Abstract
Background
Colorectal cancer remains the second leading cause of death in the United States despite improvements in incidence rates and advancements in screening. The present study evaluated the prognostic value of two tumor markers, MET and ROCK I, which have been noted in other cancers to provide more accurate prognoses of patient outcomes than tumor staging alone.
Materials and Methods
We constructed a tissue microarray from surgical specimens of adenocarcinomas from 108 colorectal cancer patients. Using immunohistochemistry, we examined the expression levels of tumor markers MET and ROCK I, with a pathologist blinded to patient identities and clinical outcomes providing the scoring of MET and ROCK I expression. We then used retrospective analysis of patients’ survival data to provide correlations with expression levels of MET and ROCK I.
Results
Both MET and ROCK I were significantly over-expressed in colorectal cancer tissues, relative to the unaffected adjacent mucosa. Kaplan-Meier survival analysis revealed that patients’ 5-year survival was inversely correlated with levels of expression of ROCK I. In contrast, MET was less strongly correlated with five-year survival.
Conclusion
ROCK I provides better efficacy in predicting patient outcomes, compared to either tumor staging or MET expression. As a result, ROCK I may provide a less invasive method of assessing patient prognoses and directing therapeutic interventions.
Keywords: Colorectal cancer, ROCK I, MET, immunohistochemistry, tissue microarray, five-year survival
The third most common cancer in both men and women, colorectal cancer, is also the second leading cause of cancer-related deaths in the United States (1). In addition, while the rates of colorectal cancer have declined modestly over the past twenty years, the rate of colorectal cancer-related deaths has remained virtually leveled over the same period (2). The best predictor of survival in colorectal cancer lies in early diagnosis, with 89.8% of patients diagnosed with Stage I colorectal cancer surviving for five years, compared to an overall 5-year survival rate of 64.7% (2). While the identification of tumor stage is most closely tied to better patient survival, the tumor stage alone provides only a crude measure of patient prognosis. A third of patients with tumor stages I or II -who should theoretically have a good prognosis- die within five years from local relapse or distal metastases (3). In contrast, some patients with voluminous masses and tumor stage III have better prognoses than some patients with tumor stage I colorectal cancer (4, 5). Fortunately, tumor markers provide more accurate indications of prognostic outcomes, including MET and ROCK I (6).
In particular, MET has proven to be a valuable indicator of prognostic outcomes. MET is valuable in wound healing in the skin (7), as well as for liver regeneration (8). However, over-activation of the MET receptor can induce cells to survive in the bloodstream without anchorage, extravasate and colonize new territories giving rise to intensive cell proliferation and, as a result, a metastatic lesion. Consequently, MET over-expression is associated with advanced tumor stages and poor patient survival, especially in breast, head and neck cancers (10, 11). Nevertheless, researchers have also identified MET as lacking a strong correlation between tumor grade and staging (12). In addition, studies have failed to establish a correlation between MET over-expression and disease recurrence (13). Perhaps most significantly, studies have also demonstrated MET as a relatively poor prognostic indicator of five-year survival outcomes in colorectal cancer (3, 11).
Another tumor marker, ROCK I, has shown promise in strong correlations between over-expression of ROCK I and poor patient survival outcomes in breast cancer (14). ROCK I, a protein kinase, belongs to the family of Rho GTPases that regulate gene transcription and adhesion (15). A downstream effector of Rho GTPase, ROCK I, is also a key regulator of the actin cytoskeleton, which plays a substantial role in the behavior of cancer cells (16). Over-expression of ROCK I may contribute to tumor angiogenesis (17), as well as to progression of tumors in bladder (18) and ovarian cancer (19). In addition, ROCK I has an association with tumor progression in colorectal cancer (20). Based on its over-expression in other cancers, we aimed to compare ROCK I and MET in the accuracy under which each tumor marker predicted patient outcomes. We hypothesized that ROCK I would prove at least as valuable a prognostic indicator of patient survival in colorectal cancer as the better-known tumor marker, MET.
Materials and Methods
Colorectal cancer tissue microarray preparation and clinical data collection
The colorectal cancer tissue microarray (TMA) was prepared using tissues collected from surgical specimens of 108 colorectal cancer patients in the Department of Pathology, University of Illinois at Chicago (UIC). Slides were arrayed with triplicate colonic tissue sections and included cores from normal tissues and adenocarcinoma. Four to eight cores were arrayed per patient. Patient survival data were obtained through the review of electronic records of patients listed on the UIC cancer registry. The Institutional Review Board (IRB) at UIC provided approval for this study.
Immunohistochemistry
The tissue microarray slides were hydrated through various xylene and alcohol gradients of 100%, 95%, 95% and 80%, then incubated with 3% hydrogen peroxide for 20 min. Antigen retrieval used a 10X concentrated retrieval solution (Cat#S1699; Dako, Carpinteria, CA, USA), then followed by incubation with primary antibody: anti-ROCK I antibody (1:50) or anti-MET antibody (1:100) for 60 minutes and treated with Biotinylated Link (Cat#K0679; Dako, Carpinteria, CA, USA) for 10 min, Streptavidin-HRP (Cat# K0679; Dako, Carpinteria, CA, USA) for 10 minutes, with all treatments at room temperature. Next, slides were incubated with 3,3’-diaminobenzidine (DAB) substrate (CAT#SK-4100; Dako, Carpinteria, CA, USA) and counterstained with hematoxylin, followed by dehydration through an alcohol gradient (95%, 100%, 100% and 50%) and xylene and mounting with Permount.
Antibodies
Anti-ROCK I antibody (C-19; catalog number: SC-6055) and anti-MET antibody (C-12; catalog number: SC-10) were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA).
Histopathological examination of tissue microarray slides
TMA slides were evaluated under a light microscope by a pathologist from the Department of Pathology at UIC, blinded to any corresponding patient information or clinical outcomes. The staining intensity was scored from 0 to 3. The score for normal and tumor tissues of each tissue sample was used for the assignment of protein expression level groups: low (0–1), medium (1–2) and high (2–3). These data were further used for correlative analysis.
Statistical analysis
Data were analyzed using the GraphPad Prism 5 software from GraphPad Software, Inc. (La Jolla, CA, USA). Kaplan-Meier survival analysis (log-rank test) evaluated the correlation of ROCK I and MET expression with patient survival. In addition, the Student’s t-test compared mean expression levels of ROCK I and MET.
Results
ROCK I and MET are over-expressed in colorectal cancer tissues
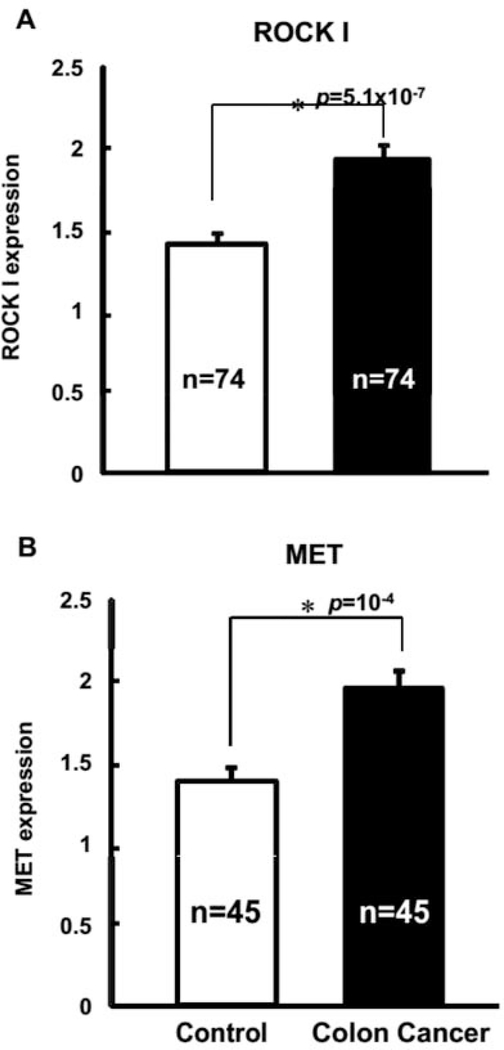
Out of the 108 patients analyzed, 107 patients had positive tumor markers for ROCK I (97.3%), with 92 patients having positive tumor markers for MET (83.6%). After histopathological examination of the TMA slides and scoring of the immunostaining intensity of ROCK I and MET proteins, we plotted data to evaluate differences in relative expression of ROCK I and MET between control and cancer tissue samples. Significantly, the data confirmed that both ROCK I and MET were over-expressed in colorectal cancer, relative to adjacent, control mucosa (Figure 1). Under this setting, MET had slightly more elevated expression values at 41% over control tissues, with ROCK I having only slightly less elevated expression at 37% in cancer tissues, compared to controls.
Figure 1.
ROCK I and MET proteins are over-expressed in colorectal cancer. Following immunostaining intensity scoring of the tissue microarray slides, the scoring data obtained from four to eight available cores per patient for ROCK I (Panel A) and MET (Panel B) expression, were analyzed. Both proteins were significantly over-expressed in colorectal cancer tissues compared to the normal, adjacent control tissues.
Expression levels of ROCK I provide associations with cancer staging
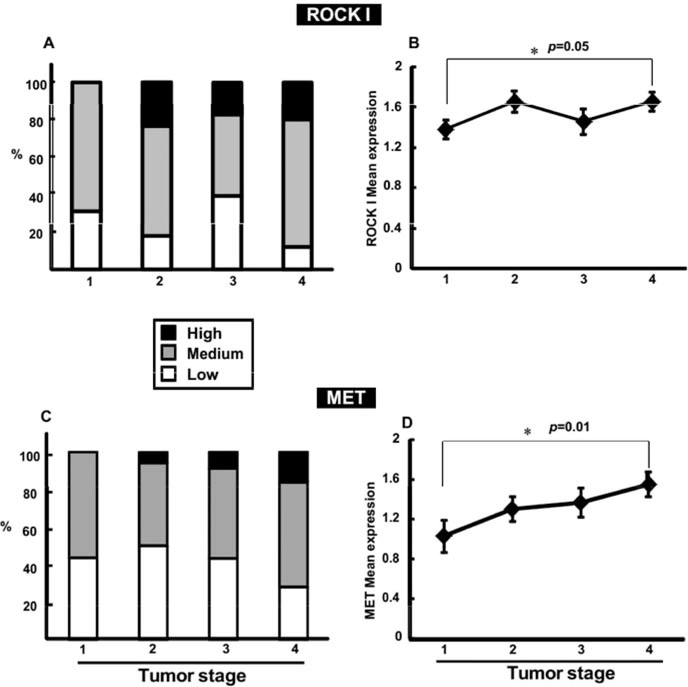
With both ROCK I and MET, positive immunostaining in TMA samples enabled the pathologist to score each cancer tissue samples (low, 0–1; medium, 1–2; high, 2–3), as shown in Figure 2. We then correlated the expression of ROCK I and MET with tumor staging data available on 100 out of the 108 patients. Surprisingly, out of the two patients with tumor Stage 0, one patient had no detectable level of either ROCK I or MET expression, while the other had no expression of MET but a high expression of ROCK I. In tissue samples of patients identified with Stage I colorectal cancer, ROCK I had low expression in 31% of samples and medium expression in 69% of samples. Notably, no patient with Stage I had high expression of ROCK I in their tissue samples. As demonstrated in Figure 3, Panel A, in patients identified with Stage II colorectal cancer, ROCK I had low expression in 18% of patients, medium expression in 59% and high expression in 23% of patients. Likewise, in Stage III, ROCK I seemed to follow a similar pattern with 39% of patients with low expression, 44% of patients with medium expression and 17% of patients with high expression. However, for patients with Stage IV, the results fail to follow the pattern established in Stages I-III. Instead, 20% of patients had low expression of ROCK I, with 52% having medium expression of ROCK I and only 28% of patients having high expression of ROCK I, despite the obvious changes to tumor progression. In contrast, further analysis did reveal a significant increase in mean expression levels of ROCK I in Stage IV compared to Stage I (Figure 3, Panel B).
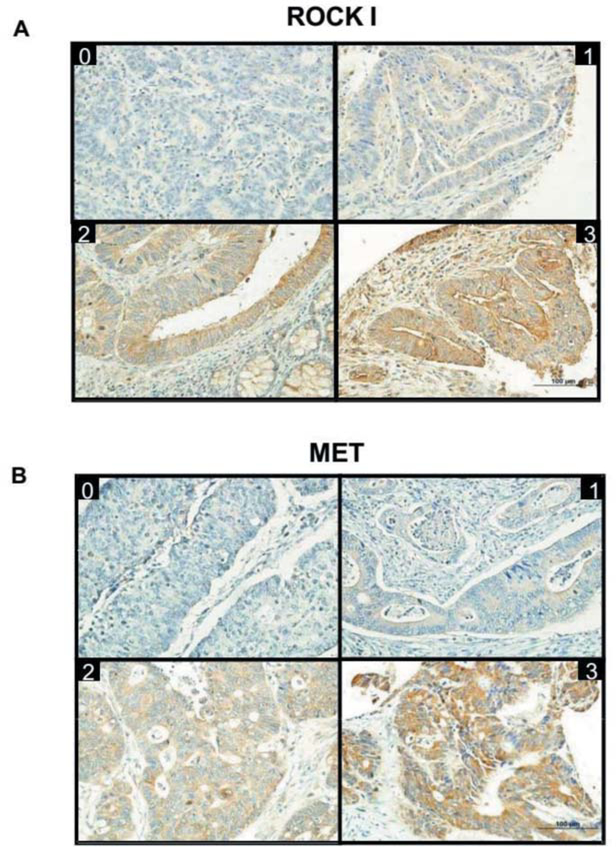
Figure 2.
Specific immunostaining for ROCK I and MET proteins in colorectal cancer. Tissue microarray slides were prepared with primary antibodies specific for ROCK I and MET. Representative images of the immunohistochemistry results for ROCK I (Panel A) and MET (Panel B) are shown. Intensity of staining is labeled as 0 (no staining detected), 1, 2 or 3 (high intensity) on the upper part of each picture.
Figure 3.
Stratification of ROCK I and MET expression in different stages of colorectal cancer. According to the protein expression levels determined through evaluation of cancer tissue staining, each patient was assigned to one of three groups: low, medium or high expression level. Panel A shows tumor stage (I, II, III and IV) stratification of ROCK I expression groups. Panel C displays MET expression groups, while Panels B and D present the mean expression data per tumor stage of ROCK I and MET, respectively. The asterisk indicates a significant mean expression difference as determined by the Student’s t-test.
MET expression provides similar associations with ROCK I in cancer staging
MET expression in tissue samples, likewise, provided some correlation with cancer staging, following a similar pattern to ROCK I expression. As seen in Figure 3, Panel C, in Stage I, 44% of patients had low MET expression compared with 56% at medium levels - and no patient showing high MET expression. Stage II patients’ MET expression levels were likewise similar to those seen with ROCK I. Fifty percent of patients with Stage II had low levels of MET expression, compared to 44% with medium and 6% with high expression. At Stage III, 43% of patients had low MET expression, 48% with medium and 9% of patients with high levels. Again, as with ROCK I, in Stage IV patients, 28% had low expression of MET, 56 medium level expression and only 16% had high expression. However, as with ROCK I, further analysis revealed increased mean expression levels of MET (Figure 3, Panel D) in tumor Stage IV over Stage I.
ROCK I provides stronger correlation with patient survival than MET
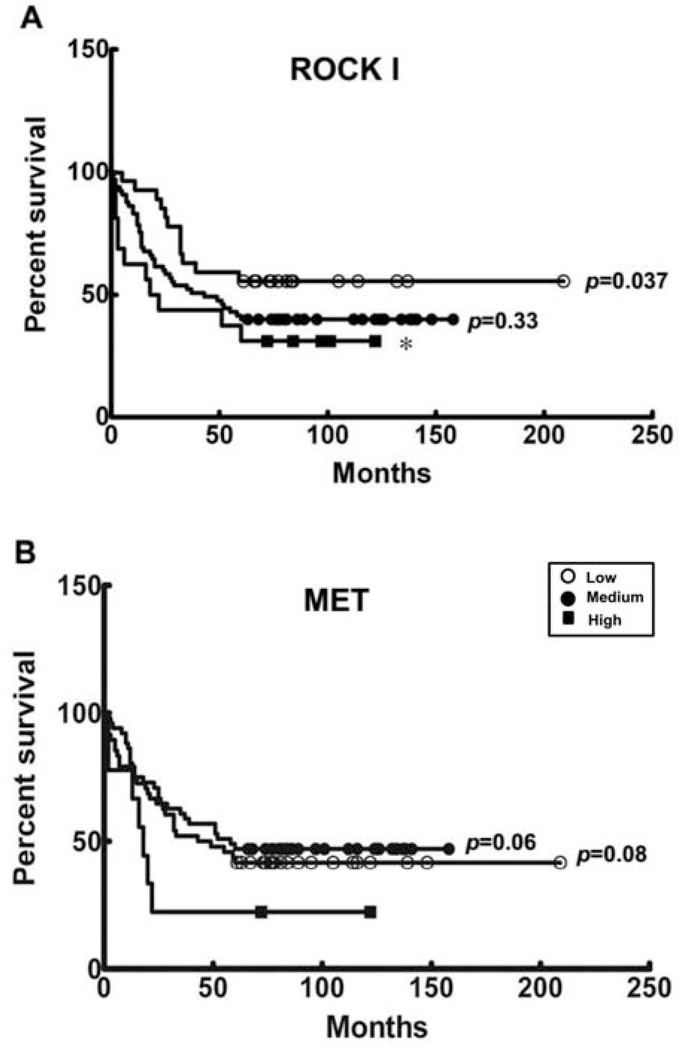
Kaplan-Meier analysis examined the potential correlation between ROCK I and MET expression levels and colorectal cancer patient survival. Patients had poorer prognoses correlated with ROCK I expression level groups, with low expression (n=12), medium (n=40) and high (n=12) having outcomes inversely correlated with their level of ROCK I expression within the 5-year follow-up period. Notably, the ROCK I high-expression group had the poorest survival rates, compared to those of patients in the low-ROCK I expression group (p=0.037) (Figure 4, Panel A).
Figure 4.
Correlation of five-year survival of colorectal cancer patients with protein expression levels. Kaplan-Meier plots show the results of the five-year survival of 108 colorectal cancer patients, stratified by protein expression levels. The correlation of expression levels (low, medium and high) of ROCK I (Panel A) and MET (Panel B) with 5-year colon cancer survival are shown. The asterisk denotes a significant difference between ROCK I high expression and low expression levels of 5-year survival. The p-values are generated from the comparisons of the low or medium with the high expression group.
In contrast, MET was less strongly associated with patient survival, with associations made more problematic by the interchangeability of medium and low levels of MET expression in 5-year patient survival outcomes (Figure 4, Panel B). In contrast, high MET expression (n=7) was more straightforwardly associated with decreased patient survival, compared to medium (n=29) and low survivals (n=28) (p=0.08).
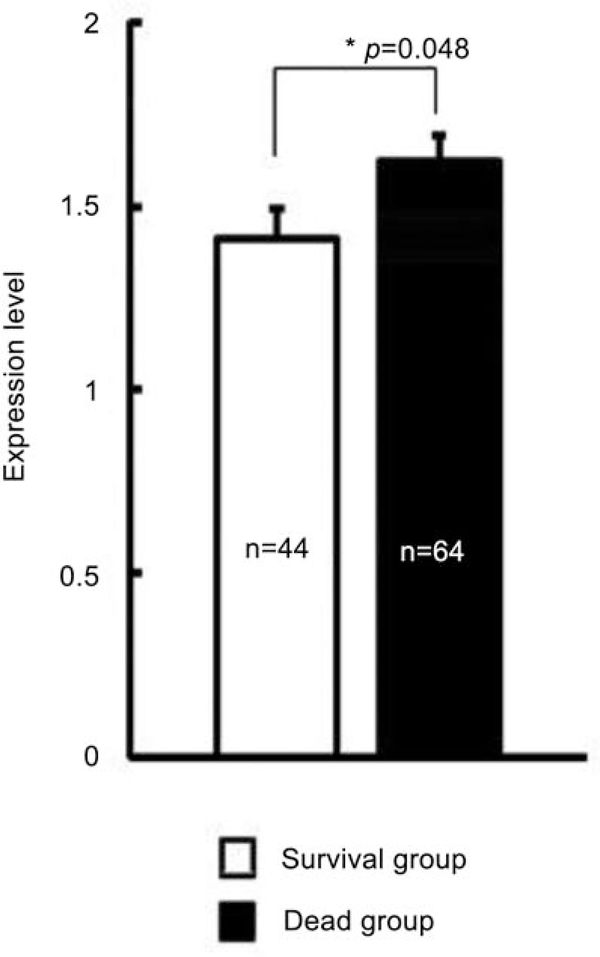
ROCK I is highly correlated with poor patient survival
ROCK I was most highly correlated with 5-year patient survival. Among the 64 patients who died during the 5-year follow-up, the mean ROCK I expression level was significantly higher in the non-survival group over the 44 patients who survived within the same period (Figure 5). In contrast, no significant difference in mean MET expression levels distinguished the survival and non-survival groups within the 5-year follow-up period. Ultimately, we used multivariate Cox proportional hazards model analysis to examine the survival and tumor staging data in combination with ROCK I expression levels. This analysis established a negative association among ROCK I expression, tumor stage and patient survival during the standard 5-year follow-up for cancer patients. The hazard ratio for tumor staging was 1.7, while the hazard ratio for ROCK I expression was 2.7 (Table I).
Figure 5.
Higher ROCK I mean expression level in the non-survival group relative to survival group. After a five-year follow-up period, 64 patients died (non-survival/dead group), whereas 44 patients survived (survival group). ROCK I mean expression was calculated for both groups and a significant difference between survival and non-survival groups, as indicated by the asterisk, was determined.
Table I.
Cox proportional hazards model (multivariate) analysis of protein expression levels, tumor stage and patient survival.
| Parameter | Hazard ratio | p-Value |
|---|---|---|
| ROCK I | 2.682 | 0.0013 |
| MET | 0.618 | 0.0465 |
| Stage | 1.665 | 0.0002 |
Discussion
This study is the first to demonstrate that ROCK I has greater efficacy than MET in predicting patient outcomes and survival in colorectal cancer. In the present study, strikingly, high levels of ROCK I expression were correlated with lower patient survival during five-year follow-up, thus providing more reliable prognostic indicators of patient survival than either tumor staging or the biomarker previously commonly used to predict disease progression, MET.
These findings support studies identifying the over-expression of ROCK I with a variety of cancers. In particular, recent clinical studies have found that ROCK I expression is significantly correlated with degree of lymph node metastasis in gastric cancer (21). In addition, ROCK I is highly expressed in pancreatic cancer tissues (22). Furthermore, consistent with this study’s findings, expression of ROCK I is much higher in breast cancer tumor tissues, compared with normal tissues, while high levels of ROCK expression were similarly significantly correlated with shorter survival in patients with breast cancer (14). In addition, high levels of expression of ROCK I also correlated with decreased patient survival in osteosarcoma (16).
ROCK I may play a key role in cancer metastasis through multiple factors. First, ROCK I signaling may contribute to morphological changes and metastasis of some tumor cells that reduce associations between tumor cells, enabling movement and penetration of tumor cells by endothelial cells, thus bringing about neovascularization (17). Second, high levels of ROCK I drive cortical actomyosin contraction, creating the rounded morphology consistent with some invasive cancer cells, as well as increased cellular motility (23). Third, ROCK I may also be involved in the adhesion of tumor cells to the vascular endothelium and invasion of tumor cells into the host tissue, all crucial steps in cancer metastasis (24).
This study provides preliminary evidence that ROCK I may prove to be a valuable biomarker in assessing patient outcomes in colorectal cancer. Other studies have found that inhibition of ROCK I may even lead to promising therapeutic targets in the treatment of pancreatic ductal adenocarcinoma and inflammatory breast cancer (25). Further studies of larger patient populations and of the specific roles played by ROCK I in the progression of colorectal cancer may equip clinicians to better identify patient prognoses from minimally invasive tissue samples and also provide better-informed interventions based on levels of ROCK I expression in tissues. Ultimately, ROCK I provides more accurate indication of patient survival than either over-expression of MET or even tumor staging.
Acknowledgments
Funding Support
NIH grant RO1 CA113975-A2; Department of Medicine and Gatorade Fund at University of Florida; and Elisa U Pardee foundation Midland, MI 48641.
References
- 1.United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. Department of Health and Human Services. Atlanta (GA): Centers for Disease Control and Prevention, and National Cancer Institute; 2014. [Google Scholar]
- 2.SEER Stat Fact Sheets: Colon and Rectum Cancer. In: Institute NC, editor. http://seer.cancer.gov/statfacts/html/colorect.html: National Cancer Institute: Surveillance, Epidemiology, and End Results Program; 2014. [Google Scholar]
- 3.De Oliveira ATT, Matos D, Logullo AF, DA Silva SR, Neto RA, Filho AL and Saad SS: MET is highly expressed in advanced stages of colorectal cancer and indicates worse prognosis and mortality. Anticancer Res 29: 4807–4811, 2009. [PubMed] [Google Scholar]
- 4.Golfinopoulos V, Salanti G, Pavlidis N and Ioannidis JP: Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol 8(10): 898–911, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Zhao DB, Wu YK, Shao YF, Wang CF and Cai JQ: Prognostic factors for 5-year survival after local excision of rectal cancer. World J Gastroenterol 15: 1242–1245, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arlt F and Stein U: Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol 41: 2356–2359, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C and Birchmeier W: c-MET is essential for wound healing in the skjn. J Cell Biol 177: 151–162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huh C-G, Factor VM, Sánchez A, Uchida K, Conner EA and Thorqeirsson SS: Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. PNAS 101: 4477–4482, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentile A, Trusolino L and Comoglio PM: The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev 27: 85–94, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Huang TJ, Wang JY, Lin SR, Lian ST and Hsieh JS: Overexpression of the c-met protooncogene in human gastric carcinoma-correlation to clinical features. Acta Oncol 40: 638–643, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Raghav KPS andEng C: Role of the MET-HGF axis in colorectal cancer: precepts and prospects. Colorectal Cancer 1: 329–341, 2012. [Google Scholar]
- 12.Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S, Plebani M, Gespach C and Comoglio M: Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res 1: 147–154, 1995. [PubMed] [Google Scholar]
- 13.Resnick MB, Routhier J, Konkin T, Sabo E and Pricolo VE: Epidermal Growth Factor Receptor, c-MET, β-Catenin, and p53 Expression as prognostic indicators in Stage II colon cancer: A Tissue Microarray study. Clin Cancer Res 10: 3069–3075, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Lane J, Martin TA, Watkins G, Mansel RE and Jiang WG: The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int J Oncol 33(3): 585–593, 2008. [PubMed] [Google Scholar]
- 15.Hall A: Rho GTPases and the actin cytoskeleton. Science 279: 509–514, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Morgan-Fisher M, Wewer UM andYoneda A: Regulation of ROCK activity in cancer. J Histochem Cytochem 61: 185–198, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft DR, Sahai E, Mavria G, Li S, Tsai J, Lee WM, Marshall CJ and Olson MF: Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res 64: 8994–9001, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Kamai T, Tsujii T, Arai K, Asami H, Ito Y and Oshima H: Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res 9: 2632–2641, 2003. [PubMed] [Google Scholar]
- 19.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T and Konishi I: Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest 83: 861–870, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Huq J, Guzman G, Elgohary B, Shakir L and Glover SC: Rho-like proteins in human colon cancers are uniquely expressed as a function of differentiation and tumor invasion. Gastroenterol 130: A679–A679, 2006. [Google Scholar]
- 21.Wu YJ, Tang Y, Li ZF, Li Z, Zhao Y, Wu ZJ and Su Q: Expression and significance of Rac1, Pak1 and Rock1 in gastric carcinoma. Asia–Pacific J Clin Oncol 10: e33–e39, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneko K, Satoh K, Masamune A, Satoh A and Shimoseqawa T: Expression of ROCK-1 in human pancreatic cancer: Its downregulation by morpholino oligo antisense can reduce the migration of pancreatic cancer cells in vitro. Pancreas 24: 251–257, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Olson MF and Sahai E: The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis 26: 273–287, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Relja B, Meder F, Wang M, Blaheta R, Henrich D, Marzi I and Lehnert M: Simvastatin modulates the adhesion and growth of hepatocellular carcinoma cells via decrease of integrin expression and ROCK. Int J Oncol 38: 879–885, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Sahai E and Marshall CJ: RHO–GTPases and cancer. Nature Rev Cancer 2: 133–142, 2002. [DOI] [PubMed] [Google Scholar]