ABSTRACT
Marrow adipose tissue (MAT) and its relevance to skeletal health during caloric restriction (CR) is unknown: It remains unclear whether exercise, which is anabolic to bone in a calorie‐replete state, alters bone or MAT in CR. We hypothesized that response of bone and MAT to exercise in CR differs from the calorie‐replete state. Ten‐week‐old female B6 mice fed a regular diet (RD) or 30% CR diet were allocated to sedentary (RD, CR, n = 10/group) or running exercise (RD‐E, CR‐E, n = 7/group). After 6 weeks, CR mice weighed 20% less than RD, p < 0.001; exercise did not affect weight. Femoral bone volume (BV) via 3D MRI was 20% lower in CR versus RD (p < 0.0001). CR was associated with decreased bone by μCT: Tb.Th was 16% less in CR versus RD, p < 0.003, Ct.Th was 5% less, p < 0.07. In CR‐E, Tb.Th was 40% less than RD‐E, p < 0.0001. Exercise increased Tb.Th in RD (+23% RD‐E versus RD, p < 0.003) but failed to do so in CR. Cortical porosity increased after exercise in CR (+28%, p = 0.04), suggesting exercise during CR is deleterious to bone. In terms of bone fat, metaphyseal MAT/ BV rose 159% in CR versus RD, p = 0.003 via 3D MRI. Exercise decreased MAT/BV by 52% in RD, p < 0.05, and also suppressed MAT in CR (−121%, p = 0.047). Histomorphometric analysis of adipocyte area correlated with MAT by MRI (R2 = 0.6233, p < 0.0001). With respect to bone, TRAP and Sost mRNA were reduced in CR. Intriguingly, the repressed Sost in CR rose with exercise and may underlie the failure of CR‐bone quantity to increase in response to exercise. Notably, CD36, a marker of fatty acid uptake, rose 4088% in CR (p < 0.01 versus RD), suggesting that basal increases in MAT during calorie restriction serve to supply local energy needs and are depleted during exercise with a negative impact on bone. © 2019 The Authors. Journal of Bone and Mineral Research published by American Society for Bone and Mineral Research.
Keywords: BONE‐FAT INTERACTIONS, EXERCISE, MARROW ADIPOSE TISSUE (MAT)
Introduction
Marrow adipose tissue (MAT) accumulation was initially detected in the 1970s by Meunier and colleagues in orthopedic surgical specimens of osteoporotic patients as well as in the setting of normal aging.1 These first findings impelled research into the significance of bone marrow adipocytes for skeletal health. MAT, derived from the differentiation of mesenchymal stem cells (MSC) into adipocytes, increases in bone‐fragility states; however, its potential role in promoting bone formation and/or resorption has not been elucidated, despite active investigation.2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Understanding of MAT has improved with quantification methods that permit exact investigations, including magnetic resonance spectroscopy (MRS) in humans12, 13, 14, 15 as well as osmium‐μCT and MRI with advanced image processing in rodents.16, 17, 18 Recent work established MAT to be suppressed by exercise, in rodents16, 18 and humans,19 suggesting that MAT may function similarly to white adipose tissue in a calorie‐replete state as an energy depot. Moreover, fatty acid β‐oxidation markers rise in bone in the setting of exercise, concomitant with increased bone quantity; this along with research9, 20 demonstrating the reliance of the osteoblast on β‐oxidation support MAT's role as an energy depot.
In addition to aging‐associated osteoporosis, MAT appears to increase in the fragile bone states of anorexia in humans and caloric restriction in mice. As nutrient stores in caloric restriction wane, gluconeogenesis provides energy21, 22, 23, 24, 25 and fat stores are mobilized as an alternate fuel source.26, 27 In late caloric restriction, white fat stores are depleted, highlighting the conundrum of persistent marrow adipocytes.28, 29 Although marrow fat accumulation in the energy‐depleted state has been shown in humans by marrow aspirate30 or MRS31 and rodents via histology and MRS,32 studies lacked 3‐dimentional valuation of MAT. Prospective caloric restriction studies are unlikely to receive institutional review board approval and thus, in humans, we largely rely on retrospective or cohort studies, limiting data quality. Imaging and bone biopsies in human studies are difficult to obtain, further dictating a need for animal studies. Thus, rigorous measures of MAT response to caloric restriction are needed.
We have shown that MAT increases with overfeeding and decreases during exercise.17, 18 This supports that MAT functions as an accessible energy depot. In addition to the paucity of data for MAT quantity and localization in states of caloric restriction, its physiology in this setting is poorly understood. The pathologic bone loss due to anorexia/caloric restriction shows a minimal anabolic‐bone response to exercise and maintains a significant fracture risk for years after successful weight gain.33, 34, 35 This stands in contrast to the exercise effect to increase bone formation while decreasing resorption in the calorie‐replete state.36, 37, 38, 39, 40, 41, 42, 43, 44, 45 We thus hypothesized that in caloric restriction, MAT's physiologic role differs from the calorie‐replete state. The reports of increased MAT in calorie restriction,28, 29 combined with increased fracture risk, suggest that the MAT energy depot may be subverted in the energy‐depleted state. Indeed, a high level of physical activity—in combination with caloric restriction—likely results in a decline in overall health,46 based on Potzner's constrained energy expenditure model.47 Accordingly, we asked if exercise might be harmful to skeletal health in the setting of caloric restriction, simultaneous with exact quantification and characterization of the physiologic response of MAT and bone.
Our findings confirmed that exercise in the setting of a calorie deficit is harmful to bone health. We observed a degradation of bone in exercised, calorically restricted mice. Interestingly, MAT decreased in CR‐exercisers compared with CR, and this was significant, suggesting an alternative purpose for the marrow fat depot in the setting of caloric restriction. Further, reduced sclerostin (Sost) and TRAP during caloric restriction reflected a low bone turnover state. Both rose during exercise in calorie restriction. Lastly, we noted that CD36, responsible for fatty acid uptake, was significantly upregulated in caloric restriction, supplying a prospective mechanism by which MAT expands in this state.
Materials and Methods
Animals, diet, and exercise intervention
Procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee. Eleven‐week‐old C57BL/6 (B6) female mice (Jackson Laboratory, Bar Harbor, ME, USA) were housed in controlled light and temperature conditions. Individually housed mice were randomly allocated to an ad libitum regular diet (RD) group or a 30% caloric restriction (CR) group for 6 weeks (#D12450J, Research Diets, New Brunswick, NJ, USA, containing 10% of the calories from fat and the corresponding, nutrient‐enriched CR diet, #D15032801). Both RD and CR diets contain 10% of the calories from fat. The CR diet is based on RD but modified as a daily allotment to provide 70% of the caloric intake as well as100% of vitamins and minerals.48 Concomitant with dietary intervention, mice were further allocated to voluntary running wheel exercise (E) for 6 weeks as previously described.16, 17, 18 Both RD‐E and CR‐E mice ran during the 6 weeks of wheel access. We did not exclude mice as all runners took part in voluntary running daily. Cyclometers record the daily distance as well as average velocity as in Styner and colleagues.17
Volumetric quantification and imaging of MAT by MRI
Imaging using a 9.4 T horizontal small‐bore MRI scanner was applied to quantify MAT volumetrically.17 Briefly, femoral water and fat maps were obtained with a 2‐dimensional RARE imaging sequence with the following parameters: RARE factor = 4, TE = 28 ms, TR = 4000 ms, number of averages = 4, number of slices = 24, slice thickness = 0.5 mm, in‐plane resolution = 100 × 100 μm2, matrix size = 130 × 130. As fat and water protons have an NMR frequency separation of 3.5 ppm, a Gaussian‐shaped 90° saturation pulse with a width of 2 ms was applied preceding the RARE sequence to suppress fat or water signal while leaving the other signal unaffected. Fat and water images were acquired by setting the saturation pulse frequency the same as the water and fat frequencies, respectively.
In our processing workflow, we manually subdivided full images containing samples into individual images for each bone. Then, we employed water images to manually contour femoral bone masks using Insight SNAP.49 Using these masks, interior bone regions were masked from other image parts in both water and fat maps. Next, a common, study‐specific reference space was established by computing an unbiased average image50 from the masked water maps using the ANTS registration software.51 Individual water and fat maps were propagated into the common space, where voxel‐wise correspondence allows direct comparison of intensities. Average fat maps for each group were computed in the common space and superimposed on the common, average water image for visualization of group fat maps. Fat map intensities were represented with a colored heat map in 3D Slicer49 for visualization (as in Fig. 3 A, B). For MAT quantification (as in Fig. 3 C), a regional label map of the femur was created, excluding cortical bone regions, for the epiphysis, metaphysis, and diaphysis. The femoral head was excluded from the final analysis due to variability in the bone shape/volume between specimens. Intensity‐weighted volume of MAT was quantified via regional fat histograms as in Styner and colleagues.17
Figure 3.
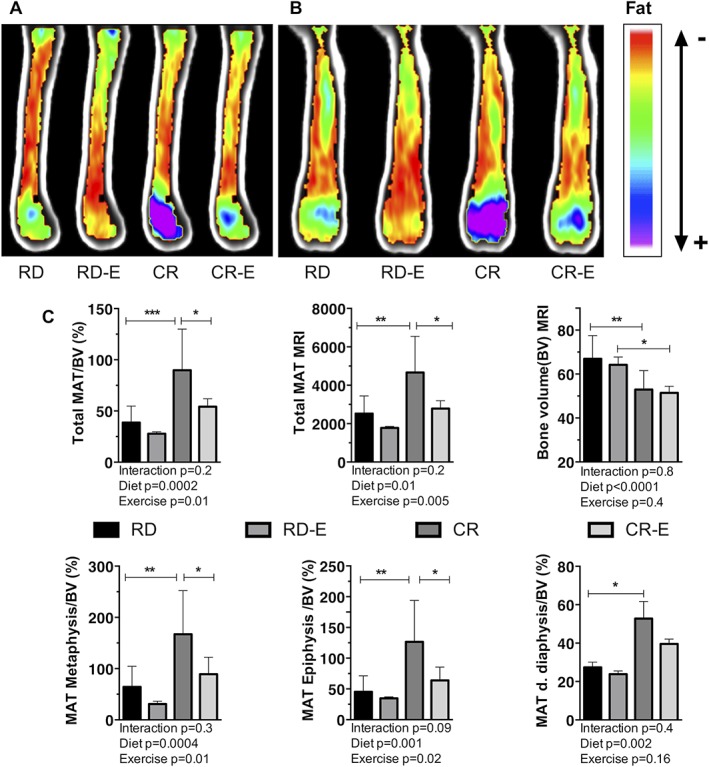
Exercise associated with reduced marrow adipose tissue (MAT), even in the setting of caloric restriction. Average MRI group images (n = 6–9/experimental group) in sagittal (A), coronal (B) planes color labeled for quantity of lipid. B6 mouse femurs analyzed 6 weeks after 30% caloric restriction (CR) or regular diet (RD) +/− running exercise (E) via MRI with advanced image processing. (C) Bone volume (BV) and marrow adipose tissue (MAT) quantification via MRI. Mean ± SD. Significance by 2‐way ANOVA. For multiple comparisons, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Histomorphometry
Fixed and decalcified femurs were imbedded in paraffin, sectioned at 5 μm, and stained with hematoxylin as previously described.17, 52 Imaging was performed via the Olympus X81 at 4× and 40× magnifications. The 40× magnification images were obtained at the distal femoral growth plate, where lipid content is maximal. ImageJ was used to isolate adipocytes and quantify adipocyte size and number.17, 53 This process was applied to 3 animals/group and 3 sections/animal, with a minimum of 300 cells analyzed per experimental group. For osteoclast quantification, sections were stained for TRAP with a Fast Green (Sigma, St. Louis, MO, USA, F7252‐5G) background stain. Analysis for osteoclast number was performed using the open source applications Image J and TrapHisto.54
Bone microarchitecture
Bone microarchitecture parameters of the proximal tibial metaphysis and mid‐diaphysis were quantified ex vivo as previously described (resolution = 12 μm, E = 55 kVa, I = 145 μA).17, 55, 56, 57
Real‐time PCR
Quantitative PCR was performed as previously described.16, 18, 58, 59, 60 Briefly, 1 μg of mRNA from whole tibia was reverse‐transcribed. Ten microliters of cDNA from each experimental condition were pooled and diluted 1:10 to 1:10,000 to generate a 5‐point standard curve. A non‐template control was added to each PCR reaction. Standards and samples were run in duplicate. PCR products were normalized to GAPDH.
Statistical analysis
Statistical significance was assessed by two‐way ANOVA with correction for multiple comparisons via a Tukey post hoc test (GraphPad Prism 7.0, GraphPad, La Jolla, CA, USA), applying exercise and dietary intervention as analysis variables. Our data sets passed the Shapiro–Wilk normality test. The p value cut‐off for significance is defined at less than or equal to 0.05.
Results
Caloric restriction attenuates white adipose tissue and body weight
To investigate marrow adiposity in caloric restriction and its relevance to skeletal health, B6 mice assigned to a 30% caloric restriction (CR) versus regular diet (RD) were further allocated to voluntary exercise (E) versus sedentary control group. Running distance was similar between the groups (Fig. 1 C, RD‐E 10.8 ± 6.6, CR‐E 10.03 ± 3.8, p = ns), with individual variability noted, consistent with other rodent studies applying a voluntary running exercise intervention.61, 62 Running speed was also likewise similar between groups (Fig. 1 C).
Figure 1.

Caloric restriction reduces body and perigonadal fat pad weight. B6 mice after 6 weeks of 30% caloric restriction (CR) versus regular diet (RD) +/− running exercise (E) (4 groups, n = 7–10/group). (A) Body weight. (B) Food intake (g/d). (C) Running distance (km/d) and average running velocity (km/hr). (D) Fat pad weight. Mean ± SD. Significance by 2‐way ANOVA or t test.
After 6 weeks, calorically restricted mice weighed 20% less than RD, p < 0.0001; exercise did not significantly affect weight (Fig. 1 A). The gonadal fat pad weight % was 31% lower in CR compared with RD (Fig. 1 D). The fat pad weight was significantly reduced applying diet as the main effect by 2‐way ANOVA (p = 0.004 for fat pad weight, p = 0.015 for fat pad weight %). Thus, CR mice had demonstrably lower fat pad weights in this analysis. Exercise, when applied as a main effect, failed to significantly affect fat pad weight or fat pad weight %.
Cortical and trabecular bone is degraded by exercise in caloric restriction
Consistent with preclinical and clinical studies, trabecular microarchitecture measured in the tibia via μCT demonstrated reduced bone quantity in caloric restriction (Tb.Th −16% in CR versus RD, p < .001; Tb.Th −45% in CR‐E versus RD‐E, p < 0.0001, Fig. 2 A). Cortical parameters such as cortical thickness and cortical area fraction were similarly decreased in CR. An anabolic response to exercise in the calorie‐replete RD mice was found, consistent with prior work.16, 17, 18 Specifically, RD mice showed a 27% increase in Tb.Th in response to exercise (p < 0.0001, Fig. 2 A). In contrast, the calorie‐restricted group demonstrated degraded bone parameters with exercise: reduced trabecular number (−45% CR‐E versus RD‐E, p = 0.002) as well as increased cortical porosity (+26% CR‐E versus RD‐E, p = .05; +28%, CR‐E versus CR p = 0.04, Fig. 2 B). Thus, the response of CR bone to exercise veers sharply from the positive anabolic response of RD runners. This suggests that a calorie‐replete state is required for exercise‐induced skeletal anabolism. Moreover, exercise‐induced suppression of MAT may mobilize bone lipid for lipolysis; however, it is unlikely to serve as an energy store for bone formation in CR.
Figure 2.
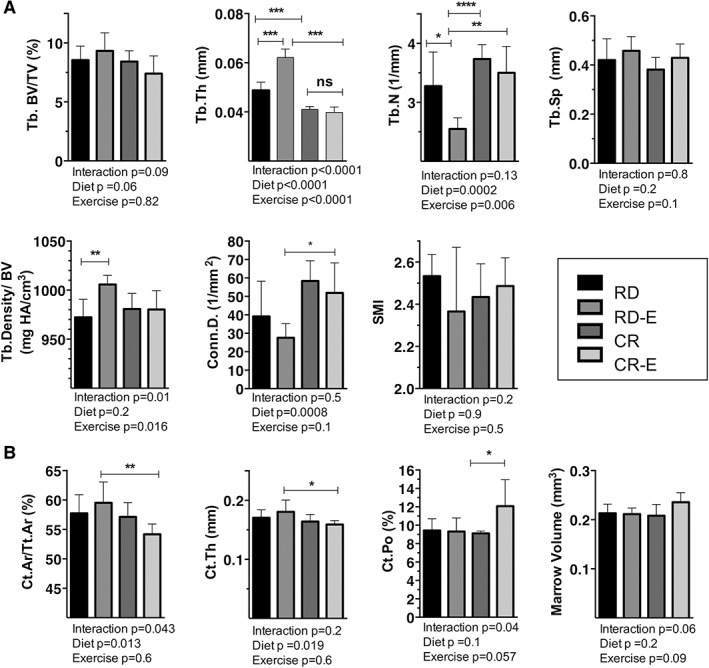
Bone quantity was degraded by exercise in caloric restriction. Tibial bone microarchitecture via μCT in B6 mice after 6 weeks of 30% caloric restriction (CR) or regular diet (RD) +/−running exercise (E) (n = 7/group). (A) Trabecular parameters. (B) Cortical parameters. Plots represent means ± SD. Significance by 2‐way ANOVA. For multiple comparisons, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001****p ≤ 0.0001.
Exercise reduced marrow adipose tissue volume in the setting of caloric restriction
We next turned to the question of whether MAT is quantifiably increased in mice in the setting of caloric restriction. We also sought to find if exercise might attenuate MAT in CR as previously shown in a calorie‐replete state.16, 18 MAT was quantified by means of volumetric magnetic resonance imaging (MRI), which allows separation of the nuclear magnetic resonance signals from water and fat, along with bone masking, allowing a precise quantification of MAT relative to bone volume in murine femurs.17 Average group femur MR images (n = 6–9/group, Fig. 3 A, B) display the distribution of MAT in the femur with a higher MAT signal in the metaphysis/epiphysis in the calorically restricted group. As expected, and corresponding to body weight measurements, bone volume (BV) measured 21% lower in CR versus RD (p = 0.04) and 19% lower in CR‐E versus RD‐E (p = 0.03, Fig. 3 C). Total femoral MAT/BV in CR, in contrast to white adipose tissue, increases (+132%, p = 0.0009), with individual regions such as the distal epiphysis, metaphysis, and diaphysis demonstrating a significant increase as well (Fig. 3 C). Notably, while the MAT content increased with CR in multiple regions, it was most evident in the metaphysis (+159%, p = 0.003, Fig. 3 C). In response to exercise, whole bone MAT/BV diminished significantly in both experimental groups (RD‐E versus RD: –28%, CR‐E versus CR: −92%, p = 0.01 for an exercise effect, Fig. 3 C), akin to prior findings demonstrating exercise‐induced diminution of MAT in non‐calorically restricted states.16, 18 In terms of regional analysis, metaphyseal MAT/BV was particularly responsive to exercise (− 52% in RD‐E versus RD and −121% in CR‐E versus CR, p = 0.01 for an exercise effect) (Fig. 3 C). Adipocyte size vis histology correlated with the MRI data: −48% in RD‐E versus RD and −20% in CR‐E versus CR, p = 0.006 for an exercise effect (for correlation R2 = 0.6233, p < 0.0001) (Fig. 5 A–C).
Figure 5.
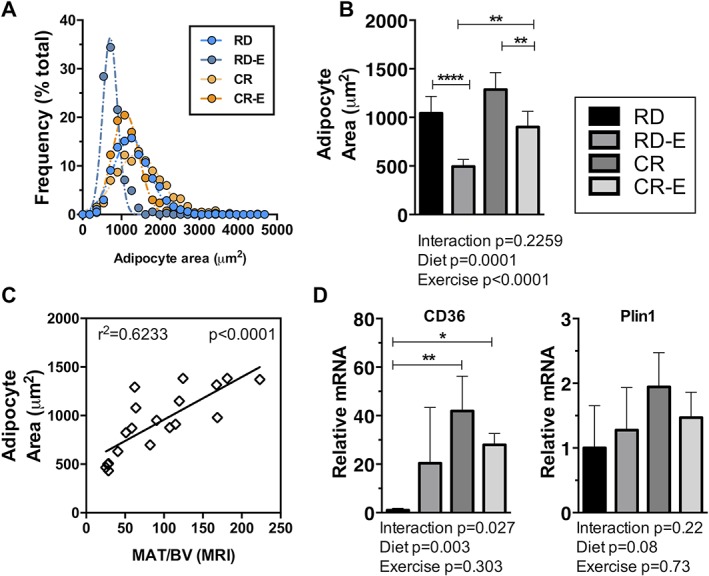
Caloric restriction, exercise attenuation of marrow adipocyte area, marker of fatty acid metabolism. Histomorphometric analysis performed on 3 sections per mouse (n = 5–6) with a minimum of 300 cells analyzed for each experimental group. (A) Marrow adipocyte area histogram. (B) Marrow adipocyte area. (C) qPCR on mRNA from tibias (n = 4). (D) Linear correlation plot of adipocyte area versus MAT/BV via MRI analysis; both performed in distal femoral metaphysis. Plots demonstrate means ± SD. Significance by 2‐way ANOVA between experimental groups. For multiple comparisons, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001.
Exercise increases markers of resorption in caloric restriction
Next, we queried whether bone resorption might be involved in the increased bone degradation noted in CR exercisers. Osteoclast number was quantified via static histomorphometry (n = 4–6 mice /group) normalized to the bone surface (N.Oc/BS). The N.Oc/BS analysis shows no statistically significant difference between the groups (Fig. 4 B). The analysis of the variance does show that more of the variance is accounted for by exercise status than by diet. It is possible that the high variability of N.Oc/BS in sedentary groups did not permit statistically significant differences to emerge in this analysis and that a larger number of animals would be required to definitively quantify a difference in N.Oc/BS.
Figure 4.
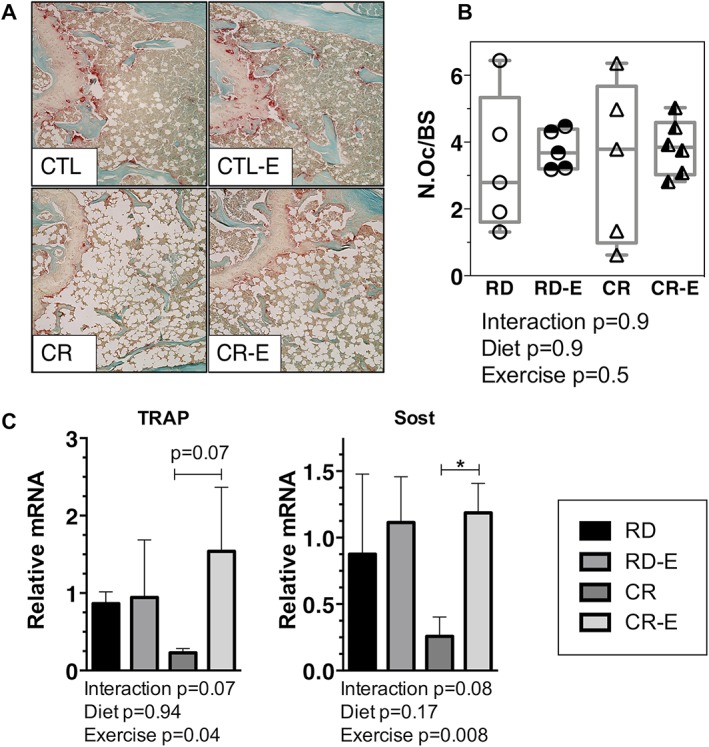
Exercise, in the setting of caloric restriction, attenuates markers of bone resorption. (A) TRAP (red) stain of osteoclasts in representative histologic sections of tibias after 6 weeks of 30% CR +/− exercise. (B) Osteoclasts quantified via semi‐automated histomorphometry in mouse femurs (n = 4–6/ group) with individual mice plotted. (C) Tibial mRNA via qPCR (n = 4/group). Means ± SD. Significance by 2‐way ANOVA between experimental groups. For multiple comparisons, *p ≤ 0.05.
In our qPCR analysis, TRAP mRNA, which was down 73% in CR versus RD, rose 150% in CR‐E versus CR (TRAP mRNA, p = 0.04 for exercise effect). Sclerostin or Sost mRNA similarly was reduced 79% in CR versus RD and rose 115% in CR‐E versus CR (p = 0.009 for exercise as main effect, Fig. 4 C). Thus, mRNA data demonstrates an increase in markers of bone resorption in the exercise groups, although staining for osteoclasts did not reach significance. Because tibias were used for PCR and microarchitecture and femorae for MAT by MRI and histomorphometry, results might not be generalizable to other long bones, and correlation of outcomes between disparate bones requires care.
Bone fatty acid uptake underlies MAT expansion in caloric restriction
Adipocyte area increased in CR compared with RD (Fig. 5 B) and correlated with MAT by MRI (correlation R2 = 0.6233, p < 0.0001), demonstrating significant fat accumulation in CR‐bone. We sought to investigate potential pathways by which marrow adipocyte accumulation occurs in this setting. Lipid droplet markers such as perilipin 1 or Plin1 was highest in the CR group (Fig. 5 C). Interestingly, caloric restriction significantly increased CD36, a marker of fatty acid uptake (+4088%, p < 0.01 CR versus RD), suggesting a mechanism for lipid accumulation in the bone in CR. Exercise attenuated CD36 in CR consistent with exercise induced suppression of MAT in this setting by MRI and histology (Fig. 5 C, −1394% CR‐E versus CR). Additionally, in RD‐exercisers, fatty acid uptake marker CD36 rose while in CR‐exercisers it declined, suggesting a divergent bone metabolic profile in these states that may dictate lipid uptake and utilization.
Discussion
Adipocytes in the bone marrow serve critical roles, regulating homeostasis in hematopoietic niches63, 64 and storing energy for use during exercise.17 An increase in marrow fat is found during conditions associated with osteoporosis—aging and estrogen deficiency12—and the same has been suggested in the unique bone fragility that accompanies caloric restriction.31, 32, 65 Here we quantified marrow adiposity with advanced image analysis during caloric restriction with an added stressor of exercise. By means of 9.4 T MRI 3D images, we demonstrated that marrow fat relative to bone volume increased during caloric restriction, most notably in the femoral metaphysis when compared with other regions of the femur. Although peripheral adipose depot size decreased due to energy utilization during caloric restriction, the size of marrow adipocytes increased. As such, adipocyte hypertrophy and significant lipid accumulation occurred in bone in the presence of an energy‐depleted state. Along with the increase in bone marrow adiposity, we demonstrated suppressed bone turnover markers in sedentary calorie‐restricted mice. Importantly, when CR mice exercised, marrow fat declined, and bone turnover markers increased. In sum, exercise requires energy for anabolism, and exercise in the absence of stored fat might result in sacrificing tissue to supply needed calories.
Our finding of increased bone turnover markers in exercised, calorically restricted mice is clinically important, as exercise is frequently proposed as a form of therapy for patients with bone fragility. Bone resorption in the calorically restricted state likely depends on several factors such as sex, severity of CR, duration of CR, and physical activity intensity. A human study of anorexic girls revealed reduced urinary N‐terminal telopeptide (NTX), a bone resorption marker,66 suggesting decreased bone turnover consistent with findings in our CR mice. Hypoestrogenism is part of anorexia in women65 and caloric restriction in female mice67 though, estrogen therapy has displayed variable efficacy in anorexia for bone density endpoints.68, 69, 70 A limitation of our study is the lack of estradiol measurements. Since exercise exacerbates hypoestrogenism,71 hormonal status might contribute to the deterioration of bone as well as to likely increased resorption in CR‐exercisers.
The mechanisms by which MAT accrues in the calorie‐restricted state continue to be an area of active investigation.3, 72 Increased glucocorticoids, Pref‐1, and MAT‐derived adiponectin28, 73, 74 as well as low IGF‐1 and leptin28, 32, 75, 76, 77 have been associated with MAT in CR; however, causality has not been established for these factors in driving MAT nor in the skeletal deterioration of CR. Systemic sclerostin inhibition was shown to reduce marrow adipocytes.78 Our data show bone Sost expression decreased during calorie restriction, potentially part of the mechanism underlying decreased bone turnover. Interestingly, Sost increased with exercise, whereas MAT decreased. Future investigation is required to determine the role of Sost in MAT accumulation.
We found a significant increase in fatty acid translocate/CD36 in bones of calorically restricted animals, suggesting an augmented cellular uptake of fatty acids.79 A study of CD36 knockout mice revealed reduced bone quantity, suggesting that CD36 is important for skeletal health.80 CD36 possesses cellular functions related to use of fat calories including functioning as a receptor for oxidized low‐density lipoprotein (LDL)81 as well as regulating fatty acid uptake in skeletal muscle and cardiomyocytes. CD36 upregulation in muscle can occur in the setting of increased dietary fat availability, driven by AMP‐activated protein kinase (AMPK) activation.82 During starvation, CD36 is upregulated in muscle and understood to be a fundamental regulator of muscle's metabolic flexibility, reducing the tissue's reliance on glucose and increasing the utilization of fatty acids for energy.83 in vitro experiments exhibited a preference for glucose as an energy source in cultured osteoblasts.84 It is unclear whether osteocytes, marrow adipocytes, and their progenitors rely on fatty acids or glucose in the calorie‐restricted state and exercised states. Recent human metabolomic data obtained after 10 days of starvation points to a shift from carbohydrate to fatty acid metabolism.27 Here the increase in CD36 in the bone of calorie‐restricted mice associates with the rise in MAT and may provide a metabolic mechanism for MAT accumulation despite the energy‐deficient state.
Exercise consumes calories from several substrates, including carbohydrates and fat, to supply energy for muscle and skeletal anabolism.17, 85 The MAT present in the CR state, and its apparent utilization during exercise, however, did not support bone formation. In fact, calorie‐restricted exercisers began with low‐turnover markers compared with calorie‐replete animals and responded to exercise with rises in resorption markers Sost and Trap in CR bone. As such, exercise‐induced cortical porosity and marrow area increases in CR‐E, along with diminished cortical thickness and cortical bone fraction, indicate bone was quantitatively reduced. Although cortical porosity is not a direct measure of strength, it is distinctly associated with reduced strength86, 87, 88 and thus reflects probable diminution of bone quality as well. Notably, low‐magnitude mechanical stimulation (LMMS), an exercise mimic, was similarly found to increase resorption in human anorexia, along with reduced markers of bone formation.89 In accordance with our findings in mice, Swift and colleagues demonstrated increased bone resorption in food‐restricted, exercised rats.67 Although studies have shown that bone loss due to anorexia in humans33 and caloric restriction in mice34, 67 is not ameliorated by exercise, ours is the first to show further degradation of bone quantity with voluntary exercise. The voluntary exercise intervention applied is distinct from prior studies, which applied forced running; indeed, mice ran despite being calorie restricted and in spite of increased energy needs. Southmayd and colleagues showed that for exercising humans, bone loss and resorption were higher in the energy‐deficient state.90 Our outcome that exercise can be harmful to the skeleton during calorie restriction is additionally in line with Pontzer's constrained energy expenditure model that suggests increasing quantities of physical activity are not necessarily additive with regard to improved health and may be constrained based on nutrient availability.46, 47
In conclusion, during the calorie‐replete state, exercise induces skeletal anabolism and alters skeletal architecture through effects on a multiplicity of cells.91 In the calorie‐replete state, data support that energy stored in marrow adipocytes are utilized for energy during exercise.2 In striking contrast, we demonstrate here that exercise appears to be harmful to bone during calorie restriction, in congruence with clinical data.90 Thus, despite MAT expansion in caloric restriction, this fat depot might not be harnessed to support energy needed to sustain bone anabolism as well as prevent bone resorption in the energy‐restricted sedentary and exercised states.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
We acknowledge the University of North Carolina Department of Medicine for generous support provided before the start of NIH grant AR073264. MS: AR073264, JR: AR066616.
Authors' roles: Study design: MS. Study conduct: CM, NMX, ZX, and BS. Data collection: CM, JSS, NMX, MAS, and XZ. Data analysis: CM and MS. Data interpretation: JR and MS. Drafting manuscript: CM and MS. Revising manuscript content: CM, JR, and MS. Approving final version of manuscript: JR and MS. MS takes responsibility for the integrity of the data analysis.
References
- 1. Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. [DOI] [PubMed] [Google Scholar]
- 2. Paccou J, Hardouin P, Cotten A, Penel G, Cortet B. The role of bone marrow fat in skeletal health: usefulness and perspectives for clinicians. J Clin Endocrinol Metab. 2015;100(10):3613–21. [DOI] [PubMed] [Google Scholar]
- 3. Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23(5):577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone—new perspectives. J Clin Endocrinol Metab. 2013;98(3):935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin J, Styner M, Uzer G. Physical signals may affect mesenchymal stem cell differentiation via epigenetic controls. Exerc Sport Sci Rev. 2018;46(1):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel VS, Ete Chan M, Rubin J, Rubin CT. Marrow adiposity and hematopoiesis in aging and obesity: exercise as an intervention. Curr Osteoporos Rep. 2018;16(2):105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pagnotti GM, Styner M. Exercise regulation of marrow adipose tissue. Front Endocrinol (Lausanne). 2016;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High‐fat diet‐induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res. 2018;33(6):1154–65. [DOI] [PubMed] [Google Scholar]
- 9. Kushwaha P, Wolfgang MJ, Riddle RC. Fatty acid metabolism by the osteoblast. Bone. 2018;115:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen A, Dempster DW, Stein EM, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2012;97(8):2782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falank C, Fairfield H, Reagan MR. Reflections on cancer in the bone marrow: adverse roles of adipocytes. Curr Mol Biol Rep. 2017;3(4):254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85. [DOI] [PubMed] [Google Scholar]
- 13. Duque G, Li W, Adams M, Xu S, Phipps R. Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporos Int. 2011;22(5):1547–53. [DOI] [PubMed] [Google Scholar]
- 14. Li X, Kuo D, Schafer AL, et al. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging. 2011;33(4):974–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ecklund K, Vajapeyam S, Mulkern RV, et al. Bone marrow fat content in 70 adolescent girls with anorexia nervosa: magnetic resonance imaging and magnetic resonance spectroscopy assessment. Pediatr Radiol. 2017;47(8):952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Styner M, Pagnotti GM, Galior K, et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology. 2015;156(8):2753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Styner M, Pagnotti GM, McGrath C, et al. Exercise decreases marrow adipose tissue through β‐oxidation in obese running mice. J Bone Miner Res. 2017;32(8):1692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belavy DL, Quittner MJ, Ridgers ND, Shiekh A, Rantalainen T, Trudel G. Specific modulation of vertebral marrow adipose tissue by physical activity. J Bone Miner Res. 2018;33(4):651–7. [DOI] [PubMed] [Google Scholar]
- 20. Kim SP, Li Z, Zoch ML, et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex‐ and diet‐dependent manner. JCI Insight. 2017;2(16):pii:92704. 10.1172/jci.insight.92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cahill G Jr, Felig P, Owen O, Wahren J. Metabolic adaptation to prolonged starvation in man. Nordisk Medicin. 1970;83(3):89. [PubMed] [Google Scholar]
- 22. Cahill GF Jr. Starvation in man. N Engl J Med. 1970;282(12):668–75. [DOI] [PubMed] [Google Scholar]
- 23. Cahill GF Jr. Starvation in man. Clin Endocrinol Metab. 1976;5(2):397–415. [DOI] [PubMed] [Google Scholar]
- 24. Soeters MR, Soeters PB, Schooneman MG, Houten SM, Romijn JA. Adaptive reciprocity of lipid and glucose metabolism in human short‐term starvation. Am J Physiol Endocrinol Metab. 2012;303(12):E1397–407. [DOI] [PubMed] [Google Scholar]
- 25. McCue MD. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol A Mol Integr Physiol. 2010;156(1):1–18. [DOI] [PubMed] [Google Scholar]
- 26. Muller MJ, Enderle J, Pourhassan M, et al. Metabolic adaptation to caloric restriction and subsequent refeeding: the Minnesota Starvation Experiment revisited. Am J Clin Nutr. 2015;102(4):807–19. [DOI] [PubMed] [Google Scholar]
- 27. Steinhauser ML, Olenchock BA, O'Keefe J, et al. The circulating metabolome of human starvation. JCI Insight. 2018;3(16):pii:121434. 10.1172/jci.insight.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cawthorn WP, Scheller EL, Parlee SD, et al. Expansion of bone marrow adipose tissue during caloric restriction is associated with increased circulating glucocorticoids and not with hypoleptinemia. Endocrinology. 2016;157(2):508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bathija A, Davis S, Trubowitz S. Bone marrow adipose tissue: response to acute starvation. Am J Hematol. 1979;6(3):191–8. [DOI] [PubMed] [Google Scholar]
- 30. Abella E, Feliu E, Granada I, et al. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol. 2002;118(4):582–8. [DOI] [PubMed] [Google Scholar]
- 31. Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Devlin MJ, Cloutier AM, Thomas NA, et al. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25(9):2078–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Waugh EJ, Woodside DB, Beaton DE, Cote P, Hawker GA. Effects of exercise on bone mass in young women with anorexia nervosa. Med Sci Sports Exerc. 2011;43(5):755–63. [DOI] [PubMed] [Google Scholar]
- 34. Mequinion M, Caron E, Zgheib S, et al. Physical activity: benefit or weakness in metabolic adaptations in a mouse model of chronic food restriction? Am J Physiol Endocrinol Metab. 2015;308(3):E241–55. [DOI] [PubMed] [Google Scholar]
- 35. Hay PJ, Delahunt JW, Hall A, Mitchell AW, Harper G, Salmond C. Predictors of osteopenia in premenopausal women with anorexia nervosa. Calcif Tissue Int. 1992;50(6):498–501. [DOI] [PubMed] [Google Scholar]
- 36. Fujimura R, Ashizawa N, Watanabe M, et al. Effect of resistance exercise training on bone formation and resorption in young male subjects assessed by biomarkers of bone metabolism. J Bone Miner Res. 1997;12(4):656–62. [DOI] [PubMed] [Google Scholar]
- 37. Avin KG, Bloomfield SA, Gross TS, Warden SJ. Biomechanical aspects of the muscle‐bone interaction. Curr Osteoporos Rep. 2015;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise‐induced bone‐forming surfaces in the adult skeleton. J Bone Miner Res. 1997;12(10):1737–45. [DOI] [PubMed] [Google Scholar]
- 39. Leichter I, Simkin A, Margulies JY, et al. Gain in mass density of bone following strenuous physical activity. J Orthop Res. 1989;7(1):86–90. [DOI] [PubMed] [Google Scholar]
- 40. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–8. [PubMed] [Google Scholar]
- 41. Snow‐Harter C, Bouxsein ML, Lewis BT, Carter DR, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women: a randomized exercise intervention trial. J Bone Miner Res. 1992;7(7):761–9. [DOI] [PubMed] [Google Scholar]
- 42. Watson SL, Weeks BK, Weis LJ, Harding AT, Horan SA, Beck BR. High‐intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J Bone Miner Res. 2018;33(2):211–20. [DOI] [PubMed] [Google Scholar]
- 43. Harding AT, Beck BR. Exercise, osteoporosis, and bone geometry. Sports (Basel). 2017;5(2):pii:E29. 10.3390/sports5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weeks BK, Young CM, Beck BR. Eight months of regular in‐school jumping improves indices of bone strength in adolescent boys and girls: the POWER PE study. J Bone Miner Res. 2008;23(7):1002–11. [DOI] [PubMed] [Google Scholar]
- 45. Ozcivici E, Luu YK, Adler B, et al. Mechanical signals as anabolic agents in bone. Nat Rev Rheumatol. 2010;6(1):50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pontzer H. Energy constraint as a novel mechanism linking exercise and health. Physiology (Bethesda). 2018;33(6):384–93. [DOI] [PubMed] [Google Scholar]
- 47. Pontzer H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc Sport Sci Rev. 2015;43(3):110–6. [DOI] [PubMed] [Google Scholar]
- 48. Lashinger LM, O'Flanagan CH, Dunlap SM, et al. Starving cancer from the outside and inside: separate and combined effects of calorie restriction and autophagy inhibition on Ras‐driven tumors. Cancer Metab. 2016;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yushkevich PA, Piven J, Hazlett HC, et al. User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28. [DOI] [PubMed] [Google Scholar]
- 50. Joshi S, Davis B, Jomier M, Gerig G. Unbiased diffeomorphic atlas construction for computational anatomy. Neuroimage. 2004;23(Suppl 1):S151–60. [DOI] [PubMed] [Google Scholar]
- 51. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Longobardi L, Li T, Myers TJ, et al. TGF‐beta type II receptor/MCP‐5 axis: at the crossroad between joint and growth plate development. Dev Cell. 2012;23(1):71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van t Hof RJ, Rose L, Bassonga E, Daroszewska A. Open source software for semi‐automated histomorphometry of bone resorption and formation parameters. Bone. 2017;99:69–79. [DOI] [PubMed] [Google Scholar]
- 55. Pagnotti GM, Adler BJ, Green DE, et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51(3):570–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gross TS, Rubin CT. Uniformity of resorptive bone loss induced by disuse. J Orthop Res. 1995;13(5):708–14. [DOI] [PubMed] [Google Scholar]
- 57. Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30(5):693–702. [DOI] [PubMed] [Google Scholar]
- 58. Styner M, Sen B, Xie Z, Case N, Rubin J. Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J Cell Biochem. 2010;111(4):1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Styner M, Meyer MB, Galior K, et al. Mechanical strain downregulates C/EBPbeta in MSC and decreases endoplasmic reticulum stress. PloS One. 2012;7(12):e51613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morton TL, Galior K, McGrath C, et al. Exercise increases and browns muscle lipid in high‐fat diet‐fed mice. Front Endocrinol (Lausanne). 2016;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type‐specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287(5):C1342–8. [DOI] [PubMed] [Google Scholar]
- 62. Hayes A, Williams DA. Beneficial effects of voluntary wheel running on the properties of dystrophic mouse muscle. J Appl Physiol. 1996;80(2):670–9. [DOI] [PubMed] [Google Scholar]
- 63. Zhou BO, Yu H, Yue R, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol. 2017;19(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mattiucci D, Maurizi G, Izzi V, et al. Bone marrow adipocytes support hematopoietic stem cell survival. J Cell Physiol. 2018;233(2):1500–11. [DOI] [PubMed] [Google Scholar]
- 65. Misra M, Klibanski A. Anorexia nervosa and bone. J Endocrinol. 2014;221(3):R163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Misra M, Miller KK, Cord J, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. J Clin Endocrinol Metab. 2007;92(6):2046–52. [DOI] [PubMed] [Google Scholar]
- 67. Swift SN, Baek K, Swift JM, Bloomfield SA. Restriction of dietary energy intake has a greater impact on bone integrity than does restriction of calcium in exercising female rats. J Nutr. 2012;142(6):1038–45. [DOI] [PubMed] [Google Scholar]
- 68. Strokosch GR, Friedman AJ, Wu SC, Kamin M. Effects of an oral contraceptive (norgestimate/ethinyl estradiol) on bone mineral density in adolescent females with anorexia nervosa: a double‐blind, placebo‐controlled study. J Adolesc Health. 2006;39(6):819–27. [DOI] [PubMed] [Google Scholar]
- 69. Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26(10):2430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Seeman E, Szmukler GI, Formica C, Tsalamandris C, Mestrovic R. Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise. J Bone Miner Res. 1992;7(12):1467–74. [DOI] [PubMed] [Google Scholar]
- 71. Javed A, Tebben PJ, Fischer PR, Lteif AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc. 2013;88(9):996–1009. [DOI] [PubMed] [Google Scholar]
- 72. Li Z, Hardij J, Bagchi DP, Scheller EL, MacDougald OA. Development, regulation, metabolism and function of bone marrow adipose tissues. Bone. 2018;110:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fazeli PK, Bredella MA, Misra M, et al. Preadipocyte factor‐1 is associated with marrow adiposity and bone mineral density in women with anorexia nervosa. J Clin Endocrinol Metab. 2010;95(1):407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scheller EL, Burr AA, MacDougald OA, Cawthorn WP. Inside out: bone marrow adipose tissue as a source of circulating adiponectin. Adipocyte. 2016;5(3):251–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Devlin MJ, Brooks DJ, Conlon C, et al. Daily leptin blunts marrow fat but does not impact bone mass in calorie‐restricted mice. J Endocrinol. 2016;229(3):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hamrick MW, Della‐Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin‐deficient ob/ob mice. J Bone Miner Res. 2005;20(6):994–1001. [DOI] [PubMed] [Google Scholar]
- 77. Khosla S. Leptin‐central or peripheral to the regulation of bone metabolism? Endocrinology. 2002;143(11):4161–4. [DOI] [PubMed] [Google Scholar]
- 78. Fairfield H, Falank C, Harris E, et al. The skeletal cell‐derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233(2):1156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vroegrijk IO, van Klinken JB, van Diepen JA, et al. CD36 is important for adipocyte recruitment and affects lipolysis. Obesity (Silver Spring). 2013;21(10):2037–45. [DOI] [PubMed] [Google Scholar]
- 80. Kevorkova O, Martineau C, Martin‐Falstrault L, Sanchez‐Dardon J, Brissette L, Moreau R. Low‐bone‐mass phenotype of deficient mice for the cluster of differentiation 36 (CD36). PLoS One. 2013;8(10):e77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268(16):11811–6. [PubMed] [Google Scholar]
- 82. Chabowski A, Coort SL, Calles‐Escandon J, et al. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by AICAR. FEBS Lett. 2005;579(11):2428–32. [DOI] [PubMed] [Google Scholar]
- 83. Nahle Z, Hsieh M, Pietka T, et al. CD36‐dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta‐mediated adaptation to metabolic stress. J Biol Chem. 2008;283(21):14317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guntur AR, Gerencser AA, Le PT, et al. Osteoblast‐like MC3T3‐E1 cells prefer glycolysis for ATP production but adipocyte‐like 3T3‐L1 cells prefer oxidative phosphorylation. J Bone Miner Res. 2018;33(6):1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J Appl Physiol. 1994;76(6):2253–61. [DOI] [PubMed] [Google Scholar]
- 86. McCalden RW, McGeough JA, Barker MB, Court‐Brown CM. Age‐related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75(8):1193–205. [DOI] [PubMed] [Google Scholar]
- 87. Popp KL, Caksa S, Martinez‐Betancourt A, et al. Cortical bone material strength index and bone microarchitecture in postmenopausal women with atypical femoral fractures. J Bone Miner Res. 2019;34(1):75–82. [DOI] [PubMed] [Google Scholar]
- 88. Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988;21(1):13–6. [DOI] [PubMed] [Google Scholar]
- 89. DiVasta AD, Feldman HA, Quach AE, Balestrino M, Gordon CM. The effect of bed rest on bone turnover in young women hospitalized for anorexia nervosa: a pilot study. J Clin Endocrinol Metab. 2009;94(5):1650–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Southmayd EA, Williams NI, Mallinson RJ, De Souza MJ. Energy deficiency suppresses bone turnover in exercising women with menstrual disturbances. J Clin Endocrinol Metab. 2019;104(8):3131–45. [DOI] [PubMed] [Google Scholar]
- 91. Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene. 2012;503(2):179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]


