Abstract
Background:
A central nosological problem concerns the etiological relationship of emotional dysregulation with ADHD. Molecular genetic risk scores provide a novel method for informing this question.
Methods:
Participants were 514 community-recruited children of Northern European descent age 7–11 defined as ADHD or non-ADHD by detailed research evaluation. Parents rated ADHD on standardized ratings and child temperament on the Temperament in Middle Childhood Questionnaire (TMCQ) and reported on ADHD and comorbid disorders by semi-structured clinical interview. Categorical and dimensional variables were created for ADHD, emotional dysregulation (implicating disruption of regulation of both anger-irritability and of positive valence surgency-sensation seeking), and irritability alone (anger dysregulation). Genome-wide polygenic risk scores (PRS) were computed for ADHD and depression genetic liability. Structural equation models and computationally derived emotion profiles guided analysis.
Results:
The ADHD PRS was associated in variable centered analyses with irritability (β = .179, 95% CI=.087–.280; ΔR2=.034, p < .0002), but also with surgency/sensation seeking (B=.146, 95%CI=.052–.240, ΔR2=.022, p=.002). In person-centered analysis, the ADHD PRS was elevated in the emotion dysregulation ADHD group versus other ADHD children (OR=1.44, 95% CI=1.03–2.20, Nagelkerke ΔR2=.013, p=.033) but did not differentiate irritable from surgent ADHD profiles. All effects were independent of variation in ADHD severity across traits or groups. The depression PRS was related to oppositional defiant disorder but not to ADHD emotion dysregulation.
Conclusions:
Irritability-Anger and Surgency-sensation-seeking, as forms of negative and positively valenced dysregulated affect in ADHD populations, both relate principally to ADHD genetic risk and not mood-related genetic risk.
Keywords: ADHD, polygenic score, irritability, temperament
Introduction
A decades-long controversy concerns whether emotional dysregulation should be included as a central or “core” part of the ADHD diagnosis, or seen as comorbidity. Reviews have noted that (a) definitions of ADHD prior to 1980s included problems in emotional dysregulation in some form (Shaw, Stringaris, Nigg, & Leibenluft, 2014) and (b) elevated emotion-related symptoms (anxiety, depression, anger; reward, excitement) remain salient clinically (Faraone et al., 2019), but (c) other disorders commonly co-occur with ADHD and could account for emotion-related symptoms, as DSM-III to DSM-5 have implicitly assumed.
In contrast to the DSM model, several authorities argue that emotion-related problems are integral to ADHD as a “core feature” (Barkley, 2010; 1997; Reimherr et al., 2005; Skirrow, McLoughlin, Kuntsi, & Asherson, 2009). The term “core feature” is evocative yet can be misconstrued. It is widely accepted that ADHD is heterogeneous, such that no symptom domain or feature is salient in all children with ADHD, as is noted in DSM-5’s different presentations. Even if emotional features are part of the ADHD syndrome, they too would be expected to be essential only in a subgroup. Thus, an empirical approach to considering heterogeneity in ADHD is critical for understanding the role of emotion dysregulation in the disorder. The key question is not whether all children with ADHD experience emotional dysregulation. It is whether ADHD should be conceptualized more broadly than it is today as a disorder of self-regulation, that encompasses not only deficits in self-regulation of attention, impulses, and activity but also self-regulation of emotion (Barkley, 1997; 2017a, 2017b). This question, along with three specific issues, sets our conceptual and scientific context.
Issue #1: The definition of the relevant emotional construct to attach to the ADHD syndrome has been challenging
Different investigators have used different terms and measures to describe the emotional symptoms of ADHD (Faraone et al., 2019). These include “deficient emotional self-regulation” (Barkley & Fischer, 2010; Surman et al., 2011), “emotional dysregulation” (Robison et al., 2010), “emotional impulsiveness” (Barkley & Fischer, 2010), and “irritability” (Riglin et al., 2017; Shaw et al., 2014). Table 1 lists specific scale items used by these investigators (Table S1 in the Supporting Information provides more details). It shows that emotional impulsivity and deficient emotional self-regulation include both positive and negative valence dysregulation (Barkley & Fischer, 2010), while anger and temper tantrums (generally congruent with negative valence, but see below) are salient in all constructs but exclusively so in irritability. Herein, therefore, irritability refers specifically to anger dysregulation, whereas, “emotional dysregulation” refers to dysregulation of both anger and positive valence affect (e.g., sensation seeking).
Table 1:
Listing of items used across the literature for constructs of irritability and related emotional dysregulation
| IRRITABILITY CONSTRUCT MEASURES | EMOTIONAL DYSREGULATION AND RELATED CONSTRUCT | CURRENT STUDY TMCQ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ODD irritable dimension | IrritabilityJ | Emotional dysregulation | Emotional impulsiveness | Emotional self-regulation | Emotional lability | Anger/ Frustration | Soothability/ Falling Reactivity | ||||
| DSM-ODD | ARIA | DAWBAB | NCDSC | SAGED | WenderE,F | Barkley et al.G | Surman et al.H | ConnersI | TMCQ | TMCQ | |
| has temper tantrums* | |||||||||||
| touchy or easily annoyed | |||||||||||
| angry /resentful | |||||||||||
| often angry or stays upset** | |||||||||||
| easily frustrated - frequently irritable*** | |||||||||||
| gets angry when… toy taken by another child | |||||||||||
| time to stop playing | |||||||||||
| can’t find something | |||||||||||
| has trouble with a task | |||||||||||
| makes a mistake | |||||||||||
| provoked by other children | |||||||||||
| argues with others | |||||||||||
| emotional over-reactivity | |||||||||||
| cries more than a couple minutes**** | |||||||||||
| affective lability | |||||||||||
| can’t settle down | |||||||||||
| difficult to tolerate waiting – impatient | |||||||||||
| easily excited | |||||||||||
| mood changes quickly and drastically | |||||||||||
| remains upset for hours… | |||||||||||
| difficult to soothe when upset | |||||||||||
| hard to go back to sleep | |||||||||||
| cheers up slowly...when troubled | |||||||||||
| feels nervous a long time … when was scared | |||||||||||
Note: Lettered superscripts refer to the following citations
See Table S1 for explanation of what nearly -identical items were combined in table.
Although irritability is operationalized in different ways (Table 1), one common approach, derived from factor analyses, relies on a subset of three items on the ODD symptom list (loses temper, touchy/easily annoyed, and angry/resentful) associated with elevated risk for depression (but not bipolar disorder) (Stringaris & Goodman, 2009). This raises the question of whether irritability relates to genetic risk for depression rather than for ADHD.
Issue #2. Use of a temperament framework can help to fulfill the goal of a neurobiologically informed nosology related to emotion/irritability in ADHD (Cuthbert & Insel, 2013)
Temperament refers to patterns of emotional response and regulation measured as dimensional traits (Rothbart, 2011). While overlapping, this approach to behavioral indicators differs conceptually from clinical approaches to ADHD’s emotional symptoms (Faraone et al., 2019), which focus on domains such as anxiety that can be episodic. Decades of empirical work have identified a hierarchical structure of child traits similar to the structure of personality in adults. The model adopted here (Rothbart, 2011; Rothbart, Sheese, Rueda, & Posner, 2011) identifies three broad, well-validated super-ordinate domains: (1) negative affect, (2) surgency, and (3) effortful control. Each domain, while including multiple lower level sub-traits, has a hypothesized neurobiological basis in the interaction of amygdala-PFC circuitry, dopaminergic reward networks, and prefrontal-striatal and cortical-cortical control networks, respectively (Posner & Rothbart, 2000; Whittle, Allen, Lubman, & Yücel, 2006).
Using a temperament framework, Nigg and colleagues (Nigg, Goldsmith, & Sachek, 2004) proposed that ADHD might comprise heterogeneity temperamentally, reflecting differential dysregulation in different children of (a) irritability and anger-proneness but also possibly component traits such as sadness and fear, and (b) dysregulated positive affect, which relates to constructs such as surgency, extraversion, and sensation seeking. Subsequent empirical work, using computational discovery methods and clinical follow-up validation, has supported this hypothesis phenotypically (Karalunas et al., 2014; Karalunas, Gustafsson, Fair, Musser, & Nigg, 2019). However, an important caveat is that children with ADHD and dysregulation of positive affect also experience moderately elevated anger-proneness (Karalunas et al., 2019). Although anger loads on the negative affect factor in the temperament trait framework, other models reframe these same traits in motivational terms (approach-avoidance) (Nigg, 2006). From that perspective, anger can also be part of the approach trait (e.g., goal-related frustration) (Vidal-Ribas, Brotman, Valdivieso, Leibenluft, & Stringaris, 2016). The field needs clarification of how these various ideas about emotional dysregulation fit together in a comprehensive picture for ADHD nosology.
Issue #3: A classic way to evaluate nosological hypotheses is with genetic research but this area is rapidly changing (Robins & Guze, 1970; Vidal-Ribas et al., 2016)
Twin and molecular studies indicate overlap in genetic risk for various psychiatric conditions, including for ADHD with both ODD (Nadder, Rutter, Silberg, Maes, & Eaves, 2002) and other psychiatric disorders (Anttila et al., 2018), suggesting pleiotropy. However, pleiotropy is not the entire story. Genetic correlations are significant but low, with more common genetic variance being unique to disorders rather than shared. Thus, improved mapping among dimensions and domains of psychopathology remains critical to improved nosology related to basic neurobiology (Cuthbert & Insel, 2013) and genetic studies play a key role.
Recent work has examined genetic association of ADHD with related constructs in Table 1, including emotional lability (Merwood et al., 2014) and irritability (Riglin et al., 2017; Stringaris, Zavos, Leibenluft, Maughan, & Eley, 2012) using both quantitative and molecular approaches. An ongoing issue is whether these measures, when associated with ADHD, reflect underlying genetic liability for ADHD, or liability for a comorbid risk, particularly mood disorder.
The genetic structure of ADHD is complex, including common polygenic variation, rare structural variants, and epigenetic effects (Faraone & Larsson, 2019). A useful approach examines the cumulative effect of common DNA variants using a polygenic risk score (PRS) (Martin, Daly, Robinson, Hyman, & Neale, 2018). Whereas no study to date has used this technique to examine the full range of emotional dysregulation in ADHD, pioneering work in the irritability aspect of this domain was reported by Riglin et al (Riglin et al., 2017). They reported that irritability correlated with an ADHD PRS and not a depression PRS. As the first PRS study to examine this critical nosological controversy, Riglin et al had several strengths, including the use of two large population samples and one clinical sample. However, limitations included a relatively small discovery cohort (limiting the accuracy of the PRS), limited assessment of ADHD in the population cohorts (reliant on one reporter and in one sample only two items), and varying, few-item definitions of irritability. Key confounds not controlled included history of mood disorder and overlap of irritability with ADHD symptom severity, which could explain any association of the PRS and irritability. A more recent study (Gisbert et al., 2019) noted that a PRS for emotional lability (negative affect related) correlated with emotional lability in ADHD, but did not examine ADHD genetic risk.
Current aims
We aimed to further map the boundaries of genetic association, to help clarify nosology related to emotion dysregulation, anger-irritability, and ADHD. We studied whether emotional dysregulation or irritability in an ADHD sample are related to (a) genetic liability for ADHD (“part of the core features of the disorder”) or (b) liability for mood disorder (“a comorbid feature”). We use both variable-centered and person-centered approaches to enhance clarity.
Method
Participants
Participants were 514 unrelated children ages 7–11 of Northern European ancestry. In this case-control design, ADHD was deliberately oversampled to ensure adequate clinical range variation to detect genetic signal as recommended by others (Benca et al., 2017), and to enable us to examine ADHD heterogeneity (e.g., related to irritability). To preserve representativeness, we did not oversample for sex or other demographics.
Recruitment and diagnostic assignment.
The local university institutional review board provided human subjects approval. A parent/legal guardian provided written informed consent, and children provided written assent. After screening, the research team conducted a diagnostic evaluation using standardized, well-normed rating scales from parent and teacher, parent semi-structured clinical interview, child intellectual testing, and clinical observation. Best-estimate research diagnoses and final eligibility were established by a team of two experienced clinicians (a child psychiatrist and a child psychologist), who independently assigned final diagnoses and comorbid disorders including ADHD, ODD, and any lifetime mood disorder (major depression, dysthymia, or other), as reported herein. Exclusion criteria included psychiatric medications other than short-acting stimulants, history of seizures or head injury, parent–teacher rating discrepancy making diagnosis uncertain, psychosis, mania, current major depressive episode, Tourette’s syndrome, autism, and IQ<80. See more recruitment details in Appendix S1; medication status in this sample is provided in Table S2.
Relatedness and final sample.
From 2144 volunteers, n=850 eligible children were identified. For the genetic analysis, related children were removed, resulting in n=656 unrelated children. Of these, n=514 (ADHD n=337) comprised the European-ancestry sample selected to minimize effects of population stratification.
Temperament measure.
A parent/guardian completed the Temperament in Middle Childhood Questionnaire (TMCQ) (Simonds & Rothbart, 2004). They were asked to try to rate how the child is when not taking any treatment or medication. The 157 TMCQ items combine into 16 scales (Simonds & Rothbart, 2004). Appendix S2 provides detailed background on the TMCQ and items included in the critical scales in the current paper noted below; Table S3-A provides the factor structure in the current sample, while Table S3-B and S3-C provide principal component results related to the combining of temperament and ODD items for what follows.
Data reduction:ADHD, irritability, and emotional dysregulation as dimensions.
The primary analysis relied on dimensional scores estimated as latent variables in a structural equation model. Because ADHD is widely seen as a polygenic trait, and because latent variables add sensitivity for detecting genetic effects (Nigg et al., 2018), we created a robust latent variable for ADHD as a quantitative traits. The ADHD indicators were the relevant inattention and hyperactivity subscale scores on the ADHD Rating Scale (ADHD-RS) (DuPaul, Power, Anastopoulos, & Reid, 1998), KSADS (parent-only) (Puig-Antich & Ryan, 1986), Conners Parent Rating Scale–3rd ed. (Conners, 2003), and Strengths and Difficulties Questionnaire (SDQ) (Goodman, 2001). Measurement models fit well (parent: RMSEA=0.057, CFI=.996, TLI=.992; teacher: RMSEA=.000, CFI=1.00, TLI=1.00).
Indicators for the irritability latent variable for SEM analysis were two TMCQ scale scores: anger and modified soothability (please see Appendix S2) (Karalunas et al., 2019) and the three-item ODD irritable total score (Stringaris & Goodman, 2009) (Figure S1). This was justified by a preliminary principle component analysis (PCA) of the TMCQ scales with and without the ODD irritability scale (recall Table S3-A, S3-B, S3-C) and an adequate CFA fit (Figure S1). Finally, based on the aforementioned PCA analyses (recall Table S3A–C), we created latent variables for surgency-approach and on conceptual grounds, having separated anger from other negative affect measures, a latent variable for sadness-anxiety (recall Table S3-C). Children with missing TMCQ scores (n=6) were included in the primary dimensional analyses via missing data procedures.
Data reduction: group profiles.
The person-centered analysis considered heterogeneity and examined putative subgroups. First, we considered three temperament profiles in the ADHD sample identified computationally using an optimization clustering method called community detection, derived from graph theory (Newman, 2006; Rubinov & Sporns, 2011), which provides an estimate of groupness (Q statistic). Procedures were identical to (Karalunas et al., 2014) and detailed in the Appendix S3. The procedure supported distinct profiles in the ADHD group (Q>.4; while Q>.2 is customarily considered good evidence of groupness in the data) labeled as Mild (normative emotion regulation) (n=108), Surgent (high sensation seeking, sociability, and activity) (n=122), and Irritable (high anger and low soothability) (n=101). We considered the TMCQ Surgent and Irritable profiles together as the emotionally dysregulated subset of ADHD (profiles depicted in more detail in Figure S2). Finally, for comparability to prior studies, we alternatively defined an Irritable group of children defined as having at least one of the three ODD irritable symptoms endorsed as present in the last six months on the parent Kiddie Schedule for Affective Disorders (KSADS) interview as suggested in the literature (Riglin et al., 2017; Stringaris & Goodman, 2009). This procedure identified 91 children as “irritable” within the ADHD group. Table S4 shows that nearly all of these children were in one of the TMCQ dysregulated groups, but split somewhat across Irritable and Surgent profiles.
Missing data.
We implemented full information maximum likelihood in MPLUS 7.4.
Genotyping and polygenic score
Genotyping.
Salivary DNA samples were genotyped at the Stanley Center for Psychiatric Research (Broad Institute of Massachusetts Institute of Technology (MIT) and Harvard, Cambridge, MA) using the PsychCHIP_v1–1 (n=603,132 single nucleotide polymorphisms [SNPs]), developed by Illumina, Inc. (San Diego, CA) in collaboration with the Psychiatric Genomics Consortium (PGC). Appendix S4 provides processing and quality control details.
PRS computation.
The polygenic risk score (PRS) for ADHD was constructed using the 2018 PGC meta-analysis (Demontis, Walters, Martin, et, & al., 2019) as the discovery data set but restricted only to the European-ancestry sub-population (19,099 individuals with ADHD and 34,194 controls) of the PGC data (full PGC data is 20,183 ADHD cases and 35,191 controls). We calculated the PRS for depression using as discovery sets the 2018 PGC MDD meta-analysis (59,851 cases and 113,154 controls, European-ancestry only) (Wray et al., 2018). Using a cutoff for SNP selection of p<.50, the number of SNPs was as follows: ADHD PRS 139,934, Depression PRS 132,213. Results using alternative p-value cutoffs for SNP selection were similar (Figure S3).
Covariates.
We covaried sex, age, lifetime mood disorder (dysthymia or depression), and the first ten genetic principal components in all models. We controlled ADHD severity secondarily to ensure its shared variance with the ADHD PRS and irritability or emotional dysregulation did not account for findings.
Data analysis
The primary analysis using SEM implemented the maximum likelihood estimator in MPLUS 7.4, with standard indices of model fit (CFI, TLI, and RMSEA) (Browne & Cudeck, 1993). The person-centered analysis then compared different group definitions of ADHD with and without irritability and emotion dysregulation using logistic regression. As these analyses aimed to discover whether results depend on a particular definition of the same construct, multiple testing correction was not appropriate. For each PRS effect we provide effect specific r2 change; for categorical models this is the Nagelkerke r2 change. Sensitivity analysis considered whether changes in latent variable indicators or covariate handling would affect results.
Results
Overview and sample description
Table 2 provides a clinical and demographic description of the sample (same sample as (Nigg et al., 2018)). Age did not differ statistically among these groups. The ADHD group had a higher proportion of boys. The ADHD sample was 72% combined presentation and 26% inattentive presentation, with 2% hyperactive presentation.
Table 2:
Sample Descriptive Data (Mean, SD, or %)
| Control | ADHD | |
|---|---|---|
| N | 177 | 337 |
| % male* | 54% | 72% |
| % non-Hispanic white | 100% | 100% |
| Age at intake | 9.4(1.5) | 9.5(1.5) |
| Income (thousands $) | 96.8 | 90.0 |
| Estimated full scale IQ* | 115(12.8) | 109.7(13.5) |
| Reading* | 113.9(11.1) | 106.4(14) |
| ADHD-RS Parent-rated T: H* | 45.2(7.5) | 67.9(14.4) |
| ADHD-RS Parent-rated T: I* | 44.5(7.1) | 72.3(12.3) |
| % ODD* | 1% | 19% |
| % lifetime Mood disorder* | 3% | 9% |
| % irritability by ODD 2* | 5% | 23% |
| ADHD med* | 0% | 39% |
Notes: Lifetime mood=dysthymia or major depressive episode. ADHD-RS parent rated T: I=inattention, H=hyperactivity-impulsivity. ODD irritability defined as any of the 3 DSM irritability symptoms are “definite” on KSADS. Age and sex covaried for all clinical measures.
ADHD-controls, p<.05.
Primary variable-centered analyses
Do irritability-anger, surgency-approach, or negative mood share polygenic effects with ADHD or MDD?
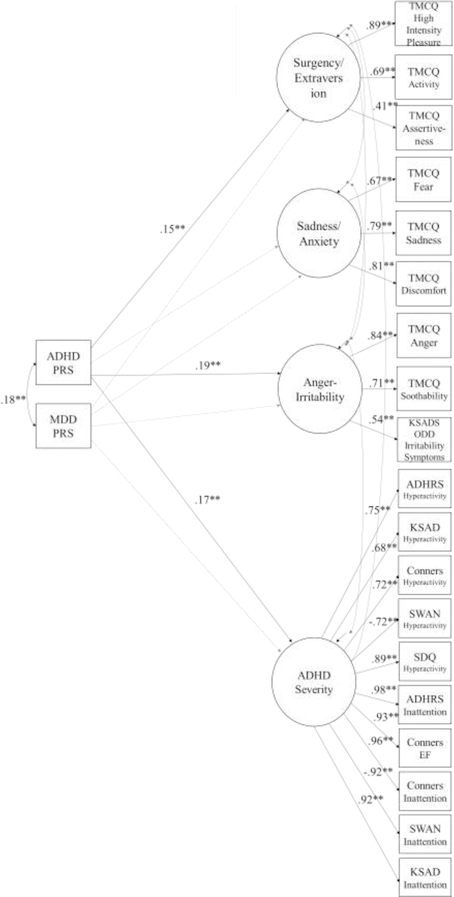
Figure 1 displays the SEM model, showing that even after adjusting for the correlations of (a) the PRS scores with one another and of (b) the emotion scores with one another and with ADHD, the ADHD PRS is related to irritability and to surgency. ADHD PRS is not reliably related to other negative affect, such as sadness, fear, or anxiety. The MDD PRS was not reliably related to these outcome variables.
Figure 1: Final SEM model showing joint PRS effects on multi-variate refined ADHD phenotype and temperament domains. Explanation:
Figure 1 shows that even allowing for their overlap with ADHD symptom severity (which encompasses the ADHD and Control group difference as well as variation within them), Surgency and Anger-irritability are related to ADHD genetic risk and not MDD genetic risk when ADHD genetic risk is in the model, whereas sadness/fear/anxiety are not related to ADHD genetic risk.
Model Fit: N=514; χ2 = 940.26; df = 392; p = .00; CFI = .95; TLI = .94; RMSEA = .05;
Correlations among latent variables (not shown in diagram to ease readability): Surgency and Sadness/Anxiety, r= −.23**; and Anger-Irritability, r= .19**; and ADHD, r= .13**; Sadness/ Anxiety and Anger-Irritability: r= .85**; and ADHD, r= .40**; Anger/Irritability and ADHD, r= .52** (all p<.01).
Covariates not shown: Age, sex, lifetime mood disorder (depression or dysthymia), first 10 genetic principal components
Key: PRS=polygenic risk score; All circles are latent variables depicting child traits as rated by multiple indicators of parent ratings (indicators listed in square boxes). Surgency=Surgency extraversion. TMCQ, KSADS, SWAN, SDQ, and Conners all measures defined in text. Paths with values and ** are reliable at p<.01; paths that are faded out are unreliable at p>.05. Alternative models are provided in the online Supplement (see text).
Person-centered (typology) analysis
Table 3 provides the ADHD and MDD PRS scores for different clinical groupings, for reference in the analyses that follow. Table 4 provides the statistical group comparisons. It shows that the above-described pattern of findings held for alternative operationalization of child ADHD, irritability, and emotional dysregulation. When considered in separate models, the ADHD PRS was associated with greater odds of being in (a) the ADHD (vs. non-ADHD), (b) the TMCQ Emotionally dysregulated versus other ADHD, and (c) the ODD-defined irritability group. However, only the former (emotionally dysregulated) survived control for ADHD symptom severity across these sub-groups. The MDD PRS generally did not correlate reliably with subgroups within ADHD, which is consistent with dimensional results suggesting that ADHD PRS related to both surgency and irritability-anger.
Table 3.
Descriptive Statistics: Polygenic Scores for Clinical and Emotion groups (Mean, SD) by Different Group Definitions
| Group | ADHD PRS | MDD PRS |
|---|---|---|
| Control (n=177) | .461(.153) | .339(.137) |
| ADHD (n=337) | .512(.139) | .351(.140) |
| ADHD-C (n=243) | .519(.137) | .360(.138) |
| ADHD-PI (n=87) | .489(.146) | .331(.146) |
| ADHD divided by TMCCQ temperament profile | ||
| ADHD-Mild (n=108) | .482(.144) | .333(.139) |
| ADHD-Surgent (n=122) | .534(.132) | .358(.134) |
| ADHD-Irritable (n=101) | .518(.139) | .360(.140) |
| ADHD divided by presence/absence of ODD | ||
| Without ODD (n=273) | .506(.140) | .345(.133) |
| With ODD (n=64) | .536(.133) | .376(.166) |
| ADHD divided by presence/absence of “irritability” within ODD items | ||
| “not ODD irritable” (n=238) | .497(.145) | .348(.129) |
| “ODD irritable” (n=99) | .541(.126) | .357(.154) |
| ADHD divided by presence/absence of lifetime MDD | ||
| ADHD never MDD (n=308) | .515(.140) | .350(.141) |
| ADHD+lifetime MDD (n=29) | .480(.134) | .366(.135) |
Notes: Group assignments are mutually exclusive within section (i.e., within TMCQ, ODD, or MDD section) but not across those sections of the table. With and without MDD and ODD as defined by diagnostic team consensus (Methods). ODD irritable defined as in “irritability ODD scale 2” in table 1, that is, at least one of the three ODD irritable items endorsed as present past 6 months on parent KSADS interview. ADHD-C=ADHD combined presentation, ADHD-PI=ADHD Predominantly inattentive presentation. 7 children with ADHD predominantly hyperactive presentation omitted from the ADHD subtype comparison. 2 Control children with ODD omitted from ODD-non-ODD comparison. 6 children missing TMCQ scores omitted from TMCQ group comparison. Polygenic scores are scaled 0–1 in this table for ease of interpretation.
Table 4:
Sensitivity analysis of different emotion and ADHD group definitions with ADHD and MDD PRS
| A: ADHD PRS Effects | ||||||||
|---|---|---|---|---|---|---|---|---|
| Full Model without ADHD-RS | Covary ADHD Severity (ADHD-RS Total) | |||||||
| OR | 95% CI | ΔR2 | p | OR | 95% CI | ΔR2 | p | |
| ADHD vs non-ADHD | 1.43 | 1.17–1.75 | 0.033 | 0.0004 | na | na | na | na |
| ODD (n=65) vs non-ODD (447) | 1.47 | 1.11–1.95 | 0.025 | 0.007 | 1.28 | 0.93–1.75 | 0.007 | 0.130 |
| ADHD-C (n=243) vs ADHD-PI (n=87) | 1.16 | 1.12–1.21 | 0.014 | <.001 | 1.09 | .79–1.51 | 0.001 | 0.585 |
| ADHD-TMCQ Irritable vs other ADHD | 1.08 | 0.83–1.40 | 0.001 | 0.578 | 0.98 | 0.74–1.30 | <.001 | 0.893 |
| ADHD-TMCQ Em Dysreg vs other ADHD | 1.50 | 1.15–1.96 | 0.022 | 0.003 | 1.44 | 1.03–2.02 | 0.013 | 0.033 |
| ADHD-ODD irritable vs other ADHD | 1.36 | 1.04–1.78 | 0.023 | 0.023 | 1.31 | 0.99–1.72 | 0.015 | 0.055 |
| B: MDD PRS Effects | ||||||||
| Full Model without ADHD-RS | Covary ADHD Severity (ADHD-RS Total) | |||||||
| OR | 95% CI | ΔR2 | P | OR | 95% CI | ΔR2 | p | |
| ADHD vs non-ADHD | 1.03 | .83–1.27 | <.001 | 0.779 | na | Na | na | na |
| ODD (n=65) vs non-ODD (n=447) | 1.46 | 1.07–2.00 | 0.020 | 0.018 | 1.50 | 1.07–2.12 | 0.018 | 0.020 |
| ADHD-C (n=243) vs ADHD-PI (n=87) | 1.27 | .94–1.71 | 0.010 | 0.123 | 1.22 | .85-.175 | 0.004 | 0.277 |
| ADHD-TMCQ Irritable vs other ADHD | 1.28 | .96–1.28 | 0.011 | 0.098 | 1.25 | .91–1.17 | 0.007 | 0.168 |
| ADHD-TMCQ Em Dysreg vs mild ADHD | 1.31 | .97–1.74 | 0.014 | 0.062 | 1.24 | .86–1.80 | 0.004 | 0.249 |
| ADHD-ODD irritable vs other ADHD | 1.06 | .80–1.39 | 0.001 | 0.692 | 1.04 | .79–1.39 | 0.001 | 0.769 |
Note: Odds ratios and p-values adjusted for age, sex, lifetime mood disorder, and the first 10 genetic principal components. The right hand model additionally controls for ADHD Severity, measured as parent ADHD Rating Scale (ADHD-RS) total score (0–27, with each item scored 0–3).ΔR2 is Nagelkerke R2 change for the PRS effect after entry of all covariates. ADHD-ODD irritable defined as at least one ODD irritable item endorsed as definite in last 6 months on KSADS. 2 control children with ODD are omitted from the ODD comparisons. TMCQ Em Dysreg is “emotion dysregulated” which combines the TMCQ Irritable and Surgent profile groups. ADHD-C=ADHD combined presentation, ADHD–PI=ADHD predominantly inattentive presentation. 7 children with ADHD hyperactive presentation are omitted from ADHD subtype comparison.
Table 4 shows results for ADHD DSM presentations, highlighting that the emotion dysregulation profile aids in detecting genetic signal compared to the DSM profiles, which mainly reflect ADHD severity.
Sensitivity analysis
In sensitivity analysis, the dimensional results in Figure 1 are essentially unchanged if MDD and ADHD PRS scores are considered alone (Table S5, see Table S6 for results; includes results for bipolar PRS for reference); if irritability is defined only by ODD items or only by TMCQ scales (Table S7); if the Conners EF score is removed as an indicator of ADHD (Table S8), or if the latent emotion/temperament variables are considered individually rather than simultaneously (Table S9). Thus results did not depend on particular methodological decisions.
Discussion
A perennial clinical and conceptual question concerns whether symptoms of emotional dysregulation are due to a comorbid condition or more central to ADHD (Barkley & Fischer, 2010; Faraone et al., 2019). Two fundamental ideas concern irritability (anger-dysregulation) (Vidal-Ribas et al., 2016) or emotion dysregulation (Barkley, 2010), the latter seen as encompassing both negative valence (e.g., anger) and positive valence (e.g., sensation seeking) dysregulation, are hypothesized as pertinent to ADHD. Differentiation from mood-related risk is of central concern due to the association of irritability with future mood disorder (Stringaris, Zavos, et al., 2012).
The present study had several strengths. We developed a carefully characterized case-control cohort, used a GWAS derived measure of genetic liability (the PRS), and well-validated and conceptually rich measures of irritability (anger-related dysregulation) and emotional dysregulation (conceived as including both approach-related and anger-related affective regulation). We conducted simultaneous modeling to ensure results were not due to correlations among traits or overlap of trait with PRS and ADHD severity, and ruled out past mood disorder as an explanation. We considered effects both from the perspective of a variable-centered, SEM-based, dimensional analyses, and two person-centered approaches—one derived from computational work to identify temperament profiles, and one taken from prior literature. Extensive sensitivity testing and convergence across analytic perspectives ensured that conclusions were not dependent on particular operational definitions of constructs or ways of structuring data.
The results consistently support three conclusions: (a) emotion dysregulation is indeed part of ADHD genetic risk not comorbidity risk per se, (b) irritability in ADHD populations is part of ADHD- and not depression-related genetic risk, and (c) the relevant emotion dysregulation domain for ADHD genetic risk includes irritability but extends beyond it to approach-related dysregulation (surgency-sensation seeking).
Conceptually and clinically, the results support a heterogeneity model of ADHD involving different etiological routes to ADHD via temperament, as proposed over a decade ago by Nigg and colleagues (Nigg et al., 2004). That model suggests that ADHD can entail early life breakdowns in regulation of both negative (anger) affect and approach (sensation seeking) motivation, in addition to primary breakdowns in control. This model is echoed in Figure 1’s three paths from ADHD genetic risk. This result also is consistent with the claim that a broader construct of emotional dysregulation or emotional impulsivity (Barkley & Fischer, 2010) is central to ADHD. The results also are largely in line with a conceptualization of ADHD as a disorder of self-regulation. In that perspective, self-regulation involves closely intertwined functioning of regulation of emotional arousal as well as cognitive abilities (e.g., focused attention, which also serves a regulatory function) (Faraone et al., 2019; Nigg, 2017a). Findings related to irritability confirm and clarify results of an earlier report (Riglin et al., 2017). Results, however, expand their conclusions to surgency-related dysregulation in ADHD.
Phenotypically, we like others found that anger-dysregulation was highly correlated with negative affect (sadness, anxiety, fear; Figure 1, Table S3). However, it is interesting that it shared ADHD genetic risk with Surgency; this is consistent with suppositions that anger dysregulation in ADHD may be associated biologically with approach-related dysregulation. Further work should reconcile these findings across biological and behavioral levels of analysis. Yet it was notable here that when irritability is defined by ODD symptoms, it detects ADHD genetic risk but encompasses both Surgent and irritable types in relation to the temperament model.
Dimensional models are strongest for detecting genetic effects on continuous polygenic trait dimensions, while person-centered approaches facilitate clinical translation. It is reassuring, here, that sensitivity analysis identified a similar pattern of findings using a clinical profile perspective. In addition, emotionally dysregulated children with ADHD had higher ADHD PRS scores than emotionally normative ADHD children, regardless of which emotionally dysregulated profile they were assigned to. Findings are consistent with suggestions ADHD genetic risk is associated with multiple facets of affective dysregulation. Neither irritable (variously defined) nor emotionally dysregulated ADHD children differ on MDD PRS after controlling ADHD severity.
We note key limitations. Polygenic scores are not direct evidence of genetic causality—like any other correlational analysis, they are vulnerable to unmeasured third variables that may explain the observed association (or, genetically, to pleiotropic effects) (Martin et al., 2018). The sample was community-recruited and selected for ADHD and non-ADHD status, but this complements prior studies (Riglin et al., 2017). The MDD PRS narrowly missed significance in some models, suggesting that with more statistical power a small additive effect might emerge, although sample size here was respectable for a case control study and results parallel Riglin et al. (2017). The surprising failure of the MDD PRS to relate to sadness/anxiety traits warrants more study but may be due to control of prior mood disorder in this sample. Finally, we only examined children; results may differ in adolescents or adults.
Overall, results provide additional molecular genetic evidence that cumulative common genetic risk loading for ADHD is shared with features of emotional dysregulation, including but not limited to irritability. They support a clinical view that emotion dysregulation, broadly, is part of the core ADHD presentation for many children and further highlight the heterogeneous nature of the disorder. They also suggest that continued refinement of clinical profiles will be fruitful to align the nosology maximally with these partially distinguishable genetic risk factors.
Supplementary Material
Supporting information
Additional methods details
Table S1. Combined items used in Table 1.
Appendix S1. Participants Recruitment and Sample Characterization.
Table S2. Medications in ADHD sample among those medicated.
Appendix S2. Evaluation of Trait-level Emotion Regulation by TMCQ.
Table S3. TMCQ factor structure current sample with and without ODD.
Figure S1. Irritability Measurement Model current study.
Appendix S3. Creation of Temperament Profiles: Methods.
Figure S2. TMCQ emotion regulation profiles description.
Table S4. Overlap of Irritability by ODD and TMCQ models.
Appendix S4. Genotyping and Polygenic score Methods details.
Sensitivity analyses
Figure S3. Effects of PRS P-value Threshold Variation.
Table S5. Effect of Multivariate Model Control versus Univariate.
Table S6. Parameter values for bipolar PRS.
Table S7. Effect of ADHD PRS on Irritability.
Table S8. If Conners EF scale removed from ADHD indicator list.
Table S9. Simple Models ADHD PRS and MDD PRS.
Key points.
Emotional dysregulation, including both irritability and surgency (e.g., sensation seeking and related traits) is salient in ADHD.
Controversy has ensued as to the best way to represent emotional and clinical heterogeneity in the ADHD population, as part of the syndrome or as comorbid.
Emotion dysregulation as a trait was related to polygenic risk for ADHD more convincingly than polygenic risk for depression.
Acknowledgments
Support was provided by NIH R01-MH099064 and R37-MH-59105. The authors thank Benjamin Neale, Ph.D. for consultation on methodology and analysis. D.A.F. is a founder of Nous Imaging, Inc. but its activities are unrelated to the current study and any potential conflict of interest has been reviewed and managed by OHSU. S.V.F. has received grant or research support from the K.G. Jebsen Centre for Research on Neuropsychiatric Disorders, the University of Bergen, Bergen, Norway, the European Union’s Seventh Framework Programme for research, technological development, and demonstration, the European Union’s Horizon 2020 research and innovation programme, and the National Institute of Mental Health. S.V.F. has received income, potential income, travel expenses, continuing education support, research support from, and/or has served on the advisory boards of/as a consultant to Lundbeck, Rhodes, Arbor, KenPharm, Ironshore, Neurovance, Impact, Takeda, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, Sunovion, Genomind, and NeuroLifeSciences. In previous years, S.V.F. has received income or research support from Shire, Neurovance, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer, and Eli Lilly and Co. S.V.F. has served as editor of the American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. S.V.F.’s institution (SUNY) has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. S.V.F. has received royalties from books published by Guilford Press (Straight Talk about Your Child’s Mental Health), Oxford University Press (Schizophrenia: The Facts), and Elsevier (ADHD: Non-Pharmacologic Interventions). S.V.F. has held stock in CogCubed and Ironshore. S.V.F. is the principal investigator of http://adhdinadults.com/. The remaining authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: See Acknowledgements for full disclosures.
References
- Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. (2018). Analysis of shared heritability in common disorders of the brain. Science, 360(6395), eaap8757 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R (2010). Deficient emotional self-regulation: a core component of attention-deficit/hyperactivity disorder. J ADHD and Related Disorders, 1, 5–37. [Google Scholar]
- Barkley RA (1997). Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin, 121(1), 65–94. 10.1037/0033-2909.121.1.65 [DOI] [PubMed] [Google Scholar]
- Barkley RA, & Fischer M (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry, 49(5), 503–513. [DOI] [PubMed] [Google Scholar]
- Benca CE, Derringer JL, Corley RP, Young SE, Keller MC, Hewitt JK, et al. (2017). Predicting cognitive executive functioning with polygenic risk scores for psychiatric disorders. Behavior Genetics, 47(1), 11–24. 10.1007/s10519-016-9814-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, & Cudeck R (1993). Alternative ways of assessing model fit Testing structural equation models (Vol. 154, pp. 136–162). Newbury Park, CA: Sage. [Google Scholar]
- Conners CK (2003). Conners’ rating scales: Revised technical manual New York, NY: Multi-Health Systems. [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine, 11(1), 126 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, et, & al. (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet, 51(1), 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul G, Power T, Anastopoulos A, & Reid R (1998). ADHD rating scale—IV: checklists, norms, and clinical interpretation NY, NY: Guilford Press. [Google Scholar]
- Faraone SV, & Larsson H (2019). Genetics of attention deficit hyperactivity disorder. Molecular Psychiatry, 24(4), 562–575. 10.1038/s41380-018-0070-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Rostain AL, Blader J, Busch B, Childress AC, Connor DF, et al. (2019). Practitioner Review: Emotional dysregulation in attention-deficit/hyperactivity disorder - implications for clinical recognition and intervention. Journal of Child Psychology and Psychiatry and Allied Disciplines, 60(2), 133–150. 10.1111/jcpp.12899 [DOI] [PubMed] [Google Scholar]
- Gisbert L, Vilar L, Rovira P, Sanchez-Mora C, Pagerols M, Garcia-Martinez I, et al. (2019). Genome-wide analysis of emotional lability in adult attention deficit hyperactivity disorder (ADHD). European Neuropsychopharmacology 10.1016/j.euroneuro.2019.04.004 [DOI] [PubMed]
- Goodman R (2001). Psychometric properties of the strengths and difficulties questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 40(11), 1337–1345. [DOI] [PubMed] [Google Scholar]
- Goodman R, Ford T, Richards H, Gatward R, & Meltzer H (2000). The Development and Well-Being Assessment: description and initial validation of an integrated assessment of child and adolescent psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines, 41(5), 645–655. [PubMed] [Google Scholar]
- Karalunas SL, Fair D, Musser ED, Aykes K, Iyer SP, & Nigg JT (2014). Subtyping attention-deficit/hyperactivity disorder using temperament dimensions: toward biologically based nosologic criteria. JAMA psychiatry, 71(9), 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Karalunas SL, Gustafsson HC, Fair D, Musser ED, & Nigg J (2019). Do we need an irritable subtype of ADHD? Replication and extension of a promising temperament profile approach to ADHD subtyping. Psychological Assessment: A Journal of Consulting and Clinical Psychology, 31(2), 236–247. 10.1037/pas0000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Martin J, Agha SS, Davies C, Stergiakouli E, Holmans P, et al. (2011). Clinical and cognitive characteristics of children with attention-deficit hyperactivity disorder, with and without copy number variants. British Journal of Psychiatry, 199(5), 398–403. 10.1192/bjp.bp.111.092130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AR, Daly MJ, Robinson EB, Hyman SE, & Neale BM (2018). Predicting Polygenic Risk of Psychiatric Disorders. Biological Psychiatry 10.1016/j.biopsych.2018.12.015 [DOI] [PMC free article] [PubMed]
- Merwood A, Chen W, Rijsdijk F, Skirrow C, Larsson H, Thapar A, et al. (2014). Genetic associations between the symptoms of attention-deficit/hyperactivity disorder and emotional lability in child and adolescent twins. Journal of the American Academy of Child and Adolescent Psychiatry, 53(2), 209–220 10.1016/j.jaac.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, Maes HH, & Eaves LJ (2002). Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (Odd/CD) symptomatologies across informant and occasion of measurement. Psychological Medicine, 32(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Newman MEJ (2006). Modularity and community structure in networks. Proceedings of the National Academy of Sciences of the United States of America, 103(23), 8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3–4), 395–422. 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017a). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry and Allied Disciplines, 58(4), 361–383. 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT (2017b). Getting Ahead of ADHD New York: Guilford Press. [Google Scholar]
- Nigg JT, Goldsmith HH, & Sachek J (2004). Temperament and attention deficit hyperactivity disorder: the development of a multiple pathway model. Journal of Clinical Child & Adolescent sychology, 33(1), 42–53. 10.1207/s15374424jccp3301_5 [DOI] [PubMed] [Google Scholar]
- Nigg JT, Gustafsson HC, Karalunas SL, Ryabinin P, McWeeney SK, Faraone SV, et al. (2018). Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 57(3), 175–182. 10.1016/j.jaac.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (2000). Developing mechanisms of self-regulation. Development and Psychopathology, 12(3), 427–441. [DOI] [PubMed] [Google Scholar]
- Power C, & Elliott J (2006). Cohort profile: 1958 British birth cohort (National Child Development Study). International Journal of Epidemiology, 35(1), 34–41. 10.1093/ije/dyi183 [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, & Ryan N (1986). Kiddie schedule for affective disorders and schizophrenia Pittsburgh, PA: Western Psychiatric Institute. [Google Scholar]
- Reimherr FW, Marchant BK, Strong RE, Hedges DW, Adler L, Spencer TJ, et al. (2005). Emotional dysregulation in adult ADHD and response to atomoxetine. Biological Psychiatry, 58(2), 125–131. 10.1016/j.biopsych.2005.04.040 [DOI] [PubMed] [Google Scholar]
- Riglin L, Eyre O, Cooper M, Collishaw S, Martin J, Langley K, et al. (2017). Investigating the genetic underpinnings of early-life irritability. Transl Psychiatry, 7(9), e1241 10.1038/tp.2017.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins E, & Guze SB (1970). Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. American Journal of Psychiatry, 126(7), 983–987. 10.1176/ajp.126.7.983 [DOI] [PubMed] [Google Scholar]
- Robison R, Marchant B, Kondo D, Gj Olsen J L, D, C., C, P., et al. (2010). The use of emotional dysregulation as an endophenotype for genetic studies in adults with attention-deficit/hyperactivity disorder (Vol. 1). [Google Scholar]
- Rothbart MK (2011). Becoming who we are : temperament and personality in development New York: Guilford Press. [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, & Posner MI (2011). Developing mechanisms of self-regulation in early life. Emotion Review, 3(2), 207–213. 10.1177/1754073910387943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2011). Weight-conserving characterization of complex functional brain networks. Neuroimage, 56(4), 2068–2079. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, & Leibenluft E (2014). Emotion dysregulation in attention deficit hyperactivity disorder. American Journal of Psychiatry, 171(3), 276–293. 10.1176/appi.ajp.2013.13070966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds J, & Rothbart MK (2004). The Temperament in Middle Childhood Questionnaire (TMCQ): A computerized self-report measure of temperament for ages 7–10. Paper presented at the Occasional Temperament Conference, Athens, GA. [Google Scholar]
- Skirrow C, McLoughlin G, Kuntsi J, & Asherson P (2009). Behavioral, neurocognitive and treatment overlap between attention-deficit/hyperactivity disorder and mood instability. Expert Review of Neurotherapeutics, 9(4), 489–503. 10.1586/ern.09.2 [DOI] [PubMed] [Google Scholar]
- Stringaris A, & Goodman R (2009). Three dimensions of oppositionality in youth. Journal of Child Psychology and Psychiatry and Allied Disciplines, 50(3), 216–223. 10.1111/j.1469-7610.2008.01989.x [DOI] [PubMed] [Google Scholar]
- Stringaris A, Goodman R, Ferdinando S, Razdan V, Muhrer E, Leibenluft E, et al. (2012). The Affective Reactivity Index: a concise irritability scale for clinical and research settings. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(11), 1109–1117. 10.1111/j.1469-7610.2012.02561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Zavos H, Leibenluft E, Maughan B, & Eley TC (2012). Adolescent irritability: phenotypic associations and genetic links with depressed mood. American Journal of Psychiatry, 169(1), 47–54. 10.1176/appi.ajp.2011.10101549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surman CB, Biederman J, Spencer T, Yorks D, Miller CA, Petty CR, et al. (2011). Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. American Journal of Psychiatry, 168(6), 617–623. 10.1176/appi.ajp.2010.10081172 [DOI] [PubMed] [Google Scholar]
- Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, & Stringaris A (2016). The status of irritability in psychiatry: a conceptual and quantitative review. Journal of the American Academy of Child and Adolescent Psychiatry, 55(7), 556–570. 10.1016/j.jaac.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Allen NB, Lubman DI, & Yücel M (2006). The neurobiological basis of temperament: Towards a better understanding of psychopathology. Neuroscience and Biobehavioral Reviews, 30(4), 511–525. [DOI] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. (2018). Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nature Genetics, 50(5), 668–681. 10.1038/s41588-018-0090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Additional methods details
Table S1. Combined items used in Table 1.
Appendix S1. Participants Recruitment and Sample Characterization.
Table S2. Medications in ADHD sample among those medicated.
Appendix S2. Evaluation of Trait-level Emotion Regulation by TMCQ.
Table S3. TMCQ factor structure current sample with and without ODD.
Figure S1. Irritability Measurement Model current study.
Appendix S3. Creation of Temperament Profiles: Methods.
Figure S2. TMCQ emotion regulation profiles description.
Table S4. Overlap of Irritability by ODD and TMCQ models.
Appendix S4. Genotyping and Polygenic score Methods details.
Sensitivity analyses
Figure S3. Effects of PRS P-value Threshold Variation.
Table S5. Effect of Multivariate Model Control versus Univariate.
Table S6. Parameter values for bipolar PRS.
Table S7. Effect of ADHD PRS on Irritability.
Table S8. If Conners EF scale removed from ADHD indicator list.
Table S9. Simple Models ADHD PRS and MDD PRS.