Abstract
Mass spectrometry is the method of choice for the characterisation of proteomes. Most proteins operate in protein complexes, in which their close association modulates their function. However, with standard MS analysis, information on protein–protein interactions is lost and no structural information is retained. To gain structural and interactome data, new crosslinking reagents are needed that freeze inter‐ and intramolecular interactions. Herein, the development of a new reagent, which has several features that enable highly sensitive crosslinking MS, is reported. The reagent enables enrichment of crosslinked peptides from the majority of background peptides to facilitate efficient detection of low‐abundant crosslinked peptides. Due to the special cleavable properties, the reagent can be used for MS2 and potentially for MS3 experiments. Thus, the new crosslinking reagent, in combination with high‐end MS, should enable sensitive analysis of interactomes, which will help researchers to obtain important insights into cellular states in health and diseases.
Keywords: click chemistry, crosslinking mass spectrometry, interactome analysis, proteomics, structure elucidation
Freezing interactions for identification: A new crosslinking reagent, with a sulfoxide fragmentation group and a click site, is reported for crosslink enrichment. Due to special cleavable properties, the reagent can be used for MS2 and potentially for MS3 experiments.
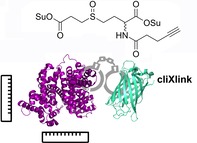
Proteins need to interact with other proteins to form functional complexes. In many cases, protein function inside cells cannot be understood without information about the protein structure and knowledge of in which complex the protein is situated.1, 2 This is, for example, of paramount importance for proteins that modulate epigenetic information on DNA. Almost all of these chromatin‐modifying proteins require intensive interaction with metabolic enzymes, which provide the cofactors needed for histone acetylation, deacetylation or methylation and demethylation.3, 4, 5 This showcases the need to study the complex environment of a given protein to analyse its function and activity state. Protein crosslinking, in combination with analysis by mass spectrometry (XL‐MS), is ideally suited as a method to gain information about protein structure and the composition of protein complexes.6, 7, 8, 9 For XL‐MS, specialised chemical reagents, crosslinkers, are required, which are able to covalently connect protein residues that are in close proximity, for example, interacting in a complex.10, 11 Characterisation of the crosslinked peptides by means of mass spectrometry, however, poses a formidable challenge for two reasons. Firstly, crosslink identification has to be achieved by analysing fragment ions of not only one, but two, connected peptides, thereby massively complicating MS2 spectra. Secondly, the crosslinked peptide species have only a very low abundance and are part of a peptide mixture that is overwhelmingly dominated by non‐crosslinked peptides. In many cases, this leads to dramatic information loss that hampers accurate crosslink identification, and therefore, interactome analysis. A few crosslinking reagents were developed, which introduced MS‐cleavable groups, and the possibility for XL enrichment to tackle these problems.12, 13, 14, 15, 16, 17, 18
Herein, we report the development of a new crosslinking reagent that is enrichable and has cleavage properties which allow accurate crosslink identification. The designed cliXlink (1) is depicted in Figure 1. The cell‐permeable reagent (Figure 1 A and Figure S1 in the Supporting Information) features two succinimidyl ester units that can react with nucleophiles in proteins and are spaced roughly 9 Å apart; thus enabling the fixation of short distances to gain structural information on proteins and to capture proteins in close proximity to each other. Efficient MS cleavability is ensured by the well‐established sulfoxide group, which can be cleaved under low‐energy collision‐induced dissociation (CID) conditions prior to peptide fragmentation. In addition, an alkyne unit allows an enrichment moiety, for example, biotin, to be attached to the crosslinked sites with the help of CuAAC reaction.19
Figure 1.
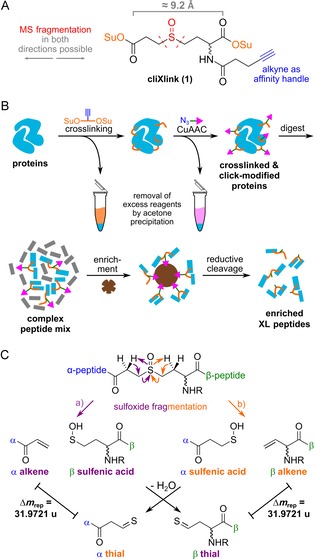
A) Structure of 1. Su: succinimidyl. B) Workflow for XL‐MS experiments. After the addition of 1, protein sites in close proximity are covalently linked. The generated crosslinks can be functionalised with a copper‐catalysed Huisgen reaction (CuAAC). Excess reagents are removed by acetone precipitations. After enzymatic digestion and enrichment on magnetic streptavidin beads, crosslinked peptides were cleaved off under mild conditions and subsequently analysed by means of LC‐MS2. C) Fragmentation pathways of the crosslinked peptides, forming two peptide pairs with a characteristic Δm rep of 31.9721 u.
Application of the crosslinker can follow the workflow depicted in Figure 1 B. It involves the addition of reagent 1 to a complex proteome, fixing the native state distance information of peptides in close proximity and preserving them throughout the further workflow for MS analysis. Attachment of the affinity group for enrichment is performed after crosslinking to avoid interference from bulky enrichment groups. By modification of the crosslinked peptides at the protein level, excess small‐molecule reagents can readily be removed by acetone precipitation. This is followed by enzymatic digestion of the proteins. The crosslinked peptides are in this way labelled, for example, with biotin, which allows for their enrichment. Afterwards, they are analysed by means of mass spectrometry. Because the individual peptide masses in a crosslink are not immediately accessible, crosslink assignment is difficult, especially in complex samples. This requires the use of MS‐cleavable reagents, which facilitate MS2 identification by forming specific fragment ions.20, 21 At best, the reagent should also enable MS3 experiments, which requires that the crosslinker is cleaved before the peptides. This allows peptides to be separated for individual MS3‐based identification. Our reagent 1 contains β‐hydrogen atoms on both sides of the sulfoxide, so that fragmentation occurs in two directions, as depicted in Figure 1 C. This leads to a clean separation of the peptides (α, β) and generates two fragments for each peptide: an alkene and a sulfenic acid fragment; the latter forms a thial upon water loss. This provides two mass pairs with a characteristic Δm/z≈32. Importantly, we observed that fragmentation pathway a (Figure 1 C) predominated. Therefore, for the α‐peptide the alkene fragment, and for the β‐peptide the thial fragment, are the main cleavage products. Due to the asymmetric cleavage properties, most of the signal intensity is retained on one fragment per peptide, which should provide excellent sensitivity in MS3 experiments.
The synthesis of reagent 1 is straightforward (Scheme 1). The starting point is the oxidised disulfide dimer of homocystein 2, which is treated with 4‐pentynoic acid to give disulfide 3. Reductive cleavage of the disulfide and alkylation of the generated thiols with methyl 3‐bromopropionate generates sulfide compound 4. Saponification of both methyl esters to 5 and conversion of 5 into activated bissuccinimidyl ester 6, followed by oxidation of the thioether to the sulfoxide, gives reagent 1 in a total yield of 16 %. Of particular importance is that the reagent is very pure and the reactive ester units are in place at both sides. Partial hydrolysis needs to be avoided. This was ensured by a final precipitation purification. Reagent 1 was dissolved in a mixture of ethyl acetate and dichloromethane and precipitated upon addition of hexanes.
Scheme 1.
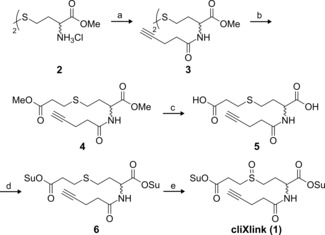
Synthesis of cliXlink (1). a) 4‐Pentynoic acid, N‐(3‐dimethylaminopropyl)‐N′‐ethylcarbodiimide hydrochloride (EDC⋅HCl), 1‐hydroxybenzotriazole hydrate (HOBt⋅H2O), NEt3, DMF, RT, 15 h, 71 %; b) over two steps: 1) tris(2‐carboxyethyl)phosphine hydrochloride (TCEP⋅HCl), NaHCO3, DMF, H2O, RT, 3 h; 2) methyl 3‐bromopropionate, 45 °C, 44 h, 55 %; c) LiOH, THF, H2O, 0 °C RT, overnight (o/n), 91 %; d) N‐hydroxysuccinimide (NHS), pyridine, trifluoroacetic anhydride, MeCN, 0 °C RT, o/n, 78 %; e) meta‐chloroperoxybenzoic acid (mCPBA), AcOEt, RT, 30 min, 57 %.
We next investigated the MS properties of 1. To this end, we added 1 to the commonly used model protein, bovine serum albumin (BSA). We followed the workflow depicted in Figure 1 B without performing the enrichment step. In short, after the addition of 1 to the protein solution and reaction for 1 h at room temperature and physiological pH, we precipitated the protein with acetone, resuspended it in buffer (see the Supporting Information) and digested the protein subsequently with a mixture of trypsin and Lys‐C.
The obtained peptide mixture was desalted with C18 Tip columns and analysed by means of HPLC‐MS2 (Figure 2). Figure 2 A shows two crosslinked BSA peptides (α+β) as an example. After determining the exact mass of the intact crosslink (m/z 677.1; Figure 2 B), we performed both CID and HCD fragmentation on the precursor to evaluate the fragmentation properties (Figure 2 C). More selective CID fragmentation (resonance excitation of the crosslink ion; Figure 2 C, top spectrum) at a low normalised collision energy of 25 % provides, as expected, only a small set of intense signals. Two prominent signal pairs, with Δm/z of 32 (Δm rep), are obtained due to crosslinker fragmentation, which results in a thial (α‐/β‐thial) and alkene (α‐/β‐alkene) fragment for each peptide. As mentioned before, fragmentation pathway a (Figure 1 C) is preferred, leading to an asymmetric intensity distribution. The dominant formation of the expected intact, separated peptides consequently makes the reagent amenable for more sophisticated MS3 experiments.
Figure 2.
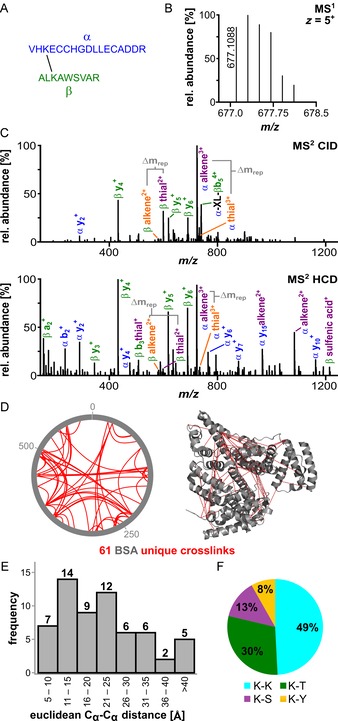
Analysis of crosslinked BSA prior to enrichment. A) Sequences of a representative crosslink within BSA: peptide α: VHKECCHGDLLECADDR (IAA‐modified on Cys) and β: ALKAWSVAR. B) The isotope envelope of the 5+ precursor is shown. C) Fragmentation of the crosslinked peptides under CID (25 %, top spectrum) and stepped higher energy collisional dissociation (HCD) conditions (25/30/32 %, bottom spectrum) showing the product ions of sulfoxide cleavage. The different fragmentation pathways are shown in orange and purple (Figure 1). D) MS2 identification of BSA crosslinking sites prior to enrichment. The xVis representation shows the positions of crosslinked amino acids as red lines (left). The corresponding Cα−Cα distances are depicted in the BSA crystal structure (PDB ID: 4F5S22). E) Histogram of the measured Euclidean Cα−Cα distances in the crystal structure; the majority are below 40 Å, with a mean measured distance of 22.1 Å. F) Distribution of the identified crosslinking sites between Lys–Lys, Lys–Thr, Lys–Ser and Lys–Tyr.
For our study, we used HCD fragmentation. This method provides simultaneous crosslinker cleavage and formation of the peptide fragments required for identification (Figure 2 C, bottom spectrum). To help with crosslink/peptide recognition, it is important that HCD fragmentation still provides the alkene and thial fragments. The HCD data, therefore, allow the identification of peptides by MS2.
Using this method, we analysed the data with the freely available MeroX software, strictly filtering all crosslink identifications (score >50, false discovery rate (FDR) 1 %) and requiring the presence of at least three out of four ions from the two characteristic mass pairs.23, 24 Without exploiting the possibility for CuAAC‐based enrichment, we were able to identify 61 unique BSA crosslinks in a single measurement (Figure 2 D).25 This result is representative for measurements conducted with purified BSA in our laboratory. Additionally, we depicted the crosslinks found in the BSA crystal stucture and noted that the majority of measured distances were reasonable. Importantly, the crosslinks show a mean Cα−Cα distance between the crosslinked amino acids of 22.1 Å and a distance distribution that is in perfect agreement of what is expected for crosslinking with a reagent that spaces the active esters by about 9 Å (Figures 2 E and S2)26 and taking into account side‐chain lengths and protein molecular dynamics.27 Crosslinking occurred mostly between two Lys residues (49 %), but we also detected Lys–Thr (30 %), Lys–Ser (13 %) and Lys–Tyr (8 %) connections, in agreement with the reactivity of the succinimidyl esters (Figure 2 F).28
This success allowed us to validate the new crosslinker in a more complex environment. We particularly wanted to show the additional value of the CuAAC‐based enrichment possibility. For this experiment, we again crosslinked the BSA protein (10 μg), precipitated it with acetone, redissolved the crosslinked protein and subsequently performed the click reaction (Figure S3 A) by adding 7 (Figure 3 A) and CuSO4/tris(3‐hydroxypropyltriazolylmethyl)amine (THPTA) followed by reduction of CuII to CuI upon addition of sodium ascorbate. After 1 h at room temperature, we performed another acetone precipitation to remove excess biotin azide. We then added trypsin and Lys‐C for digestion.
Figure 3.
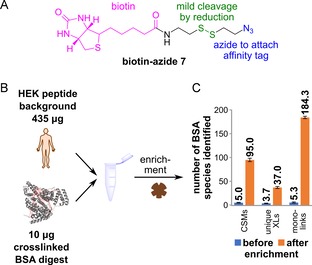
Enrichment of crosslinked BSA from a complex sample. A) Structure of 7, with the biotin group (pink), the disulfide bond (green) and the azide moiety (blue). Upon reduction of the disulfide, the biotin moiety is removed from the crosslink. B) Depiction of the spike‐in experiment. 10 μg of crosslinked and CuAAC‐modified BSA digest were added to 435 μg of HEK digest as a complex background. Magnetic streptavidin beads were used to enrich the crosslinked peptides. C) Results of the HPLC‐MS2 analysis, showing the effect of enrichment. The experiment was performed in technical triplicates; numbers show the mean value and the standard deviation is indicated by the error bar.
These peptides we next combined with a protein digest obtained from a HEK cell extract (435 μg of protein; Figure 3 B). This creates a massive background of non‐crosslinked peptides. If we now analysed the crosslinks within this large background (Figure 3 C), we identified only a very small number of crosslink spectral matches (mean CSMs=5.0), unique crosslinked sites (mean unique XLs=3.7) as well as monolinks (mean=5.3), in which one succinimidyl ester hydrolysed before reaction with the protein. However, if we performed the enrichment with streptavidin‐coated magnetic beads, the number of crosslink identifications improved dramatically. Following incubation of the peptide mixture with the magnetic particles; extensive washing (6×) of the beads with buffer (see the Supporting Information) and mild, reductive cleavage of the disulfide to liberate the “captured” crosslinks and thereby removing the biotin moiety (Figure S3 B), we now, on average, detected 95.0 CSMs and 37.0 unique XLs together with 184.3 monolinks. The number of identified unique XLs was lower than that for the purified BSA (Figure 2 D), which could be explained by inefficiences in the CuAAC reaction or interference of the non‐crosslinked complex background. However, this result shows that our new crosslinker, 1, is able to enrich crosslinked peptides in a vast excess of unmodified peptides; thus facilitating the analysis of complex samples with low‐abundant crosslinks. Originally, we were sceptical about the asymmetric structure of 1. The data, however, show that, despite this, a large number of crosslinks are identified.
In summary, our new crosslinker 1 combines the following advantages: firstly, the size of the reagent is ideally suited to gain valuable distance information for structural proteomics. In addition, the molecule is cell permeable and the alkyne unit does not cause major interference with the crosslinking reaction. Furthermore, the robust, efficient sulfoxide fragmentation simplifies crosslink identification. The possibility of functionalising peptides crosslinked with 1 by click chemistry allows diverse modifications and enrichment strategies that enable analysis of low‐abundant crosslinks to be employed. To conclude, our reagent 1 now paves the way for complex interactome analysis by using MS2 and MS3 experiments.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft for financial support through SFB1309 (TP‐A4), SFB1032 (TP‐A5) and SPP1784. Further support was obtained from the Einzelverfahren CA275‐11/1 and from the European Union through the Marie Curie International Training and Mobility Network “LightDynamics” (grant no. 765866). Research was furthermore supported by a European Research Council (ERC) advanced grant under the European Union's Horizon 2020 research and innovation programme (grant agreement no. EPiR 741912) and the Volkswagen foundation (Initiative “Life”: EcoRib). M.W. thanks the U.S. Department of Energy (DOE grant DE‐SC0018260) and the National Institutes of Health (NIH grant R35GM128813) for support. M.S. and L.S.R. thank the Fonds der Chemischen Industrie for predoctoral fellowships. The mass spectrometry proteomics data have been deposited with the ProteomeXchange Consortium through the PRIDE29 partner repository with the accession no. PXD015080.
M. Stadlmeier, L. S. Runtsch, F. Streshnev, M. Wühr, T. Carell, ChemBioChem 2020, 21, 103.
References
- 1. Berezovsky I. N., Guarnera E., Zheng Z., Eisenhaber B., Eisenhaber F., Curr. Opin. Struct. Biol. 2017, 42, 67–74. [DOI] [PubMed] [Google Scholar]
- 2. Currinn H., Wassmer T., Biochem. Soc. Trans. 2016, 44, 185–190. [DOI] [PubMed] [Google Scholar]
- 3. Allard S., Utley R. T., Savard J., Clarke A., Grant P., Brandl C. J., Pillus L., Workman J. L., Côté J., EMBO J. 1999, 18, 5108–5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xue Y., Wong J., Moreno G. T., Young M. K., Côté J., Wang W., Mol. Cell 1998, 2, 851–861. [DOI] [PubMed] [Google Scholar]
- 5. Sauvageau M., Sauvageau G., PLoS Biol. 2008, 6, e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leitner A., Walzthoeni T., Kahraman A., Herzog F., Rinner O., Beck M., Aebersold R., Mol. Cell. Proteomics 2010, 9, 1634–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sinz A., Angew. Chem. Int. Ed. 2018, 57, 6390–6396; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 6498–6504. [Google Scholar]
- 8. Rappsilber J., J. Struct. Biol. 2011, 173, 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chavez J. D., Bruce J. E., Curr. Opin. Chem. Biol. 2019, 48, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O'Reilly F. J., Rappsilber J., Nat. Struct. Mol. Biol. 2018, 25, 1000–1008. [DOI] [PubMed] [Google Scholar]
- 11. Leitner A., Faini M., Stengel F., Aebersold R., Trends Biochem. Sci. 2016, 41, 20–32. [DOI] [PubMed] [Google Scholar]
- 12. Kao A., Chiu C.-l., Vellucci D., Yang Y., Patel V. R., Guan S., Randall A., Baldi P., Rychnovsky S. D., Huang L., Mol. Cell. Proteomics 2011, 10, M110.002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Müller M. Q., Dreiocker F., Ihling C. H., Schäfer M., Sinz A., Anal. Chem. 2010, 82, 6958–6968. [DOI] [PubMed] [Google Scholar]
- 14. Burke A. M., Kandur W., Novitsky E. J., Kaake R. M., Yu C., Kao A., Vellucci D., Huang L., Rychnovsky S. D., Org. Biomol. Chem. 2015, 13, 5030–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang X., Bruce J. E., Mol. Biosyst. 2010, 6, 939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang R., Zhu W., Wu Y., Chen J., Yu J., Jiang B., Chen H., Chen W., Chem. Sci. 2019, 10, 6443–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rey M., Dupré M., Lopez-Neira I., Duchateau M., Chamot-Rooke J., Anal. Chem. 2018, 90, 10707–10714. [DOI] [PubMed] [Google Scholar]
- 18. Steigenberger B., Pieters R. J., Heck A. J. R., Scheltema R. A., ACS Cent. Sci. 2019, 5, 1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehrlich M., Gattner M. J., Viverge B., Bretzler J., Eisen D., Stadlmeier M., Vrabel M., Carell T., Chem. Eur. J. 2015, 21, 7701–7704. [DOI] [PubMed] [Google Scholar]
- 20. Liu F., Lössl P., Scheltema R., Viner R., Heck A. J. R., Nat. Commun. 2017, 8, 15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klykov O., Steigenberger B., Pektaş S., Fasci D., Heck A. J. R., Scheltema R. A., Nat. Protoc. 2018, 13, 2964–2990. [DOI] [PubMed] [Google Scholar]
- 22. Bujacz A., Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 1278–1289. [DOI] [PubMed] [Google Scholar]
- 23. Götze M., Pettelkau J., Fritzsche R., Ihling C. H., Schäfer M., Sinz A., J. Am. Soc. Mass. Spectrom. 2015, 26, 83–97. [DOI] [PubMed] [Google Scholar]
- 24. Götze M., Iacobucci C., Ihling C. H., Sinz A., Anal. Chem. 2019, 91, 10236–10244. [DOI] [PubMed] [Google Scholar]
- 25. Grimm M., Zimniak T., Kahraman A., Herzog F., Nucleic Acids Res. 2015, 43, W362–W369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Merkley E. D., Rysavy S., Kahraman A., Hafen R. P., Daggett V., Adkins J. N., Protein Sci. 2014, 23, 747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orbán-Németh Z., Beveridge R., Hollenstein D. M., Rampler E., Stranzl T., Hudecz O., Doblmann J., Schlögelhofer P., Mechtler K., Nat. Protoc. 2018, 13, 478–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalkhof S., Sinz A., Anal. Bioanal. Chem. 2008, 392, 305–312. [DOI] [PubMed] [Google Scholar]
- 29. Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D. J., Inuganti A., Griss J., Mayer G., Eisenacher M., Pérez E., Uszkoreit J., Pfeuffer J., Sachsenberg T., Yılmaz Ş., Tiwary S., Cox J., Audain E., Walzer M., Jarnuczak A. F., Ternent T., Brazma A., Vizcaíno J. A., Nucleic Acids Res. 2019, 47, D442–D450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


