Abstract
Exposure to metals may promote the risk for cancers. We aimed to evaluate the associations of a broad spectrum of metals with gallbladder cancer (GBC) and gallstones. A total of 259 GBC patients, 701 gallstone patients, and 851 population-based controls were enrolled in Shanghai, China. A metallome panel was used to simultaneously detect 18 metals in serum through inductively coupled plasma–mass spectrometry. Logistic regression models were used to estimate crude or adjusted odds ratios (ORadj) with 95% confidence intervals (CIs) for the association between metal levels and gallbladder disease. Among the 18 metals tested, 12 were significantly associated with GBC and 6 with gallstones (Pcorrected < 0.002). Boron, lithium, molybdenum, and arsenic levels were associated with GBC compared to gallstones as well as gallstones compared to population-based controls. Elevated levels of cadmium, chromium, copper, molybdenum, and vanadium were positively associated with GBC versus gallstones, and the ORadj for the highest tertile (T3) compared to the lowest tertile (T1) ranged from 1.80 to 7.28 with evidence of dose-response trends (P < 0.05). Arsenic, boron, iron, lithium, magnesium, selenium, and sulfur were inversely associated with GBC, with the T3 versus T1 ORadj ranging from 0.20 to 0.69. Arsenic, boron, calcium, lithium, molybdenum, and phosphorous were negatively associated with gallstones, with the T3 versus T1 ORadj ranging from 0.50 to 0.75 (P < 0.05).
Conclusion:
Metals were associated with both GBC and gallstones, providing cross-sectional evidence of association across the natural history of disease. Longitudinal studies are needed to evaluate the temporality of metal exposure and gallbladder diseases and investigate mechanisms of disease pathogenesis.
Gallbladder cancer (GBC) is the most common malignancy of the biliary tract.(1) Although relatively uncommon, it is the sixth most common gastrointestinal cancer. GBC is notoriously difficult to diagnose early. The prognosis is poor, and there are few treatment options. Worldwide, GBC has low incidence (less than 2 per 100,000), but there is striking geographic variation.(2) High incidence rates occur in South America, Central and Eastern Europe, and North India.(3) Even within defined geographic regions, there are notable ethnic differences in GBC incidence. The risk of GBC is highest among American Indians, Mexicans, and Latin Americans in the United States and Mapuche Indians in South America. The presence of gallstones is an important risk factor for GBC.(4) Globally, GBC rates correlate well with the prevalence of gallstones, which more commonly affect certain indigenous populations.(5, 6) More than 85% of GBC patients are diagnosed with gallstones; however, fewer than 3% of gallstone patients develop GBC,(1, 7) suggesting that additional factors are needed to drive progression to GBC.
Environmental exposures are likely important.(8–10) Metals represent one environmental exposure of increasing interest.(11) Previous studies have typically evaluated one metal or a handful of metals. Metallomics, or the study of many metals simultaneously, is an emerging field, allowing a more comprehensive analysis of metals within a biological system. Metals are quintessential to maintain various biochemical and physiological functions in humans.(12) However, metals become noxious when they exceed certain concentrations. There are wide distributions of metals in the environment as well as in diet,(13) highlighting the importance of their potential effects on human health. A few small studies have examined the relationships between metal levels and GBC.(14, 15) However, these studies assessed few metals,(14, 15) lacked sufficient statistical power to detect associations, and were unable to evaluate other potential risk factors.
In the current study, we used a metallome platform to comprehensively examine associations between metals and gallstones or GBC cross-sectionally in a large population-based, case-control study.
Methods
Shanghai Biliary Tract Cancer Case-Control Study
The Shanghai Biliary Tract Cancer Study has been described in detail.(16) Briefly, the study enrolled 368 patients with incident GBC who were identified through a rapid reporting system coordinated by the Shanghai Cancer Institute with 42 collaborative hospitals in 10 urban districts of Shanghai from June 1997 through May 2001, covering more than 95% of GBC cases in Shanghai. Concurrently, we enrolled a total of 774 patients with gallstones undergoing cholecystectomy or medical treatment at the same hospital as the index GBC patients. In this population, nearly 90% of gallstone patients had either cholesterol or mixed types of stones.(16) The 959 healthy subjects without a history of cancer were randomly selected from the 6.5 million permanent residents of Shanghai as population-based controls using the personal registry cards of all adults older than 18 years of age in urban Shanghai. The response rates of the controls were more than 83%. All of the study subjects were interviewed personally with a structured questionnaire by trained interviewers, and the blood samples were collected according to a standardized protocol. The Shanghai Cancer Institute and National Cancer Institute institutional review boards approved the study. All participants provided written informed consent for collection of biospecimens and questionnaires.
In the current study, we included 259 GBC patients, 701 patients with gallstones, and 851 population-based controls with adequate serum samples (at least 120 μL) for metallome panel testing. In order to evaluate the associations of metal levels with gallstones, we compared patients with gallstones and population-based controls. To evaluate the associations of metals with GBC in the context of gallstones, we compared patients with GBC to patients with gallstones because gallstones are a relevant risk factor for GBC.
Measurements of metals levels
A metallome panel was used to allow simultaneous evaluation of metals previously associated with human health using inductively coupled plasma–mass spectrometry (ICP-MS),(17) which measures the presence of metals at the atomic level. In total, we measured 18 detectable analytes, including arsenic, boron, cadmium, calcium, chromium, cobalt, copper, iron, lithium, magnesium, manganese, molybdenum, nickel, phosphorous, selenium, sulfur, vanadium, and zinc. This metallome panel has the capability to measure metal measurements to the part per trillion (ppt) level. Because ICP-MS measures at the elemental level, the metal measurements are not affected by processing or freeze-thawing. Results were normalized to a certified reference standard to adjust for any batch effects. We also included 30 replicate quality control samples for each group (GBC, gallstone control, population-based control) with good correlations.
Statistical analysis
We compared the baseline characteristics for patients with GBC versus gallstones or gallstones versus population-based controls using t tests for difference in means for continuous variables and chi-squared tests for categorical variables. Serum levels of metals were categorized by tertiles (Ts) and compared for differences by the two groups (GBC vs. gallstones and gallstones vs. controls) by chi-squared tests with Bonferroni corrections. P values less than 0.002 were considered significant. Metals that were statistically significant associated with either gallstones or GBC were further examined by restricted cubic spline transformation to assess nonlinear relationships with GBC or gallstone risks. The reference value was set at the 25th percentile of the distribution for each metal. Logistic regression was used to obtain the crude or adjusted odds ratios (ORadj) with 95% confidence intervals (CIs) to investigate the magnitude of the associations between metal levels and GBC or gallstones, after adjustment for age, sex, body mass index (BMI), cigarette smoking, alcohol consumption, and levels of triglycerides and cholesterol. For these analyses, we categorized the metals based on tertiles of populations in each comparison (GBC vs. gallstones and gallstones vs. population-based controls). Finally, stepwise logistic regression models were used to consider the impact of all the metals taken together after adjustment for other risk factors. In order to assess the dose-response relationships between metal levels and disease risks, Cochran-Armitage tests for trend were performed. The sensitivity analyses were performed by restricting GBC patients with evidence of gallstones when we compared them with gallstone patients. The population-based controls with history of gallstones or cholecystitis were excluded when compared to gallstones in sensitivity analyses. The metal exposures on the associations with GBC were stratified by age or gender. And the interactions of metals and age or sex were tested by adding interaction terms in the models. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC) or R package.
Results
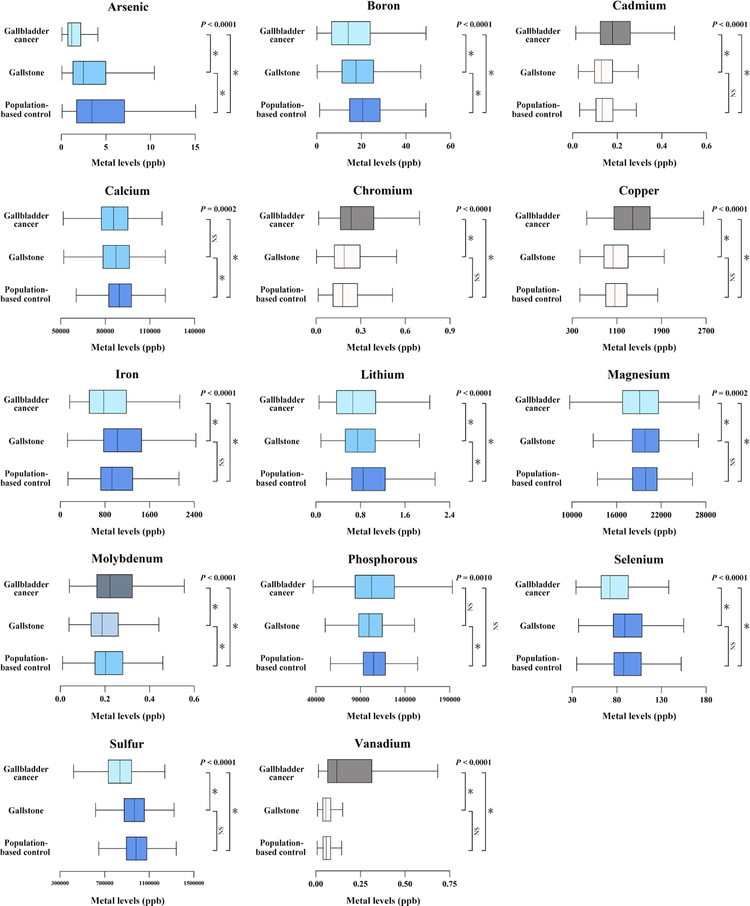
Patients with GBC were older, tended to be female, were less educated, and were more obese compared to patients with gallstones (P < 0.05) (Table 1). On the other hand, compared to population-based controls, gallstone patients were younger, more likely to be female, more highly educated, less likely to smoke or drink alcohol, and more obese (P < 0.05). The 18 metals were weakly correlated with each other in population-based controls (Supporting Fig. S1). Among the 18 metals detected, 12 were significantly, univariately associated with GBC compared to gallstones, whereas 6 were associated with gallstones compared to population-based controls (χ2 P < 0.002) (Supporting Table S1). Four metals (arsenic, boron, lithium, and molybdenum) were associated with both GBC compared to gallstones and gallstones compared to population-based controls. Chromium, cadmium, vanadium, copper, and molybdenum levels were higher among GBC compared to gallstone patients (P < 0.001) (Fig. 1). In contrast, arsenic, boron, lithium, sulfur, iron, magnesium, and selenium were lower among GBC compared to gallstone patients (P < 0.001). Compared with population-based controls, gallstone patients had lower mean levels of arsenic, boron, lithium, calcium, molybdenum, and phosphorous, suggesting that these metals are negatively associated with gallstones (P < 0.001).
TABLE 1.
Baseline Characteristics of Study Population
| Baseline centeracteristics | Gallbladder cancer patients (N = 259) |
Gallstone patient controls (N = 701) |
Population-based controls (N = 851) |
P value* | P value** |
|---|---|---|---|---|---|
| Age, mean ± SD | 64.1 ± 8.8 | 58.9 ± 10.4 | 63.7 ± 8.3 | <0.0001 | <0.0001 |
| Sex | |||||
| Female | 187 (72.2) | 458 (65.3) | 521 (61.2) | 0.0443 | 0.0947 |
| Male | 72 (27.8) | 243 (34.7) | 330 (38.8) | ||
| Education | |||||
| None | 139 (53.9) | 197 (28.1) | 347 (40.8) | <0.0001 | <0.0001 |
| Primary or junior middle | 62 (24.0) | 200 (28.5) | 214 (25.2) | ||
| Senior middle | 33 (12.8) | 175 (25.0) | 164 (19.3) | ||
| Community college or university | 24 (9.3) | 129 (18.4) | 126 (14.8) | ||
| Body mass index | |||||
| Underweight: <18.5 | 32 (12.4) | 49 (7.0) | 112 (13.2) | 0.0164 | <0.0001 |
| Normal: 18.5–24.9 | 128 (49.4) | 402 (57.4) | 499 (58.6) | ||
| Overweight: 25.0–29.9 | 79 (30.5) | 212 (30.2) | 197 (23.2) | ||
| Obese ≥30.0 | 20 (7.7) | 38 (5.4) | 43 (5.1) | ||
| Cigarette smoking | |||||
| Never | 189 (73.5) | 516 (73.6) | 581 (68.3) | 0.9831 | 0.0215 |
| Yes | 68 (26.5) | 185 (26.4) | 270 (31.7) | ||
| Alcohol drinking | |||||
| Never | 216 (83.7) | 594 (84.7) | 676 (79.4) | 0.7004 | 0.0070 |
| Yes | 42 (16.3) | 107 (15.3) | 175 (20.6) | ||
| Triglyceride levels (mmol/L) | |||||
| <1.7 | 144 (55.8) | 463 (66.4) | 631 (74.4) | 0.0025 | 0.0006 |
| ≥1.7 | 114 (44.2) | 234 (33.6) | 217 (25.6) | ||
| Total cholesterol levels (mmol/L) | |||||
| <6.2 | 224 (86.8) | 663 (95.1) | 771 (90.9) | <0.0001 | 0.0015 |
| ≥6.2 | 34 (13.2) | 34 (4.9) | 77 (9.1) |
P values for gallbladder cancer patients and gallstone controls by chi-square test.
P values for gallstone patients and population-based controls by chi-square test.
FIG. 1.
Mean levels of metals among study population.
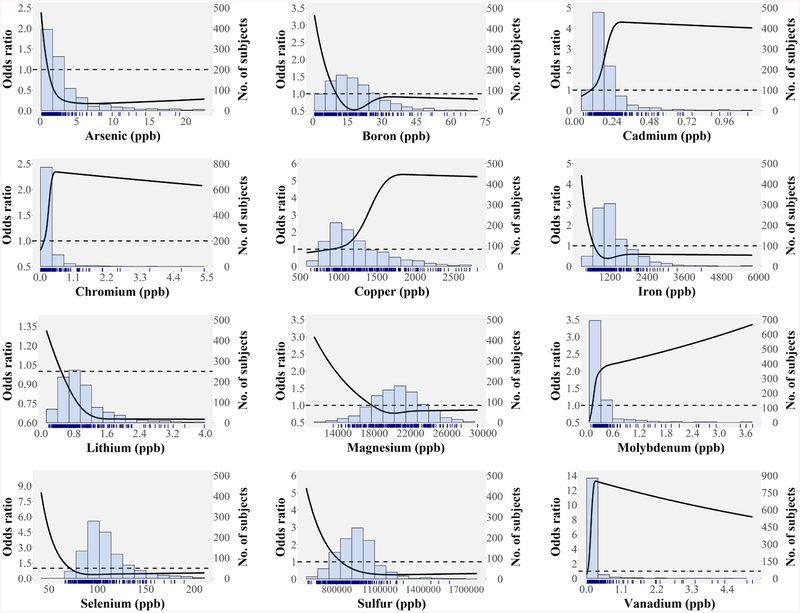
Each metal was non-linearly related to GBC (Fig. 2). Cadmium, chromium, molybdenum, and vanadium were positively associated with GBC, although they were detected at very low levels. Arsenic, lithium, magnesium, selenium, and sulfur were negatively associated with GBC, but the magnitude of association went into a plateau as the metal levels increased. Arsenic, boron, calcium, lithium, molybdenum, and phosphorous showed inverse associations with gallstones (Supporting Fig. S2).
FIG. 2.
Odds ratios of gallbladder cancer by metal levels. The bar histograms represent the frequency distribution of each metal in the study sample, including GBC and gallstone patients. The tick marks at the bottom of the histogram in each figure represent the metal levels of GBC patients.
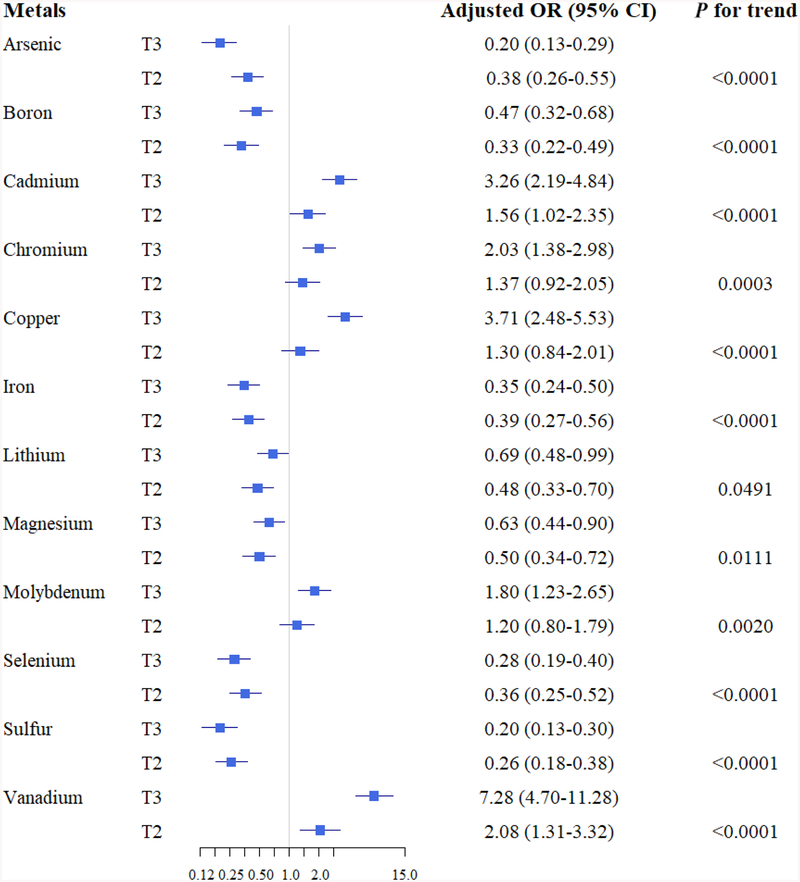
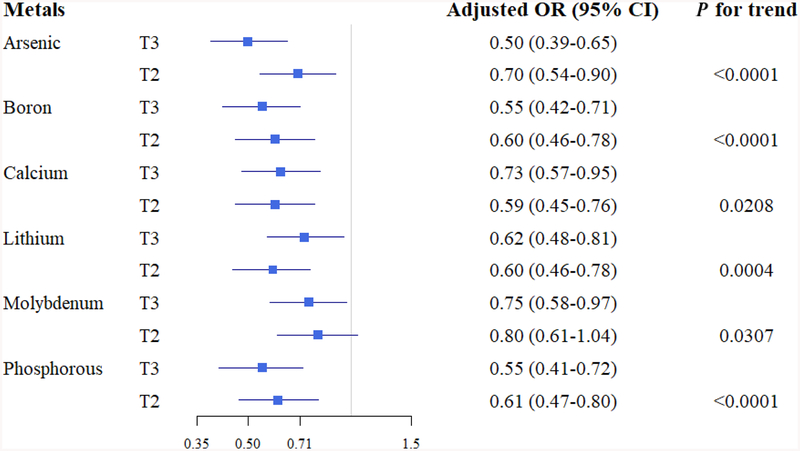
Based on these results, we further categorized the metals based on tertiles of GBC and gallstone patients and evaluated their associations with GBC or gallstones after adjustment for potential confounders. As the serum levels of cadmium, chromium, vanadium, copper, and molybdenum increased, the magnitude of the association with GBC versus gallstones increased (P trend <0.01) (Fig. 3). For example, the ORadj (95% CI) for cadmium was 1.56 (1.02–2.35) for T2 and 3.26 (2.19–4.84) for T3 compared to T1 as a reference group. Similarly, the ORadj for chromium was 1.37 (0.92–2.05) for T2 and 2.03 (1.38–2.98) for T3 compared to T1. In contrast, higher levels of arsenic, boron, lithium, sulfur, iron, magnesium, and selenium were associated with lower odds of GBC compared to gallstones. For example, arsenic levels were inversely associated with GBC, with ORadj of 0.38 (0.26–0.55) and 0.20 (0.13–0.29) for T2 and T3, respectively, versus T1 (P trend <0.001). Arsenic, boron, calcium, lithium, molybdenum, and phosphorous were found to be negatively associated with gallstones (P trend <0.05) (Fig. 4).
FIG. 3.
Adjusted odds ratios associated with gallbladder cancer by metal levels.
Adjusted for age, gender, body mass index, cigarette smoking, alcohol drinking, triglyceride level, and total cholesterol level.
FIG. 4.
Adjusted odds ratios associated with gallstones by metal levels.
Adjusted for age, gender, body mass index, cigarette smoking, alcohol drinking, triglyceride level, and total cholesterol level.
Finally, we evaluated all the metals together through stepwise logistic regression models (Table 2). Cadmium, vanadium, and copper were positively associated with GBC versus gallstones, with T2 and T3 versus T1 ORadj of 1.59 (0.97–2.58) and 2.11 (1.30–3.40) for cadmium, 1.75 (1.04–2.96) and 4.33 (2.63–7.14) for vanadium, and 2.08 (1.24–3.48) and 6.67 (3.94–11.26) for copper. In contrast, the ORadj for T2 and T3 were 0.37 (0.24–0.58) and 0.44 (0.27–0.70) for sulfur, 0.41 (0.26–0.66) and 0.27 (0.16–0.46) for selenium, 0.59 (0.38–0.90) and 0.33 (0.20–0.53) for arsenic, and 0.48 (0.30–0.76) and 0.37 (0.23–0.59) for iron. Boron, arsenic, and phosphorous were independently associated with gallstones. Compared to T1, the corresponding ORadj for T2 and T3 were 0.65 (0.50–0.86) and 0.63 (0.48–0.83) for boron, 0.76 (0.58–0.99) and 0.56 (0.42–0.73) for arsenic, and 0.68 (0.52–0.90) and 0.56 (0.42–0.74) for phosphorous.
TABLE 2.
Multivariate Analysis of Metals on the Risk of Gallbladder Cancer and Gallbladder Stones
| Gallbladder cancer | Gallbladder stones | |||
|---|---|---|---|---|
| Metals | Adjusted OR (95% CI)a | P value | Adjusted OR (95% CI)a | P value |
| Arsenic | ||||
| T1 | 1.00 (referent) | 1.00 (referent) | ||
| T2 | 0.59 (0.38–0.90) | 0.0154 | 0.76 (0.58–0.99) | 0.0410 |
| T3 | 0.33 (0.20–0.53) | <0.0001 | 0.56 (0.42–0.73) | <0.0001 |
| Boron | ||||
| T1 | 1.00 (referent) | |||
| T2 | 0.65 (0.50–0.86) | 0.0020 | ||
| T3 | 0.63 (0.48–0.83) | 0.0009 | ||
| Cadmium | ||||
| T1 | 1.00 (referent) | |||
| T2 | 1.59 (0.97–2.58) | 0.0635 | ||
| T3 | 2.11 (1.30–3.40) | 0.0024 | ||
| Copper | ||||
| T1 | 1.00 (referent) | |||
| T2 | 2.08 (1.24–3.48) | 0.0052 | ||
| T3 | 6.67 (3.94–11.26) | <0.0001 | ||
| Iron | ||||
| T1 | 1.00 (referent) | |||
| T2 | 0.48 (0.30–0.76) | 0.0019 | ||
| T3 | 0.37 (0.23–0.59) | <0.0001 | ||
| Phosphorous | ||||
| T1 | 1.00 (referent) | |||
| T2 | 0.68 (0.52–0.90) | 0.0067 | ||
| T3 | 0.56 (0.42–0.74) | <0.0001 | ||
| Selenium | ||||
| T1 | 1.00 (referent) | |||
| T2 | 0.41 (0.26–0.66) | 0.0002 | ||
| T3 | 0.27 (0.16–0.46) | <0.0001 | ||
| Sulfur | ||||
| T1 | 1.00 (referent) | |||
| T2 | 0.37 (0.24–0.58) | <0.0001 | ||
| T3 | 0.44 (0.27–0.70) | 0.0006 | ||
| Vanadium | ||||
| T1 | 1.00 (referent) | |||
| T2 | 1.75 (1.04–2.96) | 0.0362 | ||
| T3 | 4.33 (2.63–7.14) | <0.0001 | ||
Adjusted for age, gender, body mass index, and triglyceride levels.
Among the 851 population-based healthy controls, 146 (17%) had gallstone history and 77 (9%) had cholecystitis history. In sensitivity analyses, the metals (arsenic, boron, calcium, lithium, molybdenum, and phosphorous) were still significantly associated with gallstones when the healthy controls with history of either gallstones or cholecystitis were excluded (P < 0.05). On the other hand, among GBC patients, 189 (73%) and 101 (39%) had history of gallstones or cholecystitis, respectively. All of the metals remained consistent when we compared GBC versus gallstone patients if we restricted our comparison to all of the patients who had history of gallstones. We also compared the GBC patients with gallstone patients and population-based controls (n = 1,552), and the results were consistent. The significant metals associated with gallstones or GBC remained the same if we stratified the analyses by gender. There were no significant interactions of metals and gender on the associations with GBC or gallstones (P > 0.05), suggesting that the relationships of metals with GBC or gallstones did not differ by genders. Age was stratified by the median age of GBC patients (66 years) to evaluate metals on the associations of GBC. All of the results were similar except lithium and molybdenum. Interestingly, the associations between lithium with GBC were found only among patients younger than 66 years of age (P for interaction = 0.0256), whereas the associations between molybdenum and GBC were only found among those older than 66 years of age. Subjects older than 66 years of age and with a high (T3) molybdenum level had a 2.89-fold (1.76–4.77) likelihood of GBC compared to subjects with a low level of molybdenum (T1). Age and molybdenum showed statistical interactions on the associations with GBC (P for interaction = 0.0353).
Discussion
To date, this study is the largest population-based study evaluating the relationship between levels of multiple serum metals with respect to risk of gallstones and GBC. We found there were several metals associated with either gallstones or GBC. Among them, arsenic, boron, lithium, and molybdenum were significantly associated with both gallbladder diseases. These metals provide insights for futures studies on gallbladder carcinogenesis and preventive strategies.
The inclusion of both the GBC and gallstone groups offered the unique opportunity to assess whether metals are associated across the natural history of disease because gallstones are the major risk factor for GBC. Identification of additional risk factors for gallstones is useful for both gallstone and GBC prevention. In addition, because not all gallstone patients progress to GBC, discovering risk factors for GBC among gallstone patients may provide insights into the pathogenesis of GBC as well as identifying high-risk patients to prioritize for intervention (e.g., cholecystectomy).(18)
Some of the studied metals have been associated with human physiological functions or the occurrence of diseases by epidemiological studies(19–21) or in vitro studies.(22, 23) Consistent with our findings, small case-control studies showed GBC cases had higher copper, cadmium, and chromium levels compared to gallstone patients.(14, 15) Interestingly, one study found that the metal levels that were elevated or decreased in serum samples from GBC patients compared to gallstone patients were similarly elevated or decreased in bile and gallbladder tissue samples,(15) suggesting that serum metal levels reflect exposure to metals in the gallbladder itself. Metals may be released from the gallbladder into the bloodstream, suggesting that measuring metal levels in serum can provide etiological insights into gallstone and GBC pathogenesis.
We found that magnesium was inversely associated with GBC but not with gallstones. A prospective study found that men in the highest quintile of dietary magnesium consumption had a 28% decreased risk for symptomatic gallstone disease compared to those with the lowest quintile(21); however, decreased magnesium on the risk of gallstones without symptoms was not found, suggesting that magnesium intake might be associated with most advanced stages of gallstone disease. Therefore, the inverse associations with GBC were found in our study. The potential biological mechanism may result from the fact that higher magnesium intake has been associated with improved insulin sensitivity and lower risk of diabetes in middle-aged adults.(19) Given that metabolic syndrome has been significantly associated with GBC with a dose relationship as the number of metabolic syndrome components increases,(24) the inverse association between magnesium and GBC in our study could reflect the impact of magnesium on diabetes, a component of metabolic syndrome.
Arsenic, which causes oxidative DNA damage and genomic instability, has been classified as a human carcinogen by the International Agency for Research on Cancer.(25) In our study, however, we observed an inverse association between arsenic and both gallstones and GBC. There are two major groups of arsenic compounds: inorganic and organic. Human exposure and toxicological assessments have focused on the inorganic form through studies of occupational workers who inhaled air contaminated by arsenic or other agents(26) or studies in locations where people ingested arsenic in drinking water over prolonged periods of time.(27) Although organic arsenic has generally been considered to be nontoxic, to date there have been no epidemiological studies of the cancer risks associated with arsenic exposure from seafood intake.(28) Marine-derived food, including finfish, shellfish, and seaweed, is the primary contributor to organic arsenic exposure in human populations.(29) Seafood is a normal part of the Shanghainese diet. It is possible that study subjects made dietary changes prior to sample collection due to symptoms from their gallbladder disease. Unfortunately, ICP-MS can only detect total arsenic and cannot distinguish organic from inorganic arsenic. However, it is possible that patients with gallstones or GBC may have decreased their seafood intake and therefore their organic arsenic exposure, which may have resulted in lower arsenic levels compared to population controls. Although we found that preserved or salted food increased the risk of GBC,(30) we lacked a detailed dietary questionnaire, including the amount, frequency, and types of seafood intake, which makes the inverse associations of arsenic and gallbladder diseases difficult to interpret. It needs further investigation.
Alternatively, it is possible that arsenic may have a protective effect. A study conducted in Chile found a 70% reduction of breast cancer mortality after high exposure to inorganic arsenic in drinking water.(23) The investigators found that arsenic may impair cancer cell viability by inducing apoptotic effects.(23) Arsenic trioxide is currently used to treat acute promyelocytic leukemia. However, the temporality between arsenic exposures and gallbladder diseases in this case-control study was uncertain. In the future, more basic studies to elucidate the effect of arsenic on gallbladder disease pathogenesis need to be conducted. Moreover, urine samples may be helpful to measure metabolites of inorganic arsenic compounds, including methyl and phenyl derivatives of arsenic, such as monomethylarsonic acid (MMA) and dimethyl arsenic acid (DMA). Approximately 70% of inorganic arsenic is secreted in urine, of which 50% appears as DMA and 25% as MMA.(31) We had stored urine samples of these subjects, and they may provide insights to further understanding the associations between arsenic levels and gallbladder diseases in future studies.
In our study, we found that boron levels were negatively associated with gallstones and GBC. There is accumulating evidence that boron may have beneficial effects on human health.(32) Boron is abundant in vegetables, fruits, and nuts, and it has roles in lipid metabolism, healthy bone development, and cell membrane maintenance. Boron is absorbed from the gastrointestinal tract completely and is present in the body as boric acid, which was been found to decrease body weight, visceral fat, insulin, and interleukin-7 levels.(22) Given that both gallstones and GBC are associated with obesity(33–35) as well as inflammation, there is biologic plausibility of boron’s role as a protective factor.
Molybdenum, which is abundant in rice and soybean products, is an essential trace element in human nutrition. Interestingly, the molybdenum levels among our study population were within the suggested range of 0.1 to 4.73 ppb.(36) Molybdenum has been shown to be correlated positively with female mortality from pancreatic cancer.(20) Xanthine oxidase is a molybdenum containing metalloenzyme, which is thought to catalyze the action of various carcinogens and mutagens into DNA adducts that induce mutation of microorganisms.(37) However, there are scant studies investigating biological mechanisms of molybdenum on GBC, and the roles of xanthine oxidase in gallbladder pathogenesis still need further studies. In our study, increased molybdenum levels were inversely associated with gallstones but positively associated with GBC, potentially suggesting that limited levels of molybdenum may have a beneficial impact on gallstone formation but may increase the risk of GBC in the context of gallstones. Interestingly, the positive association of molybdenum levels on GBC was confined to patients older than 66 years of age, suggesting that the elements needed in the human body may be different among various age groups. Additional research is needed to investigate the potential mechanisms to establish safe levels.
Lithium has been used as a drug for treatment of mood disorders. It could alter the biochemical properties of a variety of transcription factors and play important roles in cancer development. Lithium was found as a specific noncompetitive inhibitor for glycogen synthase kinase-3β as well as an inhibitor of the nuclear factor kappa B (NF-κB) pathway.(38) It was suggested that lithium has potentials to inhibit cell cycle progression and proliferation. A population-based study found that lithium use was associated with significantly reduced cancer risk among patients with bipolar disorders.(39) Although the study did not evaluate the effects of lithium on specific cancer sites, a dose-response relationship was found for overall cancer risk.
Generally, females had a higher incidence of GBC than males.(40) Previously, we found increased risks of GBC associated with higher parity, younger age of first birth, and late age at menarche, particularly among women with gallstones.(41) These reproductive risk factors suggested the need for further study into hormonal and inflammatory mechanisms underpinning the development of GBC. It will be interesting to examine serum hormonal levels on the risk of GBC. In this study, we additionally evaluated whether gender modifies the associations between metal levels on gallbladder diseases. However, we did not find differences of the metals on the risk of gallstones or GBC by males or females.
The major limitation of this study is the uncertainty of whether the observed metal levels reflect exposure prior to the occurrence of gallbladder disease or whether the levels were affected by the presence of gallbladder disease. It is possible that gallstones or gallbladder tumors may lead to an imbalance in metal levels in the body. Metal levels may be affected by changes in dietary patterns or other behaviors due to symptoms from the disease. Case-control studies can be affected by reverse causality and cannot establish temporality. Thus, the associations between metal levels and gallbladder diseases should be interpreted cautiously. However, in our study, we found that four common metals were associated with both gallstones and GBC, which provides some cross-sectional evidence of temporality given that gallstones precede GBC. Cumulatively, our findings suggest that these metals might warrant further investigation. In addition, humans are exposed to metals through several different routes and locations (e.g., industrial, domestic agriculture, medical, and technological applications), which we were unable to thoroughly explore in the present study. Our study only measured one time point of metals, which may only reflect a short period of exposure. Therefore, long-term serial measurements of metals may be helpful to delineate their associations with the progression of gallstone diseases.
In conclusion, in this large, population-based, case-control study, we found several metals significantly associated with gallstones or GBC. This study demonstrates the value of metallomics as a novel approach to further explore the role of metals, which have an extensive and essential role in the pathophysiology and pathogenesis of gallbladder diseases. In order to clarify the potential causal role of these metals on gallbladder disease occurrence, longitudinal studies are warranted.
Supplementary Material
Funding/Support:
This study was supported by research grants from the Ministry of Science and Technology, Taipei, Taiwan (105–2628-B-010–003-MY4, 107–2314-B-010–004-MY2 and 107–2918-I-010–004 to M.H.L.), and the National Cancer Institute Intramural Research Funding.
Abbreviations:
- CI
confidence interval
- GBC
gallbladder cancer
- ICP-MS
inductively coupled plasma–mass spectrometry
- OR
odds ratio
- ORadj
adjusted odds ratio
- T
tertile
Footnotes
Potential conflict of interest: Nothing to report.
REFERENCES
- 1.Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol 2014;6:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6:172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koshiol J, Ferreccio C, Devesa S, et al. Biliary tract cancers In: Thun M, Linet M, Cerhan J, Haiman C, Schottenfeld, eds. Cancer Epidemiology and Prevention. New York: Oxford University Press, 2017. [Google Scholar]
- 4.Nogueira L, Freedman ND, Engels EA, et al. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol 2014;179:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer 2004;4:695–706. [DOI] [PubMed] [Google Scholar]
- 6.Lammert F, Gurusamy K, Ko CW, et al. Gallstones. Nat Rev Dis Primers 2016;2:16024. [DOI] [PubMed] [Google Scholar]
- 7.Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167–76. [DOI] [PubMed] [Google Scholar]
- 8.Koshiol J, Gao YT, Dean M, et al. Association of Aflatoxin and Gallbladder Cancer. Gastroenterology 2017;153:488–494 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koshiol J, Wozniak A, Cook P, et al. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med 2016;5:3310–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nogueira L, Foerster C, Groopman J, et al. Association of aflatoxin with gallbladder cancer in Chile. JAMA 2015;313:2075–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nemunaitis JM, Brown-Glabeman U, Soares H, et al. Gallbladder cancer: review of a rare orphan gastrointestinal cancer with a focus on populations of New Mexico. BMC Cancer 2018;18:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaishankar M, Tseten T, Anbalagan N, et al. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 2014;7:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turconi G, Minoia C, Ronchi A, et al. Dietary exposure estimates of twenty-one trace elements from a Total Diet Study carried out in Pavia, Northern Italy. Br J Nutr 2009;101:1200–8. [DOI] [PubMed] [Google Scholar]
- 14.Shukla VK, Prakash A, Tripathi BD, et al. Biliary heavy metal concentrations in carcinoma of the gall bladder: case-control study. BMJ 1998;317:1288–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu S, Singh MK, Singh TB, et al. Heavy and trace metals in carcinoma of the gallbladder. World J Surg 2013;37:2641–6. [DOI] [PubMed] [Google Scholar]
- 16.Hsing AW, Gao YT, Han TQ, et al. Gallstones and the risk of biliary tract cancer: a population-based study in China. Br J Cancer 2007;97:1577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauchemin D Inductively Coupled Plasma Mass Spectrometry. Analytical Chemistry 2010;82:4786–4810. [DOI] [PubMed] [Google Scholar]
- 18.Koshiol J, Gao YT, Corbel A, et al. Circulating inflammatory proteins and gallbladder cancer: Potential for risk stratification to improve prioritization for cholecystectomy in high-risk regions. Cancer Epidemiol 2018;54:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hruby A, Meigs JB, O’Donnell CJ, et al. Higher Magnesium Intake Reduces Risk of Impaired Glucose and Insulin Metabolism and Progression From Prediabetes to Diabetes in Middle-Aged Americans. Diabetes Care 2014;37:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakadaira H, Endoh K, Yamamoto M, et al. Distribution of selenium and molybdenum and cancer mortality in Niigata, Japan. Arch Environ Health 1995;50:374–80. [DOI] [PubMed] [Google Scholar]
- 21.Tsai CJ, Leitzmann MF, Willett WC, et al. Long-Term Effect of Magnesium Consumption on the Risk of Symptomatic Gallstone Disease Among Men. The American Journal Of Gastroenterology 2008;103:375. [DOI] [PubMed] [Google Scholar]
- 22.López-Cabrera Y, Castillo-García EL, Altamirano-Espino JA, et al. Profile of three boron-containing compounds on the body weight, metabolism and inflammatory markers of diabetic rats. Journal of Trace Elements in Medicine and Biology 2018;50:424–429. [DOI] [PubMed] [Google Scholar]
- 23.Smith AH, Marshall G, Yuan Y, et al. Rapid reduction in breast cancer mortality with inorganic arsenic in drinking water. EBioMedicine 2014;1:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shebl FM, Andreotti G, Meyer TE, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer 2011;105:1424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straif K, Benbrahim-Tallaa L, Baan R, et al. A review of human carcinogens--Part C: metals, arsenic, dusts, and fibres. Lancet Oncol 2009;10:453–4. [DOI] [PubMed] [Google Scholar]
- 26.Lubin JH, Pottern LM, Stone BJ, et al. Respiratory cancer in a cohort of copper smelter workers: results from more than 50 years of follow-up. Am J Epidemiol 2000;151:554–65. [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Hsu LI, Chiou HY, et al. Ingested arsenic, cigarette smoking, and lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan. JAMA 2004;292:2984–90. [DOI] [PubMed] [Google Scholar]
- 28.IARC monographs on the evaluation of carcinogenic risks to humans. Volume 100C Lyon, France: IARC, 2012. [Google Scholar]
- 29.Taylor V, Goodale B, Raab A, et al. Human exposure to organic arsenic species from seafood. Science of The Total Environment 2017;580:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson SM, Gao YT, Nogueira LM, et al. Diet and biliary tract cancer risk in Shanghai, China. PLoS One 2017;12:e0173935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keil DE, Berger-Ritchie J, McMillin GA. Testing for Toxic Elements: A Focus on Arsenic, Cadmium, Lead, and Mercury. Laboratory Medicine 2011;42:735–742. [Google Scholar]
- 32.Nielsen FH, Meacham SL. Growing Evidence for Human Health Benefits of Boron. Journal of Evidence-Based Complementary & Alternative Medicine 2011;16:169–180. [Google Scholar]
- 33.Campbell PT, Newton CC, Kitahara CM, et al. Body Size Indicators and Risk of Gallbladder Cancer: Pooled Analysis of Individual-Level Data from 19 Prospective Cohort Studies. Cancer Epidemiol Biomarkers Prev 2017;26:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engeland A, Tretli S, Austad G, et al. Height and body mass index in relation to colorectal and gallbladder cancer in two million Norwegian men and women. Cancer Causes Control 2005;16:987–96. [DOI] [PubMed] [Google Scholar]
- 35.Stender S, Nordestgaard BG, Tybjaerg-Hansen A. Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology 2013;58:2133–41. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Ôta S, Fukunaga K, et al. Serum molybdenum concentration in healthy Japanese adults determined by inductively coupled plasma-mass spectrometry. Journal of Trace Elements in Medicine and Biology 2006;20:19–23. [DOI] [PubMed] [Google Scholar]
- 37.Howard PC, Heflich RH, Evans FE, et al. Formation of DNA adducts in vitro and in Salmonella typhimurium upon metabolic reduction of the environmental mutagen 1-nitropyrene. Cancer Res 1983;43:2052–8. [PubMed] [Google Scholar]
- 38.Hoeflich KP, Luo J, Rubie EA, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000;406:86–90. [DOI] [PubMed] [Google Scholar]
- 39.Huang RY, Hsieh KP, Huang WW, et al. Use of lithium and cancer risk in patients with bipolar disorder: population-based cohort study. Br J Psychiatry 2016;209:393–399. [DOI] [PubMed] [Google Scholar]
- 40.Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591–602. [DOI] [PubMed] [Google Scholar]
- 41.Andreotti G, Hou L, Gao YT, et al. Reproductive factors and risks of biliary tract cancers and stones: a population-based study in Shanghai, China. British Journal Of Cancer 2010;102:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.