Abstract
Different types of in vitro blood-brain barrier (BBB) models have been constructed and applied for drug transport to evaluate the efficacy of nano-carrier based drug delivery. However, the effectiveness of different types of BBB models has not been reported. In this paper, we developed two types of in vitro models: one-cell type BBB model developed using only endothelial cells and three-cell type BBB model obtained by co-culturing endothelial, pericyte and astrocyte cells. The nanoparticle transport mechanisms through BBB and transport efficiencies of the Lactoferrin attached silica nanoparticles were studied using both types of the in vitro BBB models. Compared with one-cell type model, the PSi-Lf NPs exhibit relatively lower transport efficiency across three-cell type BBB system. Moreover, the effects of the nanoparticle size on the transport efficacies are consistent for both models. For both types of BBB models, the transport efficacies of the NPs are size dependent, and the highest efficacies are achieved for NPs with 25 nm in diameter. Our experimental results indicate that the one-cell type and three-cell type BBB models are equivalent for evaluating and optimizing nanoparticle transport across BBB.
Keywords: Lactoferrin attached silica nanoparticles, blood brain barrier, one-cell type, three-cell type, transcytosis
Graphical Abstract
Introduction
Blood-brain-barrier (BBB) is a fundamental structure for precise regulation of biomolecules transportation between the blood and the central nervous system (CNS).1–3 Generally, it composes of various brain endothelial cells. These cells express the low amount of leukocytes binding molecules and capable of forming tight junctions, which limits the paracellular transportation.4 However, several drug molecules or protein-based therapeutics could transport through BBB freely except some low molecular weight lipophilic molecules and selective ions.5 All of them is because of the protection of the BBB, which exclude the brain away from the environmental invasion.6–7 On the other hand, it often obstructs the delivery of brain therapeutic drugs for neurological diseases treatment. Therefore, great efforts have been devoted to develop novel nano-carriers for directly brain drug delivery and improve the prognosis of patients with CNS diseases.
For nano-carrier based targeted drug-delivery, it is crucial to construct an in vitro model of the BBB biological system for studying the interaction between nano-carriers and BBB. Nowadays, various kinds of in vitro BBB models, by culturing brain cells, have been developed by culturing brain cells and investigated to understand the biological mechanisms of BBB and evaluate the performance of nano-carriers.8–10 These BBB models are usually formed using transwell apparatus to separate the luminal side from the abluminal side.11
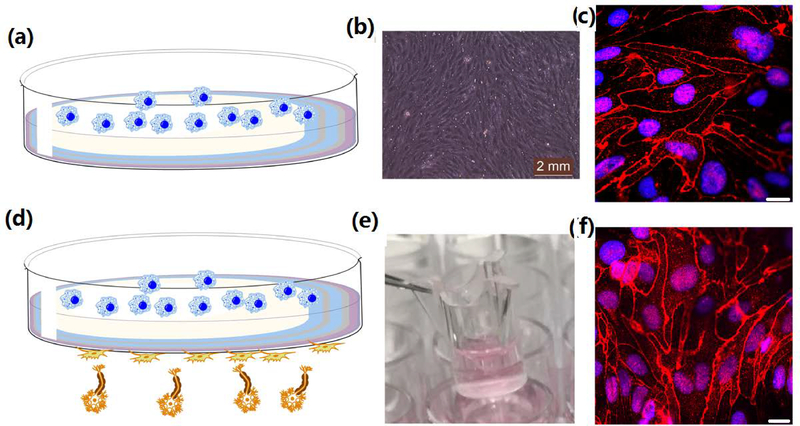
The most common model is based on growing a monolayer of BCECs on the transwell membranes (Fig. 1a).12 The monolayer BBB model can be obtained with a relatively simple procedure and low cost.10 However, this type of in vitro model is a simplified version of real BBB structure. In human and animal brains, BBB is formed from brain pericyte and astrocyte cells in addition to endothelial cells. Some researchers have developed in vitro BBB models by co-culturing three types of cells (Fig. 1d) to mimic the human brain.13–16 These works demonstrated that the addition of astrocyte cells could induce more stringent inter-endothelial tight junctions which improves the overall expression of proprietary BBB features. The three-cell type BBB models can better mimic the BBBs in brains.12 Despite the clear advancement in the three-cell type BBB models, these systems suffer from some disadvantages, including in expensive and the quality of the three-cell type BBB model is more difficult to control.
Figure 1.
(a) Schematic of the one-cell type BBB model. (b) Microscopy image of the brain endothelial cells grew on the transwell membrane after day 13. (c) Immunofluorescence of membrane proteins (Claudin-5) for one-cell type BBB model. Scale bar 20 μm. (d) Schematic of the three-cell type BBB model. (e) Photograph of a three-cell type BBB model. Reproduced with permission from Ref.16. Copyright 2017, American Chemical Society. (f) Immunofluorescence of membrane proteins (Claudin-5) on three-cell type BBB model. Scale bar 20 μm.
In this paper, we developed two types of in vitro BBB models: one-cell type formed using only BCECs and three-cell type syngeneic BBB model obtained by co-culturing BCECs, pericyte and astrocyte cells. Transport of silicon nanoparticles is studied through these BBB models. Since BBB endothelial cells carry a number of receptors, various ligands have been successfully used to help to transporting drug through the BBB research.23–25 Among them, lactoferrin (Lfs) is a mammalian cationic iron-binding glycoprotein belonging to the transferrin (Tf) family, which can bind the lactoferrin receptor on the BBB to transport across the BBB.22 Lactoferrin attached silica nanoparticles were used to evaluate these different BBB models. By using a combination of fluorescence imaging and permeability assay, we characterized the spatiotemporal behavior of lactoferrin attached silicon nanoparticle transcytosis through different BBBs and identified relevant efficiencies. The results showed that NPs exhibited relatively lower transport efficiency across three-cell type BBB system. However, the effects of the nanoparticle sizes on the transport efficacies were consistent for different models. It indicated that both types of BBB models were equally effective to optimize and select nano-carriers.
Experimental section
Preparation of NPs
Silica nanoparticles (Si NPs) were synthesized according to the previous work16. Briefly, 7.5 mL of cyclohexane was mixed with a relative amount of Triton X-100 and n-hexanol. For synthesis of different sizes of Si NPs, different amount of DI water (160, 400, and 1120 μL) was added and stirring for another 20 min. Then, 80 μL of Rubpy dye (0.1 M) was injected slowly. After stirred for 5 min, 200 μL of tetraethyl orthosilicate (TEOS) and 100 μL of NH4OH were injected. After 24h incubation, the Si NPs were washed with DI water for 3 times. Then the polyethylene glycol (PEG) labeled Si NPs (PSi NPs) and lactoferrin (Lf) attached Si NPs (Psi-Lf NPs) were synthesized according to previous report.16
Construction of BBB models
In this study, the one-cell type in vitro BBB model was formed according to previous work.17 This BBB model is developed by culturing the bEnd.3 endothelial cell line on the transwell membrane of micro plate wells. Briefly, bEnd.3 endothelial cells were incubating on the transwell (0.4 μm pore size) with DMEM medium. Cells were grown 13 days with changing medium every day until a compact monolayer was formed on the transwell. On the other hand, the three-cell type in vitro BBB model was purchased from PharmaCo-Cell Co. Ltd (Nagasaki, Japan, http://www.pharmacocell.co.jp/, BBB kit (RBT-24H)). This BBB kit was developed by co-culturing primary wistar rat BCECs, brain pericytes and astrocytes on transwell membranes,16 which was stored at −80 °C and can be used during one month. According to the protocol, the BBB kit has to be thawed and activated before use. The medium was de-frozen in 37 °C water bath, and quickly added to the brain-side chamber and blood-side chamber. Next the BBB kit was placed in the incubator and the medium was changed every day. After 4 days of incubation, the kit was functionally active.
Measurement of Trans-endothelial Electrical Resistance of BBB models
The trans-endothelial electrical resistance (TEER) can be determined according to our previous study.16 Briefly, an EVOM voltammeter with two probes were placed at both sides of the transwells. The relative resistance value was recorded as follow:
| (1) |
where RA and RZ represent the relative resistance value and resistance of medium alone, respectively.
Transport Efficiency of Nanoparticles across BBB models
The transport efficiency of PSi NPs or PSi-Lf NPs was calculated according to our previous study.16 Briefly, the medium containing PSi NPs and PSi-Lf NPs were incubated in the two BBB models for 12 h. Then, the medium was collected from the basolateral side and the fluorescence intensity was recorded using fluorescence spectrometry. The transport efficiency of NPs could be determined according to this equation:
| (2) |
where Ib and It represent the collective fluorescence intensity in the basolateral side and the original medium containing PSi NPs in the apical side. Ic is calculated from autofluorescence of control.
Results and Discussion
Characterization of BBB models
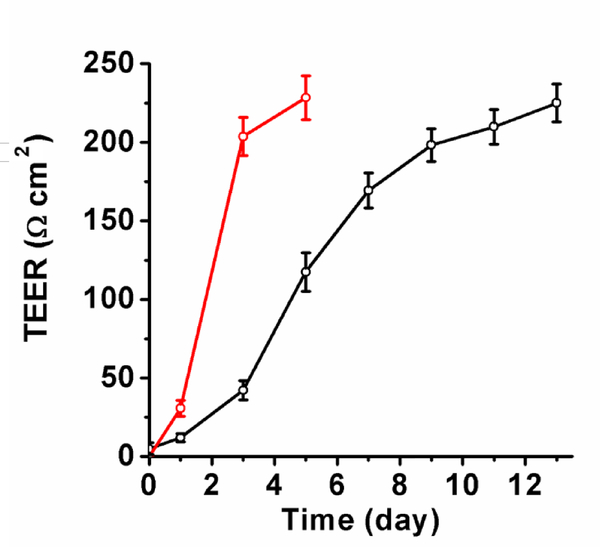
As shown in Figure 1b, the brain endothelial cells, in the one-cell type BBB model, formed tight junctions with spindle-shaped optical morphology after 13 days of culture. The trans-endothelial electrical resistance (TEER) value is used to estimate the tightness of the BBB model. Usually, TEER value is related with the permeability of the intercellular molecule across the BBB.18 The strong tight junctions might eliminate all intercellular transport mechanisms, which can result in extremely high electric resistance.19 Previous works have demonstrated that incubation of endothelial cells on transwell could achieve a TEER value of ~200 Ω cm2 within 12 days.20 In this study, a TEER value of ~225 Ω cm2 was obtained after 13-day of incubation for the one-cell type BBB model, indicating that this BBB model was suitable for in vitro study of the permeability assay (Figure 2). The TEER value of BBB model formed from three types of cells is larger than 200 Ω cm2.21 The immunostained of the relative protein (Claudin-5) on one cell type and three cell type BBB models, as shown in Figure 1c and 1f, showed that the endothelial cells formed spindle like junctions for both models.
Figure 2.
Measured TEER values of two types of BBB models. Black: monolayer BBB model. Red: three-cell type BBB model.
Characterization of Nanoparticles
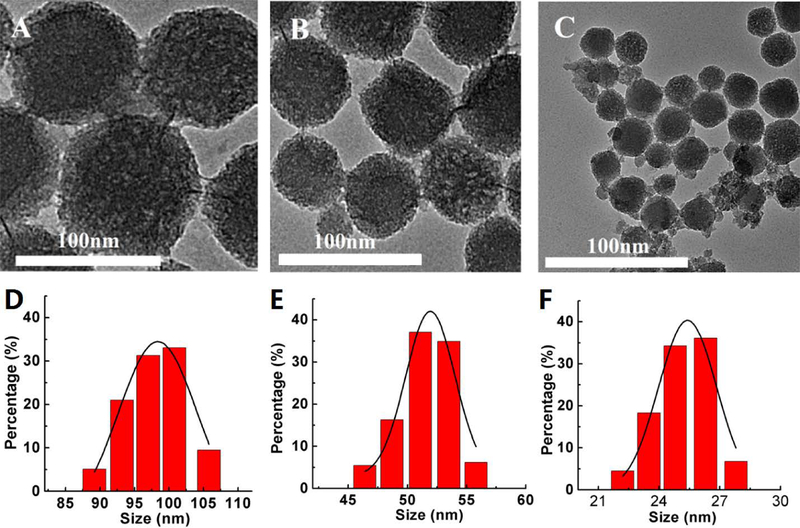
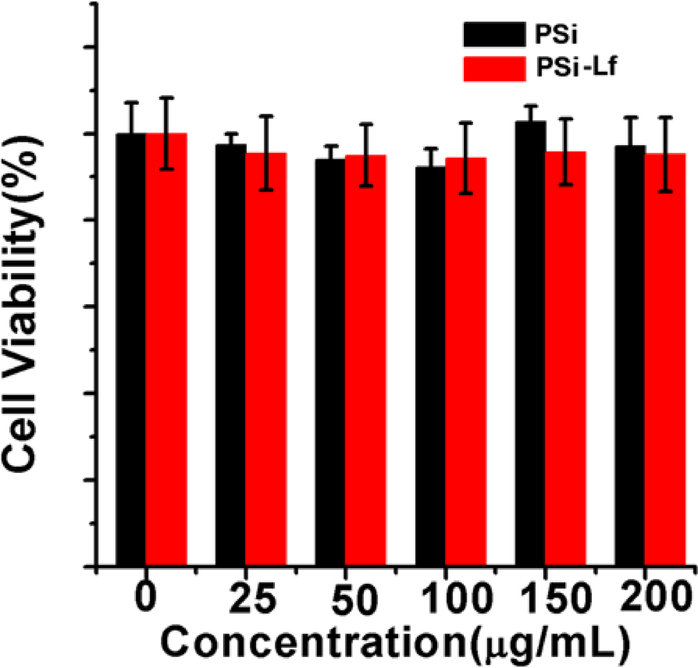
In Figure 3a–c, the TEM images show that PSi NPs with different diameters can be successfully synthesized. The average diameter of PSi NPs is presented in the size distribution histogram (Figure 3d–f). In addition, the cytotoxicity of these NPs was evaluated. As shown in Figure 4, more than 90% cell viability on bEnd.3 cell can be achieved after incubation with PSi NPs or PSi-Lf NPs with various concentrations for 24 hours, indicating negligible cytotoxicity for these NPs.
Figure 3.
TEM images of (A) 100 nm, (B) 50 nm, and (C) 25 nm diameter PEG labeled Silicon nanoparticles (PSi NPs). Size distribution for (D) 100 nm, (E) 50 nm, and (F) 25 nm diameter PSi NPs.
Figure 4.
Cellular cytotoxicity assessment of bare PSi and lactoferrin bound PSi nanoparticles on one-cell type BBB model.
Transcytosis Mechanism for Different BBB models
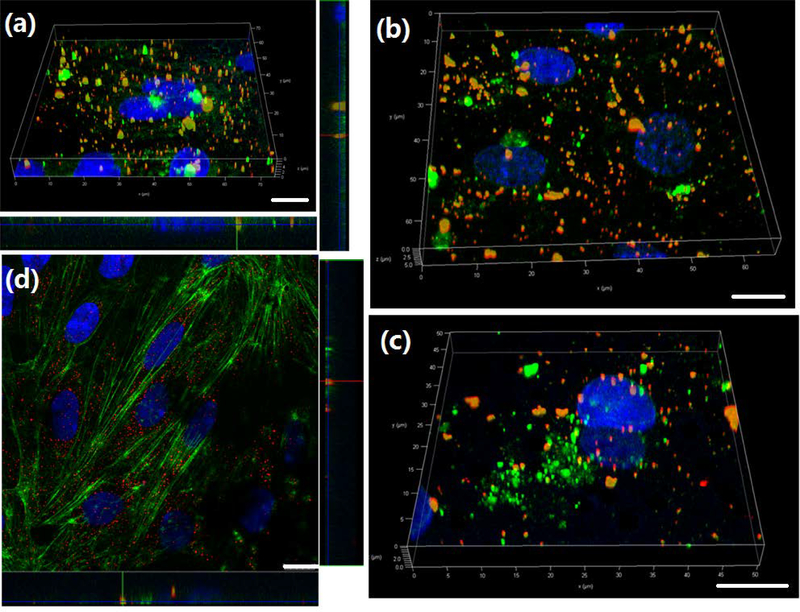
In earlier work,16 we have studied the mechanism of intercellular transcytosis in the three-cell type BBB model. Briefly, we have demonstrated the transport pathway of PSi-Lf NPs during the transcytosis. The three-cell type BBB models were first incubated with transferrin (Tf). Figure S1a shows that endothelial cells can uptake transferrin bound PSi-Lf NPs indicating the entry of PSi-Lf NPs through the endocytosis. After the BBB was stained with Rab 5/Rab 7, it was further incubated with PSi-Lf NPs. As shown in Figure S2a and S2b, the PSi-Lf NPs demonstrated less co-localization with early endosomes (EEs) and late endosomes (LEs), indicating the NPs trafficked backward from the EEs and LEs during the endocytosis. However, the PSi-Lf NPs showed high co-localization in the sorting endosomes (SEs) as seen in Figure S1b and S1d. In addition, the PSi-Lf NPs containing compartment also showed less co-localization with lysosomes (Figure S1c and S1d), which indicated that the endocytosis of PSi-Lf NPs will not involve the lysosome pathway. Z-stacks reconstructed CLSM imaging further showed transcellular movement across the BBB monolayer (Figure S2c).
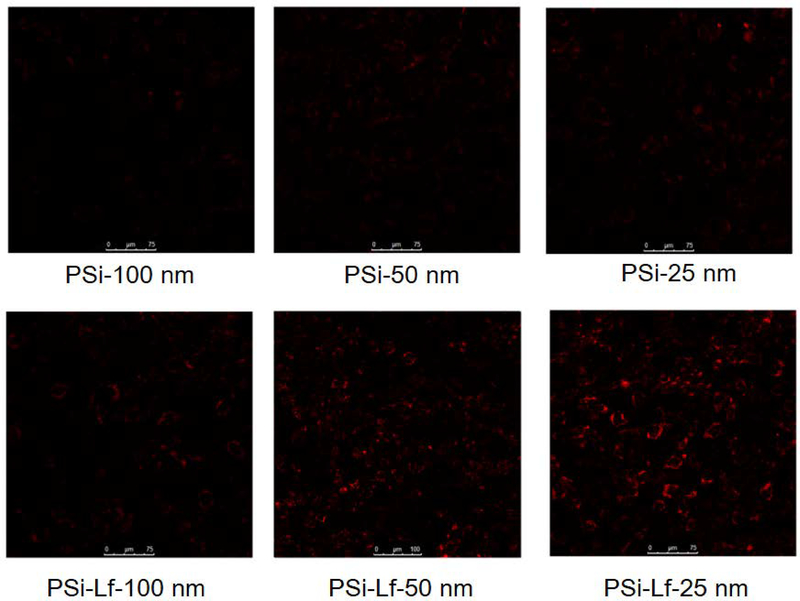
In this work, we studied whether PSi-Lf NPs penetrating one-cell BBB model exhibited the same trafficking pathway and transcytosis mechanism that observed in three-cells co-cultured BBB model. To do so, we treated cells with multiplex endocytic markers. As shown in Figure 7, the NPs can co-locate with the EEs after 1 h of incubation (Figure 5a) but show low colocalization with LEs after 2 h of incubation (Figure 5b), indicating PSi-Lf NPs trafficked out from EEs. Compartments containing PSi-Lf NPs were confirmed to have a low degree of association with LEs. In addition, the confocal laser scanning microscopy (CLSM) image has shown less co-localization of the PSi-Lf NPs with lysosome trackers (Figure 5c). The CLSM imaging indicated the co-localization of PSi-Lf NPs with the Actin network after 4 h of incubation, implying that Actin is required for efficient transcytosis and delivery in apical recycling pathways (Figure 5d). They indicated that the transcellular mechanism of NPs on one-cell BBB model is similar to that found in the three-cell type model.
Figure 7.
Confocal fluorescence images of the one cell type BBB model in vitro with NPs for 2 h.
Figure 5.
(a) Representative 3D confocal image showed co-localization of PSi-Lf NPs (red) and EEs marker Rab-5 (green) on one-cell type BBB model. (b) Co-localization study of PSi-Lf NPs (red) with late endosome marker Rab-7a (green) on one-cell type BBB model. (c) Z-stacks reconstructed into 3D images of co-localization profiles of PSi-Lf NPs (red) with Lyso-Tracker (green) on one-cell type BBB model. (d) Co-localization study of PSi-Lf NPs (red) with Actin (green) on one-cell type BBB model. Scale bar: 10 μm.
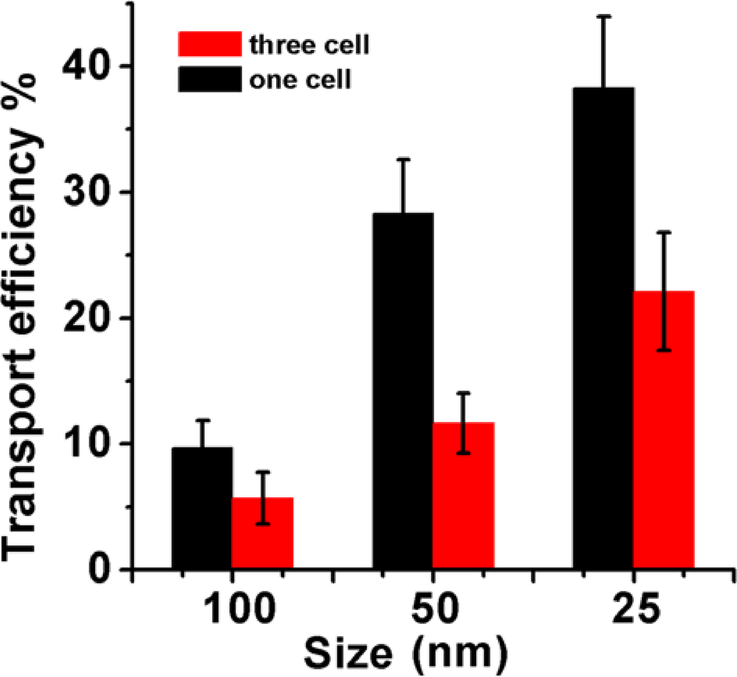
Transport efficiencies of Nanoparticle across different BBB models
As shown in Figure 6, the transport efficiency of the three-cell type BBB model demonstrated a particle size dependent transport behavior. The maximum transport efficiency was achieved for 25 nm PSi-Lf among different nanoparticles studied here (Figure 6). In the one-cell type BBB model, the 25 nm PSi-Lf NPs also achieved the highest efficiency (38%), which was 1.8 fold higher than that achieved in the three-cell type BBB model. In addition, as shown in Figure 7, the red color intensity in the BBB incubated with 25 nm PSi-Lf NPs is stronger than others (50 nm and 100 nm), indicated that NPs with such size achieved the most effective uptake by the endothelial cell. It is important to note that after conjugation of Lf with PSi NPs, the intensity of red fluorescence in the endothelial cell was strongly enhanced. Aforementioned results conclude that transport behavior of PSi-Lf NPs for one-cell type BBB model is also size and ligand-dependent.
Figure 6.
Transport efficiencies of PSi-Lf NPs across the monolayer and co-cultured BBB models.
Overall, the three-cell type BBB model showed a lower permeability for NPs compared with the one-cell type BBB model. This phenomenon might be caused by the more complex astrocyte interaction and protein expression in the three-cell type BBB model. However, our results also indicate that the size-dependent nanoparticle transport trend remains the same for both BBB models. Thus, the one-cell type and three-cell type BBB models are equivalent for evaluating and optimizing nanoparticles across BBB. Considering the low cost and the simplicity of the construction procedure, one-cell type BBB model is more favorable for high-throughput drug permeability test and ligand binding affinity measurement.
Conclusions
In conclusion, we compared the one-cell type and three-cell type in vitro BBB models. The results indicated that three-cell type co-cultured BBB model offers lower permeability compared to the one-cell type BBB model. However, our results showed that the transport behavior of Psi-Lf NPs through BBB is similar for both types of BBB models. Both BBB models could be used for studying the molecular mechanisms responsible for drug permeability test and ligand binding affinity measurement.
Supplementary Material
ACKNOWLEDGMENT
The research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM122081. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website. Additional details regarding transcytosis of lactoferrin bound PSi nanoparticles in three-cell type BBB model and intracellular localization of PSi-Lf nanoparticles in three-cell type BBB model.
Reference
- 1.Ballabh P; Braun A; Nedergaard M The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol Dis 2004, 16 (1), 1–13. [DOI] [PubMed] [Google Scholar]
- 2.Farrall AJ; Wardlaw JM Blood-Brain Barrier: Ageing and Microvascular Disease-Systematic Review and Meta-Analysis. Neurobiol Aging 2009, 30 (3), 337–352. [DOI] [PubMed] [Google Scholar]
- 3.Hawkins BT; Davis TP The Blood-Brain Barrier/Neurovascular Unit in Health and Disease. Pharmacol Rev 2005, 57 (2), 173. [DOI] [PubMed] [Google Scholar]
- 4.Abbott NJ; Ronnback L; Hansson E Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat Rev Neurosci 2006, 7 (1), 41–53. [DOI] [PubMed] [Google Scholar]
- 5.Calvo P; Gouritin B; Chacun H; Desmaële D; D’Angelo J; Noel J-P; Georgin D; Fattal E; Andreux JP; Couvreur P Long-Circulating PEGylated Polycyanoacrylate Nanoparticles as New Drug Carrier for Brain Delivery. Pharmaceut Res 2001, 18 (8), 1157–1166. [DOI] [PubMed] [Google Scholar]
- 6.Begley DJ Delivery of Therapeutic Agents to the Central Nervous System: the Problems and the Possibilities. Pharmacol Therapeut 2004, 104 (1), 29–45. [DOI] [PubMed] [Google Scholar]
- 7.Orive G; Ali OA; Anitua E; Pedraz JL; Emerich DF Biomaterial-Based Technologies for Brain Anti-Cancer Therapeutics and Imaging. Biochim. Biophys. Acta. Reviews on Cancer 2010, 1806 (1), 96–107. [DOI] [PubMed] [Google Scholar]
- 8.Bramini M; Ye D; Hallerbach A; Nic Raghnaill M; Salvati A; Åberg C; Dawson KA Imaging Approach to Mechanistic Study of Nanoparticle Interactions with the Blood-Brain Barrier. ACS Nano 2014, 8 (5), 4304–4312. [DOI] [PubMed] [Google Scholar]
- 9.Schiera G; Sala S; Gallo A; Raffa MP; Pitarresi GL; Savettieri G; Liegro ID Permeability Properties of a Three-Cell Type In Vitro Model of Blood-Brain Barrier. J. Cell. Mol. Med 2005, 9 (2), 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik P; Cucullo L In Vitro Blood–Brain Barrier Bodels: Current and Perspective Technologies. J Pharm Sci 2012, 101 (4), 1337–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin LL; Hall DE; Porter S; Barbu K; Cannon C; Horner HC; Janatpour M; Liaw CW; Manning K; Morales J A Cell Culture Model of the Blood-Brain Barrier. J Cell Biol 1991, 115 (6), 1725–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott NJ; Rönnbäck L; Hansson E Astrocyte-Endothelial Interactions at the BloodBrain Barrier. Nat Rev Neurosci 2006, 7, 41. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi K; Nakao S; Nakaoke R; Nakagawa S; Kitagawa N; Niwa M Effects of Hypoxia on Endothelial/Pericytic Co-culture Model of the Blood-Brain Barrier. Regul Peptides 2004, 123 (1), 77–83. [DOI] [PubMed] [Google Scholar]
- 14.Stanness KA; Neumaier JF; Sexton TJ; Grant GA; Emmi A; Maris DO; Janigro D A New Model of the Blood-Brain Barrier: Co‐culture of Neuronal, Endothelial and Glial Cells under Dynamic Conditions. Neuroreport 1999, 10 (18), 3725–3731. [DOI] [PubMed] [Google Scholar]
- 15.Megard I; Garrigues A; Orlowski S; Jorajuria S; Clayette P; Ezan E; Mabondzo AS A Co-culture-based Model of Human Blood-Brain Barrier: Application to Active Transport of Indinavir and In Vivo-In Vitro Correlation. Brain Res 2002, 927 (2), 153–167. [DOI] [PubMed] [Google Scholar]
- 16.Song Y; Du D; Li L; Xu J; Dutta P; Lin Y In Vitro Study of Receptor-Mediated Silica Nanoparticles Delivery across Blood-Brain Barrier. ACS Appl. Mater. Interfaces 2017, 9 (24), 20410–20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong AD; Ye M; Levy AF; Rothstein JD; Bergles DE; Searson PC The Blood-Brain Barrier: An Engineering Perspective. Front. Neuroeng 2013, 6, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh AV; Batuwangala M; Mundra R; Mehta K; Patke S; Falletta E; Patil R; Gade WN Biomineralized Anisotropic Gold Microplate-Macrophage Interactions Reveal Frustrated Phagocytosis-like Phenomenon: A Novel Paclitaxel Drug Delivery Vehicle. ACS Appl. Mater. Interfaces 2014, 6 (16), 14679–14689. [DOI] [PubMed] [Google Scholar]
- 19.Lohmann C; Hüwel S; Galla HJ Predicting Blood-Brain Barrier Permeability of Drugs: Evaluation of Different In Vitro Assays. J Drug Target 2002, 10 (4), 263–276. [DOI] [PubMed] [Google Scholar]
- 20.Hanada S; Fujioka K; Inoue Y; Kanaya F; Manome Y; Yamamoto K Cell-Based in Vitro Blood-Brain Barrier Model Can Rapidly Evaluate Nanoparticles’ Brain Permeability in Association with Particle Size and Surface Modification. Int. J. Mol. Sci 2014, 15, 1812–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nurunnabi M; Khatun Z; Huh KM; Park SY; Lee DY; Cho KJ; Lee YK In Vivo Biodistribution and Toxicology of Carboxylated Graphene Quantum Dots. ACS Nano 2013, 7 (8), 6858–6867. [DOI] [PubMed] [Google Scholar]
- 22.Hu K; Li J; Shen Y; Lu W; Gao X; Zhang Q; Jiang X Lactoferrin-Conjugated PEG-PLA Nnanoparticles with Improved Brain Delivery: In Vitro and In Vivo Evaluations. J. Control. Release 2009, 134 (1), 55–61. [DOI] [PubMed] [Google Scholar]
- 23.Tian X; Nyberg S; Sharo PS; Madsen J; Daneshpour N; Steven PA; Berwick J; Azzouz M; Shaw P, Abbott NJ; Battaglia G, LRP-1-Mediated Intracellular Antibody Delivery to the Central Nervous System. Sci. Rep 2015, 5, 11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang R; Ke W; Liu Y; Jiang C; Pei Y, The Use of Lactoferrin as A Ligand for Targeting the Polyamidoamine-Based Gene Delivery System to the Brain. Biomaterials 2008, 29, 238–246. [DOI] [PubMed] [Google Scholar]
- 25.Huang R; Ke W; Han L; Liu Y; Shao K; Jiang C; Pei Y, Lactoferrin-Modified Nanoparticles Could Mediate Efficient Gene Delivery to the Brain In Vivo. Brain Res. Bull 2010, 81, 600–604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.