Abstract
Background:
The US CDC and the Institute of Medicine recommend that women capable of becoming pregnant consume ≥400 μg synthetic folic acid/d to prevent neural tube defects (NTDs). The United States has 3 sources of folic acid: fortified enriched cereal grain products (ECGPs), fortified ready-to-eat (RTE) cereals, and dietary supplements.
Objective:
Our objectives were as follows: 1) to estimate the usual daily folic acid intake and distributions of red blood cell (RBC) folate concentrations among women consuming folic acid from different sources; 2) to assess the usual daily total folic acid intake associated with optimal RBC folate concentrations for NTD prevention; 3) to predict NTD prevalence; and 4) to estimate the number of pre-ventable folate-sensitive NTDs.
Design:
NHANES data (2007–2012) for nonpregnant women of re-productive age (12–49 y) were used to estimate usual daily intakes of synthetic folic acid and natural food folate. We applied existing models of the relation between RBC folate concentrations and NTD risk to predict NTD prevalence.
Results:
Based on the distribution of overall RBC folate concentrations (4783 women), the predicted NTD prevalence was 7.3/10,000 live births [95% uncertainty interval (UI): 5.5–9.4/10,000 live births]. Women consuming folic acid from ECGPs as their only source had lower usual daily total folic acid intakes (median: 115 μg/d; IQR: 79–156 μg/d), lower RBC folate concentrations (median: 881 nmol/L; IQR: 704–1108 nmol/L), and higher predicted NTD prevalence (8.5/10,000 live births; 95% UI: 6.4–10.8/10,000 live births) compared with women consuming additional folic acid from diet or supplements. If women who currently consume folic acid from ECGPs only (48% of women) consumed additional folic acid sources, 345 (95% UI: 0–821) to 701 (95% UI: 242–1189) additional NTDs/y could be prevented.
Conclusions:
This analysis supports current recommendations and does not indicate any need for higher intakes of folic acid to achieve optimal NTD prevention. Ensuring 400 μg/d intake of folic acid prior to pregnancy has the potential to increase the number of babies born without an NTD.
Keywords: folic acid, neural tube defects, public health, folate, fortification, congenital anomalies
INTRODUCTION
The US CDC and the Institute of Medicine (IOM) recommend that women capable of becoming pregnant consume ≥400 μg synthetic folic acid/d, in addition to a diet rich in natural food folate, to prevent neural tube defects (NTDs) (1, 2). This recommendation is based on data from randomized controlled trials that showed that folic acid intake before and during pregnancy reduced NTD prevalence (3). These results were later confirmed in community intervention studies and evaluations of food fortification programs (4–8). Currently, there are 3 sources of folic acid in the United States, each of which are regulated differently: mandatory fortification of enriched cereal grain products (ECGPs), voluntary fortification of ready-to-eat (RTE) cereals, and vitamin supplements. In 1996, the Food and Drug Administration ruled that grain products labeled as enriched, i.e., ECGPs, must contain 140 μg folic acid/100 g of product (9). Fortification of this staple food was implemented because there is a high rate of unplanned pregnancy in the United States (~50%) (10), and the neural tube closes by day 28 of pregnancy— before many women know that they are pregnant (11). RTE cereals are allowed, but not required, to contain ≤400 μg of folic acid/serving (9). The third source of folic acid, vitamin supplements, generally contain 400–1000 μg folic acid in adult formulations (12). Recently, the US Preventive Services Task Force affirmed its previous recommendation that all women capable of becoming pregnant consume 400–800 μg folic acid/d from supplements for the prevention of NTDs with a grade of A, indicating the highest level of confidence in the recommendation based on available literature (13).
NTDs (including anencephaly, encephalocele, and spina bifida) have devastating effects on infants and their families. Data from birth defect surveillance systems show that NTD prevalence decreased 35% after the implementation of mandatory folic acid fortification, from 10.7 to 7.0 NTDs/10,000 live births (14, 15). It is estimated that folic acid fortification in the United States has averted > 1300 NTDs/y (14,15) and that >$600 million are saved annually in the United States due to the postfortification reduction in spina bifida alone (16). In 2015, the WHO recommended a population-level threshold for red blood cell (RBC) folate concentration of >400 ng/mL (>906 nmol/L) for optimal prevention of NTDs (17).
This analysis had the following objectives: 1) to estimate daily total folic acid and natural food folate intake and the distribution of RBC folate concentrations among women who consume folic acid from different sources; 2) to assess the usual daily total folic acid intake associated with optimal RBC folate concentrations for NTD prevention; 3) to predict the NTD prevalence of women who consume folic acid from different sources based on their RBC folate concentrations; and 4) to estimate the number of potentially folate-sensitive NTDs that could be prevented if women consumed folic acid from additional sources, including RTE cereals and dietary supplements.
METHODS
Demographic characteristics, RBC folate concentration distributions, and usual intakes
We used data from the 2007–2012 NHANES to describe the demographic characteristics, RBC folate concentrations, and dietary intake of natural food folate and synthetic folic acid among nonpregnant women of reproductive age [12–49 y, consistent with the age range used in the WHO guidelines for Optimal Blood Folate Concentrations (17)]. The survey design and procedures have been described in detail elsewhere (18–20). Briefly, RBC folate concentrations in the 2007–2012 NHANES were measured through the use of a microbiologic assay with chloramphenicol-resistant Lactobacillus rhamnosus and 5-methyltetrahydrofolate as the calibrator (21). RBC folate concentrations were log transformed to normalize the distribution of this variable. As was done in a previous analysis (22), we considered the following categories of RBC folate concentrations in this analysis: high risk, associated with an NTD prevalence of >14/10,000 live births, ≤585 nmol/L in the CDC assay used in NHANES (equivalent to ≤699 nmol/L in the Molloy assay); elevated risk, the concentrations associated with 9–14 NTDs/10,000 live births, 586–747 nmol/L in the CDC assay (700–905 nmol/L in the Molloy assay); optimal, associated with <9 NTDs/10,000 live births, 748–1215 nmol/L in the CDC assay (906–1500 nmol/L in the Molloy assay); and limited additional benefit, ≥1216 nmol/L in the CDC assay (>1500 nmol/L in the Molloy assay). Analyses on demographic variables and RBC folate distributions were performed with SAS-callable SUDAAN (version 11.0, Research Triangle Institute) with corresponding NHANES sample weights (combined 6-y mobile examination center and dietary 2-d sampling weights) to account for unequal probability of selection and non-participation. Weighted proportions with 95% CIs were reported for categoric variables.
Dietary information was collected from two 24-h recalls. The first was administered in person and the second was administered by telephone 3–10 d later. Participants were asked about their use of supplements in the previous 30 d (yes or no), and those who reported supplement use were asked to report the name of the supplement(s) and the corresponding frequency of use. We created 4 mutually exclusive groups based on source(s) of folic acid intake: ECGP only (mandatory fortification), ECGP and RTE cereals (ECGP + RTE) (mandatory and voluntary fortification), ECGP and use of supplements containing folic acid (ECGP + SUP), and all 3 sources (ECGP + RTE + SUP) (Supplemental Figure 1).
Intakes of total synthetic folic acid, natural food folate, and total folate were estimated for each individual on each day of their dietary recall. Total folic acid intake was defined as the sum of an individual’s intake of synthetic folic acid from all fortified foods on the day of the recall and the average daily folic acid intake from supplements in the previous 30 d. Total natural food folate was defined as the sum of an individual’s intake of natural food folate in all foods consumed on the day of the recall. We defined total folate intake as the sum of the individual’s daily total folic acid intake and their daily total natural food folate intake. To account for the higher bioavailability of synthetic folic acid compared with natural food folate, total folate intake was measured in dietary folate equivalents (DFEs). DFEs are equal to the total natural food folate intake plus 1.7 times the total folic acid from fortified foods and supplements [total folate DFE = μg of natural food folate + (1.7 × μg folic acid)] (2).
Usual daily folic acid, natural food folate, and total folate intake were estimated with the use of the Software for Intake Distribution Estimation (PC-SIDE), taking into account the between- and within-person variations in intake (23). The distributions of the usual daily intakes of these nutrients were estimated, adjusting for age, race/ethnicity, day 1 or 2 of dietary recall, and the day of the week on which the dietary recall was administered. Only nonpregnant women aged 12–49 y with an RBC folate concentration measurement, supplement use information, and reliable day 1 and 2 dietary recall data were included in the analyses (n = 4783).
Predicted NTD prevalence
We conducted simulations to predict the prevalence of NTDs among US women of reproductive age (12–49 y) stratified by source of folic acid. We predicted prevalence within each strata by a 2-step process. First, we simulated a distribution of RBC folate concentrations for a hypothetical population of 100,000 women for each folic acid source strata. The simulated RBC folate concentration values within each intake group were generated through the use of a log-normal distribution with mean and variance corresponding to that observed in the NHANES data for that folic acid source group. In the second step, we used these simulated RBC folate concentrations to estimate the NTD prevalence within each source group. To do this, we used the estimated coefficients of a logistic regression model relating RBC folate concentration to the risk of NTDs developed in a previous analysis of a large prospective data set from China (24). In that analysis, observed NTD outcomes were matched to each woman’s estimated RBC folate concentration, based on observed folic acid intake, through the use of a logistic regression model with 2 parameters: an intercept term and the estimated log odds ratio relating the increase in the odds of an NTD to a unit increase in RBC folate concentration. Because the original analysis of the Chinese data utilized a Bayesian approach, the result of the analyses was a collection of 20,000 posterior samples for the parameters of the logistic model relating NTD risk and RBC folate concentration. These parameters correspond to the intercept of the logistic model and the increase in the log odds of an NTD for every increase of 1 unit of the log RBC folate concentration. Further analyses of these posterior samples indicated that their joint posterior distribution was well modeled by a bivariate normal distribution. To develop the NTD prevalence estimates for this analysis, we sampled one set of possible parameter values from this bivariate normal posterior distribution and combined this with the simulated RBC folate concentrations to estimate the probability of having a child with an NTD for each of the 100,000 women in the hypothetical population. Thus, each simulation corresponds to selecting one possible set of values for the logistic model parameters and combining that selected set with the simulated distribution for RBC folate concentrations within the population of interest.
Given this estimated NTD probability, occurrence of an NTD was simulated by comparing the estimated probability to a uniform random variate such that, if the estimated probability exceeded the generated uniform random variable, the woman was assigned to have a child with an NTD, whereas she was modeled to not have a child with an NTD otherwise. We summed the number of NTD outcomes and divided by 100,000 to generate an NTD prevalence estimate for the given hypothetical population.
This simulation process was repeated 10,000 times for each folic acid source group (ECGP only, ECGP + RTE, ECGP + SUP, and ECGP + RTE + SUP), resulting in 10,000 estimates of NTD prevalence. Note that the simulated NTD prevalence estimates within each group reflect both the sampling variability in RBC folate concentrations within each population of hypothetical women and the uncertainty associated with the true values of the parameters of the NTD risk model. The distribution of the 10,000 estimated NTD prevalence values within each source population was summarized as the median of the 10,000 estimates with uncertainty represented by a 95% uncertainty interval (UI) with bounds defined by the 2.5th and 97.5th percentiles of the 10,000 generated values.
At higher RBC folate concentrations, there is limited additional NTD prevalence reduction above 1500 nmol/L based on the modeled Chinese data; and in the Daly data there were limited data points above this RBC folate concentration (24). Thus, for the purpose of this analysis, if RBC folate concentrations were > 1500 nmol/L, the predicted prevalence was set at the median prevalence of 1500 nmol/L in the model (24).
We evaluated the differences in the NTD prevalence among the folic acid source groups by comparing the differences in the estimated NTD prevalences. To illustrate, suppose we are comparing the 10,000 predicted NTD prevalence values generated for the ECGP + RTE population with the 10,000 generated for the ECGP only group. To evaluate the magnitude of the difference in predicted NTD prevalence between these 2 populations, we calculated the difference in the estimates, ECGP + RTE minus ECGP only for all 10,000 NTD prevalence estimates from each population. Thus, we had 10,000 possible values for the difference in NTD prevalence between ECGP + RTE and ECGP-only populations. These estimated distributions for the difference in NTD prevalence due to folic acid intake source were summarized as the median and a 95% UI bounded by the 2.5th and 97.5th percentiles of the 10,000 difference estimates.
Estimating the number of NTDs among live-born infants that could be prevented through increasing sources of folic acid intake
We predicted the number of NTDs among live-born infants that could be prevented if women who consume folic acid from ECGP only increased their folic acid intake to include either RTE, SUP, or both (e.g., ECGP + RTE, ECGP + SUP, or ECGP + RTE + SUP) such that they had the same RBC distribution as the women currently in these groups. To do this, we first estimated the proportion of live births that occurred among women with ECGP as their only source of folic acid. Based on NHANES data, we estimated that 48.4% ± 1.1% of women of re-productive age in the United States had ECGP as their only source of folic acid. If we assume that the sampling variability in the proportion of women who consume folic acid from ECGP only can be represented as a normal distribution with a mean of 0.484 and an SD of 0.011, and that the proportion of women of reproductive age with ECGP as their only folic acid source is the same as the proportion of women with a live-born infant with ECGP as their only folic acid source, then the estimated number of live births among women in the ECGP group, nECGP, is:
| (1) |
where p is a sampled value from the assumed normal uncertainty distribution for the ECGP proportion and 3,990,876 is the total live births per year (25).
Next, we let rECGP be the predicted NTD prevalence among women who consume folic acid only from ECGP derived as described above, and rECGP+RTE be the predicted NTD prevalence among those women consuming ECGP + RTE. With these values, we estimated the number of NTDs among live births in the ECGP-only group as nECGP × rECGP and the number of NTDs expected if these women increase their consumption to include RTE cereals as nECGP × rECGP+RTE. The number of NTDs estimated to be prevented if women in the ECGP group moved to the ECGP + RTE intake level is then given by:
| (2) |
Analogous methods were used to estimate the number of NTDs that could be prevented among women consuming folic acid from ECGP only if they added supplements alone, and if they added RTE cereals and supplements.
Estimates of the number of prevented NTDs were developed for each of the 10,000 samples of rECGP, rECGP+RTE, rECGP+SUP, and rECGP+RTE+SUP produced by the modeling described above. We summarize these 10,000 estimates for the number of pre-vented NTDs as the median and a 95% UI. To reflect our assumption that the number of NTDs will not likely increase due to increased consumption of folic acid, the lower limit of the UIs was truncated to zero if the 2.5th percentile of the distribution of estimates was negative.
RESULTS
Among the 4783 women of reproductive age in NHANES aged 12–49 y with no missing information on supplement use, and who had RBC folate concentrations and reliable dietary data, 28.9% (95% CI: 26.4%, 31.6%) reported using a supplement containing folic acid, and 48.2% (95% CI: 45.9%, 50.6%) reported ECGP as their only source of folic acid (Table 1). About three-fourths of the women had RBC folate concentrations in the optimal range or higher (≥748 nmol/L).
TABLE 1.
Demographic characteristics of nonpregnant women aged 12–49 y, NHANES, 2007–20121
| Characteristics | n | Weighted % (95% CI) |
|---|---|---|
| Total | 4783 | |
| Race/ethnicity | ||
| Non-Hispanic white | 1793 | 61.7 (56.9, 66.4) |
| Non-Hispanic black | 1067 | 13.8 (11.3, 16.8) |
| Hispanic | 1473 | 17.3(14.2,21.0) |
| Other | 450 | 7.1 (5.9, 8.5) |
| Age, y | ||
| 12–24 | 1944 | 33.8(31.0,36.8) |
| 25–34 | 1041 | 24.2(22.1,26.4) |
| 35–49 | 1798 | 42.0 (39.4, 44.6) |
| Use of supplements containing folic acid | ||
| None | 3629 | 71.1 (68.4,73.6) |
| Any | 1154 | 28.9(26.4,31.6) |
| <400 μg/d | 605 | 14.6(12.9, 16.5) |
| <200 μg/d | 305 | 7.2 (5.8, 8.9) |
| 200 to <400 μg/d | 300 | 7.4 (6.3, 8.8) |
| ≥400 μg/d | 549 | 14.3 (12.8, 16.0) |
| Folic acid source(s) | ||
| ECGP only | 2421 | 48.2 (45.9, 50.6) |
| ECGP + RTE | 1208 | 22.8 (20.9, 24.9) |
| ECGP + SUP | 761 | 18.6(17.0, 20.2) |
| ECGP + RTE + SUP | 393 | 10.4 (8.9, 12.0) |
| RBC folate concentration concentrations by risk category2 | ||
| High ≤585 nmol/L3 (699 nmol/L4) | 450 | 7.9 (6.7, 9.2) |
| Elevated 586–747 nmol/L3 (700–905 nmol/L4) | 746 | 14.6(13.0, 16.4) |
| Optimal 748–1215 nmol/L3 (906–1500 nmol/L4) | 2377 | 47.7 (45.6, 49.9) |
| Limited additional benefit ≥ 1216 nmol/L3 (> 1500 nmol/L4) | 1210 | (27.3, 32.4) |
All women attended the mobile examination center, had reliable day 1 and 2 dietary data, and had no missing RBC folate concentration or supplement use data. RBC, red blood cell; ECGP, enriched cereal grain product; RTE, ready-to-eat cereal; SUP, supplements containing folic acid.
Risk categories defined based on neural tube defect risk at specific RBC folate concentrations (22).
NHANES method–derived RBC folate concentration [= (Molloy method RBC folate concentration × 0.7876) + 34.2802].
Molloy method.
Median usual daily total folic acid intake was lowest among women for whom ECGP was their only source of folic acid, 115 μg/d (IQR: 79, 156 μg/d) (Table 2). Women who consumed folic acid from all 3 sources had the highest median usual daily total folic acid intake, 661 μg/d (IQR: 519, 830 μg/d). Although intake of natural food folate was similar for women who consumed folic acid from ECGP only and for women who consumed folic acid from ECGP + RTE (162 DFEs; IQR: 122, 204 DFEs and 164 DFEs; IQR: 124, 213 DFEs, respectively), it was slightly higher among consumers of supplements (ECGP + SUP: 189 DFEs; IQR: 144, 245 DFEs and ECGP + RTE + SUP: 187 DFEs; IQR: 146, 241 DFEs). Women who had optimal RBC folate concentrations (748–1215 nmol/L) had a median usual daily total intake of 236 μg of folic acid (IQR: 181, 304 μg) and 174 DFEs of natural food folate (IQR: 147, 206 DFEs) (Table 2).
TABLE 2.
Usual daily intake of folic acid, natural food folate, and total folate by folic acid source and optimal RBC folate concentrations among nonpregnant women aged 12–49 y, NHANES, 2007–20121
| Folic acid, μg | Natural food folate (DFE) | Total folate (DFE)2 | Total μg folic acid3 | |||||
|---|---|---|---|---|---|---|---|---|
| median | IQR | median | IQR | median | IQR | median | IQR | |
| Total | 237 | 146, 370 | 170 | 129, 222 | 591 | 415, 830 | 355 | 249, 498 |
| ECGP | 115 | 79, 156 | 162 | 122, 204 | 367 | 281, 471 | 220 | 169, 283 |
| ECGP + RTE | 239 | 161,339 | 164 | 124, 213 | 582 | 433, 772 | 349 | 260, 463 |
| ECGP + SUP | 461 | 349, 577 | 189 | 144, 245 | 980 | 784, 1221 | 588 | 470, 733 |
| ECGP + RTE + SUP | 661 | 519, 830 | 187 | 146, 241 | 1341 | 1063, 1624 | 805 | 638, 974 |
| RBC folate concentration 748–1215 nmol/L | 236 | 181,304 | 174 | 147, 206 | 581 | 474, 711 | 349 | 284, 427 |
DFE, dietary folate equivalent; ECGP, enriched cereal grain product; RBC, red blood cell; RTE, ready-to-eat cereal; SUP, supplement containing folic acid.
Total folate (DFE) = natural food folate (DFE) + [folic acid (μg) × 1.7].
Total folate DFE was converted to total in μg folic acid; total in μg folic acid = total folate DFE × 0.6.
RBC folate concentrations were lowest among women in the ECGP-only group at every percentile in the RBC folate concentration distribution, and highest among the ECGP + RTE + SUP group (Table 3). Based on the overall RBC folate concentration distributions from all nonpregnant women of reproductive age combined, we predicted an NTD prevalence of 7.3 NTDs/10,000 live births (95% UI: 5.5, 9.4; Table 3). We estimated that women who consume only ECGP had an overall NTD prevalence of 8.5/10,000 live births (95% UI: 6.4,10.8/10,000 live births; Table 3). This prevalence was significantly higher than the predicted prevalence for both of the groups that included supplement use, with a statistically significant prevalence difference of –2.6 (UI: –0.2, –5.0) for the ECGP + SUP group comparison and –3.6 (UI: –1.3, –6.1) for the ECGP + RTE + SUP group comparison (Table 3). The highest predicted NTD prevalence, 20.3/10,000 live births (95% UI: 17, 24.3/10,000 live births), was for women who consumed folic acid only from ECGP, and had RBC folate concentrations in the 5th percentile (RBC folate concentration 484 nmol/L), (Figure 1, Table 3). By comparison, women in the 5th percentile of RBC folate concentrations (749 nmol) among the ECGP + RTE + SUP group had a predicted NTD prevalence of 9.3/10,000 live births (95% UI: 7.8, 10.9/10,000 live births).
TABLE 3.
Predicted NTD prevalence by folic acid source and number of folate-sensitive NTDs preventable by increasing folic acid intake among nonpregnant women aged 12–49 y consuming enriched cereal grain products, NHANES 2007–20121
| Total | ECGP only | ECGP + RTE | ECGP + SUP | ECGP + RTE + SUP | |
|---|---|---|---|---|---|
| n | 4783 | 2421 | 1208 | 761 | 393 |
| 50th percentile RBC folate concentration nmol/L (5th, 25th, 75th, 95th percentile) | 993 (532, 771,1282, 1799) | 881 (484, 704, 1108,1601) | 1003 (596, 806, 1224,1723) | 1157 (603, 875, 1431, 2090) | 1376 (749, 1019, 1606, 2155) |
| Predicted median NTD prevalence per 10,000 live births (95% UI) | 7.3 (5.5, 9.4) | 8.5 (6.4, 10.8) | 6.7 (5.0, 8.7) | 5.9 (4.3, 7.7) | 4.9 (3.5, 6.4) |
| Predicted median difference in NTD prevalence per 10,000 live births (95% UI) | ref. | −1.8 (−4.2, 0.7) | −2.6 (−5.0, −0.2)2 | −3.6 (−6.1, −1.3)2 | |
| Predicted median difference in NTD prevalence per 10,000 live births (95% UI) | ref. | −1.0 (−3.1, 1.0) | −1.8 (−4.0, 0.3) | ||
| Predicted median number of NTDs prevented per year if women consuming only ECGP increased their sources of folic acid intake (95% UI) | 345 (0, 821) | 503 (39, 966)2 | 42, 1189)2 | ||
NTD prevalence is based on modeling with RBC folate concentrations (24). ECGP, enriched cereal grain product; NTD, neural tube defect; ref., reference; RBC, red blood cell; RTE, ready-to-eat cereal; SUP, supplement containing folic acid; UI: uncertainty interval.
Statistically significant as 95% UIs do not include zero.
FIGURE 1.
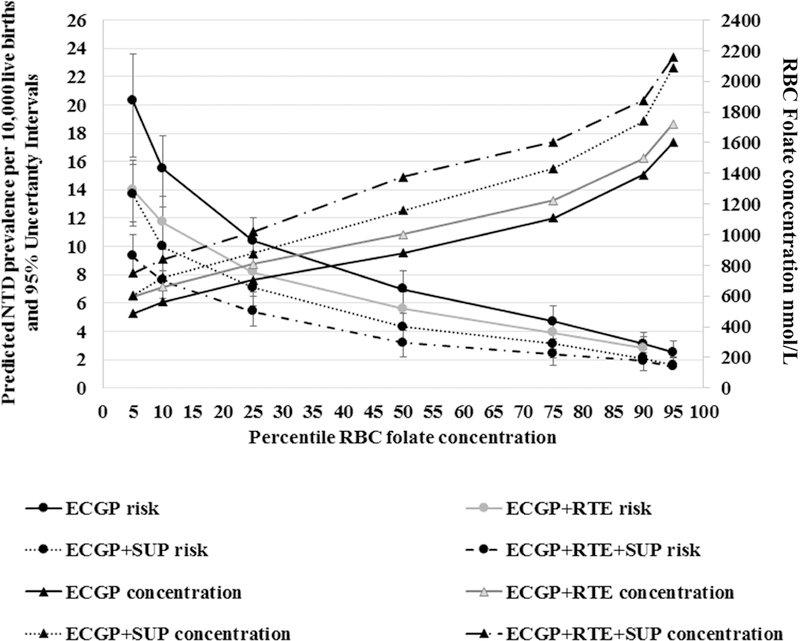
Predicted risk of NTDs by RBC folate concentrations by folic acid source among nonpregnant women aged 12–49 y, NHANES, 2007–2012. y axis: median NTD prevalence and 95% uncertainty intervals were predicted based on RBC folate concentrations obtained from previously reported associations between RBC folate concentrations and NTD prevalence (18) at the 5th–95th percentile stratified by source of folic acid intake: ECGP only, ECGP + RTE, ECGP + SUP, and all 3 sources ECGP + RTE + SUP. z axis: RBC folate concentrations at the 5th–95th percentile by source of folic acid. EGCP, enriched cereal grain product; NTD, neural tube defect; RBC, red blood cell; RTE, ready-to-eat cereal; SUP, supplement containing folic acid.
If those consuming only ECGP were to add RTE cereals to their diets, median usual folic acid intake would increase from 115 to 239 μg/d (Table 2), median RBC folate concentration would increase from 881 to 1003 nmol/L, and the model predicts a (median) reduction of 345 NTDs/y (95% UI: 0, 821 NTDs/y). ECGP plus supplements would increase the usual daily total folic acid intake to 461 μg/d, median RBC folate concentration to 1157 nmol/L, and would be predicted to prevent 503 NTDs/y (median; 95% UI: 39, 966 NTDs/y). Adding both RTE cereal and supplements containing folic acid would be predicted to prevent 701 NTDs/y (95% UI: 242, 1189 NTDs/y) among women who currently consume folic acid from ECGP only. Statistically significant decreases in estimated NTD prevalence were observed only with the addition of supplements.
Among women of reproductive age, only 28.9% (95% CI: 26.4%, 31.6%) reported consuming any supplement containing folic acid (Table 1). Women who reported no supplement use had an RBC folate concentration distribution that predicts an NTD prevalence of 7.9/10,000 live births (95% UI: 6.0, 10.2/10,000 live births). Among women who reported use of a supplement containing folic acid, those who reported consuming <200 μg folic acid/d from supplements had a predicted prevalence of 6.0/10,000 live births (95% UI: 4.4, 7.9/10,000 live births). For women who reported consuming >200 to <400 μg folic acid/d from supplements, we predicted an NTD prevalence of 5.7/10,000 live births (95% UI: 4.2, 7.5/10,000 live births), and for women consuming ≥400 μg folic acid/d from supplements, we predicted an NTD prevalence of 5.1/10,000 live births (95% UI: 3.7, 6.7/10,000 live births). Regardless of supplement dose, the predicted NTD prevalence for women who used supplements was statistically significantly lower than for women who consume folic acid from only ECGP. However, only women with reported intakes ≥400 μg folic acid/d from supplements had statistically significantly lower estimated NTD prevalence than women who did not use supplements, regardless of RTE intake (median difference 2.8/10,000 live births; 95% UI: 0.6, 5.2/10,000 live births).
DISCUSSION
Almost half of this study population (48.2%) was women whose only source of folic acid was ECGP. This group had lower median usual daily total folic acid intake, lower RBC folate concentrations, and higher predicted NTD prevalence than women who consumed folic acid from multiple sources. The RBC folate concentration distributions we observed suggest that adding additional sources of folic acid among those consuming only ECGP would prevent a median of 345–701 NTDs each year in the United States, depending on the additional source(s) of folic acid. Although NTD rates decreased 35% after mandatory fortification of ECGP with folic acid (15), some women who consumed folic acid only from ECGP did not have sufficient folic acid intake to increase RBC folate concentrations and optimally prevent NTDs.
Optimal RBC folate concentration and folic acid intake for NTD prevention
The WHO has established an RBC folate concentration threshold for optimal NTD prevention of 906 nmol/L (calculated with the Molloy method of microbiologic assay, equivalent to 748 nmol/L calculated with the CDC microbiologic assay method) (17, 21, 24). This threshold is based in part on a case-control study from Ireland in which 906 nmol/L was the lower bound of a stratum in which the median RBC folate concentration was 1292 nmol/L (1052 nmol/L CDC microbiological method) and the NTD prevalence was 8/10,000 live births (26). It is important to note that 906 nmol/L is a threshold of insufficiency for NTD prevention at the population level, not a recommended population median. We have previously reported that 22.8% of US women of reproductive age have insufficient RBC folate concentrations for optimal NTD prevention (concentrations under the WHO threshold for optimal NTD prevention) (22). In this analysis, we found that women who had RBC folate concentrations in the optimal range (748–1215 nmol/L) reported a median usual daily total folic acid intake of 236 μg (IQR: 181, 304 μg). This optimal range was used to determine the minimal amount of folic acid intake needed, as there is no established additional benefit to blood folate concentration above this range (22, 24). When combined with their intake of natural food folate (median 174 DFEs; IQR: 147, 206 DFEs), their estimated median daily total folate intake was 581 DFEs (IQR: 474, 711 DFEs), equivalent to 349 μg of folic acid (IQR: 284, 427 μg) (Table 2). This intake level is striking in its similarity to the folic acid intake level of 400 μg recommended by the CDC and IOM, particularly given that the optimal RBC folate concentration was not determined based on its relation to consuming 400 μg folic acid/d, but rather a direct relation with NTD prevalence. In addition, we looked at the impact of different reported folic acid intake dosage from supplements and found that with increasing folic acid supplement intake the median predicted risk decreased compared with those consuming no supplements; however, it did not reach statistical significance until ≥400 μg/d. These data provide further support that women should consume 400 μg/d folic acid before and during pregnancy to prevent NTDs (4) and that both the WHO threshold for optimal RBC folate concentration and recommeded intakes are decribing simliar conditions (intake and biomarker concentrations) for optimal NTD prevention.
The usual intakes of dietary nutrients are limited by the imprecision of the tools used to collect the food intake information, concerns about recall validity, and the variability in food content not represented in food composition databases (27–31). Use of biomarkers, such as RBC folate concentrations, can reduce or eliminate many of these concerns, as presented in a recent analysis of NHANES data (32). We estimate that those consuming folic acid from all 3 sources (ECGP + RTE + SUP) have median usual daily total folic acid intake consistent (Table 2) with the daily intake of 400 μg/d recommended by the CDC and IOM and 400–800 μg/d recommended by the US Preventive Services Task Force. This group had the lowest predicted NTD prevalence [4.9/10,000 live births (IQR: 3.5, 6.4/10,000 live births)] based on their RBC folate concentrations [median 1376 nmol/L (IQR: 1019, 1606 nmol/L)] and median folic acid intake of 661 μg/d (IQR: 519, 830 μg/d), which suggests that current recommendations are associated with RBC folate concentration distributions within and above the optimal range. These data do not support the need for higher levels of folic acid intake in women of reproductive age.
Natural food folate
Although natural food folate intake was similar between women who only consumed ECGP and women who consumed both ECGP and RTE, it was higher among women consuming supplements containing folic acid. The median usual daily total intakes of natural food folate were less than half of the recommended daily allowance of 400 DFEs recommended for the prevention of megaloblastic anemia for all groups (Table 2) (12). These low intakes highlight the importance of consuming folic acid in the US diet not only for NTD prevention but also for the prevention of megaloblastic anemia. There are currently no recommendations for natural food folate intake for the optimal prevention of NTDs or recommendations for intake of any folate vitamin other than folic acid. There are also no randomized control trials to show that increased consumption of only natural food folate at any dose would prevent NTDs. The IOM recommends that women consume a diet rich in folate-dense foods; however, it is important to note that it is difficult for women to consume enough natural food folate to reach RBC folate concentrations for optimal NTD prevention (33). In addition, low-carbohydrate diets were recently associated with increased NTD risk likely through decreased intake of folic acid from fortified enriched cereal grain products (34).
Limitations
The NHANES data on RBC folate concentrations are weighted to represent the overall US population of women aged 12–49 y (18–20); however, the likelihood for women of different ages within this age range becoming pregnant was not taken into account. Although women with folate insufficiency for NTD prevention identified in our analysis are of reproductive age, they are not all equally likely to become pregnant. Thus, the predicted NTD prevalence should be considered not an estimate of actual prevalence but an estimated prevalence among women aged 12–49 y at risk for an NTD should they become pregnant.
The dose-response relation between RBC folate concentrations and NTD prevalence used in this analysis was based on data from Chinese and Irish populations (24). Non-Hispanic black populations in the United States tend to have lower RBC folate concentrations (35–38), but do not have increased NTD prevalence (14, 15); therefore, caution should be used in applying these models to non-Hispanic black populations. However, currently the rates of spina bifida and anencephaly are similar for non-Hispanic whites and blacks, whereas Hispanics have higher rates (15). In addition, although RBC folate concentrations are affected by genetic variation in MTHFR (~8% reduction per T allele rs1801133) (39), genotypes are not currently available for NHANES participants. However, data show that the rate of increase in RBC folate concentration associated with folic acid intake is not affected by MTHFR genotype (24). Although the MTHFR genotype likely affects the amount of folic acid needed to achieve optimal RBC folate concentrations, once optimal RBC folate concentrations are achieved, the same level of NTD prevention is predicted.
Despite these limitations, our approach predicted an NTD prevalence remarkably similar to the current reported overall prevalence. We predict an NTD prevalence in the United States of about 7.3/10,000 live births (95% UI: 5.5,9.4/10,000 live births), consistent with the 2004–2006 NTD prevalence of 6.9/10,000 live births reported from birth defect surveillance systems in the United States that include NTDs that are prenatally ascertained (14).
Conclusions
Currently in the United States, RBC folate concentrations are largely consistent with optimal NTD prevention. However, among women of reproductive age whose only source of folic acid is ECGP, there remains a predicted increased risk of having an NTD-affected pregnancy. Additional folic acid intake is needed among these women to achieve the recommended 400 μg/d (2) and to achieve RBC folate concentrations consistent with optimal prevention of NTDs (17).
Supplementary Material
Abbreviations used:
- DFE
dietary folate equivalent
- ECGP
enriched cereal grain products
- IOM
Institute of Medicine
- NTD
neural tube defect
- RBC
red blood cell
- RTE cereals
ready-to-eat cereals
- SUP
supplements containing folic acid
- UI
uncertainty interval
Footnotes
The authors’ responsibilities were as follows: KSC, OD, SCT, and RJB: designed the research; OD: developed the statistical analysis; OD, KSC, and YPQ: analyzed the data; KSC: wrote the paper and had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors have no conflicts of interest to report. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental Figure 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
REFERENCES
- 1.Centers for Disease Control and Prevention. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 2.Institute of Medicine . Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academy Press; 1998. [PubMed] [Google Scholar]
- 3.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council vitamin study. Lancet, 1991:131–7. https://www.ncbi.nlm.nih.gov/pubmed/1677062. [PubMed] [Google Scholar]
- 4.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LY, Gindler J, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med 1999;341:1485–90. [DOI] [PubMed] [Google Scholar]
- 5.Chen LT, Rivera MA. The Costa Rican experience: reduction of neural tube defects following food fortification programs. Nutr Rev 2004;62 (6 Pt 2):S40–3. [DOI] [PubMed] [Google Scholar]
- 6.De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, Crowley M, Uh SH, Zimmer P, Sibbald B, et al. Spina bifida before and after folic acid fortification in Canada. Birth Defects Res A Clin Mol Teratol 2008;82:622–6. [DOI] [PubMed] [Google Scholar]
- 7.De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, Evans JA, Van den Hof MC, Zimmer P, Crowley M, et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 2007;357:135–42. [DOI] [PubMed] [Google Scholar]
- 8.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res A Clin Mol Teratol 2008;82:211–6. [DOI] [PubMed] [Google Scholar]
- 9.Food US and Administration Drug. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Final rule. 21 CFR Parts 136, 137, and 139. Fed Regist, 1996;61:8781–9. [Google Scholar]
- 10.Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011;84:478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branum AM, Ahrens KA. Trends in timing of pregnancy awareness among US women. Matern Child Health J 2017;21:715–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey LB, ed. Folate in health and disease. Boca Raton (FL): Taylor & Francis; 2009. [Google Scholar]
- 13.US Preventive Services Task Force. Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:183–9. [DOI] [PubMed] [Google Scholar]
- 14.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 2010;88: 1008–16. [DOI] [PubMed] [Google Scholar]
- 15.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS. Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep 2015;64:1–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Grosse SD, Ouyang L, Collins JS, Green D, Dean JH, Stevenson RE. Economic evaluation of a neural tube defect recurrence-prevention program. Am J Prev Med 2008;35:572–7. [DOI] [PubMed] [Google Scholar]
- 17.Cordero AM, Crider KS, Rogers LM, Cannon MJ, Berry RJ. Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects: World Health Organization guidelines. MMWR Morb Mortal Wkly Rep 2015;64:421–3. [PMC free article] [PubMed] [Google Scholar]
- 18.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) 2007–2008. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2007.20.2009-2010.
- 19.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) 2009–2010. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2009.
- 20.National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) 2011–2012. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011.
- 21.Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, Rogers LM, Bailey RL, Yetley EA. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. Am J Clin Nutr 2016;104:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinker SC, Hamner HC, Qi YP, Crider KS. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res A Clin Mol Teratol 2015;103:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carriquiry AL. Estimation of usual intake distributions of nutrients and foods. J Nutr 2003;133:S601–8. [DOI] [PubMed] [Google Scholar]
- 24.Crider KS, Devine O, Hao L, Dowling NF, Li S, Molloy AM, Li Z, Zhu J, Berry RJ. Population red blood cell folate concentrations for prevention of neural tube defects: Bayesian model. BMJ 2014;349:g4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2012. Natl Vital Stat Rep 2013;62:1–68. [PubMed] [Google Scholar]
- 26.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention. JAMA 1995;274:1698–702. [DOI] [PubMed] [Google Scholar]
- 27.Bacardí-Gascón M, Ley y de Góngora S, Castro-Vázquez BY, Jiménez-Cruz A. Validation of a semiquantitative food frequency questionnaire to assess folate status. Results discriminate a high-risk group of women residing on the Mexico-U.S. border. Arch Med Res 2003;34:325–30. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–87. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki S, Ishihara J, Tsugane S. Reproducibility of a self-administered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess food and nutrient intake. J Epidemiol 2003;13(1 Suppl):S115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subar AF, Midthune D, Kulldorff M, Brown CC, Thompson FE, Kipnis V, Schatzkin A. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol 2000;152:279–86. [DOI] [PubMed] [Google Scholar]
- 31.Willett W, Reynolds R, Cottrell-Hoehner S, Sampson L, Browne M. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87:43–7. [PubMed] [Google Scholar]
- 32.Bailey RL, Fulgoni VL, Taylor CL, Pfeiffer CM, Thuppal SV, McCabe GP, Yetley EA. Correspondence of folate dietary intake and biomarker data. Am J Clin Nutr 2017;105:1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchetta CM, Devine OJ, Crider KS, Tsang BL, Cordero AM, Qi YP, Guo J, Berry RJ, Rosenthal J, Mulinare J, et al. Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 2015;7:2663–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desrosiers TA, Siega-Riz AM, Mosley BS, Meyer RE. Low carbohydrate diets may increase risk of neural tube defects. Birth Defects Res 2018. doi: 10.1002/bdr2.1198. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer CM, Caudill SP, Gunter EW, Osterloh J, Sampson EJ. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999–2000. Am J Clin Nutr 2005;82:442–50. [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr 2015;145:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tinker SC, Hamner HC, Berry RJ, Bailey LB, Pfeiffer CM. Does obesity modify the association of supplemental folic acid with folate status among nonpregnant women of childbearing age in the United States? Birth Defects Res A Clin Mol Teratol 2012;94:749–55. [DOI] [PubMed] [Google Scholar]
- 38.Yeung LF, Cogswell ME, Carriquiry AL, Bailey LB, Pfeiffer CM, Berry RJ. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children: National Health and Nutrition Examination Survey (NHANES), 2003–2006. Am J Clin Nutr 2011;93:172–85. [DOI] [PubMed] [Google Scholar]
- 39.Tsang BL, Devine OJ, Cordero AM, Marchetta CM, Mulinare J, Mersereau P, Guo J, Qi YP, Berry RJ, Rosenthal J, et al. Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: a systematic review and meta-analysis of trials and observational studies. Am J Clin Nutr 2015;101:1286–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


