) | 1.442 (0.211) |
| Moderate | 23.6±24.2 | 36.4±37.6* | 52.3±59.2* | 38.6±41.9 | 44±47.3 | 44.6±49.2 | |||
| Lymphocytes (×103 cells/μL) | |||||||||
| Vigorous | 1.6±0.4 | 3.4±0.6* | 3.7±0.7* | 1.2±0.2 | 1.3±0.3 | 1.5±0.4 | 139.667 (<0.001) | 22.588 (<0.001) | 18.272 (<0.001) |
| Moderate | 1.5±0.3 | 2.6±0.6* | 2.7±0.8*# | 1.4±0.2 | 1.5±0.4 | 1.66±0.5 | |||
| Monocytes (×103 cells/μL) | |||||||||
| Vigorous | 0.4±0.1 | 1.0±0.4* | 0.9±0.3* | 0.3±0.1 | 0.4±0.2 | 0.577±0.6 | 16.503 (<0.001) | 0.089 (0.766) | 4.480 (0.001) |
| Moderate | 0.4±0.1 | 0.7±0.3* | 0.7±0.3# | 0.5±0.2 | 0.5±0.3 | 0.7±0.3 | |||
| CD34+ Cells (cells/μL) | |||||||||
| Vigorous | 2.5±1.4 | 4.2±1.4*# | 4.4±1.4*# | 2.3±1.3 | 2.7±1.3 | 3.1±2.9 | 11.377 (<0.001) | 14.120 (<0.001) | 4.080 (0.002) |
| Moderate | 2.5±1.0 | 3.3±1.4 | 2.8±1.2 | 2.5±1.4 | 2.0±0.8 | 2.3±1.7 | |||
| CD133+ Cells (cells/μL) | |||||||||
| Vigorous | 2.1±1.2 | 3.2±2.4 | 3.5±2.0 | 1.6±0.7 | 2.2±1.9 | 2.7±2.7 | 3.932 (0.001) | 13.586 (0.060) | 2.327 (0.045) |
| Moderate | 1.7±0.5 | 2.3±0.9 | 2.2±1.0 | 2.2±1.8 | 2.1±1.83 | 2.2±1.8 | |||
There was a significant main effect of exercise time [F (1,179.027)= 3.932, p=0.001], but no main effect of exercise trial for the number of circulating CD133+ HSCs (p>.05). There was also a significant interaction effect [F (5, 179.029)=2.327, p=.045) between exercise time x exercise trial for circulating CD133+ HSCs. This was driven by a significant (p<.05) increase in the number of CD133+ HSCs at post-exercise (p<.05) for the vigorous intensity but not the moderate intensity trial. When comparing the effects of exercise intensity on circulating CD133+ HSCs at the +15-min time point, there was no statistical difference (p>.05) between the vigorous and moderate intensity trials (2.36±2.36 vs 2.46 vs 0.79; p>.05).
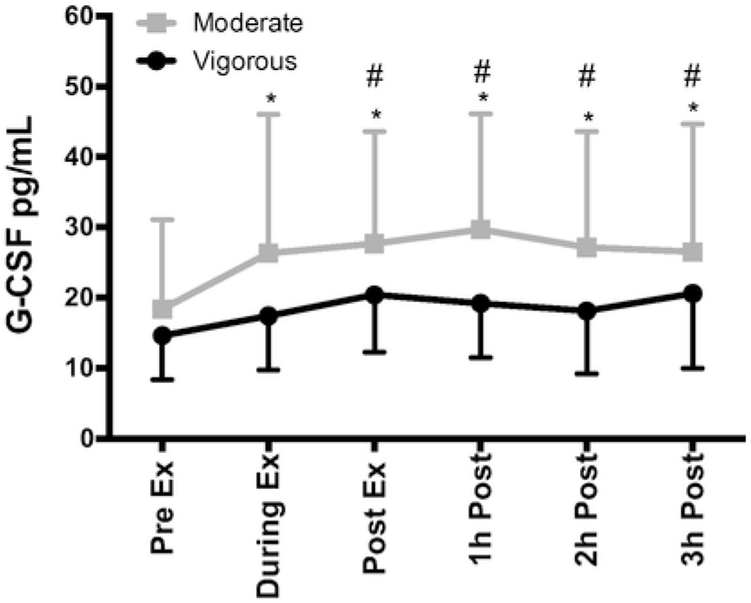
3.2. The mobilization of CD34+ and CD133+ HSCs is independent of circulating G-CSF levels
The kinetic response of circulating G-CSF levels in response to vigorous and moderate intensity running exercise is shown in Fig. 2. A main effect of exercise trial was observed, driven by higher levels of circulating G-CSF (pg/ml) in the moderate intensity trial compared to the vigorous intensity trial [F (1, 180)=44.017, p<.001]. Circulating levels of G-CSF did not change significantly during or after the vigorous intensity trial (p>.05). Differences in G-CSF were seen in the moderate trial +1h during (p = .036), post (p = .005), 1h-post (p<.001), 2h-post (p = .011) and 3h-post (p = .027) when compared to pre-exercise. Differences between trials occurred at post-exercise (p = .004), 1h- post (p<.001), 2h-post (p<.001) and 3h-post (p =.017). When controlling for G-CSF levels as a covariate in the LMM, the exercise time effect seen with CD34+ and CD133+ HSCs did not change, indicating that the mobilization of HSCs is independent of circulating G-CSF levels [F (1,167.029)= 14.120, p<.001].
Fig. 2.
The effects of exercise intensity (Vigorous or Moderate) on serum granulocyte colony stimulating factor (G-CSF) (pg/mL) in the Part 1 cohort. During-exercise measures were obtained at +15-min for the vigorous trial and +60-min for the moderate trial. Values are mean±SD. Differences from baseline and exercise trials (Vigorous and Moderate) indicated by * and #, respectively, p<.05.
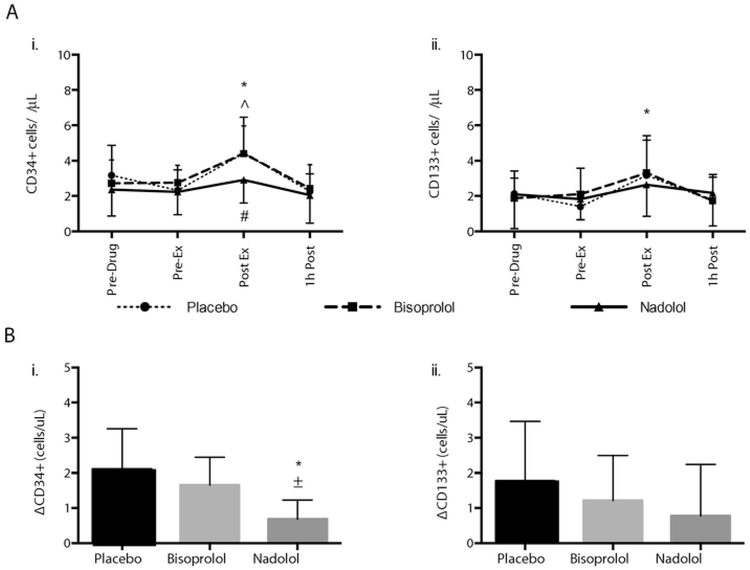
3.3. The mobilization of CD34+ HSCs with exercise is dependent on β2-AR signaling
The effects of exercise on the numbers of CD34+ and CD133+ HSCs in peripheral blood in response to the bisoprolol, nadolol and placebo trials are shown in Fig. 3, panel A. Changes in circulating leukocyte, lymphocyte and monocyte counts in response to these trials are described in Table 5. A main effect of time [F (3, 121)= 19.224, p<.001] and trial [F (2, 121) = 7.008, p =.001] was observed for CD34+ HSCs. Specifically, the CD34+ cell count (cells/μL) increased immediately after exercise in both the placebo and bisoprolol trials (p<.001), but not in the nadolol trial (p = 1.00). A main effect of time [F (3132)=7.967, p = .001] was observed for CD133 cells/μL Specifically, the CD133+ cell count (cells/μL) increased immediately after exercise in the placebo trial (p=.004), but not in the bisoprolol (p=.104) or nadolol (p=.648) trials.
Fig. 3.
The effects on nadolol and bisoprolol administration relative to placebo on the mobilization of CD34+ and CD133+ HSCs with exercise. Panel A shows the effects of exercise under placebo, bisoprolol and nadolol trials on (i) CD34+ and (ii) CD133+ HSCs (cells/μL) in the Part 2 cohort. Values are mean±SD. Differences from pre-exercise were found for placebo (*) and bisoprolol (^), but not for nadolol, p<.05. Differences from the placebo condition were found for CD34+ HSCs at post-exercise for nadolol (#), p < .05. Panel B shows the absolute number of CD34+ (i) and CD133+ (ii) HSCs mobilized by exercise (Post-Ex – Pre-Ex; Δ) in the placebo, bisoprolol and nadolol trials in the Part 2 cohort. Values are mean±SD. Differences from the placebo and bisoprolol conditions are noted by * and ±, respectively, p<.05.
Table 5.
The total number of leukocytes, lymphocytes, monocytes and HSCs after exercise under placebo, bisoprolol and nadolol conditions for the Part 2 cohort. Data are mean±SD. Main effects of time (pre-drug, pre-exercise, post-exercise, 1 h post-exercise), trial (placebo, bisoprolol, nadolol) and time × trial interactions are shown. Differences between Pre-Drug and Pre-Exercise are indicated by * and #, respectively (p<.05).
| Pre-Drug | Pre-Exercise | Post-Exercise | 1 h Post-Exercise | Time | Trial | Interaction | |
|---|---|---|---|---|---|---|---|
| F (p-value) | F (p-value) | F (p-value) | |||||
| Leukocytes | |||||||
| Placebo | 5911.1±1002.6 | 6076.4±1278.7 | 9838.9±1894.8*# | 7394.4±1303*# | 90.886 (0.001) | 2.868 (0.060) | 4.209 (0.001) |
| Bisoprolol | 5608.3±607.4 | 6911.1±1577.1* | 10801.4±2205.2*# | 8105.6±2315.5* | |||
| Nadolol | 6056.9±1153.3 | 6415.3±1355.9 | 9114.2±1892.2*# | 9140.3±2009*# | |||
| Lymphocytes | |||||||
| Placebo | 2193.1±813.6 | 2116.7±889.3 | 3768.1±959.6*# | 2225±1613.1 | 51.717 (0.001) | 3.450 (0.035) | 1.22 (0.299) |
| Bisoprolol | 2080.6±574.1 | 2427.8±1188.5 | 4113.9±1149.6*# | 2501.4±2280.9 | |||
| Nadolol | 2026.4±725.3 | 2202.8±1150.1 | 3272.5±1262.5*# | 2290.3±1687 | |||
| Monocytes | |||||||
| Placebo | 423.3±200.0 | 431.7±189.6 | 871.7±356.1 | 341.7±114.7 | 80.346 (0.001) | 6.918 (0.001) | 4.210 (0.001) |
| Bisoprolol | 421.7±170.2 | 428.3±239.5 | 1028.3±297.7 | ||||
| Nadolol | 435±154.5 | 348.3±166.0 | 678.7±226.7 | 388.3±63.4 | |||
| CD34+ Cells | |||||||
| Placebo | 3.17±1.69 | 2.32±1.15 | 4.42±2.03*# | 2.39±0.93 | 19.224 (<0.001) | 7.008 (0.001) | 1.626 (0.146) |
| Bisoprolol | 2.72±1.32 | 2.75±0.99 | 4.40±1.55*# | 2.43±1.34 | |||
| Nadolol | 2.35±1.51 | 2.22±1.29 | 2.91±1.31 | 2.04±1.57 | |||
| CD133+ Cells | |||||||
| Placebo | 2.1±1.3 | 1.4±0.9 | 3.2±2.2# | 1.7±1.5 | 7.967 (<0.001) | 0.180 (0.835) | 0.814 (0.561) |
| Bisoprolol | 1.9±1.1 | 2.1±1.5 | 3.3±1.9* | 1.7±1.4 | |||
| Nadolol | 2.1±2.0 | 1.8±1.2 | 2.6±1.8 | 2.2±1.9 | |||
We also compared the absolute number of HSCs mobilized in response to exercise under the placebo, bisoprolol and nadolol conditions (post-exercise HSCs – pre-exercise HSCs; Δ cells/μl) (Fig. 3, panel B). We did this due to low numbers of HSCs in peripheral blood and were also concerned that the within-subject variation observed across the three drug trials that would potentially affect the power of the LMM to detect meaningful differences across the three trials when all four exercise time points were included. A main effect of trial [F (2,22)=13.283, p<.001) was observed for CD34+ HSCs. The absolute number of CD34+ HSCs mobilized under the nadolol trial was lower than the placebo trial (p<.001) and the bisoprolol trial (p=.007). There were no differences in the number of cells mobilized between the placebo and bisoprolol trials (p=.378). No main effect for trial was observed for the absolute number of CD133+ HSCs mobilized in response to exercise (p>.05).
4. Discussion
Acute dynamic exercise has been purported as a potential adjuvant to boost the collection of CD34+ HSCs from the peripheral blood of healthy donors for alloHSCT (Baker et al., 2017; Emmons et al., 2016; Niemiro et al., 2017; Simpson et al., 2017). This study compared the effects of vigorous to moderate intensity exercise on the mobilization of CD34+ and CD133+ HSCs to the bloodstream in healthy volunteers, and examined the temporal response of HSC mobilization and plasma G-CSF levels both during and after exercise. We also determined, for the first time, the effects of in vivo β1+β2-AR blockade on HSC mobilization during exercise while controlling for simultaneous β1-AR mediated alterations in hemodynamic shear stress. We report here that the mobilization of HSCs is dependent on exercise intensity and β2-AR signaling and occurs even when plasma G-CSF levels are unaffected by exercise. Blocking β1+β2-AR signaling with nadolol was found to lower exercising blood pressure, heart rate and the mobilization of HSC to the blood. In contrast, bisoprolol, which blocks signaling through the β1-AR while leaving β2-AR signaling intact (Lipworth et al., 1991), similarly lowered exercising blood pressure and heart rate, but failed to block HSC mobilization with exercise. We conclude that β2-AR signaling plays a pivotal role in the mobilization of CD34+ HSCs with exercise.
It is now evident that acute dynamic exercise reliably mobilizes CD34+ and CD133+ HSCs to the bloodstream (Baker et al., 2017; Bonsignore et al., 2010; Emmons et al., 2016; Krüger et al., 2014; Mobius-Winkler et al., 2009; Niemiro et al., 2017; Zaldivar et al., 2007). This has resulted in exercise being touted as a potential clinical adjuvant to boost the collection of HSCs from healthy doors, potentially eliminating the need for expensive and toxic pharmaceutical agents to mobilize HSCs to the bloodstream from the bone marrow (Emmons et al., 2016; Niemiro et al., 2017; Simpson et al., 2017). We found that the mobilization of HSCs with exercise is rapid and dependent on the intensity of the bout, as greater numbers of CD34+ HSCs were found in blood after just 15-min of vigorous compared to 15-min of moderate intensity exercise. Increasing the duration of the moderate intensity exercise bout, despite elevating circulating G-CSF levels, did not further the mobilization of CD34+ HSCs. Our findings are corroborated by a recent study showing that CD34+ HSCs are mobilized with exercise in an intensity dependent manner. Baker et al. (2017) reported that CD34+ cells in blood increased ~2.5-fold after an exhaustive bout of cycling at 70% of peak work rate, but observed no change after an exhaustive bout of cycling at 30% of peak work rate (Baker et al., 2017). While we saw no differences in the mobilization of CD133+ HSCs between our vigorous and moderate intensity trials, Bonsignore et al. (2010) found greater CD133+ HSC mobilization after 1.5km field race but no changes after a marathon. Although there is some dubiety in the literature regarding the absolute number of HSCs that can feasibly be mobilized with exercise (Baker et al., 2017; Krüger et al., 2014; Mobius-Winkler et al., 2009; Niemiro et al., 2017; Zaldivar et al., 2007), our results are consistent with those of Krüger et al. (2014) in that exercise typically mobilizes 3–4 CD34+ cells/μL, which, similar to other studies, is equivalent to a 2–3-fold increase above resting levels (Baker et al., 2017; Bonsignore et al., 2010; Krüger et al., 2014; Niemiro et al., 2017). The equivocal findings reported in the literature could be due to different methodologies used for the isolation and enumeration of CD34+ HSCs (Baker et al., 2017; Bonsignore et al., 2010; Krüger et al., 2014; Mobius-Winkler et al., 2009; Riddell et al., 2015; Zaldivar et al., 2007).
The mobilization of several lymphocyte and monocyte subtypes with exercise are known to be dependent on the actions of catecholamines signaling through the β2-AR, which is expressed at high levels on the exercise-responsive cell types (Simpson et al., 2015). Because the β2-AR could be a viable pharmaceutical target for HSC mobilization in healthy donors, we sought to determine if the β2-AR subtype was responsible for HSC mobilization during exercise. We found that administration of the non-selective β1+β2-AR antagonist, nadolol, blunted the mobilization of HSCs in response to vigorous exercise. However, nadolol also alters hemodynamic shear forces and reduces systolic blood pressure and heart rate, due mostly to its β1-AR antagonist activity (Wheeldon et al., 1994). Therefore, we also administered bisoprolol, the most preferential β1-AR antagonist clinically available, as a control to account for potential effects of β1-AR and/or hemodynamic perturbations on HSC mobilization with exercise. Importantly, exercising heart rate and blood pressure responses did not differ between the nadolol and bisoprolol trials, indicating that both drugs caused similar perturbations in hemodynamic shear forces with exercise. However, only nadolol, not bisoprolol or placebo, blunted the mobilization of CD34+ HSCs during exercise. These results clearly implicate β2-AR signaling as responsible for the mobilization of CD34+ HSCs seen with exercise. In contrast, a recent study by Riddell et al. (2015) showed that direct infusion with a synthetic non-preferential β-AR agonist (isoproterenol) failed to mobilize HSCs into the bloodstream, despite finding that HSCs were mobilized to the bloodstream in response to an acute psychological stress task. Although our findings and those of Riddell et al. (2015) may at first seem contradictory, it must be recalled that the endogenous β-AR agonists, epinephrine and norepinephrine also activate contractile α-ARs on blood vessels. We hypothesize that in addition to β2-AR activation, increased vascular forces are also required to physically mobilize CD34+. Supporting this hypothesis is that while both the isoproterenol infusion and the acute psychological stress task in the study by Riddell et al. (2015) elevated heart rate to similar extents (~80 bpm), systolic blood pressure was markedly lower during the infusion trial (~120 mmHg) compared to the stress task (~140 mmHg). In the current study, despite bisoprolol and nadolol lowering exercising blood pressure and heart rate, the levels of vascular shear stress during the two drug trials were still elevated as exercising heart rate and systolic blood pressure was ~120 bpm and ~140 mmHg, respectively, during both drug trials. Moreover, exercise performed during β-AR blockade results in substantial elevations in cardiac stroke volume to maintain the required levels of cardiac output to complete the exercise task (Van Baak, 1988) which may have also further increased the levels of shear stress in the blood vessels and tissues in close proximity to the heart (Silke et al., 1995). Taken together, these data suggest that CD34+ HSCs are likely mobilized with exercise due to the combined effects of epinephrine acting on β2-ARs expressed by the mobilized cells, and on α-ARs within the vasculature that cause profound increases in hemodynamic forces.
As exercise is known to increase circulating G-CSF levels (Emmons et al., 2016; Krüger et al., 2014; Zaldivar et al., 2007), we hypothesized that elevations in plasma G-CSF would precede HSC mobilization with exercise, and that peak HSC numbers would occur during the recovery phase of exercise. Contrary to this, we found peak HSC mobilization to occur during vigorous intensity exercise even when G-CSF levels did not change. Conversely, long duration moderate-intensity exercise elevated plasma G-CSF levels but did not evoke the mobilization of HSCs. Taken together, these findings indicate that elevations in circulating G-CSF are not responsible for HSC mobilization during exercise. Moreover, the rapid mobilization of HSCs (~15-min) in response to exercise suggests they do not originate directly from the bone marrow, as it can take up to several days for the numbers of bone-marrow derived HSCs to increase in blood following G-CSF therapy (Greenbaum and Link, 2011). Rather, HSCs are likely mobilized from marginal pools within the vasculature under the influence of β2-AR signaling and hemodynamic forces that accompany sustained elevations in cardiac output and blood flow during steady state dynamic exercise (Simpson et al., 2017). It is possible, however, that G-CSF released during long duration moderate-intensity exercise caused a ‘delayed’ mobilization of HSCs from the bone marrow. For-instance, bone marrow-derived neutrophils have been shown to reach peak numbers in the bloodstream hours after very prolonged exercise bouts such as marathon running (Kakanis et al., 2010; Nieman et al., 1995). Unfortunately, we did not examine the temporal response of circulating G-CSF and HSC mobilization beyond 3h of exercise recovery. While other growth factors might be involved in the mobilization of HSCs by exercise, Niemiro et al. (2017) recently showed that HSC mobilization was unrelated to exercise-induced changes in plasma levels of CXC chemokine ligand 12 (CXCL12) or stem cell factor (SCF). They did find, however, that HSC mobilization was positively correlated with circulating endothelial cells (marker of endothelial damage) (Niemiro et al., 2017), which is consistent with our notion that hemodynamic shear stress within the vasculature, in combination with β2-AR activation, are required to mobilize HSCs during exercise.
We found that the mobilization of HSCs with exercise is relatively modest compared to pharmaceutical interventions such as G-CSF and/or the CXCR4 antagonist/CXCR7 agonist, Plerixafor. The receptor for G-CSF is expressed on many hematopoietic progenitor cells and these cells will increase their proliferation rate in response to G-CSF (Panopoulos and Watowich, 2008). G-CSF also disrupts the CLCX4/SDF-1 matrix and releases stem cells from their bone marrow niche (within 4–24 h) (Panopoulos and Watowich, 2008), indicating that the immediate HSC response seen with exercise is likely not due to increases in systemic G-CSF. Rather, increases in G-CSF post-exercise may be causing a delayed proliferation/elevation of CD34+ HSCs to repair/replace damaged cells and tissues caused by exercise hours after exercise cessation. The mobilization of ~4 CD34+cells/μl in response to even vigorous exercise reported here pales in comparison to the 10–20 CD34+cells/μl that are mobilized in response to just a few days of exogenous G-CSF therapy (Vaughn and Waller, 2012b) or the ~40 CD34+ cells/μl achieved after G-CSF therapy with the addition of Plerixafor (Cashen et al., 2008). Moreover, G-CSF therapy elicits sustained elevations in the circulating CD34+ count, whereas exercise only transiently increases HSC numbers in blood. However, a limitation of using G-CSF mobilized HSCs is the increased risk of GvHD (Anasetti et al., 2012), which could result in exercise-mobilized HSCs being preferred if they are found to cause fewer complications after transplant. It is also possible that exercise performed either during G-CSF administration followed by apheresis, or during apheresis (after G-CSF administration) will facilitate HSC collections from G-CSF treated donors due to its effects on increasing hemodynamic shear stress and activating the β2-AR. Indeed, the β2-AR has been shown to play a role in HSC mobilization from the bone marrow in combination with G-CSF infusions (Cancelas and Williams, 2006; Katayama et al., 2006), suggesting that exercise performed during the G-CSF treatment phase could facilitate HSC mobilization prior to apheresis. The effects of acute exercise on the mobilization of HSCs may also help reduce the duration of apheresis required to reach target HSC numbers required for transplant especially for situations when there is a large discrepancy between donor and recipient weights. These potential benefits of exercise could lead to lower doses of G-CSF, potentially reducing the severity of the side effects and prevent the need for further pharmaceutical interventions (i.e. Plerixafor) (Pantin et al., 2017; Schroeder et al., 2017). Future studies should determine if exercise performed during G-CSF administration followed by apheresis will increase HSC yields and lower the burden placed on alloHSCT donors. It will also be important to show that these effects can be reproduced in actual HSCT donors, who are likely to have lower fitness levels than the participants in this study.
A notable limitation of our study is that we did not further phenotype the CD34+ and CD133+ cells to differentiate HSCs from endothelial progenitor cells (EPCs) or mesenchymal stem cells (MSCs). We rather focused our attention on the total number of CD34+ and CD133+ cells, as this is often the criterion used when isolating HSCs from healthy donors for allogeneic HSCT (Barrett and Treleaven, 1998; Bonig and Papayannopoulou, 2012; Hosing, 2012; Vaughn and Waller, 2012a). However, other researchers reported that 1h of running at 70% VO2peak mobilized CD34+, CD34 + CD45dim, and CD34+CD45dimCD38- HSCs, whereas the number of CD34+CD45-CD31+ and CD45-CD31+ EPCs and CD34-CD45-CD31-CD105+ and CD34+CD45-CD31-CD105+ MSCs did not change (Niemiro et al., 2017). Therefore, it is very likely that the progenitor cells mobilized by exercise in the present study are predominantly HSCs and not EPCs or MSCs. There are several available β-AR antagonists we could have used for the β-AR blockade experiments in this study. We selected bisoprolol because of its high affinity for the β1-AR and nadolol because of its greater affinity for the β2-AR (Baker, 2005; Schnabel et al., 2000). We considered using a ‘selective’ β2-AR antagonist, but drugs such as butaxamine are not commercially available and have α-AR agonistic activity that would increase vascular shear stress and confound our results (Arnold et al., 1985; Reid, 1986). Furthermore, the experimental ‘selective’ β2-AR antagonist ICI 118,551, despite now being considered unsafe for human use (Salpeter et al., 2004; Yang et al., 2013), only marginally lowers exercising blood pressure and heart rate compared to ‘selective’ β1-AR blockers such as atenolol (Arnold et al., 1985; Gullestad et al., 1991) and this would have prevented us from comparing the effects of ‘selective’ β1-AR blockade with ‘selective’ β2-AR blockade on HSC mobilization under similar conditions of hemodynamic shear stress.
In summary, we report that steady state vigorous but not moderate intensity exercise mobilizes CD34+ and CD133+ HSCs to the peripheral blood compartment in healthy participants. We are the first to report that CD34+ HSCs mobilized to the blood during exercise are done so via a β2-AR mediated pathway, independently of circulating G-CSF levels. Our findings, in conjunction with those reported by Riddell et al. (2015) that β-AR agonist infusions alone fail to mobilize CD34+ HSCs, would indicate that a combination of β2-AR activation and α-AR-induced increases in hemodynamic forces are required to mobilize CD34+ HSCs into the main axial flow of the bloodstream during exercise. We conclude that CD34+ HSC mobilization with exercise is transient and insufficient to completely replace exogenous G-CSF therapy as a clinical adjuvant for mobilizing HSCs in the healthy alloHSCT donor population. However, exercise may facilitate HSC mobilization during G-CSF treatment and/or increase HSC collections if performed during apheresis. Future studies are needed to determine the feasibility of exercising during G-CSF treatment and HSC collections, and the impact this may have on reducing donor burden in the alloHSCT setting.
Acknowledgments
This work was supported by NASA Grants NNJ10ZSA003N, NNJ14ZSA001N-FLAGSHIP and NNJ14ZSA001N-MIXEDTOPICS to R.J. Simpson, NIH Grant R21 CA197527-01A1 to R.J. Simpson, ACSM NASA Foundational Research Grant to N. Agha, and NIH grant P01 CA148600-01A1 to C.M. Bollard.
References
- Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S, Ehninger G, Johnston L, Maziarz RT, Pulsipher MA, Porter DL, Mineishi S, McCarty JM, Khan SP, Anderlini P, Bensinger WI, Leitman SF, Rowley SD, Bredeson C, Carter SL, Horowitz MM, Confer DL, Blood, Marrow Transplant, Clinical Trials, N., 2012. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl. J. Med 367, 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JM, O'Connor PC, Riddell JG, Harron DW, Shanks RG, McDevitt DG, 1985. Effects of the beta 2-adrenoceptor antagonist ICI 118,551 on exercise tachycardia and isoprenaline-induced beta-adrenoceptor responses in man. Br. J. Clin. Pharm 19, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG, 2005. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br. J. Pharmacol 144, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JM, Nederveen JP, Parise G, 2017. Aerobic exercise in humans mobilizes HSCs in an intensity-dependent manner. J. Appl. Physiol 122, 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Treleaven J, 1998. The Clinical Practice of Stem-cell Transplantation. Isis Medical Media. [Google Scholar]
- Beaver WL, Wasserman K, Whipp BJ, 1986. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 60, 2020–2027. [DOI] [PubMed] [Google Scholar]
- Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B, Bollard CM, Simpson RJ, 2014. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav. Immun. 39, 160–171. [DOI] [PubMed] [Google Scholar]
- Bonig H, Papayannopoulou T, 2012. Mobilization of Hematopoietic Stem/Progenitor Cells: General Principles and Molecular Mechanisms In: Kolonin MG, Simmons PJ (Eds.), Stem Cell Mobilization: Methods and Protocols. Humana Press, Totowa, NJ, pp. 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore MR, Morici G, Riccioni R, Huertas A, Petrucci E, Veca M, Mariani G, Bonanno A, Chimenti L, Gioia M, Palange P, Testa U, 2010. Hemopoietic and angiogenetic progenitors in healthy athletes: different responses to endurance and maximal exercise. J. Appl. Physiol 109, 60–67. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA, 2003. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat. Med 9, 1520–1527. [DOI] [PubMed] [Google Scholar]
- Cancelas JA, Williams DA, 2006. Stem cell mobilization by beta2-agonists. Nat. Med 12, 278–279. [DOI] [PubMed] [Google Scholar]
- Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, DiPersio J, 2008. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biol. Blood Marrow Transp. 14, 1253–1261. [DOI] [PubMed] [Google Scholar]
- Copelan EA, 2006. Hematopoietic stem-cell transplantation. N. Engl. J. Med 354, 1813–1826. [DOI] [PubMed] [Google Scholar]
- Emmons R, Niemiro GM, De Lisio M, 2016. Exercise as an adjuvant therapy for hematopoietic stem cell mobilization. Stem Cells Int. 2016, 7131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England TJ, Sprigg N, Alasheev AM, Belkin AA, Kumar A, Prasad K, Bath PM, 2016. Granulocyte-colony stimulating factor (G-CSF) for stroke: an individual patient data meta-analysis. Sci. Rep. 6, 36567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum AM, Link DC, 2011. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 25, 211–217. [DOI] [PubMed] [Google Scholar]
- Grigg AP, Roberts AW, Raunow H, Houghton S, Layton JE, Boyd AW, McGrath KM, Maher D, 1995. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood 86, 4437–4445. [PubMed] [Google Scholar]
- Gullestad L, Birkeland K, Nordby G, Larsen S, Kjekshus J, 1991. Effects of selective beta 2-adrenoceptor blockade on serum potassium and exercise performance in normal men. Br. J. Clin. Pharmacol 32, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtick U, Albrecht M, Chemnitz JM, Theurich S, Skoetz N, Scheid C, von Bergwelt-Baildon M, 2014. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Syst Rev, CD010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosing C, 2012. Hematopoietic Stem Cell Mobilization with G-CSF In: Kolonin MG, Simmons PJ (Eds.), Stem Cell Mobilization: Methods and Protocols. Humana Press, Totowa, NJ, pp. 37–47. [Google Scholar]
- Kakanis MW, Peake J, Brenu EW, Simmonds M, Gray B, Hooper SL, Marshall-Gradisnik SM, 2010. The open window of susceptibility to infection after acute exercise in healthy young male elite athletes. Exercise Immunol. Rev 16, 119–137. [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS, 2006. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421. [DOI] [PubMed] [Google Scholar]
- Kostis JB, Lacy CR, Krieger SD, Cosgrove NM, 1984. Atenolol, nadolol, and pindolol in angina pectoris on effort: effect of pharmacokinetics. Am. Heart J 108, 1131–1136. [DOI] [PubMed] [Google Scholar]
- Kruger K, Pilat C, Schild M, Lindner N, Frech T, Muders K, Mooren FC, 2014. Progenitor cell mobilization after exercise is related to systemic levels of G-CSF and muscle damage. Scandinavian journal of medicine & science in sports. [DOI] [PubMed] [Google Scholar]
- Le Coz F, Sauleman P, Poirier JM, Cuche JL, Midavaine M, Rames A, Lecocq B, Jaillon P, 1991. Oral pharmacokinetics of bisoprolol in resting and exercising healthy volunteers. J. Cardiovasc. Pharmacol 18, 28–34. [DOI] [PubMed] [Google Scholar]
- Leopold G, Pabst J, Ungethum W, Buhring KU, 1986. Basic pharmacokinetics of bisoprolol, a new highly beta 1-selective adrenoceptor antagonist. J. Clin. Pharmacol. 26, 616–621. [DOI] [PubMed] [Google Scholar]
- Lipworth BJ, Irvine NA, McDevitt DG, 1991. A dose-ranging study to evaluate the beta 1-adrenoceptor selectivity of bisoprolol. Eur. J. Clin. Pharmacol 40, 135–139. [DOI] [PubMed] [Google Scholar]
- Mobius-Winkler S, Hilberg T, Menzel K, Golla E, Burman A, Schuler G, Adams V, 2009. Time-dependent mobilization of circulating progenitor cells during strenuous exercise in healthy individuals. J. Appl. Physiol 107, 1943–1950. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Simandle S, Henson DA, Warren BJ, Suttles J, Davis JM, Buckley KS, Ahle JC, Butterworth DE, Fagoaga OR, et al. , 1995. Lymphocyte proliferative response to 2.5 hours of running. Int. J. Sports Med 16, 404–409. [DOI] [PubMed] [Google Scholar]
- Niemiro GM, Parel J, Beals J, van Vliet S, Paluska SA, Moore DR, Burd NA, De Lisio M, 2017. Kinetics of circulating progenitor cell mobilization during submaximal exercise. J. Appl, Physiol, jap 00936, 02016. [DOI] [PubMed] [Google Scholar]
- Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM, 2005. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev. Biol 279, 336–344. [DOI] [PubMed] [Google Scholar]
- Panch SR, Szymanski J, Savani BN, Stroncek DF, 2017. Sources of Hematopoietic Stem and Progenitor Cells and Methods to Optimize Yields for Clinical Cell Therapy. Biol. Blood Marrow Transp. 23, 1241–1249. [DOI] [PubMed] [Google Scholar]
- Panopoulos AD, Watowich SS, 2008. Granulocyte colony-stimulating factor: molecular mechanisms of action during steady state and ‘emergency’ hematopoiesis. Cytokine 42, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin J, Purev E, Tian X, Cook L, Donohue-Jerussi T, Cho E, Reger R, Hsieh M, Khuu H, Calandra G, Geller NL, Childs RW, 2017. Effect of high-dose plerixafor on CD34+ cell mobilization in healthy stem cell donors: results of a randomized crossover trial. Haematologica 102, 600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passweg JR, Halter J, Bucher C, Gerull S, Heim D, Rovo A, Buser A, Stern M, Tichelli A, 2012. Hematopoietic stem cell transplantation: a review and recommendations for follow-up care for the general practitioner. Swiss Med Wkly 142, w13696. [DOI] [PubMed] [Google Scholar]
- Reid JL, 1986. Alpha-adrenergic receptors and blood pressure control. Am. J. Cardiol 57, 6E–12E. [DOI] [PubMed] [Google Scholar]
- Riddell NE, Burns VE, Wallace GR, Edwards KM, Drayson M, Redwine LS, Hong S, Bui JC, Fischer JC, Mills PJ, Bosch JA, 2015. Progenitor cells are mobilized by acute psychological stress but not beta-adrenergic receptor agonist infusion. Brain Behav. Immun 49, 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE, 2004. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann. Intern. Med 140, 802–813. [DOI] [PubMed] [Google Scholar]
- Schafer-Korting M, Bach N, Knauf H, Mutschler E, 1984. Pharmacokinetics of nadolol in healthy subjects. Eur. J. Clin. Pharmacol 26, 125–127. [DOI] [PubMed] [Google Scholar]
- Schnabel P, Maack C, Mies F, Tyroller S, Scheer A, Bohm M, 2000. Binding properties of beta-blockers at recombinant beta1-, beta2-, and beta3-adrenoceptors. J. Cardiovasc. Pharmacol 36, 466–471. [DOI] [PubMed] [Google Scholar]
- Schroeder MA, Rettig MP, Lopez S, Christ S, Fiala M, Eades W, Mir FA, Shao J, McFarland K, Trinkaus K, Shannon W, Deych E, Yu J, Vij R, Stockerl-Goldstein K, Cashen AF, Uy GL, Abboud CN, Westervelt P, DiPersio JF, 2017. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood 129, 2680–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke B, Thompson A, Leitch A, Riddell JG, 1995. A placebo controlled comparison of the effects of metoprolol and celiprolol on echo-Doppler measurements of cardiovascular function in normal volunteers. Br. J. Clin. Pharmacol 40, 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Bigley AB, Agha N, Hanley PJ, Bollard CM, 2017. Mobilizing immune cells with exercise for cancer immunotherapy. Exercise Sport Sci. Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ, Kunz H, Agha N, Graff R, 2015. Exercise and the regulation of immune functions. Progr. Mol. Biol. Transl. Sci 135, 355–380. [DOI] [PubMed] [Google Scholar]
- Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC, 2006. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell 5, 495–503. [DOI] [PubMed] [Google Scholar]
- Treleaven J, Barrett AJ, 2009. Hematopoietic Stem Cell Transplantation. In Clinical Practice, Churchill Livingstone/Elsevier. [Google Scholar]
- Van Baak MA, 1988. Beta-adrenoceptor blockade and exercise. An update. Sports Med. 5, 209–225. [DOI] [PubMed] [Google Scholar]
- Vaughn ML, Waller EK, 2012. Monitoring blood for CD34+ cells to determine timing of hematopoietic progenitor cells apheresis. Methods Mol. Biol. 904, 79–83. [DOI] [PubMed] [Google Scholar]
- Vaughn ML, Waller EK, 2012. Monitoring Blood for CD34+ Cells to Determine Timing of Hematopoietic Progenitor Cells Apheresis In: Kolonin MG, Simmons PJ (Eds.), Stem Cell Mobilization: Methods and Protocols, Humana Press, Totowa, NJ, pp. 79–83. [DOI] [PubMed] [Google Scholar]
- Wahl P, Bloch W, Schmidt A, 2007. Exercise has a positive effect on endothelial progenitor cells, which could be necessary for vascular adaptation processes. Int. J. Sports Med. 28, 374–380. [DOI] [PubMed] [Google Scholar]
- Wheeldon NM, McDevitt DG, Lipworth BJ, 1994. The effects of lower than conventional doses of oral nadolol on relative beta 1/beta 2-adrenoceptor blockade. Br. J. Clin. Pharmacol 38, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Liang B, Srivastava K, Zeng J, Zhan J, Brown L, Sampson H, Goldfarb J, Emala C, Li XM, 2013. The Sophora flavescens flavonoid compound trifolirhizin inhibits acetylcholine induced airway smooth muscle contraction. Phytochemistry 95, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldivar F, Eliakim A, Radom-Aizik S, Leu SY, Cooper DM, 2007. The effect of brief exercise on circulating CD34+ stem cells in early and late pubertal boys. Pediatr. Res 61, 491–495. [DOI] [PubMed] [Google Scholar]