Abstract
BACKGROUND:
Peak lung function and rate of decline predict future airflow obstruction and non-respiratory comorbid conditions. Associations between lung function trajectories and emphysema have not been explored.
METHODS:
Using data from the population-based CARDIA Study, we sought to describe the prevalence of visually ascertained emphysema at multiple time points and contextualize its development based upon participant’s adult life course measures of lung function. 3,171 men and women were enrolled at a mean age of 25 years and underwent serial spirometric examinations through a mean age of 55. Trajectories for the change in percent-predicted forced expiratory volume in one second (FEV1) were determined by fitting a mixture model via maximum likelihood. Emphysema was visually identified on computed tomographic scans and its prevalence reported at mean ages of 40, 45 and 50.
RESULTS:
We identified 5 trajectories describing peak and change in FEV1: “Preserved Ideal”, “Preserved Good”, “Preserved Impaired”, “Worsening”, and “Persistently Poor”. Ever smokers comprised part of all 5 trajectories. The prevalence of emphysema was 1.7% (n=46; mean age of 40), 2.5% (n=67; mean age of 45) and 7.1% (n=189; mean age of 50). Of those with emphysema at a mean age of 50, 18.0% were never smokers. Worsening and Poor lung health trajectories were associated with increased odds of future emphysema independent of chronic tobacco smoke exposure (OR 5.06, CI 1.84–13.96; OR 4.85, CI 1.43–16.44).
CONCLUSIONS:
Lower peak and accelerated decline in FEV1 are risk factors for future emphysema independent of smoking status.
ClinicalTrials.gov Identifier:
INTRODUCTION:
The adult life-course of the respiratory system is characterized by attainment of maximal lung function in young adulthood and then decline with advancing age. The magnitude of this peak and the rate of subsequent decline have implications for overall health. Both lower peak lung function and accelerated loss of lung function are associated with future expiratory airflow obstruction as well as non-respiratory comorbid conditions such as diabetes and cardiovascular disease.(1–5) Little is known though about the association of life-course trajectories of lung function and the development of emphysema.
Emphysema is defined as abnormal, permanent dilation of the distal airspaces and a major risk factor for the development of such pathology is chronic exposure to tobacco smoke.(6) Much of our knowledge of the prevalence and clinical implications of emphysema has, however, been learned from cohorts of elderly individuals over the age of 60 in whom we know little about their respiratory history other than what is self-reported at the time of study enrollment. The absence of earlier standardized serial measures of lung function and repeated CT scans in a sizable cohort has limited our understanding of the age at which emphysema tends to become evident radiologically and who is at greatest risk for developing it.
The Coronary Artery Risk Development in Young Adults (CARDIA) study(7) is a longitudinal US population-based cohort study of young adults aged 18–30 years old at baseline in 1985–86. CARDIA participants have been followed for 30 years and have undergone serial measures of both lung function and standardized CT scans of the chest. Using these data, we sought to describe the prevalence of visually ascertained emphysema at multiple time points and contextualize the development of this parenchymal condition based upon participant’s adult life course measures of lung function. We hypothesized that independent of smoking status, life course trajectories of lung function characterized by lower peak or more rapid rates of decline would be differentially associated with the presence of emphysema at a younger age.
METHODS:
Study design and participants
CARDIA is a longitudinal cohort study of the evolution of cardiovascular disease risk factors and clinical risk that started in young adults.(7) From 1985 to 1986 (year 0), 5,115 black and white individuals aged 18 to 30 were recruited in Birmingham, AL, Chicago, IL, Minneapolis, MN, and Oakland, CA. Recruitment achieved nearly equal numbers at each site of race, sex, educational attainment (more than high school or high school or less) and age (18–24 years or 25–30 years). Fifty percent of invited individuals contacted were examined. Re- examination occurred after 2, 5, 7, 10, 15, 20, 25, and 30 years with 71% of the surviving cohort returning for the Year 30 examination. At each examination, participants completed several questionnaires including those related to smoking, respiratory symptoms, and medical history (www.CARDIA.dopm.UAB.edu). Aggregate tobacco smoke exposure is reported in pack-years and the designation of ever (current and former) vs never smoker is based upon self- reported smoking up to and including the Year 30 exam. CARDIA is approved by the Institutional Review Boards of each participating center.
Lung function and computed tomographic scan measurements
Lung function was measured at years 0, 2, 5, 10, 20, and 30. Standard spirometry procedures as recommended by the American Thoracic Society were followed at all examinations.(8–11) Measures were obtained at all time points without the administration of a short acting inhaled bronchodilator. A ratio of the forced expiratory volume in the first second (FEV1) to the forced vital capacity (FVC) less than 0.7 was used to define the presence of expiratory airflow obstruction. CT scans were obtained at three study visits, Year 15, Year 20, and Year 25.(12)
We began by performing a visual assessment of the Year 25 CT scans to detect emphysema and classify its subtype was performed using methods we have described previously.(13–16) There was no panacinar emphysema identified on visual review. Briefly, Reader 1 reviewed all the CT scans and categorized them as having centrilobular emphysema and/or paraseptal emphysema, or neither. Reader 2 then reviewed all those CT scans that had emphysema (centrilobular, paraseptal, both) as well as a random sample of 10% of the remaining CT scans. A third reader then adjudicated cases in which there was a disagreement between Reader 1 and 2. The kappa for agreement between Readers 1 and 2 for centrilobular emphysema was 0.74 and for paraseptal emphysema was 0.70. Visual review of the Year 15 and Year 20 CT scans was then performed on the subset of CARDIA participants who had emphysema on their Year 25 scans. These Year 15 and Year 20 CT scans were independently reviewed using the same 3 reader system to provide final binary classifications of centrilobular emphysema, paraseptal emphysema or both at either timepoint.
Statistical Analysis
We generated lifetime trajectories of percent predicted lung function, specifically the FEV1, using a group-based trajectory modeling approach (SAS PROC TRAJ). To ensure that trajectories reflected lung function to middle age in all participants in the analysis, we required an individual participant to have lung function measured at Year 30 and at least one other time point to be included in the analysis (Supplement Figure 1). SAS PROC TRAJ analyzes longitudinal data by fitting a mixture model via maximum likelihood.(17–19) The model assumes that the population is made up of multiple trajectory groups and allows us to simultaneously estimate the probabilities for multiple trajectories rather than simply fitting the population mean. The time scale for the FEV1 models was age at lung function examination. Model fit was assessed using the Bayesian Information Criterion (BIC) as well as visual inspection of group plots. We considered models ranging in class number from 2 to 9. Because the BIC continually improved with each increase in class size, we visually identified that the model with 8 classes was more parsimonious than that with 9, and from this model calculated the posterior predicted probability for each participant’s probability of being a member in each of the eight classes. Participants were assigned to the trajectory for which they had the greatest posterior probability (Supplement Figure 2 and Supplement Table 1): the mean probability of group membership was 0.88 indicating good discrimination. After trajectory group assignment, we qualitatively examined the pattern of FEV1 change, the demographic and clinical features of each group, and combined similar groups to result in five final FEV1 (expressed as a percent predicted based upon sex and race(20)) trajectory groups (Supplement Table 1, Supplement Figures 2–3).
Demographic characteristics, prevalence of airflow obstruction and emphysema (the time point when thoracic CT scans were obtained in CARDIA) were determined across the five FEV1% trajectory groups. We then tested the association between trajectory membership and the presence of emphysema at Year 25 using multivariable logistic regression. Covariates in the multivariable model included Year 0 age, race, sex, field center, baseline body mass index (BMI) and its change from baseline to Year 25, and lifetime pack-years of cigarette smoking at Year 25 for emphysema.
RESULTS:
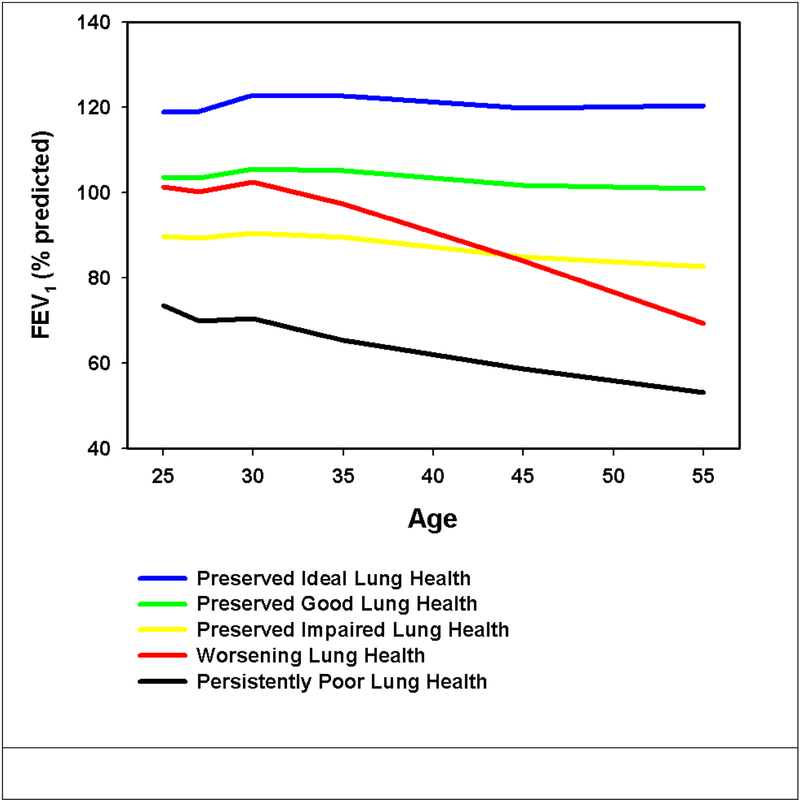
Three thousand one hundred and seventy-one (3,171) participants (Supplement Figure 1) were represented by 5 trajectories of FEV1 that differed in baseline lung function as well as rate of subsequent decline (Figure 1). To facilitate reference to these trajectories within this manuscript, these plots were named Preserved Ideal lung health (n=187; 5.9%), Preserved Good lung health (n=1,478; 46.6%), Preserved Impaired lung health (n=1,264; 39.9%), Worsening lung health (n=180; 5.7%) and Persistently Poor lung health (n=62; 2%) with the first three differing primarily in peak lung function. Those participants with Worsening lung health had a mean baseline FEV1 of 101.4% with a more rapid decline in lung function compared to the Preserved trajectories. The Persistently Poor lung health trajectory had the lowest mean baseline FEV1 of all 5 groups (73.4%) and like the declining subgroup exhibited a more rapid decline in lung function. The prevalence of self-reported asthma at enrollment varied from 1.6% in the Preserved Ideal lung health to 21% of those with Persistently Poor lung health (Table 1)
Figure 1:
Lung function trajectories in CARDIA. These plots were named Preserved Ideal Lung Health (Blue: n=187; 5.9%), Preserved Good Lung Health (Green: n=1,478; 46.6%), Preserved Impaired Lung Health (Yellow: n=1,264; 39.9%), Worsening Lung Health (Red: n=180; 5.7%) and Persistently Poor Lung Health (Black: n=62;
Table 1:
Demographic and spirometric data for the CARDIA cohort. Data presented are mean (SD) and N (percent) unless otherwise specified.
| Trajectories of FEV1% (n=3,171) | |||||
|---|---|---|---|---|---|
| Baseline | Preserved Ideal Lung Health |
Preserved Good Lung Health |
Preserved Impaired Lung Health |
Worsening Lung Health |
Persistently Poor Lung Health |
| N (in whom Year 30 lung function available) | 187 | 1478 | 1264 | 180 | 62 |
| Sex – Female, N (%) | 121 (64.7%) | 880 (59.5%) | 669 (52.9%) | 115 (63.9%) | 33 (53.2%) |
| Race- African American, N (%) | 108 (57.8%) | 654 (44.3%) | 592 (46.8%) | 119 (66.1%) | 37 (59.7%) |
| Age – years (SD) (Baseline) | 25.0 (3.7) | 25.2 (3.5) | 25.1 (3.6) | 24.3 (3.9) | 24.2 (3.9) |
|
Smoking (Baseline) Never, N (%) Former, N (%) Current, N (%) |
121 (64.7%) 29 (15.5%) 37 (19.8%) |
916 (62.4%) 239 (16.3%) 313 (21.3%) |
758 (60.2%) 135 (10.7%) 365 (29.0%) |
89 (49.7%) 25 (14.0%) 65 (36.3%) |
34 (55.7%) 6 (9.8%) 21 (34.4%) |
| Lifetime pack-years of cigarettes (Baseline) | 1.23 (2.99) | 1.66 (3.80) | 2.15 (4.35) | 2.95 (4.89) | 2.94 (5.16) |
|
Smoking (Y30) Never, N (%) Former, N (%) Current, N (%) |
125 (67.9%) 39 (21.2%) 20 (10.9%) |
959 (65.5%) 340 (23.2%) 165 (11.3%) |
792 (63.3%) 292 (23.3%) 168 (13.4%) |
78 (44.3%) 37 (21.0%) 61 (34.7%) |
32 (53.3%) 14 (23.3%) 14 (23.3%) |
| Lifetime pack-years of cigarettes (Y30) | 3.53 (7.07) | 4.49 (9.04) | 6.39 (11.81) | 11.00 (13.48) | 11.15 (16.82) |
| BMI - kg/m2(Baseline) | 24.4 (4.3) | 24.0 (4.5) | 24.5 (4.9) | 25.7 (5.2) | 25.9 (6.8) |
| BMI - kg/m2(Y30) | 29.3 (6.1) | 29.5 (6.5) | 31.0 (7.3) | 35.1 (9.3) | 32.1 (7.6) |
| Asthma, N %(Baseline) | 3 (1.6%) | 25 (1.7%) | 71 (5.6%) | 4 (2.2%) | 13 (21.0%) |
| Baseline FVC -% predicted | 118.0 (8.4) | 104.7 (8.6) | 93.7 (8.7) | 105.5 (10.4) | 84.5 (10.9) |
| Baseline FEV1 -% predicted | 118.8 (7.1) | 103.6 (7.0) | 89.7 (7.1) | 101.4 (10.4) | 73.4 (8.8) |
| BaselineFEV1/FVC (%) | 86 (4.4) | 84 (5.4) | 82 (6.8) | 82 (6.3) | 75 (10.4) |
| Year 30 FVC -% predicted | 118.7 (10.5) | 101.0 (9.5) | 85.4 (10.0) | 78.3 (14.2) | 65.8 (12.1) |
| Year 30 FEV1 -% predicted | 120.4 (9.3) | 100.9 (8.7) | 82.6 (8.7) | 69.3 (15.2) | 53.1 (12.0) |
| Year 30 FEV1/FVC | 80 (4.4) | 78 (5.4) | 76 (6.5) | 70 (11.5) | 65 (14.7) |
| Decline FVC(baseline to Year30) – mL/y | −19 (15) | −22 (13) | −26 (14) | −48 (17) | −36 (21) |
| Decline FEV1 (baseline to Year30) – mL/y | −25 (11) | −26 (11) | −27 (11) | −51 (13) | −36 (16) |
The sex and race composition of the five trajectories of lung function are presented in Table 1. In total, 1,818 of the 3,171 participants (57.3%) were female and 1,510 (47.6%) were African American. Ever (current or former) smokers were present in all 5 trajectories and ranged from 32.1% of those in the Ideal lung health group to 55.7% of those in the Worsening lung health group.
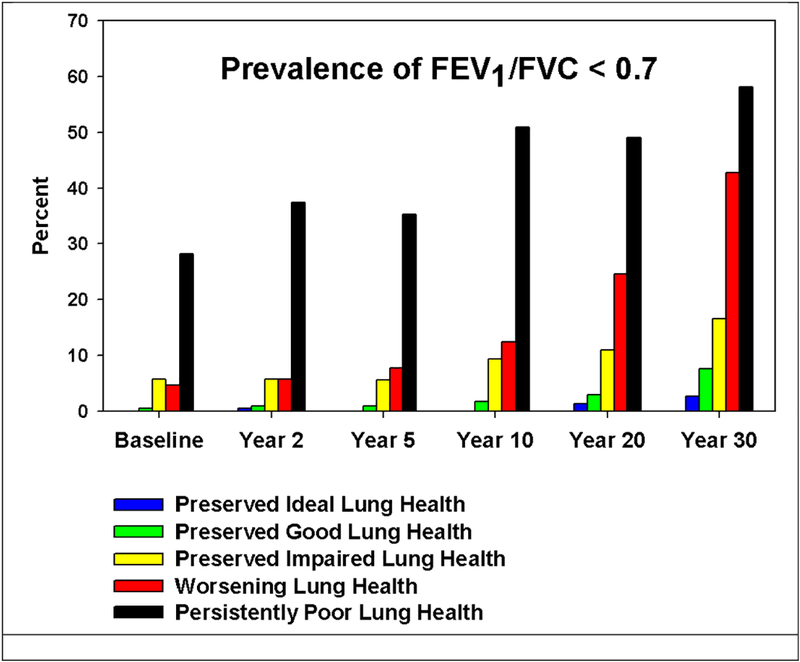
We next explored the prevalence of expiratory airflow obstruction at the Year 30 CARDIA exam (mean age 55 years). The prevalence of a pre-bronchodilator FEV1/FVC ratio less than 0.7 at the Year 30 exam is graphically presented for all time points of ascertainment by trajectory membership in Figure 2. The percentage of participants with spirometrically defined expiratory airflow obstruction is greatest at all time points in those participants designated as belonging to the Persistently Poor lung health group followed by the Worsening lung health and then the Preserved Impaired lung health groups.
Figure 2.
Prevalence of pre-bronchodilator obstruction (defined as a FEV1 to FVC ratio < 0.70) at multiple time points by spirometric trajectory.
A subset of 2,660 participants had serial thoracic CT scans acquired at years 15, 20 and 25 (mean age 40, 45 and 50 years respectively) which were available for review. The prevalence of emphysema was 1.7% (n=46) at Year 15, 2.5% (n=67) at Year 20 and 7.1% (n=189) at Year 25. At all time points there was a trend for a greater prevalence of emphysema in those with the more impaired lung function trajectories.
The prevalence of emphysema by subtype (centrilobular or paraseptal) is provided in Tables 2, 3 and 4 for the Year 15, Year 20 and Year 25 CT scan respectively. These data are further broken down by smoking status (ever vs never) and both the prevalence of emphysema by subtype and by smoking status by year of CT are provided in Figures S4 and S5 of the on-line supplement. At all time points, the prevalence of emphysema was greater in the ever vs never smokers. Visual inspection of the data in Tables 2–4 further suggests that while emphysema develops in never smokers it appears to be delayed by approximately 10 years from that which is observed in the those with a history of chronic tobacco smoke exposure. The prevalence of emphysema in the ever smokers at Year 15 (mean age of 40) is comparable to the prevalence of emphysema in the never smokers observed at Year 25 (mean age of 50).
Table 2:
Prevalence of emphysema on the CT scan by lung function trajectory group in the Year 15 CT scan.
| Trajectories of FEV1% (Y15 CT scan Data: n=2,660) | |||||
|---|---|---|---|---|---|
| Year 15 | Preserved Ideal Lung Health |
Preserved Good Lung Health |
Preserved Impaired Lung Health |
Worsening Lung Health |
Persistently Poor Lung Health |
| N (in whom Year 15 CT scan available) | 160 | 1214 | 1084 | 153 | 49 |
| Centrilobular N (%) | 1 (0.6%) | 9 (0.7%) | 9 (0.8%) | 2 (1.3%) | 2 (4.1%) |
| Paraseptal | 0 (0.0%) | 13 (1.1%) | 13 (1.2%) | 6 (3.9%) | 3 (6.1%) |
| Centrilobular or paraseptal | 1 (0.6%) | 18 (1.5%) | 16 (1.5%) | 7 (4.6%) | 4 (8.2%) |
| Ever Smoker | |||||
| N (ever smokers in whom Year 105 CT scan available) | 47 | 413 | 392 | 83 | 19 |
| Centrilobular N (%) | 0 (0.0%) | 7 (1.7%) | 5 (1.3%) | 2 (2.4%) | 2 (10.5%) |
| Paraseptal | 0 (0.0%) | 12 (2.9%) | 11 (2.8%) | 4 (4.8%) | 1 (5.3%) |
| Centrilobular or paraseptal | 0 (0.0%) | 16 (3.9%) | 11 (2.8%) | 5 (6.0%) | 1 (10.5%) |
| Never Smoker | |||||
| N (never smokers in whom Year 15 CT scan available) | 111 | 790 | 682 | 68 | 29 |
| Centrilobular N (%) | 1 (0.9%) | 2 (0.3%) | 4 (0.6%) | 0 (0.0%) | 0 (0.0%) |
| Paraseptal | 0 (0.0%) | 1 (0.1%) | 2 (0.3%) | 1 (1.5%) | 2 (6.9%) |
| Centrilobular or paraseptal | 1 (0.9%) | 2 (0.3%) | 5 (0.7%) | 1 (1.5%) | 2 (6.9%) |
Note that a subject may have centrilobular, paraseptal or both types of emphysema so the rows designated as “Centrilobular or paraseptal” are not a direct sum of their prevalence.
Table 3:
Prevalence of emphysema on the CT scan by lung function trajectory group in the Year 20 CT scan.
| Trajectories of FEV1% (Y20 CT scan Data: n=2,660) | |||||
|---|---|---|---|---|---|
| Year 20 | Preserved Ideal Lung Health |
Preserved Good Lung Health |
Preserved Impaired Lung Health |
Worsening Lung Health |
Persistently Poor Lung Health |
| N (in whom Year 20 CT scan available) | 160 | 1214 | 1084 | 153 | 49 |
| Centrilobular N (%) | 1 (0.6%) | 12 (1.0%) | 16 (1.5%) | 5 (3.3%) | 2 (4.1%) |
| Paraseptal | 0 (0.0%) | 17 (1.4%) | 18 (1.7%) | 11 (7.2%) | 3 (6.1%) |
| Centrilobular or paraseptal | 1 (0.6%) | 24 (2.0%) | 25 (2.3%) | 13 (8.5%) | 4 (8.2%) |
| Ever Smoker | |||||
| N (ever smokers in whom Year 20 CT scan available) | 47 | 413 | 392 | 83 | 19 |
| Centrilobular N (%) | 0 (0.0%) | 9 (2.2%) | 11 (2.8%) | 5 (6.0%) | 2 (10.5%) |
| Paraseptal | 0 (0.0%) | 16 (3.9%) | 12 (3.1%) | 7 (8.4%) | 1 (5.3%) |
| Centrilobular or paraseptal | 0 (0.0%) | 21 (5.1%) | 16 (4.1%) | 9 (10.8%) | 2 (10.5%) |
| Never Smoker | |||||
| N (never smokers in whom Year 20 CT scan available) | 111 | 790 | 682 | 68 | 29 |
| Centrilobular N (%) | 1 (0.9%) | 3 (0.4%) | 5 (0.7%) | 0 (0.0%) | 0 (0.0%) |
| Paraseptal | 0 (0.0%) | 1 (0.1%) | 6 (0.9%) | 3 (4.4%) | 2 (6.9%) |
| Centrilobular or paraseptal | 1 (0.9%) | 3 (0.4%) | 9 (1.3%) | 3 (4.4%) | 2 (6.9%) |
Note that a subject may have centrilobular, paraseptal or both types of emphysema so the rows designated as “Centrilobular or paraseptal” are not a direct sum of their prevalence.
Table 4:
Prevalence of emphysema on the CT scan by lung function trajectory group in the Year 25 CT scan.
| Trajectories of FEV1% (Y25 CT scan Data: n=2,660) | |||||
|---|---|---|---|---|---|
| Year 25 | Preserved Ideal Lung Health |
Preserved Good Lung Health |
Preserved Impaired Lung Health |
Worsening Lung Health |
Persistently Poor Lung Health |
| N (in whom Year 25 CT scan available) | 160 | 1214 | 1084 | 153 | 49 |
| Centrilobular N (%) | 4 (2.5%) | 43 (3.5%) | 45 (4.2%) | 17 (11.1%) | 7 (14.3%) |
| Paraseptal | 4 (2.5%) | 51 (4.2%) | 58 (5.4%) | 25 (16.3%) | 7 (14.3%) |
| Centrilobular or paraseptal | 6 (3.8%) | 68 (5.6%) | 75 (6.9%) | 30 (19.6%) | 10 (20.4%) |
| Ever Smoker | |||||
| N (ever smokers in whom Year 25 CT scan available) | 47 | 413 | 392 | 83 | 19 |
| Centrilobular N (%) | 2 (4.3%) | 37 (9.0%) | 35 (8.9%) | 15 (18.1%) | 5 (26.3%) |
| Paraseptal | 1 (2.1%) | 44 (10.7%) | 43 (11.0%) | 19 (22.9%) | 5 (26.3%) |
| Centrilobular or paraseptal | 3 (6.4%) | 58 (14.0%) | 56 (14.3%) | 23 (27.7%) | 7 (36.8%) |
| Never Smoker | |||||
| N (never smokers in whom Year 25 CT scan available) | 111 | 790 | 682 | 68 | 29 |
| Centrilobular N (%) | 1 (0.9%) | 5 (0.6%) | 9 (1.3%) | 2 (2.9%) | 2 (6.9%) |
| Paraseptal | 2 (1.8%) | 6 (0.8%) | 12 (1.8%) | 5 (7.4%) | 2 (6.9%) |
| Centrilobular or paraseptal | 2 (1.8%) | 8 (1.0%) | 15 (2.2%) | 6 (8.8%) | 3 (10.3%) |
Note that a subject may have centrilobular, paraseptal or both types of emphysema so the rows designated as “Centrilobular or paraseptal” are not a direct sum of their prevalence.
Finally, we examined the association between trajectory group and odds of emphysema. After adjustment for covariates including pack-years smoking, baseline BMI, and change in BMI from baseline to Year 25, compared to the Ideal lung health trajectory, membership in the Worsening and the Persistently Poor lung health groups was associated with increased odds of emphysema on CT (OR 5.06: 1.84–13.96; OR 4.85: 1.43–16.44 respectively). (Appendix Table 2)
DISCUSSION
In a sample of adults followed from young adulthood and into middle-age, we created five trajectories to represent baseline FEV1 and change in predicted FEV1 over 30 years of follow-up. These trajectories were not intended to be the definitive description of the variable life courses of lung function that people may experience but rather a schema upon which we could interpret susceptibility to lung disease. In view of this, membership in each of these trajectories was associated with a differential burden of expiratory airflow obstruction and emphysema on CT. The novel findings of our work include the demonstration that chronic tobacco smoke exposure is not necessary to develop emphysema and almost 20% of the people with emphysema in the general population may have never smoked. We will begin by discussing the characteristics of these 5 trajectories and then focusing on their relation to emphysema and its subtypes.
The prevalence of self-reported ever smoking was greatest in those assigned to the Persistently Poor lung health trajectory. Chronic tobacco smoke exposure, however, was not solely responsible for trajectory membership since over 40% of those in the Worsening lung health or Persistently Poor lung health trajectories were never smokers. Conversely, approximately 30% of those with Preserved Ideal lung health smoked. These data further support widely held beliefs that there is a range of noxious exposures such as those found at home, work, and in the environment as well as innate structural, genetic and childhood factors that determine the life course of respiratory health. That all smokers do not develop expiratory airflow obstruction has been well documented previously.(21) Our data extend these findings by the inclusion of never smokers from a population-based cohort with an expanded definition of respiratory disease (including emphysema) in middle age.
Over 8% of the CARDIA cohort had expiratory airflow obstruction at their Year 30 (mean age 55 years) exam. This approached or exceeded half of the participants in the Worsening and Persistently Poor lung health trajectories and increased in prevalence in all subgroups from initial ascertainment at study enrollment. The prevalence of obstruction at the Year 30 CARDIA visit is less than the approximate 20% prevalence reported at each of the 12 international sites participating in the population based Burden of Obstructive Lung Disease (BOLD) Study.(22) Part of this difference may be explained by the nature of the investigation. Our data analyses were contingent upon continued participant participation through Year 30 while the initial prevalence estimates reported in BOLD were determined by cross-sectional sampling. Those CARDIA participants with progressive respiratory dysfunction, self-reported symptoms or other medical co-morbidities may be more prevalent in those not attending follow-up exams(16) leading us to underestimate the true burden of respiratory disease.
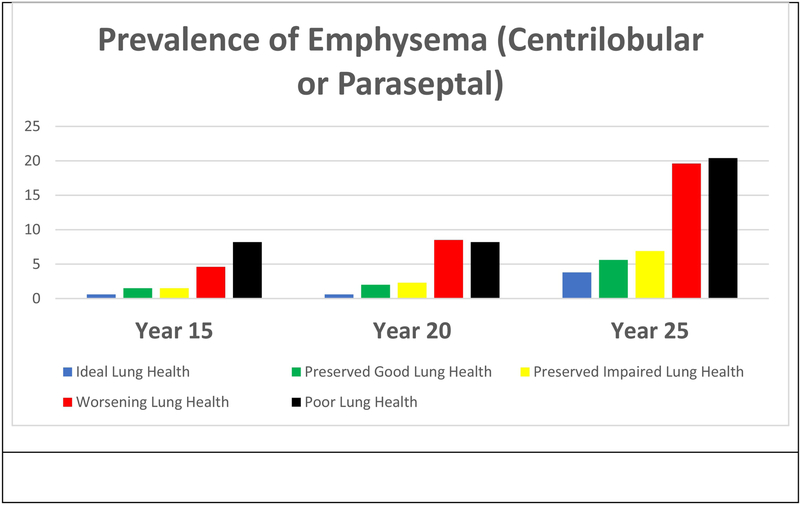
Approximately 2% of CARDIA participants had visually ascertained emphysema at the Year 15 CT scan and that prevalence more than doubled from almost 3% at Year 20 to 7% by the Year 25 CT. These overall estimates, however, belie the disparate likelihood of having emphysema in these age groups. While there is little to no emphysema in those assigned to the Preserved Ideal Lung Health trajectory until people are in their 50s, 10% of people in the Persistently Poor Lung Health group may manifest emphysema a decade earlier at a mean age of 40.
Age did not appear to have a substantial effect on the differential development of centrilobular or paraseptal emphysema. While these two distinct patterns of parenchymal remodeling are associated with differential burdens of symptoms and expiratory airflow obstruction(23), their general co-occurrence in the same trajectories at a time well after the life course of lung function has been established suggest that they may not be the cause of reduced lung function or accelerated spirometric decline but rather more generally portend poorer lung health.
Our data also demonstrate that smoking does not fully account for these parenchymal processes. As shown in Table 4, while only 15% of smokers developed emphysema by the age of 50 years, almost 20% of those who developed emphysema were never smokers. Visual inspection of our data also suggests that smoking does not appear to differentially increase the risk of developing either centrilobular or paraseptal emphysema. Smokers do, however, appear to develop emphysema up to a decade earlier age than never smokers.
There are limitations to this investigation that must be acknowledged. These longitudinal CARDIA data may underestimate the true prevalence of respiratory disease because of non-attendance at follow-up exams over the 30 years of observation. These data also represent assessments of susceptibility to respiratory disease in participants in their 40s to 50s which is generally younger than the age of cohorts included in such investigations as COPDGene(24), SPIROMICS(25) and ECLIPSE(26) where more detailed assessments of respiratory symptoms and emphysema have been performed. However, these latter cohorts were largely comprised of smokers, which may bias estimates of disease susceptibility. Additionally, smoking status (current, former, never) in our study was ascertained by self-report and not confirmed by more objective measures of cotinine. Finally, the trends of worsening expiratory airflow obstruction reported herein represent pre-bronchodilator measures that cannot discern reversible and fixed airflow obstruction. The utilization of pre- bronchodilator measures does not, however, preclude our ability to examine or define trends in lung function over the adult life-course.
There are multiple pathways that define the adult life course of lung function. As first suggested by Fletcher and Peto(21), these pathways represent differences in peak lung function as well as its subsequent rate of decline. We found that those with lower peak lung function or more rapid rates of decline are more likely to experience emphysematous destruction of the lung parenchyma. Further work is needed to identify participants with reduced lung health at the earliest time point and change respiratory care from our current focus on the treatment of established disease to one focused on preventive strategies that preserve respiratory health.
Supplementary Material
Figure 3:
Prevalence of emphysema (centrilobular or paraseptal) at Year 15, 20 and 20.
Clinical Significance:
There are several life course trajectories of lung function characterized by differences in peak lung function and subsequent rate of decline
Those with a low peak lung function and/or rapid rate of decline are at increased risk for the development of emphysema independent of smoking status
Up to 20% of people with emphysema never smoked.
Acknowledgments
Funding Sources: The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). Additional funding was provided by NHLBI R01 HL122477 (CARDIA Lung Study). This manuscript has been reviewed by CARDIA for scientific content.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors had access to these data, had a role in writing this manuscript and approve of its content.
References
- 1.Agusti A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–45. [DOI] [PubMed] [Google Scholar]
- 2.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med. 2010;123(5):468 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lange P, Celli B, Agusti A. Lung-Function Trajectories and Chronic Obstructive Pulmonary Disease. N Engl J Med. 2015;373(16):1575. [DOI] [PubMed] [Google Scholar]
- 4.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018. [DOI] [PubMed] [Google Scholar]
- 5.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souef PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018. [DOI] [PubMed] [Google Scholar]
- 6.Snider GL. Emphysema: the first two centuries--and beyond. A historical overview, with suggestions for future research: Part 1. Am Rev Respir Dis. 1992;146(5 Pt 1):1334–44. [DOI] [PubMed] [Google Scholar]
- 7.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–16. [DOI] [PubMed] [Google Scholar]
- 8.ATS statement--Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119(5):831–8. [DOI] [PubMed] [Google Scholar]
- 9.Standardization of spirometry−−1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis. 1987;136(5):1285–98. [DOI] [PubMed] [Google Scholar]
- 10.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144(5):1202–18. [DOI] [PubMed] [Google Scholar]
- 11.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–36. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ, Jacobs DR, Jr, Terry JG, Shay CM, Sidney S, Liu K, et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years With Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017;2(4):391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, et al. Identification of Early Interstitial Lung Disease in Smokers from the COPDGene Study(1). Acad Radiol. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR Jr, Thyagarajan B, et al. Respiratory Symptoms in Young Adults and Future Lung Disease: The CARDIA Lung Study. Am J Respir Crit Care Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagin DS, Odgers CL. Group-Based Trajectory Modeling (Nearly) Two Decades Later. J Quant Criminol. 2010;26(4):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. [DOI] [PubMed] [Google Scholar]
- 19.Jones BL ND, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–93. [Google Scholar]
- 20.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. [DOI] [PubMed] [Google Scholar]
- 21.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741–50. [DOI] [PubMed] [Google Scholar]
- 23.Smith BM, Austin JH, Newell JD, Jr, D’Souza BM, Rozenshtein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1):94 e7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vestbo J, Anderson W, Coxson HO, Crim C, Dawber F, Edwards L, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31(4):869–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.