Abstract
Research studies suggest that neonatal seizures, which are most commonly associated with hypoxic-ischemic injury, may contribute to brain injury and adverse neurologic outcome. Unfortunately, neonatal seizures are often resistant to treatment with current anticonvulsants. In the present study, we evaluated the efficacy of flupirtine, administered at clinically relevant time-points, for the treatment of neonatal seizures in an animal model of hypoxic-ischemic injury that closely replicates features of the human syndrome. We also compared the efficacy of flupirtine to that of phenobarbital, the current first-line drug for neonatal seizures. Flupirtine is a KCNQ potassium channel opener. KCNQ channels play an important role in controlling brain excitability during early development. In this study, hypoxic-ischemic injury was induced in neonatal rats, and synchronized video-EEG records were acquired at various time-points during the experiment to identify seizures. The results revealed that flupirtine, administered either 5 min after the first electroclinical seizure, or following completion of 2 h of hypoxia, i.e., during the immediate reperfusion period, reduced the number of rats with electroclinical seizures, and also the frequency and total duration of electroclinical seizures. Further, daily dosing of flupirtine decreased the seizure burden over 3 days following HI-induction, and modified the natural evolution of acute seizures. Moreover, compared to a therapeutic dose of phenobarbital, which was modestly effective against electroclinical seizures, flupirtine showed greater efficacy. Our results indicate that flupirtine is an extremely effective treatment for neonatal seizures in rats and provide evidence for a trial of this medication in newborn humans.
Keywords: Neonatal seizures, EEG, Video, Electroclinical, Hypoxia, Ischemia
1. Introduction
The probability of occurrence of seizures during the neonatal period is higher than any other age group (Hauser et al., 1993). The incidence of seizures in newborns is estimated at 1.8–3.5 per 1000 live births (Lanska et al., 1995; Saliba et al., 1999). Approximately 60% of neonatal seizures are associated with a hypoxic-ischemic event (Ronen et al., 2007; Tekgul et al., 2006). Survivors of neonatal hypoxic-ischemic encephalopathy (HIE) often develop brain injury and neurologic disabilities (e.g. cognitive deficits and epilepsy) in later life (Bergamasco et al., 1984; Robertson and Finer, 1988). Studies in humans and animal models suggest that seizures contribute to brain damage and adverse neurologic outcome (Dzhala et al., 2000; Glass et al., 2009; McBride et al., 2000; Miller et al., 2002; Wirrell et al., 2001). In clinics across the world, phenobarbital is the drug of choice for the treatment of neonatal seizures (Blume et al., 2009; Carmo and Barr, 2005). However, phenobarbital, an agonist of γ-aminobutyric acid (GABA), is not completely effective in controlling seizures; it does not stop seizures in 50% of patients. Moreover, it has only a limited efficacy in treating electrographic seizures (Foster and Lewis, 2007; Painter et al., 1999; Sankar and Painter, 2005). Frequently, clinical seizures will respond to phenobarbital but then electrical seizures will persist. In neonates, electrographic seizure activity has been shown to correlate with poor neurodevelopmental outcome (McBride et al., 2000), and treatment of electrographic seizures in addition to clinical seizures has been demonstrated to significantly reduce the severity of brain injury (van Rooij et al., 2010). Further, in developing rats and children, phenobarbital treatment has been linked to neuronal and white matter apoptosis, chronic changes in gene expression, and synaptic and cognitive impairment (Bittigau et al., 2002; Farwell et al., 1990; Forcelli et al., 2012; Kaushal et al., 2016; Raol et al., 2005). The GABAergic inhibitory system of the immature brain is underdeveloped as compared to that of the adult brain (Ben-Ari, 2002; Brooks-Kayal and Pritchett, 1993; Gibbs et al., 1996; Kapur and Macdonald, 1999), which may partly explain the suboptimal efficacy of phenobarbital in the treatment of neonatal seizures. Thus, development of a safe treatment that is effective against both clinical and purely electrographic seizures is critical.
In the kainic acid and flurothyl models of neonatal seizures, flupirtine is very effective and more efficacious than phenobarbital and diazepam in stopping seizures (Raol et al., 2009). Flupirtine (ethyl-N-[2-amino-6-(4-fluorophenylmethyl-amino)pyridin-3-yl] carbamate) has been used as an analgesic in Europe for the last 30 years. Flupirtine shifts the voltage required to open KCNQ type of potassium channels to a more negative potential, resulting in an increased threshold for generating a neuronal action potential (Klinger et al., 2012; Martire et al., 2004; Wladyka and Kunze, 2006). KCNQ channels are voltage gated, depolarization activated potassium channels whose expression in the brain begins before birth (Brown and Passmore, 2009; Devaux et al., 2004; Okada et al., 2003). These channels play a very important role in controlling over excitation during early life when the GABA-mediated inhibition is weak (Pena and Alavez-Perez, 2006; Peters et al., 2005). Mutations in genes encoding KCNQ2/3 channels result in benign familial neonatal epilepsy (BFNE), a relatively mild condition in which seizures resolve spontaneously within a few weeks after the onset and majority of patients have normal outcome, and KCNQ2 encephalopathy, in which seizures are usually pharmacoresistant and patients have an epileptic encephalopathy with severe to moderate intellectual disability (Kato et al., 2013; Saitsu et al., 2012; Singh et al., 2003; Weckhuysen et al., 2012). Flupirtine has been shown also to activate G-protein-regulated inwardly rectifying K+ channels (GIRK) (Jakob and Krieglstein, 1997; Kornhuber et al., 1999; Montandon et al., 2016; Sattler et al., 2008) (but see (Klinger et al., 2012)), which has been suggested to indirectly inhibit NMDA receptor activity (Klinger et al., 2012; Kornhuber et al.,1999) (but see (Jakob and Krieglstein, 1997)). Recent studies suggest that flupirtine may also shift the gating of GABAA receptors to lower GABA concentration, an action that is more pronounced in dorsal horn neurons than in hippocampal neurons (Klinger et al., 2012). In our recent study, we showed that flupirtine given to 10-day-old rats before exposure to global hypoxia prevented development of electroclinical seizures (behavioral seizures with an EEG correlate) during a hypoxic episode (Sampath et al., 2015). In humans, however, the treatment is usually started after a seizure is observed. In neonates with HIE, the median age for the detection of purely electrographic seizures has been reported as 13.1 h (Lynch et al., 2015), but clinical seizures have been observed as early as 4 h of age in babies in whom the injury occurred before labor (Filan et al., 2005). These seizures often continue to occur for 48 h or more (Ahn et al., 1998; Filan et al., 2005).
In the current study, we examined the efficacy of flupirtine to stop seizures when given at clinically relevant time points. The rat model of HIE that we used replicates the etiology and characteristics of seizures in human newborns (Sampath et al., 2014). We also compared the efficacy of flupirtine with phenobarbital, the first-line choice for the treatment of neonatal seizures. Our result suggests that flupirtine is highly efficacious against HI-induced neonatal seizures, and is effective even when given following 2 h of hypoxia and multiple seizures. In our study, phenobarbital at a clinically therapeutic dose was only modestly effective in reducing and preventing seizures. This is similar to the situation in human neonates, supporting the use of our experiment as a model for the human case.
2. Methods
2.1. Animals
All procedures involving animals were performed in accordance with the NIH guidelines for the care and use of laboratory animals, and according to the protocol approved by the Institutional Animal Care and use Committee (IACUC) of the University of Colorado Anschutz Medical Campus (UC-AMC). In addition, all efforts were made to reduce animal suffering and the number of animals used. Timed pregnant Sprague-Dawley rats were obtained from Charles River laboratories (Wilmington, MA). The pregnant rats were at the 14th day of gestation (E14) on arrival at the vivarium and delivered the pups at E22 or E23. Only male pups were used for the study.
2.2. Hypoxia-ischemia protocol
HI was induced in postnatal day 7 (P7) rats by Rice-Vannucci method (Rice et al., 1981; Sampath et al., 2014). Under isoflurane anesthesia (2–4% for induction and 1–1.5% maintenance), the right common carotid artery was identified and double ligated with a 4–0 polyglycolic acid suture. The neck incision was closed with 4–0 nylon Dermalon sutures. The entire surgical procedure lasted for 10–12 min. Following the ligation, the pups were housed with the dam in a warm cage for 1.5–2.25 h before they were exposed to hypoxia. The pups were exposed to hypoxic environment (8–8.3% oxygen) for 2 h. The oxygen content of the chamber was monitored using an oxygen sensor (Dräger Pac 7000, Pittsburg, PA). During the hypoxia, the temperature was maintained at 36.5° Celsius and humidity between 60 and 70%. Following HI-induction, the pups were treated with analgesic (0.1 mg/kg buprenorphine hydrochloride) once every 12 h for 48 h.
2.3. Electrode implantation
To record EEG, the electrode implantation was performed according to our published protocol (Sampath et al., 2014). Briefly, P6 rats were implanted bilaterally in the parietal cortex with a silver electrode (A-M Systems, Carlsborg, WA). The active electrode in each hemisphere was referenced to a separate silver electrode positioned near lambda in the same hemisphere. The implantation procedure was performed under isoflurane anesthesia (2–4% for induction and 1–1.5% for maintenance). After the surgery, the rats were returned to the dam and treated with analgesic (0.1 mg/kg buprenorphine hydrochloride) once every 12 h for 48 h.
2.4. Video-EEG monitoring
Synchronized, time locked video and EEG signals were recorded using the Stellate Harmonie system (Natus Medical, San Carlos, CA, U.S.A.). The EEG signal was digitized at 1000 Hz and stored on a hard disk for offline analysis. The video-EEG monitoring began at P7. A 30 min baseline recording was obtained before any manipulation was done to induce HI. The pups were monitored continuously by video and EEG during the entire 2 h hypoxia period. The monitoring of the pups by video-EEG continued for two more hours after completion of the hypoxic period, i.e., during the immediate reperfusion period. The pups were reconnected for video-EEG recording sessions 24, 48 and 72 h after HI-induction.
2.5. Seizure scoring
The video-EEG records were analyzed by the first author of the current paper (DS) who was aware of the identity of the record. Randomly selected video-EEG records analyzed by DS, and whose identity was masked, were reevaluated by YHR and AMW to confirm the accuracy of the analysis. Electroclinical seizures were defined by an EEG pattern that differed from background in either amplitude, frequency, or both, evolved over time, and contained spikes or sharp waves lasting for 10 s or more and were associated with a change in the rat’s behavior. The behavioral seizure consisted of clonic seizures, tonic posturing of the trunk, tonic-clonic seizures, facial twitching, and stiffening of the tail. Electrographic seizures were defined as seizures observed in the EEG records that were not associated with a behavioral correlate on video (Sampath et al., 2014).
2.6. Drug treatment
All drugs were administered to the pups via intraperitoneal injection. The pups were selected randomly for a specific drug treatment on the day of the experiment, and whenever possible, pups from each litter were divided equally among the treatment groups. Three drug treatment strategies were used to evaluate the efficacy of flupirtine to treat HI-induced neonatal seizures: (1) a single dose of flupirtine given 5 min after the first electroclinical seizure, which occurred within a few minutes into the hypoxic period (Sampath et al., 2014), (2) a single dose of flupirtine given 5 min after the rats were reintroduced to room air following 2 h of hypoxia, i.e., 5 min into the reperfusion period (Qiao et al., 2004), and (3) a single dose of flupirtine given five minutes after the end of the 2 h of hypoxia, and then every 24 h up to 72 h (total of 4 doses). Each treatment group in all three treatment strategies consisted of pups from multiple litters. The twelve pups in the treatment group where they were given drug five minutes after the first electroclinical seizure study were obtained from 7 litters. Eleven litters were used to obtain 29 pups for the study in which the treatment was started five minutes into the reperfusion period. For the multiple dose study, 12 pups came from 6 litters. Twelve mg of flupirtine maleate (Sigma, St. Louis, MO) was dissolved in 1 ml dimethylsulfoxide (Sigma) on the day of use. Phenobarbital injection vials were purchased from the University of Colorado Hospital pharmacy (Aurora, CO). The equivalent volume of flupirtine, phenobarbital and vehicle were injected to pups (e.g. ~0.03 ml to P7 pups).
2.7. Statistical analysis
GraphPad Prism 5 statistical software (GraphPad Software Inc., San Diego, CA, U.S.A.) was used for statistical analysis. A Student’s t-test was used to compare two sets of data. One-way analysis of variance (ANOVA), followed by the Tukey’s post-hoc test was used to determine significance of difference in data values involving three study groups. The two-sided Fisher’s exact test was used to compare the difference in the number of rats with seizures between treatment groups. Differences were considered significant at p ≤ 0.05. The results are presented as the mean ± standard deviation (SD).
3. Results
3.1. Flupirtine given after the first electroclinical seizure prevents development of further seizures during hypoxia and the immediate reperfusion period
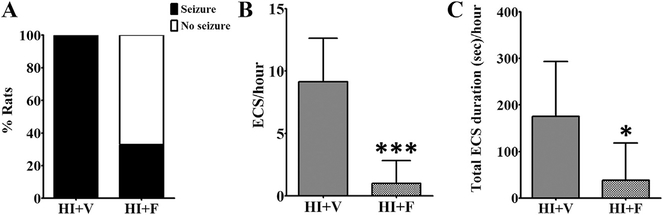
In a clinical setting, treatment with an anticonvulsant is often initiated following observation of a clinical seizure. To replicate this treatment protocol, a single dose of 25 mg/kg flupirtine was given to the pup 5 min after the occurrence of first electroclinical seizure (a seizure on the EEG that correlated with change in rat’s behavior). Our decision to use 25 mg/kg flupirtine dose for the current study was based on our earlier studies, in which we had carried out a dose response study to examine efficacy of flupirtine to block kainic acid (Raol et al., 2009) and hypoxia (Raol et al., 2009; Sampath et al., 2015) induced neonatal seizures. A single dose of 25 mg/kg flupirtine given 5 min after the first electroclinical seizure significantly reduced the number (Student’s two-tailed t-test, t (10) = 5.1, p = 0.0005) and total duration (Student’s two-tailed t-test, t (10) = 2.3, p = 0.04) of subsequent seizures during hypoxia (Fig. 1, Table 1) and the immediate period following the end of the hypoxia period (reperfusion period, Table 1; Student’s two-tailed t-test, t (10) = 3.0, p = 0.01 for seizure number and t (10) = 2.9, p = 0.01 for seizure duration). The flupirtine treatment showed a trend toward a decreased number of animals that developed electroclinical seizures during hypoxia (Fig. 1) and the immediate reperfusion period (Table 1, p = 0.06 for both the time points, two-sided Fisher’s exact test). However, the single dose of flupirtine given after the first electroclinical seizure was not sufficient to prevent occurrence of seizures 24 h onwards after the HI-induction (Table 1).
Fig. 1. Flupirtine effectively treats HI-induced electroclinical seizures.
Flupirtine given after the 1st electroclinical seizure reduces (A) the percent of rats with electroclinical seizures and (B) the number and (C) total duration of electroclinical seizures during the remaining hypoxia period. The data shown in the B and C histograms are mean ± SD. *p < 0.05, ***p < 0.0005, Student’s two-tailed t-test. ECS = electroclinical seizure, HI = hypoxia-ischemia, V = vehicle, F = flupirtine.
Table 1.
The effect of flupirtine on HI-induced seizures when administered five minutes after first electroclinical seizure.
| Parameter | During Hypoxia |
Immediate Reperfusion Period |
24 h after HI |
48 h after HI |
72 h after HI |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HI + V (n = 6) | HI + F (n = 6) | HI + V (n = 6) | HI + F (n = 6) | HI + V (n = 6) | HI + F (n = 6) | HI + V (n = 6) | HI + F (n = 6) | HI + V (n = 5) | HI + F (n = 6) | |
| Frequency of ECS/hour | 9.2 ± 3.5 | 1.0 ± 1.8*** | 13.2 ± 10.3 | 0.4 ± 0.7* | 14.8 ± 8.6 | 9.0 ± 11.5 | 12.7 ± 17.6 | 6.8 ± 7.8 | 5.0 ± 4.9 | 4.0 ± 8.8 |
| Total duration of ECS (secs)/hour | 175.5 ± 117.7 | 38.3 ± 80.0* | 200.3 ± 164.2 | 4.5 ± 7.0* | 233.5 ± 147.5 | 299.0 ± 350.2 | 219.2 ± 320.7 | 118.2 ± 142.7 | 68.2 ± 68.8 | 160.7 ± 375.5 |
| % rats with ECS | 100 | 33.3 | 100 | 33.3 | 100 | 66.6 | 66.6 | 66.6 | 60 | 50 |
Because of the poor EEG quality, the 72 h time-point data from one vehicle treated HI rat was not included in the final analysis. ECS = electroclinical seizures. HI = hypoxia-ischemia, V = vehicle, F = flupirtine. Data shown are mean ± SD.
p < 0.05
p < 0.0005.
3.2. Flupirtine given after HI-induction is very effective in decreasing the number and duration of neonatal seizures during the immediate reperfusion period
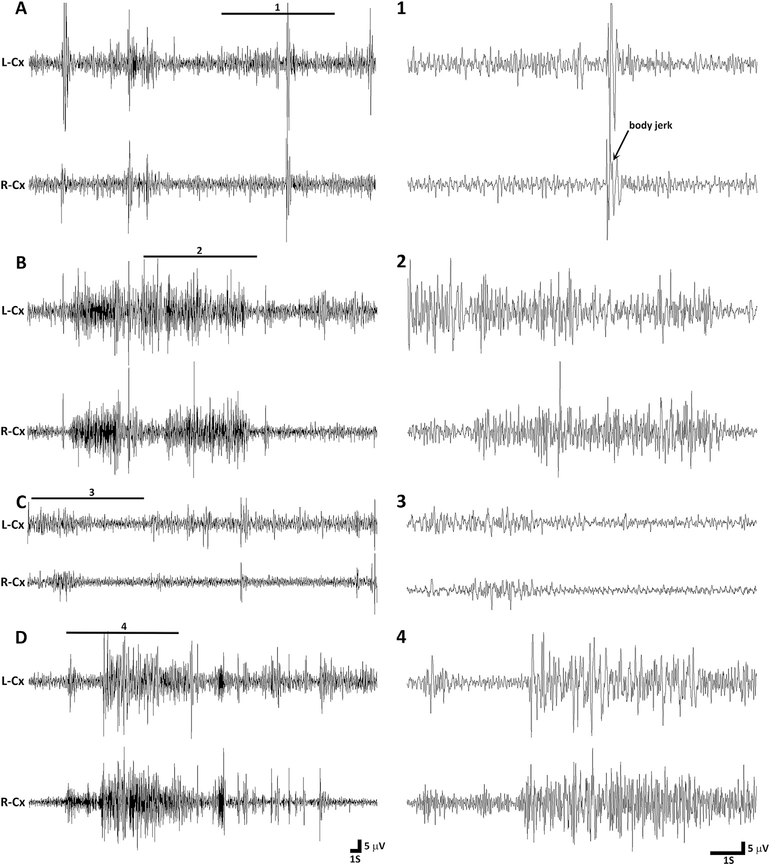
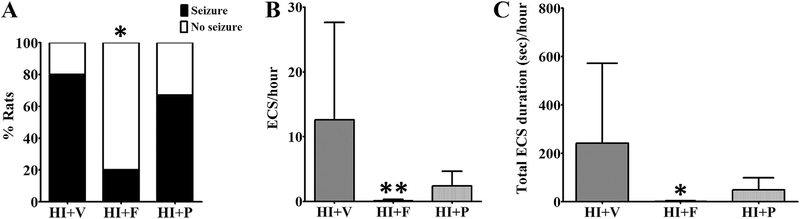
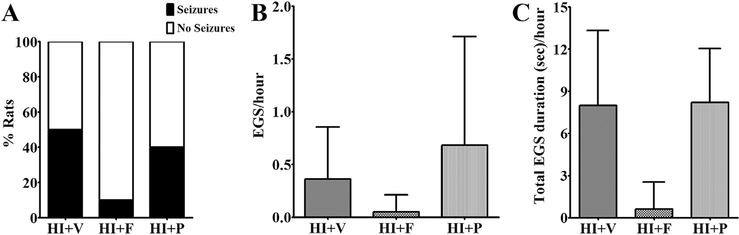
In newborns with hypoxic-ischemic injury, the seizures are typically (these can be observed if the hypoxia is still ongoing, but that is less likely) first observed after initial treatment to resume oxygen and blood supply to the brain, i.e. during the reperfusion period. To test the efficacy of flupirtine to treat neonatal seizures under a similar clinical condition, a single dose of 25 mg/kg was administered 5 min after the rats were reintroduced to the room air following 2 h of the hypoxic period (after HI-induction), i.e. during the immediate reperfusion phase (Qiao et al., 2004). The efficacy of flupirtine was compared with phenobarbital, the current first-line treatment for neonatal seizures. We selected a 25 mg/kg phenobarbital dose for comparison, because in our earlier study, we observed that this dose in neonatal rats results in clinically relevant therapeutic drug levels in serum within 30 min of drug injection via the intraperitoneal route (Raol et al., 2009). The data analysis revealed that flupirtine very effectively prevents HI-induced seizures during the immediate reperfusion period (Fig. 2, Table 2). Compared to vehicle treated HI rats, a significantly lower number of flupirtine treated rats developed electroclinical seizures during the immediate reperfusion period (Two-sided Fisher’s exact test, p = 0.02; Fig. 3, Table 2). Furthermore, compared to vehicle treated rats, flupirtine treated rats had significantly lower number (One-way ANOVA, F (2, 26) = 5.46, p = 0.01, Tukey post-hoc test showed p < 0.05) and total duration (One-way ANOVA, F (2, 26) = 4.14, p = 0.03, Tukey post-hoc test showed p < 0.05) of electroclinical seizures (Fig. 3, Table 2). On average, flupirtine reduced the number and total duration of electroclinical seizures by greater than 99% when compared to vehicle treated HI rats, and prevented the occurrence of seizures during immediate reperfusion period in 80% of HI rats (Table 2). The two flupirtine treated rats (out of 10) that developed electroclinical seizures during the reperfusion period seized at the rate of 0.5 ± 0.01 seizures/hour (mean ± SD) and spent 5.75 ± 0.01 s/hour (mean ± SD) seizing whereas, vehicle treated rats with seizures (8 out of 10) seized at the rate of 15.7 ± 15.3 seizures/ hour (mean ± SD) and spent 301.3 ± 346.1 s/hour (mean ± SD) seizing. Additionally, fewer flupirtine treated rats were observed to develop purely electrographic seizures than vehicle treated rats (seizures on EEG without a change in the rat’s behavior), resulting in an overall reduction in the number and duration of electrographic seizures (Fig. 4). However, the very low frequency of purely electrographic seizures in our model (Sampath et al., 2014) (Fig. 4), is likely a factor for not achieving statistical significance for this parameter (One-way ANOVA, F (2, 27) = 2.27, p = 0.1, Tukey post-hoc test showed p > 0.05).
Fig. 2. Representative EEG tracings from P7 rats during immediate reperfusion period.
(A) The tracing on the left shows an example of inter-ictal EEG in a vehicle treated rat during the immediate reperfusion period. A magnified excerpt of a part of the EEG, marked with a bar above the tracing (1), is provided on the right side of the compressed EEG tracing. During this epoch the rat is not moving, except for some forelimb twitches and a whole body jerk preceded by a spike. (B) The tracing shows EEG activity during an electroclinical seizure that occurred approximately one hour in the reperfusion period in the same vehicle treated rat as above. The EEG ictal activity was associated with clonic movements of limbs. The enlarged excerpt of a part of the EEG (2) is provided next to compressed EEG tracing. (C) The representative tracing shows EEG activity in a flupirtine treated rat one hour in the reperfusion period. Except for the slight hindlimb movement at the start of the epoch, the rat was lying motionless during the entire epoch. The magnified excerpt of a part of the EEG (3) is provided on the right side of the compressed EEG tracing. (D) The tracing shows EEG activity during an electroclinical seizure in the phenobarbital treated HI rat. The EEG ictal activity was associated with tonic-clonic movement of limbs and thrashing-like motion of both hindlimbs. The enlarged excerpt of a part of the EEG (4) is provided next to compressed EEG tracing. L-Cx = left cortex, R-Cx = right cortex, S = second, V = volts.
Table 2.
The effect of flupirtine on HI-induced seizures when administered five minutes into the reperfusion period.
| Parameter | During Hypoxia |
Immediate Reperfusion Period |
24 h after HI |
48 h after HI |
72 h after HI |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HI + V (n = 10) | HI + F (n = 10) | HI + P (n = 9) | HI + V (n = 10) | HI + F (n = 10) | HI + P (n = 9) | HI + V (n = 10) | HI + F (n = 10) | HI + P (n = 9) | HI + V (n = 10) | HI + F (n = 10) | HI + P (n = 9) | HI + V (n = 7) | HI + F (n = 5) | HI + P (n = 5) | |
| Frequency of ECS/hour | 12.4 ± 4.5 | 10.2 ± 3.2 | 10.2 ± 2.9 | 12.6 ± 15.1 | 0.1 ± 0.2** | 2.4 ± 2.3 | 7.0 ± 5.6 | 12.7 ± 13.6 | 6.1 ± 8.1 | 7.8 ± 8.2 | 18.7 ± 17.2 | 11.8 ± 12.2 | 1.8 ± 2.5 | 0 | 0.4 ± 0.9 |
| Total duration of ECS (secs)/hour | 267.9 ± 118.9 | 265.6 ± 108.1 | 224.4 ± 55.1 | 241.1 ± 330.6 | 1.1 ± 2.4* | 49.2 ± 50.1 | 109.3 ± 95.1 | 235.3 ± 260.0 | 101.1 ± 127.2 | 146.4 ± 162.0 | 319.7 ± 310.4 | 199.3 ± 216.8 | 30.7 ± 39.8 | 0 | 7.4 ± 16.5 |
| % rats with ECS | 100 | 100 | 100 | 80 | 20* | 66.7 | 100 | 100 | 66.7 | 80 | 70 | 55.5 | 42.8 | 0 | 20 |
The numbers of rats in all treatment groups at the 72 h time-point is less than the previous recording time-points because a few rats from each group were sacrificed after completion of recording at the 48 h time-point to obtain data for other studies. ECS = electroclinical seizures. HI = hypoxia-ischemia, V = vehicle, F = flupirtine, P = phenobarbital. Data shown are mean ± SD.
Statistically different from HI + V group; **p < 0.01, *p < 0.0.
Fig. 3. Flupirtine, unlike phenobarbital, very effectively reduces seizure activity during the immediate reperfusion period.
A single dose of flupirtine administered during the immediate reperfusion period (5 min after the rat is reintroduced to the room air after a 2 h hypoxia period) reduced (A) the percent of rats developing electroclinical seizures and (B) the number and (C) total duration of electroclinical seizures during the reperfusion period. The data shown in the B and C histograms are mean ± SD. *, **Statistically different from HI + V group; *p < 0.05, **p < 0.01, Fisher’s exact test was used to compare data in A, and One-way ANOVA and Tukey’s post-hoc test was used to compare data in B and C. ECS = electroclinical seizure, HI = hypoxia-ischemia, V = vehicle, F = flupirtine, P = phenobarbital.
Fig. 4. Efficacy of flupirtine and phenobarbital against purely electrographic seizures.
A single dose of flupirtine administered during the immediate reperfusion period appears to reduce (A) the percent of rats with purely electrographic (subclinical) seizures and (B) the number and (C) total duration of electrographic seizures during the reperfusion period. However, a single dose of phenobarbital was completely ineffective against purely electrographic seizures. The data shown in the B and C histograms are mean ± SD. EGS = electrographic seizure, HI = hypoxia-ischemia, V = vehicle, F = flupirtine, P = phenobarbital.
The 25 mg/kg phenobarbital dose was not as effective as 25 mg/ kg flupirtine dose in reducing and preventing HI-induced electroclinical seizures during the immediate reperfusion period. Compared to 80% vehicle and 20% flupirtine treated rats, 66.7% phenobarbital treated HI rats developed electroclinical seizures during the immediate reperfusion period (Two-sided Fisher’s exact test, p = 0.62 for HI + P vs HI + V, p = 0.07 for HI + P vs HI + F; Fig. 3, Table 2). In comparison to vehicle treated rats, phenobarbital treated rats had lower, but statistically not significant, mean number and duration of electroclinical seizures during the reperfusion period (Fig. 3, Table 2). In contrast to its modest effect on electroclinical seizures, 25 mg/kg phenobarbital dose was completely ineffective in preventing purely electrographic seizures (One-way ANOVA, F (2, 27) = 2.27, p = 0.1, Tukey post-hoc test showed p > 0.05; Fig. 4). Four out of 10 phenobarbital treated rats developed purely electrographic seizures, compared to 5 out of 10 vehicle treated and only 1 out of 10 flupirtine treated rats, during the immediate reperfusion period (Fig. 4). A one-time dose of flupirtine or phenobarbital was not sufficient to prevent the occurrence of seizures greater than 24 h after the HI-induction (Table 2).
3.3. Daily dose of flupirtine reduces HI-induced acute seizure burden
In newborns with HIE, seizures may persist for 48 h or more following the precipitating injury (Ahn et al., 1998; Filan et al., 2005). As described above, a single dose of flupirtine given after the 1st seizure, or after 2 h of hypoxia during the reperfusion period, was not sufficient to prevent seizures at 24 h and later. To determine whether repeated dosing of flupirtine is effective in reducing seizures over 24–72 h period following hypoxic-ischemic injury, a separate group of rats were given a single dose of flupirtine (25 mg/kg) or vehicle 5 min after completion of exposure to 2 h of hypoxia (during the immediate reperfusion phase), and an additional dose of 25 mg/kg at 24, 48 and 72 h after HI. A 2 h Video-EEG record was acquired before and after the animal was dosed with the drug. Both, vehicle and flupirtine treated, groups of rats experienced similar number and duration of electroclinical seizures during hypoxia (frequency: 14.17 ± 4.87 for HI + V and 15.33 ± 3.25 for HI + F seizures/hour; 320.2 ± 126 s for HI + V and 394.8 ± 65 s for HI + F seizure duration/hour). Similar to the previous experiment, a single dose of 25 mg/kg flupirtine given 5 min after the rats were reintroduced to room air following 2 h of the hypoxic period (after HI-induction) was very effective in reducing electroclinical seizures during the immediate 2 h reperfusion period (frequency: 14.67 ± 9.77 for HI + V and 0.75 ± 1.6 for HI + F seizures/hour; 222.9 ± 141.9 s for HI + V and 14.07 ± 27.92 s for HI + F seizure duration/hour). Further, pups treated with an additional dose of flupirtine at 24, 48 and 72 h after HI overall experienced a significantly lower number of electroclinical seizures (Student’s two-tailed t-test, t (10) = 2.3, p = 0.046; Fig. 5A), and spent significantly less time seizing over the 3 days (Student’s two-tailed t-test, t (10) = 2.4, p = 0.035; Fig. 5B) following the hypoxic-ischemic injury.
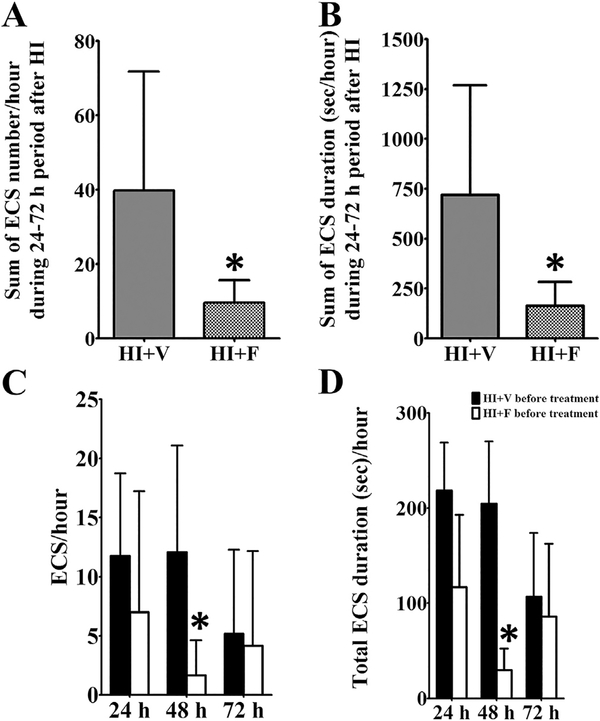
Fig. 5. Repeated flupirtine administration reduces overall seizure burden and modifies disease progression during the acute phase of hypoxic-ischemic injury.
Flupirtine treatment initiated during the immediate reperfusion period and then continued for 3 more days (a daily dose of 25 mg/kg flupirtine), significantly reduced (A) the total number and (B) duration of electroclinical seizures over a 72 h period following hypoxia-ischemia (HI)-induction. Further, multiple flupirtine doses altered the evolution of disease and reduced (C) number and (D) duration of electroclinical seizures during the acute phase of hypoxic-ischemic injury. Data shown are mean ± SD. *p < 0.05, Student’s two-tailed t-test. ECS = electroclinical seizure, V = vehicle, F = flupirtine.
To examine the effect of flupirtine treatment on the natural progression of seizures over three days following HI-induction, i.e., to determine whether flupirtine treatment has a disease modifying effect, we compared the seizure data of recording acquired before each treatment dose at 24 (second dose), 48 (third dose), and 72 h (fourth dose) after the initial dose (first dose) given 5 min after HI during the immediate reperfusion period. The analysis revealed that at 24 h after HI and the first dose of flupirtine, a trend towards lower number and duration of electroclinical seizures was observed in flupirtine treated rats as compared to vehicle treated rats (Fig. 5 C, D). This suggests that a single dose of flupirtine is not sufficient to significantly alter disease progression. However, at 48 h, after two doses of flupirtine, a significantly lower number and duration of seizures was observed in pre-treatment recordings of flupirtine treated rats than the vehicle treated rats (Student’s two-tailed t-test, t (10) = 2.4, p = 0.03; Fig. 5 C, D). These results suggest that two doses of flupirtine modify the injury to reduce occurrence of acute seizures. At 72 h time period, no significant difference in seizure number and duration was observed between flupirtine and vehicle treated rats (Fig. 5C and D). This could be because, by 72 h a spontaneous reduction in the number of rats with seizure (50% decrease from 24 h), and frequency and duration of seizures occurred in vehicle treated HI rats.
4. Discussion
The current study shows that flupirtine is very effective against hypoxia-ischemia-induced neonatal seizures. Furthermore, it is more effective than phenobarbital at a clinically relevant dose. We designed the current study to closely replicate clinical conditions for easy translation of the test drug to clinical use. Since neonatal seizures are most commonly (~60%) associated with hypoxic-ischemic injury in human newborns (Ronen et al., 2007; Tekgul et al., 2006), we used an animal model that closely replicates the etiology, neonatal seizure characteristics, and other pathological features associated with hypoxic-ischemic injury in human babies (Sampath et al., 2014). We used time synchronized video and EEG records to accurately determine behavioral seizures and to identify purely electrographic, subclinical seizures. Further, the treatment with flupirtine was initiated at a clinically relevant time-point, and its ability to treat neonatal seizures was compared with the current first-line drug phenobarbital.
In clinical settings, treatment with an anticonvulsant is often initiated after a seizure is observed. In neonates with HIE, the injury most commonly occurs before labor, or at the time of delivery, and seizures are first observed during the reperfusion period. Flupirtine administered either 5 min after the first electroclinical seizure, which occurred during hypoxia exposure, or following completion of the hypoxic period, was very effective in preventing subsequent seizures, reducing the overall seizure burden in the first few hours of the reperfusion period, and appeared to be effective against purely electrographic seizures. Similar to what has been observed in human neonatal studies, phenobarbital was modestly effective in stopping electroclinical seizures and ineffective against purely electrographic or subclinical seizures. For the dose used, it was less effective than flupirtine. However, the results regarding the effect of test drugs on purely electrographic seizures should be interpreted carefully as the frequency and duration of electrographic seizures in our model is very low (Sampath et al., 2014). Beyond the first few hours following the treatment, a single dose of flupirtine was unable to sustain the prevention of seizures at later times, necessitating repeated dosing to significantly reduce the overall seizure burden. The half-life of flupirtine in adult rats ranges from 2.5 to 3.5 h (Schuster et al., 1998), which may explain recurrence of seizures following a single dose of flupirtine. However, interestingly, a significant reduction in electroclinical seizures was observed at 48 h after HI and two doses of flupirtine, and before the third dose was administered. This suggests that flupirtine treatment has a disease modifying effect that alters the evolution of seizures during the acute phase of hypoxic-ischemic injury.
The strong efficacy of flupirtine against HI-induced neonatal seizures observed in the current study is consistent with its equally strong ability to prevent and stop chemoconvulsant and global hypoxia-induced neonatal seizures (Raol et al., 2009; Sampath et al., 2015). In a flurothyl model of neonatal seizures, flupirtine blocked the induction of seizures, whereas, phenobarbital and diazepam delayed the occurrence of seizures. In the kainic acid model of neonatal seizures, flupirtine given 15 min after continuous behavioral and electrographic seizures immediately and completely stopped the seizures. Although phenobarbital and diazepam stopped the seizures, unlike flupirtine, the effect was not complete and prolonged. Phenobarbital treated rats were observed to develop breakthrough seizures within a few minutes of drug injection, and in the rats treated with diazepam, the seizures reappeared ~20 min after the treatment. Two recent studies made similar observations as to that of the current study regarding the efficacy of phenobarbital to treat hypoxia/ischemiainduced neonatal seizures (Cleary et al., 2013; Morin et al., 2016). Cleary and colleagues observed that treatment with 15 mg/kg phenobarbital prevented neonatal seizures in 23% rats during hypoxia and reduced seizure incidence by 50%, but it did not completely attenuate seizure activity. In a different model of ischemia than ours, Morin and colleagues observed that 20 mg/kg phenobarbital administered prior to ischemia-induction prevented seizures in 41% rats and delayed the onset of seizure, but it did not change the total duration of seizures during the reperfusion period. In all the various studies that have been performed, flupirtine have been observed to be very effective against neonatal seizures, and much more efficacious than the current first-line anticonvulsant drugs.
Flupirtine was approved in 1984 in Europe for the treatment of pain and is marketed for use in many European countries, China, Brazil, and India (Douros et al., 2014; Harish et al., 2012; Szelenyi, 2013). However, it is not approved for any indications in the USA. Flupirtine has been shown to affect the function of KCNQ potassium channels (Klinger et al., 2012; Martire et al., 2004; Wladyka and Kunze, 2006), the G protein-coupled inwardly rectifying potassium (GIRK) channels (Jakob and Krieglstein,1997; Kornhuber et al., 1999; Montandon et al., 2016; Sattler et al., 2008) (but see (Klinger et al., 2012)), and GABAA receptors (Klinger et al., 2012, 2015). KCNQ channels are voltage gated, depolarization activated potassium channels. Flupirtine reduces membrane depolarization required for activation, increases opening rates, and slows closing rates of KCNQ channels. Flupirtine has also been found to potentiate hippocampal GABA responses of the delta subunit containing GABAA receptor, but not of the gamma subunit containing GABAA receptors (Klinger et al., 2015). The exact mechanism through which flupirtine meditates its anti-neonatal-seizure activity is not known, but it is less likely to be via potentiation of GABAA receptor activity for following reasons. Unlike KCNQ channel activity which is inhibitory throughout the development, GABA is depolarizing during early development (Ben-Ari, 2002; Ganguly et al., 2001). Further, compared to adult brain, the immature brain has fewer GABAA receptors (Brooks-Kayal and Pritchett, 1993; Brooks-Kayal et al., 2001; Swann et al., 1989), lower GABA-mediated currents (Brooks-Kayal and Pritchett, 1993), and different GABAA receptor subunit composition (Didelon et al., 2000; Fritschy et al., 1994; Laurie et al., 1992). The hippocampal neurons of neonatal brain express very low levels of the delta subunit, the subunit through which flupirtine potentiates GABAA receptor activity. The underdeveloped GABAergic system in the neonatal brain may also explain phenobarbital’s decreased efficacy against neonatal seizures in our and other’s studies in animal models, and the human patient population. Further, in contrast to the potentiating effect of flupirtine on GABAA receptor under normal conditions, flupirtine has been shown to inhibit depolarization-induced release of GABA and glutamate from presynaptic terminals via KCNQ2 activation (Martire et al., 2004), prevent 4-AP-induced increase in GABA and glutamate levels (Kapetanovic et al., 1995), and decrease new GABA and glutamate synthesis (Kapetanovic et al., 1995). However, further studies using specific activators and antagonists of KCNQ channels will be required to unravel the involvement of KCNQ channels in antineonatal-seizure activity.
The common side-effects associated with flupirtine are dizziness, dry mouth, nausea, pruritis, fatigue, and heartburn (Klawe and Maschke, 2009; Schuster et al., 1998). In recent years, however, concerns have been raised regarding the association of long-term use of flupirtine and hepatotoxicity (Douros et al., 2014; Michel et al., 2012). Flupirtine has been used in children (Cialone et al., 2011; Harish et al., 2012; Schuster et al., 1998), and it is available in injection dosage formulation for intravenous administration (Siegmund et al., 2015), an important necessity for neonatal patients, specifically, ones with seizures and hypoxic-ischemic encephalopathy. In neonates, the gastrointestinal functions are not fully developed and the hypoxic-ischemic injury further reduces its functionality. As a result, the bioavailability of the drug given orally is compromised; hence, the intravenous route is the most commonly used route to deliver drugs in neonatal patients. It is not known whether flupirtine treatment during development increases apoptosis as has been observed with retigabine, an analogue of flupirtine, and many currently available anticonvulsants (Bittigau et al., 2002; Brown et al., 2016). A single dose of phenobarbital, diazepam, and phenytoin, the current first-line drugs for the treatment of neonatal seizures, given to neonatal rats cause apoptotic neurodegeneration (Bittigau et al., 2002). In the case of retigabine, multiple doses, but not a single dose, causes apoptosis in the neonatal brain (Brown et al., 2016). Phenobarbital treatment during development is also associated with reduced IQ in human patients (Farwell et al., 1990) and learning impairment in rats (Frankel et al., 2016). Repeated administration of retigabine during early development increases anxiety-like behavior, but does not impair learning and memory in adult rats (Frankel et al., 2016). In our laboratory, we observed that repeated treatment of sham control neonatal rats (treated exactly like HI rats, except for ligation of the artery and exposure to hypoxia) with flupirtine (25 mg/kg, once a day for four days) did not cause learning and memory deficits in later life (data not shown, unpublished observations). However, it is important to note that all these studies were conducted in normal rats, and a drug may have a completely different effect under different brain conditions and neuronal milieu (e.g., under normal conditions vs. in the presence of an injury or abnormal background). As discussed earlier in the paper, flupirtine potentiates GABA activity under normal conditions (Klinger et al., 2015), but reduces GABA activity under hyperexcitable conditions (Kapetanovic et al., 1995; Martire et al., 2004). A single dose of topiramate administered to normal P7 rat causes apoptosis (Kim et al., 2007), but provides protection against neuronal death when given to P7 rats with hypoxic-ischemic injury (Noh et al., 2006). Repeated doses of flupirtine (25 mg/kg) administered to P10 rats, unlike phenobarbital (25 mg/kg), provide protection against repetitive febrile seizures-induced cell death and cognitive impairment in later life (Yu et al., 2011). Flupirtine has also been shown to exhibit neuroprotective activity in an animal model of infantile neuronal ceroid lipofuscinosis (Dhar et al., 2002), and to provide protection against glutamate-induced toxicity in 8 days-old hippocampal cultures prepared from 1 day-old rats (Rupalla et al., 1995).
One of the deficits in the current study is that we tested the efficacy of flupirtine and phenobarbital in the treatment of neonatal seizures in only hypoxic-ischemic male rats. It is now well known that significant differences exist in development of GABA mediated inhibition and susceptibility to neonatal hypoxic-ischemic injury between males and females, and as a result, response to both the drugs may differ between males and females. As discussed above, the immature brain has a depolarizing GABAergic system due to developmentally regulated expression of transporters that maintain the chloride gradient in the neuron (Ben-Ari, 2002; Ganguly et al., 2001). In rat CA1 pyramidal neurons, the hyperpolarizing reversal potentials of GABAergic postsynaptic current appear earlier in females (before P4) than in the males (13.7) (Galanopoulou, 2008). Similarly, in the substantia nigra pars reticulate, the switch of GABAA receptor-induced synaptic responses to hyperpolarization occurs much earlier in female (at P10) than in male (at P17) rats (Kyrozis et al., 2006). Treatment of neonatal male and female rats with muscimol, an agonist for the GABAA receptors, led to a significant reduction in the volume of the male hippocampus but not female hippocampus later in life (Nunez and McCarthy, 2007). Substantial damage to hippocampus and substantia nigra has been observed following neonatal hypoxia-ischemia in humans and animal models (Chao et al., 2006; Wideroe et al., 2012). Further, males have a higher incidence of neonatal HI and increased severity of effects following the injury (Hill and Fitch, 2012). It is likely that immaturity of the GABAergic inhibitory system contributes to this heightened susceptibility of neonatal males to hypoxic-ischemic injury. The expression of KCNQ channels begins before birth and rapidly increases over the early developmental period (Chege et al., 2012; Kanaumi et al., 2008; Safiulina et al., 2008; Tinel et al., 1998). However, to the best of our knowledge, the developmental pattern of KCNQ channel expression in male and female have never been compared.
Overall, we believe that the results of the present study combined with earlier observations regarding its efficacy and superiority over phenobarbital in the treatment of neonatal seizures strongly suggest that flupirtine is likely to be a successful antineonatal-seizure medication in clinics as well. Its long history of human use and its availability in injectable dosage form will help expedite its translation to clinical use.
Acknowledgement
We thank the University of Colorado Anschutz Medical Campus Rodent In Vivo Neurophysiology Core for providing facilities to acquire and review video-EEG data.
Funding
This work was supported by the NIH/NICHD HD065534 grant and Citizens United for Research in Epilepsy (AWD-111740) Prevention of Acquired Epilepsy award to Yogendra Raol. The funders had no role in study design, data collection and analysis, in the writing of the manuscript, and in the decision to submit the article for publication.
Abbreviations
- GABA
γ-aminobutyric acid
- h
hour
- HI
hypoxia-ischemia
- HIE
hypoxic-ischemic encephalopathy
- min
minutes
- P7
postnatal day 7
- sec
second
Footnotes
Disclosure of conflicts of interest
None of the authors have any conflict of interest to disclose.
References
- Ahn MO, Korst LM, Phelan JP, Martin GI, 1998. Does the onset of neonatal seizures correlate with the timing of fetal neurologic injury? Clin. Pediatr. (Phila) 37, 673–676. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, 2002. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci 3, 728–739. [DOI] [PubMed] [Google Scholar]
- Bergamasco B, Benna P, Ferrero P, Gavinelli R, 1984. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia 25, 131–136. [DOI] [PubMed] [Google Scholar]
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C, 2002. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc. Natl. Acad. Sci. U. S. A 99, 15089–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume HK, Garrison MM, Christakis DA, 2009. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J. Child. Neurol 24, 148–154. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Pritchett DB, 1993. Developmental changes in human gamma-aminobutyric acidA receptor subunit composition. Ann. Neurol. 34, 687–693. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA, 2001. gamma-Aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J. Neurochem. 77, 1266–1278. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM, 2009. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 156, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Gutherz S, Kulick C, Soper C, Kondratyev A, Forcelli PA, 2016. Profile of retigabine-induced neuronal apoptosis in the developing rat brain. Epilepsia 57, 660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo KB, Barr P, 2005. Drug treatment of neonatal seizures by neonatologists and paediatric neurologists. J. Paediatr. Child. Health 41, 313–316. [DOI] [PubMed] [Google Scholar]
- Chao CP, Zaleski CG, Patton AC, 2006. Neonatal hypoxic-ischemic encephalopathy: multimodality imaging findings. Radiographics 26 (Suppl. 1), S159–S172. [DOI] [PubMed] [Google Scholar]
- Chege SW, Hortopan GA, M TD, Baraban SC, 2012. Expression and function of KCNQ channels in larval zebrafish. Dev. Neurobiol 72, 186–198. [DOI] [PubMed] [Google Scholar]
- Cialone J, Augustine EF, Newhouse N, Adams H, Vierhile A, Marshall FJ, de Blieck EA, Kwon J, Rothberg PG, Mink JW, 2011. Parent-reported benefits of flupirtine in juvenile neuronal ceroid lipofuscinosis (Batten disease; CLN3) are not supported by quantitative data. J. Inherit. Metab. Dis 34, 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary RT, Sun H, Huynh T, Manning SM, Li Y, Rotenberg A, Talos DM, Kahle KT, Jackson M, Rakhade SN, Berry G, Jensen FE, 2013. Bumetanide enhances phenobarbital efficacy in a rat model of hypoxic neonatal seizures. PLoS One 8, e57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS, 2004. KCNQ2 is a nodal K+ channel. J. Neurosci 24, 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S, Bitting RL, Rylova SN, Jansen PJ, Lockhart E, Koeberl DD, Amalfitano A, Boustany RM, 2002. Flupirtine blocks apoptosis in batten patient lymphoblasts and in human postmitotic CLN3- and CLN2-deficient neurons. Ann. Neurol 51, 448–466. [DOI] [PubMed] [Google Scholar]
- Didelon F, Mladinic M, Cherubini E, Bradbury A, 2000. Early expression of GABA(A) receptor delta subunit in the neonatal rat hippocampus. J. Neurosci. Res 62, 638–643. [DOI] [PubMed] [Google Scholar]
- Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Orzechowski HD, Kreutz R, Garbe E, 2014. Flupirtine-induced liver injury - seven cases from the Berlin case-control surveillance study and review of the German spontaneous adverse drug reaction reporting database. Eur. J. Clin. Pharmacol 70, 453–459. [DOI] [PubMed] [Google Scholar]
- Dzhala V, Ben-Ari Y, Khazipov R, 2000. Seizures accelerate anoxia-induced neuronal death in the neonatal rat hippocampus. Ann. Neurol 48, 632–640. [PubMed] [Google Scholar]
- Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB, 1990. Phenobarbital for febrile seizures - effects on intelligence and on seizure recurrence. N. Engl. J. Med 322, 364–369. [DOI] [PubMed] [Google Scholar]
- Filan P, Boylan GB, Chorley G, Davies A, Fox GF, Pressler R, Rennie JM, 2005. The relationship between the onset of electrographic seizure activity after birth and the time of cerebral injury in utero. BJOG 112, 504–507. [DOI] [PubMed] [Google Scholar]
- Forcelli PA, Janssen MJ, Vicini S, Gale K, 2012. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann. Neurol 72, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster M, Lewis A, 2007. The treatment of neonatal seizures: a critical review of the evidence. Neonatal Paediatr. Child. Health Nurs. 10, 11–19. [Google Scholar]
- Frankel S, Medvedeva N, Gutherz S, Kulick C, Kondratyev A, Forcelli PA, 2016. Comparison of the long-term behavioral effects of neonatal exposure to retigabine or phenobarbital in rats. Epilepsy Behav. 57, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J-M, Paysan J, Enna A, Mohler H, 1994. Switch in the expresson of rat GABAA- receptor subtypes during postnatal development: an immunohistochemical study. J. Neurosci. 14, 5302–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS, 2008. Sexually dimorphic expression of KCC2 and GABA function. Epilepsy Res. 80, 99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M, 2001. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105, 521–532. [DOI] [PubMed] [Google Scholar]
- Gibbs JW 3rd, Schroder GB, Coulter DA, 1996. GABAA receptor function in developing rat thalamic reticular neurons: whole cell recordings of GABA-mediated currents and modulation by clonazepam. J. Neurophysiol 76, 2568–2579. [DOI] [PubMed] [Google Scholar]
- Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP, 2009. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J. Pediatr 155, 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish S, Bhuvana K, Bengalorkar GM, Kumar T, 2012. Flupirtine: clinical pharmacology. J. Anaesthesiol. Clin. Pharmacol 28, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT, 1993. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 34, 453–468. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fitch RH, 2012. Sex differences in mechanisms and outcome of neonatal hypoxia- ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurol. Res. Int 2012, 867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob R, Krieglstein J, 1997. Influence of flupirtine on a G-protein coupled inwardly rectifying potassium current in hippocampal neurones. Br. J. Pharmacol 122, 1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaumi T, Takashima S, Iwasaki H, Itoh M, Mitsudome A, Hirose S, 2008. Developmental changes in KCNQ2 and KCNQ3 expression in human brain: possible contribution to the age-dependent etiology of benign familial neonatal convulsions. Brain Dev. 30, 362–369. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Yonekawa WD, Kupferberg HJ, 1995. The effects of D-23129, a new experimental anticonvulsant drug, on neurotransmitter amino acids in the rat hippocampus in vitro. Epilepsy Res. 22, 167–173. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL, 1999. Postnatal development of hippocampal dentate granule cell gamma-aminobutyric acidA receptor pharmacological properties. Mol. Pharmacol. 55, 444–452. [PubMed] [Google Scholar]
- Kato M, Yamagata T, Kubota M, Arai H, Yamashita S, Nakagawa T, Fujii T, Sugai K, Imai K, Uster T, Chitayat D, Weiss S, Kashii H, Kusano R, Matsumoto A, Nakamura K, Oyazato Y, Maeno M, Nishiyama K, Kodera H, Nakashima M, Tsurusaki Y, Miyake N, Saito K, Hayasaka K, Matsumoto N, Saitsu H, 2013. Clinical spectrum of early onset epileptic encephalopathies caused by KCNQ2 mutation. Epilepsia 54, 1282–1287. [DOI] [PubMed] [Google Scholar]
- Kaushal S, Tamer Z, Opoku F, Forcelli PA, 2016. Anticonvulsant drug-induced cell death in the developing white matter of the rodent brain. Epilepsia 57, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kondratyev A, Gale K, 2007. Antiepileptic drug-induced neuronal cell death in the immature brain: effects of carbamazepine, topiramate, and levetiracetam as monotherapy versus polytherapy. J. Pharmacol. Exp. Ther. 323, 165–173. [DOI] [PubMed] [Google Scholar]
- Klawe C, Maschke M, 2009. Flupirtine: pharmacology and clinical applications of a nonopioid analgesic and potentially neuroprotective compound. Expert Opin. Pharmacother 10, 1495–1500. [DOI] [PubMed] [Google Scholar]
- Klinger F, Bajric M, Salzer I, Dorostkar MM, Khan D, Pollak DD, Kubista H, Boehm S, Koenig X, 2015. Delta subunit-containing GABA-A receptors are preferred targets for the centrally acting analgesic flupirtine. Br. J. Pharmacol 172, 4946–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger F, Geier P, Dorostkar MM, Chandaka GK, Yousuf A, Salzer I, Kubista H, Boehm S, 2012. Concomitant facilitation of GABAA receptors and KV7 channels by the non-opioid analgesic flupirtine. Br. J. Pharmacol 166, 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber J, Bleich S, Wiltfang J, Maler M, Parsons CG, 1999. Flupirtine shows functional NMDA receptor antagonism by enhancing Mg2+ block via activation of voltage independent potassium channels. Rapid Commun. J. Neural Transm 106, 857–867. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Chudomel O, Moshe SL, Galanopoulou AS, 2006. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci. Lett. 398, 1–5. [DOI] [PubMed] [Google Scholar]
- Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ, 1995. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 45, 724–732. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH, 1992. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci 12, 4151–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch NE, Stevenson NJ, Livingstone V, Mathieson S, Murphy BP, Rennie JM, Boylan GB, 2015. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure 33, 60–65. [DOI] [PubMed] [Google Scholar]
- Martire M, Castaldo P, D’Amico M, Preziosi P, Annunziato L, Taglialatela M, 2004. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J. Neurosci 24, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride MC, Laroia N, Guillet R, 2000. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 55, 506–513. [DOI] [PubMed] [Google Scholar]
- Michel MC, Radziszewski P, Falconer C, Marschall-Kehrel D, Blot K, 2012. Unexpected frequent hepatotoxicity of a prescription drug, flupirtine, marketed for about 30 years. Br. J. Clin. Pharmacol 73, 821–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, Weiss J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ, 2002. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology 58, 542–548. [DOI] [PubMed] [Google Scholar]
- Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL, 2016. G-protein-gated inwardly rectifying potassium channels modulate respiratory depression by opioids. Anesthesiology 124, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L, Enderlin J, Leger PL, Perrotte G, Bonnin P, Dupuis N, Baud O, Charriaut- Marlangue C, Auvin S, 2016. Different response to antiepileptic drugs according to the type of epileptic events in a neonatal ischemiareperfusion model. Neurobiol. Dis. 99, 145–153. [DOI] [PubMed] [Google Scholar]
- Noh MR, Kim SK, Sun W, Park SK, Choi HC, Lim JH, Kim IH, Kim HJ, Kim H, Eun BL, 2006. Neuroprotective effect of topiramate on hypoxic ischemic brain injury in neonatal rats. Exp. Neurol 201, 470–478. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM, 2007. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev. Neurobiol 67, 1879–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Zhu G, Hirose S, Ito KI, Murakami T, Wakui M, Kaneko S, 2003. Age- dependent modulation of hippocampal excitability by KCNQ-channels. Epilepsy Res. 53, 81–94. [DOI] [PubMed] [Google Scholar]
- Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, Paneth N, Minnigh B, Alvin J, 1999. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N. Engl. J. Med 341, 485–489. [DOI] [PubMed] [Google Scholar]
- Pena F, Alavez-Perez N, 2006. Epileptiform activity induced by pharmacologic reduction of M-current in the developing hippocampus in vitro. Epilepsia 47, 47–54. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D, 2005. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci 8, 51–60. [DOI] [PubMed] [Google Scholar]
- Qiao M, Latta P, Foniok T, Buist R, Meng S, Tomanek B, Tuor UI, 2004. Cerebral blood flow response to a hypoxic-ischemic insult differs in neonatal and juvenile rats. MAGMA 17, 117–124. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lapides DA, Keating JG, Brooks-Kayal AR, Cooper EC, 2009. A KCNQ channel opener for experimental neonatal seizures and status epilepticus. Ann. Neurol 65, 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR, 2005. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience 132, 399–407. [DOI] [PubMed] [Google Scholar]
- Rice JE 3rd, Vannucci RC, Brierley JB, 1981. The influence of immaturity on hypoxic- ischemic brain damage in the rat. Ann. Neurol 9, 131–141. [DOI] [PubMed] [Google Scholar]
- Robertson CM, Finer NN, 1988. Educational readiness of survivors of neonatal encephalopathy associated with birth asphyxia at term. J. Dev. Behav. Pediatr 9, 298–306. [PubMed] [Google Scholar]
- Ronen GM, Buckley D, Penney S, Streiner DL, 2007. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 69, 1816–1822. [DOI] [PubMed] [Google Scholar]
- Rupalla K, Cao W, Krieglstein J, 1995. Flupirtine protects neurons against excitotoxic or ischemic damage and inhibits the increase in cytosolic Ca2+ concentration. Eur. J. Pharmacol 294, 469–473. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Zacchi P, Taglialatela M, Yaari Y, Cherubini E, 2008. Low expression of Kv7/M channels facilitates intrinsic and network bursting in the developing rat hippocampus. J. Physiol 586, 5437–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Koide A, Goto T, Fujita T, Nishiyama K, Tsurusaki Y, Doi H, Miyake N, Hayasaka K, Matsumoto N, 2012. Whole exome sequencing identifies KCNQ2 mutations in Ohtahara syndrome. Ann. Neurol 72, 298–300. [DOI] [PubMed] [Google Scholar]
- Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM, 1999. Incidence of neonatal seizures in Harris county, Texas, 1992–1994. Am. J. Epidemiol 150, 763–769. [DOI] [PubMed] [Google Scholar]
- Sampath D, Shmueli D, White AM, Raol YH, 2015. Flupirtine effectively prevents development of acute neonatal seizures in an animal model of global hypoxia. Neurosci. Lett 607, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath D, White AM, Raol YH, 2014. Characterization of neonatal seizures in an animal model of hypoxic-ischemic encephalopathy. Epilepsia 55, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar R, Painter MJ, 2005. Neonatal seizures: after all these years we still love what doesn’t work. Neurology 64, 776–777. [DOI] [PubMed] [Google Scholar]
- Sattler MB, Williams SK, Neusch C, Otto M, Pehlke JR, Bahr M, Diem R, 2008. Flupirtine as neuroprotective add-on therapy in autoimmune optic neuritis. Am. J. Pathol 173, 1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G, Schwarz M, Block F, Pergande G, Schmidt W, 1998. Flupirtine: a review of its neuroprotective and behavioral properties. CNS drug Rev. 4, 149–164. [Google Scholar]
- Siegmund W, Modess C, Scheuch E, Methling K, Keiser M, Nassif A, Rosskopf D, Bednarski PJ, Borlak J, Terhaag B, 2015. Metabolic activation and analgesic effect of flupirtine in healthy subjects, influence of the polymorphic NAT2, UGT1A1 and GSTP1. Br. J. Clin. Pharmacol 79, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Westenskow P, Charlier C, Pappas C, Leslie J, Dillon J, Anderson VE, Sanguinetti MC, Leppert MF, 2003. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: expansion of the functional and mutation spectrum. Brain 126, 2726–2737. [DOI] [PubMed] [Google Scholar]
- Swann JW, Brady RJ, Martin DL, 1989. Postnatal development of GABA-mediated synaptic inhibition in rat hippocampus. Neuroscience 28, 551–561. [DOI] [PubMed] [Google Scholar]
- Szelenyi I, 2013. Flupirtine, a re-discovered drug, revisited. Inflamm. Res. 62, 251–258. [DOI] [PubMed] [Google Scholar]
- Tekgul H, Gauvreau K, Soul J, Murphy L, Robertson R, Stewart J, Volpe J, Bourgeois B, du Plessis AJ, 2006. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics 117, 1270–1280. [DOI] [PubMed] [Google Scholar]
- Tinel N, Lauritzen I, Chouabe C, Lazdunski M, Borsotto M, 1998. The KCNQ2 potassium channel: splice variants, functional and developmental expression. Brain localization and comparison with KCNQ3. FEBS Lett. 438, 171–176. [DOI] [PubMed] [Google Scholar]
- van Rooij LG, Toet MC, van Huffelen AC, Groenendaal F, Laan W, Zecic A, de Haan T, van Straaten IL, Vrancken S, van Wezel G, van der Sluijs J, Ter Horst H, Gavilanes D, Laroche S, Naulaers G, de Vries LS, 2010. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics 125, e358–366. [DOI] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, de Jonghe P, 2012. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann. Neurol. 71, 15–25. [DOI] [PubMed] [Google Scholar]
- Wideroe M, Havnes MB, Morken TS, Skranes J, Goa PE, Brubakk AM, 2012. Doxycycline treatment in a neonatal rat model of hypoxia-ischemia reduces cerebral tissue and white matter injury: a longitudinal magnetic resonance imaging study. Eur. J. Neurosci 36, 2006–2016. [DOI] [PubMed] [Google Scholar]
- Wirrell EC, Armstrong EA, Osman LD, Yager JY, 2001. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr. Res 50, 445–454. [DOI] [PubMed] [Google Scholar]
- Wladyka CL, Kunze DL, 2006. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J. Physiol 575, 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Liu Y, Wang Y, Yin J, Wang H, Liu W, Peng B, He X, 2011. Protective effect of the KCNQ activator flupirtine on a model of repetitive febrile seizures. Epilepsy Res 97, 64–72. [DOI] [PubMed] [Google Scholar]