Abstract
Background:
Preterm delivery and current nutritional strategies result in deficiencies of critical long chain fatty acids and lipophilic nutrients increasing the risk of preterm morbidities. We sought to determine the efficacy of preventing postnatal deficits in fatty acids and lipophilic nutrients using an enteral concentrated lipid supplement in preterm piglets.
Methods:
Preterm piglets were fed a baseline diet devoid of arachidonic acid (AA) and docosahexaenoic acid (DHA) and randomized to enteral supplementation as follows: (1) Intralipid®; (2) complex lipid supplement 1 (CLS1) with an AA:DHA ratio of 0.25; or (3) complex lipid supplement 2 (CLS2) with an AA:DHA ratio of 1.2. On day 8, plasma and tissue levels of fatty acids and lipophilic nutrients were measured and ileum histology performed.
Results:
Plasma DHA levels decreased in the Intralipid® group by day 2. In contrast, DHA increased by day 2 compared to birth levels in both CLS1 and CLS2 groups. The Intralipid® and CLS1 groups demonstrated a continued decline in AA levels during the 8-day protocol, while AA levels in the CLS2 group on day 8 were comparable to birth levels. Preserving AA levels in the CLS2 group was associated with greater ileal villus height and muscular layer thickness. Lipophilic nutrients were effectively absorbed in plasma and tissues.
Conclusions:
Enteral administration of CLS1 and CLS2 demonstrated similar increases in DHA levels compared to birth levels. Only CLS2 maintained AA birth levels. Providing a concentrated complex lipid emulsion with an AA:DHA ratio greater than 1 is important in preventing postnatal AA deficits.
1.0. Introduction
During the third trimester, there is enhanced placental transfer of long chain polyunsaturated fatty acids (LCPUFAs) and in particular docosahexaenoic acid (DHA, 22:6, n-3) and arachidonic acid (AA, 20:4, n-6) from the maternal circulation to the fetus during this critical period of gestation.1 Early delivery at or before the third trimester and inadequate postnatal provision of DHA and AA make the preterm infant particularly vulnerable to systemic deficits of critical LCPUFAs. Intervention and cohort studies have documented that within the first postnatal week there are significant reductions in blood AA and DHA levels compared to birth levels with a concomitant increase in linoleic acid (LA, 18:2, n-6).2–4 Altered fatty acid levels in the postnatal period are associated with an increased risk of bronchopulmonary dysplasia and neonatal sepsis.2 Recent studies have shown that reduced serum levels of AA in the postnatal period were associated with retinopathy of prematurity.5
With no significant fat stores of their own, preterm infants are entirely reliant on external sources of nutrition to supplement the necessary essential fatty acids and LCPUFAs in the Neonatal Intensive Care Unit (NICU). The early use of currently available parenteral soybean and fish-oil based lipid emulsions fails to correct the postnatal deficits of AA and DHA.4 Although, AA and DHA are present in breastmilk, the variability of fatty acids in breastmilk and the practice of slow gradual introduction of enteral feedings with low enteral volumes are not adequate to prevent the decreases in AA and DHA. The difficulty in maintaining in utero or birth levels in LCPUFAs extends to other lipophilic nutrients such as lutein, tocopherol, and Vitamin D where deficits and or inadequate accrual compared to term infants have been documented.6–8
Our primary hypothesis was that early, enteral delivery of a highly emulsified, concentrated complex lipid emulsion would prevent postnatal deficits in plasma and tissue levels of the critical LCPUFAs DHA and AA. Our secondary hypotheses were that the lipid supplement would result in tissue accretion of lipophilic nutrients and would be well-tolerated with safety determined by growth and morphological assessment of the small intestine. To evaluate our hypotheses, the preterm piglet model was used. We have shown previously that LCPUFA levels at birth and over the first postnatal week in the preterm pig model mirror the changes seen in preterm humans.9
2.0. Materials and Methods
2.1. Animal care
All studies were approved by the Institutional Animal Care and Use Committee at Boston, Children’s Hospital. Three litters from 3 sows comprised the study cohort. Timed pregnant sows were obtained from Parsons (Hadley, MA). At gestational day 105–107, the sow was anesthetized, and piglets delivered by Caesarian section and placed in 35o C incubators to stabilize. Two to 3 hours after delivery, the piglets were anesthetized and a 5 French naso- or orogastric tube (Bard, Covington, GA) was placed and secured with sutures to the adjacent skin. For the first and second litter, a central line was place in the external jugular vein (3Fr, ARROW® Midline Catheterization kit 3FR x 8” trimmable catheter CDC-02031-MK1A) and secured for repeated blood draws. To minimize complications during placement of an indwelling central line, the third litter had baseline labs drawn through the umbilicus at birth, then percutaneous blood draws thereafter.
On the day of birth all piglets received 100 mcg vitamin B12 intramuscularly (IM), 100 mg iron dextran IM, and 15 mL/kg of maternal plasma intravenously (IV) divided in three doses for passive immunization. Prior to central line placement and initiation of enteral feedings, piglets were hydrated with 10% dextrose with 0.45N saline and 50% dextrose boluses as needed. Piglets were group housed (3–4 per cage) in cubicles maintained at 35° C for the first 3 days and at 30o C, thereafter. Piglets were monitored continuously for temperature and general well-being, including twice daily weights, fluid balance, and activity.
2.2. Nutrition protocol and dietary composition
Piglets were fed a liquid diet of LitterLife™ (Merrick’s Inc, Middleton, WI) which is devoid of any LCPUFAs including DHA and AA. LitterLife™ was prepared according to the manufacturer’s instructions. The enteral feeding protocol was designed to mimic the common practice in the NICU of gradual advancement to full volume feedings. Enteral feedings were started 6–9 hours after delivery through the naso- or orogastric tube. Over the course of the following 8 days the piglets progressed from 42% to 100% of their daily energy requirements on a per kilogram basis with the feeding interval increased from every 1 to every 3 hours (Table S1). 100% of daily calories and fluid requirements were estimated at 2838 kcal/kg/day and 240 mL/kg/day, respectively.10. Feeding volumes <10 mL were administered as a bolus delivered over 1–2 minutes. Once the volume reached 10 mL, it was delivered by continuous infusion using a Medfusion 3500 pump (Smith Medical, St. Paul, MN) at 3 mL/min flow rate. After feeding, the naso- or orogastric tube was flushed with 2 mL of water.
2.3. Provision of experimental lipid emulsions
The complex enteral lipid emulsion supplement (CLS) was manufactured by Abbott Nutrition, Columbus, OH. Initial goal was to create a concentrated (>40% lipid) shelf stable oil in water emulsion prototype with neutral pH (pH 6.2) and relatively low osmolality value (40 mOsm). Piglets were randomly assigned to one of three groups: (1) Intralipid® (IL, Fresenius, Uppsala Sweden) which is an n-6 dominant emulsion lacking DHA and AA; (2) complex lipid supplement 1 (CLS1) with an AA:DHA ratio of 0.25; and, (3) complex lipid supplement 2 (CLS2) with an AA:DHA ratio of 1.2. Dosing for CLS1 and CLS2 was on a per kilogram basis and targeted to provide 80 mg/kg/day of DHA. This target was selected based on the current understanding of the range of DHA accretion during the fetal periods in the human with the simultaneous goal of minimizing the postnatal decline in DHA in the first postnatal week.11,12 The total daily lipid dose was divided into 4 aliquots given enterally on an every 6 hour interval (Table S1). The lipid dose was given as a bolus just prior to the Litterlife feeding.
Fatty acid composition of the base diet and the three enteral lipid emulsions used in this study is shown in Table 1 along with the other lipophilic nutrients. CLS1 and CLS2 were similar in its composition except for the amount of AA relative to DHA. CLS1 had an AA:DHA ratio of 0.25 (3 mol% AA to 12 mol% DHA) and CLS1 had an AA:DHA ratio of 1.2 (12 mol% AA to 10 mol% DHA). Intralipid®, the current standard of care intravenous lipid emulsion that is devoid of AA and DHA, served as the control lipid and was provided in an amount that matched the kilocalorie content of the experimental lipid emulsion.
Table 1.
Nutritional composition in Soweena (base diet) and lipid supplements1
| LitterLife | Intralipid | Complex Lipid Supplement 1 (CLS1) | Complex Lipid Supplement 2 (CLS2) | |||||
| Fatty Acids3 | nmol/mL | mol% | nmol/mL | mol% | nmol/mL | mol% | nmol/mL | mol% |
| Palmitic acid | 9,580 | 26 | 52,037 | 13 | 20,138 | 6 | 24,496 | 7 |
| Oleic acid | 12,981 | 35 | 113,845 | 27 | 60,662 | 19 | 71,358 | 21 |
| Linoleic acid (LA) | 4,278 | 12 | 205,550 | 49 | 40,945 | 13 | 38,315 | 12 |
| α-Linolenic acid (ALA) | 357 | 1 | 28,478 | 7 | 923 | 0.3 | 774 | 0.2 |
| γ-Linolenic acid (GLA) | 0 | 0 | 0 | 0 | 21,830 | 7 | 19,695 | 6 |
| Arachidonic acid (AA) | 0 | 0 | 0 | 0 | 9,491 | 3 | 40,632 | 12 |
| Eicosapentaenoic acid (EPA) | 0 | 0 | 0 | 0 | 6,117 | 2 | 4,354 | 1 |
| Docosahexaenoic acid (DHA) | 0 | 0 | 0 | 0 | 39,120 | 12 | 32,130 | 10 |
| AA:DHA Ratio | 0 | 0 | 0 | 0 | 0.24 | 0.25 | 1.26 | 1.2 |
| Lipophilic Micronutrients3 | LitterLife | Intralipid | CLS2 | |||||
| Vitamin D (IU/kg) | 12,600 | <40 | 97,900 | |||||
| Vitamin E forms | ||||||||
| Alpha-tocopherol (mg/kg) | <20.6 | <2.6 | 584 | |||||
| Dl-alpha tocopherol acetate (IU/kg) | 344 | <2.5 | 1700 | |||||
| Lutein (mcg/100g) | 10.6 | <5 | 2950 | |||||
Quantification by gas chromatograpy - mass spectroscopy (GCMS);
CLS represents both CLS1 and CLS2;
Sources of fatty acids and lipohilic micronutrients are listed in Supplemental Table 8 (Table S8)
CLS, complex lipid supplement
2.4. Monitoring of growth and sample collection
Piglets were weighed twice daily and growth velocities calculated over the eight-day protocol. Growth velocity (g/kg/day) was calculated by: [(final weight, grams – birth weight, grams)/(birth weight, kg)/8]. Plasma was collected at birth and one hour post-feeding on days 2, 4, 6, 8 for litters one and two, and at birth and days 2 and 8 for litter three, the latter as a result of a lack of central venous access. Tissue samples were harvested on day 8 and fixed in formalin. Samples were stored at −80o C until further analysis.
2.5. Pancreatic lipase quantification
Pancreatic lipase levels were quantified to confirm relative pancreatic insufficiency in this preterm piglet model as seen in preterm infants. For comparison, we harvested pancreas from 14-month old piglets from our core animal facility. Room temperature was maintained between 68–72 degrees with humidity between 30–70%. Animals were fed LabDiet Porcine Lab Grower Diet # 5084.
15 ug of protein extracted from approximately 50–100mg of pancreas tissue were run on SDS-PAGE gel, transferred to Polyvinylidene difluoride membrane, immunoblotted with pancreatic lipase antibody (Proteintech, Rosemont, IL, USA, catalog 11209–1-AP) and blots developed by Clarity™ Western ECL Blotting Substrate (Bio-Rad) according to the manufacturer’s instructions. The same membrane was then incubated with beta-actin antibody (Cell Signaling, Danvers, MA, catalog #4970). Densitometric quantification of bands and normalization to β-actin was completed using ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/).
2.6. Plasma and tissue fatty acid levels
Fatty acids were isolated and methylated using a modified Folch method as described previously.2,13 Samples were quantified by gas chromatography–mass spectroscopy (GC-MS) with a Supelcowax-10 column (Sigma-Aldrich). Peak identification was based upon comparison of both retention time and mass spectra of the unknown peak to that of known standards. Fatty acid methylated ester (FAME) mass was determined by comparing areas of unknown FAMEs to that of a fixed concentration of 17:0 internal standard. Response factors were determined for each individual FAME to correct for GC-MS total ion chromatogram discrepancies in quantification. These factors were determined through the use of a GLC reference standard which contained known masses of FAMEs ranging from 14–24°C. The response ratio of each FAME is corrected to a fixed amount ratio for each FAME relative to 17:0. Individual FA is expressed as a percent of the total FA mass (mol%).
2.7. Plasma and tissue lutein, tocopherol, 25 hydroxyvitamin D3, and Vitamin D3 levels
A plasma volume of 0.5 mL and up to 0.5 g of homogenized tissue was used for all measurements. For lutein, analysis was performed by positive electrospray liquid chromatography/tandem mass spectrometry (Xevo® TQ-S, Waters Corp, Milford, MA). Quantification was performed by stable isotope dilution using uniformly 13C-labelled lutein as internal standard.
For tocopherol, plasma and tissue samples were treated with 0.4 mL ethanol containing 0.03% BHT, followed by extraction into 1 mL hexane. The hexane extract was evaporated, and the residue was reconstituted in isooctane. Chromatographic separation and quantitation was carried out on a Chromegabond Diol 3μ, 100 × 2.1 mm analytical column with a 10 × 3.2mm guard column used for separation (ES Industries) using a mobile phase system consisting of 95:5 hexane:methyl tert-butyl ether. Detection was performed by fluorescence with excitation and emission wavelengths of 295 nm and 330 nm respectively and quantified by external calibration. 25-hydroxyvitamin D3 and vitamin D3 levels were quantified using a method adapted from AOAC official method 2016.05 ((Official Methods of Analysis of AOAC International, 20th Edition (2016) Method 2016.05 reference)). Analysis was performed by positive electrospray liquid chromatography/tandem mass spectrometry (Xevo® TQ-S, Waters Corp). Chromatographic separation was carried out on an Waters Acquity® BEH C18 column (2.1 × 50 mm, 1.7 μm particle size) using a mobile phase system consisting of 5 mM ammonium formate in water/methanol. Quantitation was performed by stable isotope dilution using 26,26,26,27,27,27-d6-labelled 25-hydroxyvitamin D3 and vitamin D3 as internal standards.
2.8. Ileum morphometry measurements
The distal ileum (10 cm proximal to the cecum), was fixed in 10% formalin. Tissues were embedded in paraffin with the orientation allowing cross sectional images to be obtained with H&E staining. Height of intact villi, depth of crypts, and muscular thickness were measured using the Aperio ImageScope software (Leica Biosystems, Buffalo Grove, IL). For every section at least 5 randomly selected areas were counted. Villous height was measured in continuously visible villi that ended with a rounded tip using a 20x objective from the tip of villous to the entrance of the crypt opening. Crypt depth was measured from crypt base to villus-crypt junction.
2.9. Ileum immunohistochemistry
Apoptosis was quantitated using the terminal transferase-mediated dUTP-nick end labeling (TUNEL) technique according to the instructions of the kit manufacturer (Millipore, Waltham, MA).
2.10. Statistical analyses
All reported outcome measures were evaluated for normality. Measures following a normal distribution were summarized as means ± standard deviation (SD); while, non-normal data were summarized using median ± interquartile range (IQR). Hypothesis testing was performed using 1 or 2-way ANOVA depending on the number of independent variables in the model. For non-normal data, statistical significance was determined using Kruskal-Wallis test followed by Mann Whitney U pairwise comparisons adjusted for multiple comparisons. Two-way ANOVA using generalized linear modeling following rank normal transformation of the data was used to compare unbalanced, repeated measures of fatty acid levels over the duration of the protocol across groups. If significant, this was followed by Tukey post-hoc analysis adjusting for multiple comparisons.
All analyses were performed using R software (version 3.5.1, R Core Team 2018a) within RStudio (Version 1.1.453, RStudio, Inc.) using the tidyverse (Wickham 2017), RNOmni (McCaw 2018), and multcomp (Hothorn, Bretz and Westfall 2008), packages. Results were considered significant at p<0.05.
3.0. Results
3.1. Piglet cohort, growth, and mortality
The final piglet cohort consisted of a total of three litters across three separate experiments (Table S2). Of the viable piglets following delivery, four piglets from litter one and seven piglets from litter two were randomized to the IL and CLS1 groups. Ten piglets from litter three were randomized to the IL, CLS1, and CLS2 groups. The final cohort consisted of seven piglets in the IL group, ten in the CLS1 group, and four in the CLS2 group.
Although there was variation in birth weight and growth velocity by litter, overall there were no statistically significant differences in these parameters by study group (Table S2). The median ± IQR birth weight across all litters was 920 ± 290 grams in the Intralipid® group, 1068 ± 428 grams in the CLS1 group, and 1402 ± 79 grams in the CLS2 group (p=0.1). The median ± IQR growth velocity across all litters was 31.4 ± 16.7 grams/k/d in the Intralipid® group, 23.4 ± 15.1 grams/k/d in the CLS1 group, and 15.9 ± 12.8 grams/k/d in the CLS2 group (p=0.2). During the eight-day protocol, there were three deaths, two in the IL group and 1 in the CLS1 group. The causes of death are stated in Table S2.
Quantification of lipase protein normalized to β-actin in pancreatic homogenates was 3-fold lower from preterm piglets compared to older, 14-month old pigs (p=0.0008) confirming a relative exocrine pancreatic insufficiency seen with prematurity (Figure S1).
3.2. Plasma fatty acid levels
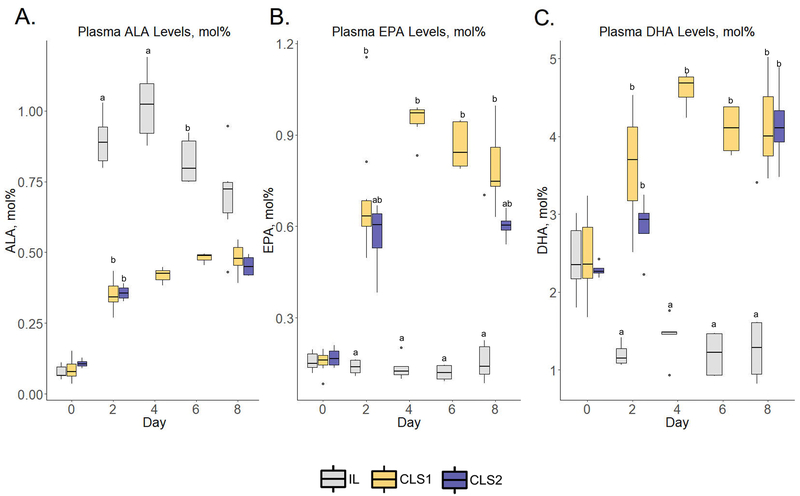
Despite the IL group demonstrating an increase in median plasma ALA by postnatal day 2 compared to birth levels in the n-3 pathway (0.9 ± 0.1 vs. 0.07 ±0.03 mol%, respectively; p=<.001) (Figure 1, panel A, Table S3) downstream plasma DHA levels were decreased (Figure 1, panel C), (2.4 ± 0.6 vs. 1.2 ± 0.2 mol%, respectively; p= 0.01). EPA levels were unchanged (Figure 1, panel B). In contrast, in the CLS1 group there were increases in both EPA and DHA by postnatal day two compared to birth levels (Figure 1, panels B and C). Specifically, median plasma DHA on day two was 3.7 ± 0.9 mol% representing an increase from birth levels of 2.4 ± 0.7 mol percent (p=0.03). Median plasma EPA levels increased from 0.2 ± 0.03 at birth to 0.6 ± 0.08 at day 2 (p <0.001). These levels in the CLS1 group remained significantly greater than the IL levels at each timepoint throughout the eight-day protocol period. In the CLS2 group, EPA levels increased from birth to day 2 by over 3-fold (Figure 1, panel B) and DHA levels increased by 30% (Figure 1, panel C), however, these temporal changes were not statistically significant. DHA levels at day 2 in the CLS2 group were significantly greater than the IL group (p=0.009). Similar to the IL and CLS1 group, there was an increase in ALA levels from birth (Figure 1, panel A), but peak levels were less than those in the IL control group.
Figure 1.
Plasma n-3 fatty acid profiles determined by gas chromatography–mass spectrometry in the IL (gray), CLS1 (yellow), and CLS2 (blue) groups. The concentration of alpha-linolenic acid (ALA, panel A), eicosapentaenoic acid (EPA, panel B) and docosahexaenoic acid (DHA, panel C) are presented as mol% in the form of box plots. Labeled points without a common letter represent a statistically significant difference of P˂0.05. ALA, alpha-linolenic acid; CLS, concentrated lipid supplement; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IL, Intralipid®-control.
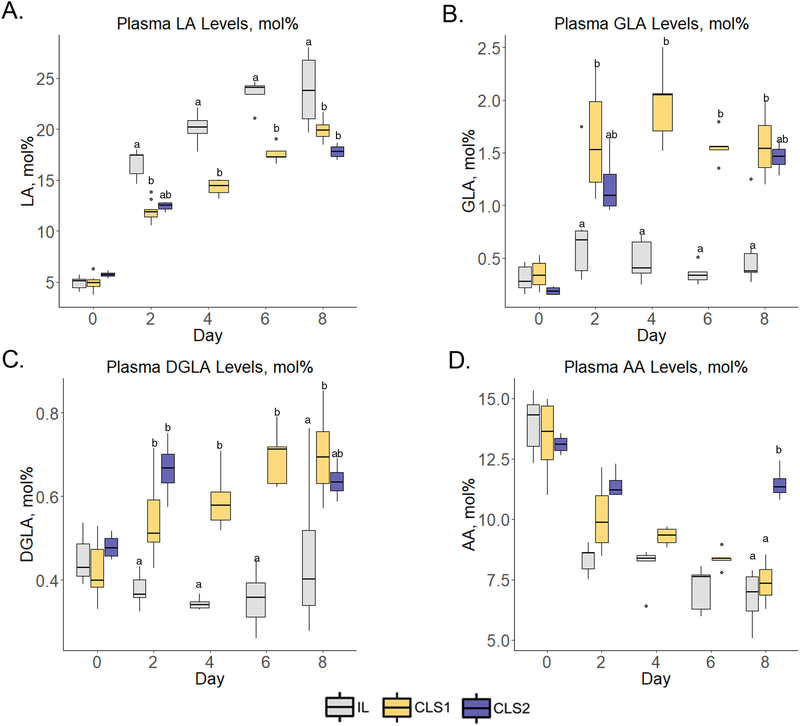
By day two, all groups demonstrated an increase in median LA levels (Figure 2, panel A, Table S4) with levels greater in the IL group versus the CLS1 and CLS2 groups (17.4±1.8, 11.9±0.7, and 12.5±0.6 mol%, respectively). The CLS1 levels were significantly lower compared to the IL group (p=0.3). On days 4, 6 and 8, LA levels continued to rise in all groups with levels in the IL group significantly greater compared to the CLS1 group on days 4 (p=0.003) and 6 (p<0.0001) and both CLS1 and CLS2 groups on day 8 (p<0.01 and p<0.0001, respectively). As expected from the composition of the experimental lipid, plasma median γ-linolenic acid (GLA) (Figure 2, panel B) and dihomo-γ-linolenic acid (DGLA) (Figure 2, panel C) levels were greater in the CLS1 group versus the IL group. Although levels were greater in the CLS2 group versus the IL group, the difference was not statistically different on day 8. Compared to birth levels, AA declined by day 2 for both the IL and CLS1 groups (Figure 2, panel D). In the IL group, levels declined to 8.6±0.7 mol% at day 2 from 14.3±1.7 mol% at birth (p<0.0001). Similar to the control IL group, AA levels decreased from birth in the CLS1 group with levels on day 2 of 9.9±2.0 mol% from birth levels of 13.6±2.2 mol% (p<0.0001). In contrast, the CLS2 group did not experience a significant decline from birth levels on day 2. Both IL and CLS1 groups demonstrated a continued decline in AA levels during the 8-day protocol, while the AA levels in the CLS2 on day 8 were comparable to levels at birth, thus uniquely maintaining birth levels of AA for the duration of the protocol.
Figure 2.
Plasma n-6 fatty acid profiles determined by gas chromatography–mass spectrometry in the IL (gray), CLS1 (yellow), and CLS2 (blue) groups. The concentration of linoleic acid (LA, panel A), gamma-linolenic acid (GLA, panel B), dihomo-gamma linolenic acid (DGLA, panel C) and arachidonic acid (AA, panel D) are presented as mol% in the form of box plots. Labeled points without a common letter represent a statistically significant difference of P˂0.05. AA, arachidonic acid; CLS, concentrated lipid supplement; DGLA, dihomo-gamma linolenic acid; GLA, gamma-linolenic acid; IL, Intralipid®-control; LA, linoleic acid.
3.3. Distal ileum and brain tissue fatty acids
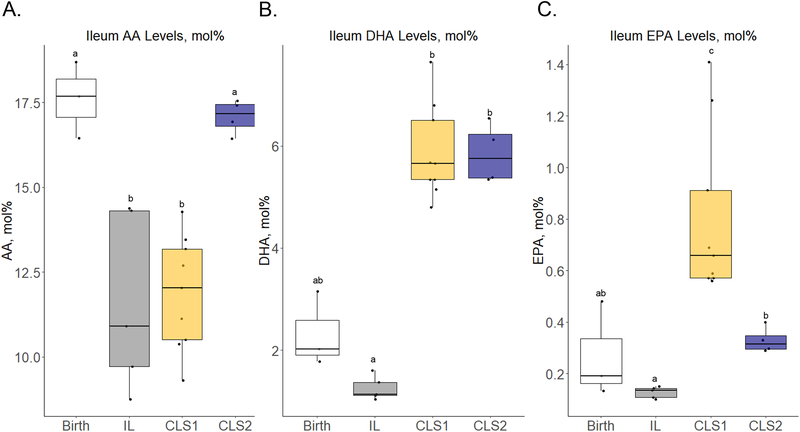
Changes in tissue levels of key fatty acids from birth in the distal ileum and brain are depicted in Figure 3 and Table 2, respectively. As in plasma, only the CLS2 group was able to maintain birth levels of AA in the ileum with median tissue levels significantly lower in the IL and CLS1 groups compared to birth and to the CLS2 group (Figure 3, panel A, Table S5). In contrast to the IL group which maintained birth levels of DHA, the CLS1 and CLS2 groups were able to significantly increase ileum DHA levels by 2.8-fold from birth and by 5-fold when compared to the control IL group (Figure 3, panel B). Although EPA levels increased in the CLS1 group compared to birth and the IL and CLS2 groups, the magnitude of this change and total abundance in mol% were low (Figure 3, panel C).
Figure 3.
Select fatty acid profiles in the distal ileum (A-C) determined by gas chromatography–mass spectrometry presented as mol% in the form of box plots at birth (white) and in the IL (gray), CLS1 (yellow), and CLS2 (blue) groups. Labeled points without a common letter represent a statistically significant difference of P˂0.05. AA, arachidonic acid; CLS, concentrated lipid supplement; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IL, Intralipid®-control.
Table 2.
Median brain tissue levels of select fatty acids in the n-3 and n-6 pathways, expressed as mol% (± IQR)
| Group | AA, 20:4,n-6 | DHA, 22:6,n-3 | EPA, 20:5,n-3 |
|---|---|---|---|
| Birth | 11.6 ± 0.2a | 7.2 ± 0.8a | 0.1 ± 0.03a |
| Intralipid | 12.0 ± 0.3a | 8.1 ± 0.5a | 0.06 ± 0.05b |
| CLS1 | 11.9 ± 0.3a | 9.7 ± 0.5b | 0.05 ± 0.01b |
| CLS2 | 12.0 ± 0.0a | 9.7 ± 0.2b | 0.06 ± 0.01ab |
Medians in a column for each group without a common letter differ, P < 0.05.
AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range
Unlike plasma, median tissue AA levels in the brain appear tightly controlled with little change in levels across the three groups compared to birth levels (Table 2; Table S6). The CLS1 and CLS2 groups increased tissue brain DHA levels by 35% when compared to birth. In contrast, the levels in the control IL group were comparable to birth. As in the ileum, any post-birth changes in EPA across groups were of low magnitude.
3.4. Lutein, Tocopherol, 25-hydroxyvitamin D3, and Vitamin D3
Lutein, tocopherol, 25-hydroxyvitamin D3, and vitamin D3 quantitative analyses were performed in plasma, liver, spleen, eye, frontal cortex, occipital cortex, and cerebellum. Values of these micronutrients are summarized in Table S7. For each of the tissues and for all micronutrients, there was a dose response moving from birth values to values in the IL group, then CLS1 group, and then the CLS2 group. In plasma, CLS2 demonstrated a statistically significant change from levels at birth and in the IL group. Compared to the IL control group there was a 3.3-fold increase in tocopherol (p=0.02) and 25-hydroxyvitamin D3 (p=0.02) and a 2-fold increase vitamin D3 in the CLS2 group (p=0.04).
In the liver, compared to IL controls the CLS2 group had a 20-fold increase in lutein (p=0.2), a 2.5-fold increase in tocopherol (p=0.04) and a 3-fold increase in 25-hydroxyvitamin D3 (p=0.04). Only with lutein did the CLS1 group also show a statistically significant increase in levels compared to the IL group (p=0.01).
Both CLS1 and CLS2 groups demonstrated an increase in select micronutrients in the neurological tissues. Lutein was increased by approximately 2.6-fold in the eye in the CLS1 and CLS2 groups compared to the IL group (p=0.004). In the frontal cortex tocopherol was increased 2.0 to 2.5-fold in the CLS1 (p=0.03) and CLS2 (p=0.02) groups, respectively, compared to the IL group. Similarly, there was a 2.3 to 3.1-fold increase in 25-hydroxyvitamin D3 in the CLS1 (p=0.03) and CLS2 (p=0.02) groups, respectively, compared to IL controls.
3.5. Intestinal morphometry and TUNEL
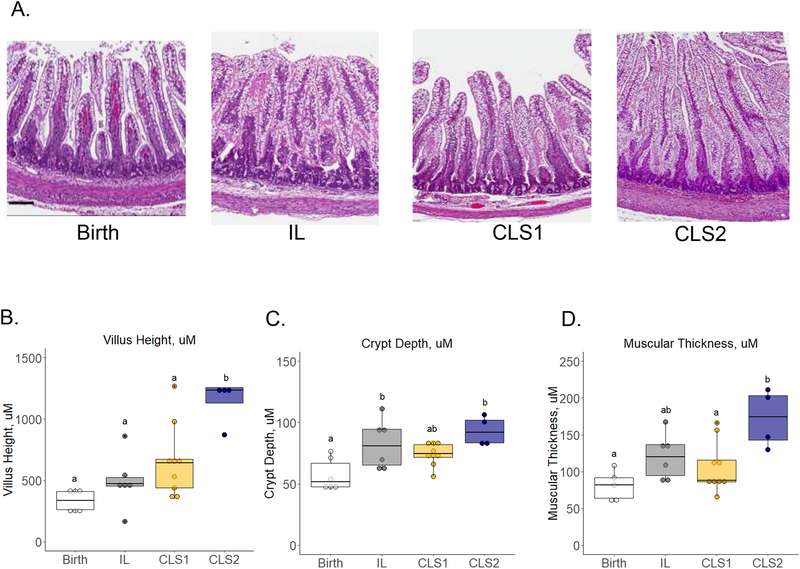
Representative images of the distal ileum are shown in Figure 4, panel A. The median villous height was not statistically different between the birth, IL and CLS1 groups (334.0±149.3, 472.2±71.3, and 644.0±231.6 μm, respectively) (Figure 4, panel B). In the CLS2 group, villous height was significantly longer at 1234.9±126.0 μm when compared to all three groups (p<0.01). Compared to a crypt depth of 51.7±19.4 μm at birth, the IL group and the CLS2 group had significantly greater values (Figure 4, panel C; 81.0±29.4 and 92.1±18.4 μm, respectively; p<0.05). Crypt depth of the CLS1 group did not differ from the three other groups. The CLS2 group was the only group that demonstrated a significant increase in muscular thickness when compared to birth (Figure 4, panel D; 174.1±60.7 versus 81.9±27.7 μm, respectively; p<0.01). There were no statistical differences in TUNEL staining among the three groups (Figure S2).
Figure 4.
Histologic evaluation of the distal ileum at birth (white) and in the IL (gray), CLS1 (yellow), and CLS2 (blue) groups. Representative hematoxylin and eosin-stained images at 5x magnification (A) are shown with quantification of villous height (B), crypt depth (C), and muscular thickness (D). Results shown as box plots. Labeled points without a common letter represent a statistically significant difference of P˂0.05. Scale bar =100um. CLS, concentrated lipid supplement; IL, Intralipid®-control.
4.0. Discussion
Our data demonstrate that the preterm piglet model mirrors the pancreatic insufficiency and postnatal fatty acid changes observed in the preterm infant. Similar to the human preterm infant, fatty acid profiles when IL is the primary source of lipid delivery, leads to an increase in the n-6 fatty acid LA, with a concomitant decline in critical LCPUFAs, in particular AA and DHA within the first postnatal week in the preterm piglet model. Additionally, we confirmed that the preterm piglet has a pancreatic lipase production 3-fold less than that of older, more mature pigs which also mimics the human experience.14 As a result, the preterm piglet serves as a valid model to study the impact of parenteral and enteral therapies to prevent the critical LCPUFA deficits that accrue during the postnatal period.
Two variations of a complex enteral lipid emulsion were studied with the goal of preventing DHA and AA deficits in the postnatal period. Both lipid emulsions were well-tolerated without concerns in overall growth or signs of intestinal pathology. The only difference between the two variations was the ratio of AA:DHA. The first (CLS1) had 4-fold greater level of DHA than AA with an AA:DHA ratio of 0.25. In contrast, the second (CLS2) had 20% higher levels of AA than DHA for an AA:DHA ratio of 1.2. While both demonstrated similar increases in postnatal DHA levels compared to birth levels, the second experimental emulsion was the only one able to maintain postnatal levels of AA in both plasma and ileum. Thus, maintaining an AA:DHA ratio greater than at least 1 appears to be important in preventing postnatal AA deficits. To address the decreased lipase levels present as a result of developmental consequences, we found that this enteral lipid emulsion was able to be utilized suggesting that the emulsification of this compound may be an important determinant of its bioavailability.
During the fetal period the fetus is provided AA and DHA through the placenta via a process known as biomagnification thus relying on the parent fatty acid rather than active biosynthesis of downstream fatty acids from precursors. After birth, the failure to maintain AA with the provision of AA fatty acid precursors suggests that the ability to biosynthesize AA is inefficient and/or the consumption of AA for growth and development outpace substrate availability. Maintaining adequate levels of AA is of significant clinical importance. In early studies evaluating supplementation of fatty acids in preterm infant formulas, the lack of AA supplementation resulted in lower growth attainment.15 Studies have shown that a balance of both n-3 and n-6 LCPUFAs by sustaining AA to DHA ratios of approximately 2:1 allows for optimal expression of enzymes and proteins related to growth and neurodevelopment.16,17 In a clinical cohort of preterm infants, for every 1 mol% drop in AA, this was associated with a 40% increase in the risk of nosocomial sepsis.2 More recently, reduced serum levels of AA in the postnatal period was associated with retinopathy of prematurity.5
Although one has to be cautious in extrapolating our enteral data to parenteral lipid emulsions, the data raise the question of the adequacy of n3-dominant parenteral emulsions as a maintenance lipid emulsion for the preterm infant. The CLS1 group reflects an n-3 enriched lipid as seen in fish oil emulsions, such as SMOFlipidⓇ, which has an AA:DHA ratio of 0.23.19 The piglets in the CLS1 group failed to maintain postnatal levels of AA and this is also true in the human preterm infant experience when IntralipidⓇ or SMOFlipidⓇ is given parenterally.4,20 In the preterm infant, the decline in AA in fish oil containing lipid emulsions is even greater than the decline from using IntralipidⓇ (ref)Whether CLS2 can attenuate the postnatal decline in AA plasma and tissue levels in preterm infants with a background diet containing DHA is unknown and requires further study. Enteral supplementation strategies of fatty acids in preterm infants have also relied on a n-3 dominant approach, similar to the evolving intravenous lipid emulsions. These DHA-centric strategies have not been successful in preventing postnatal changes and may in fact be harmful.21 Although the failure of these strategies may be due to multiple reasons such as dosing, timing, digestion, absorption, and emulsification, the lack of supplemental AA in these strategies may be of critical importance.
Preserving AA levels in the CLS2 group was associated with greater ileal villus height and muscular layer thickness. This is consistent with the role of these fatty acids not only in inflammatory signaling but also in organogenesis as has been shown with administration of their terminal metabolites Resolvin D1 (RvD1) and Lipoxin A4 (LXA4) in a mouse model of hyperoxia induced lung injury.22 While both RvD1 and LXA4 reduced inflammatory signaling during hyperoxia exposure, the addition of LXA4, a metabolite of AA, uniquely improved alveologenesis. Increasing villi height and muscular thickness are expected adaptive responses after birth and are observed post-intestinal resection. Intestinal adaptation in the latter scenario has been linked to improved clinical outcomes in studies of short bowel syndrome.23–25 Furthermore, prostaglandin E2 (PGE2), a cyclooxygenase metabolite of AA, is known to function as a mitogen and transactivate epidermal growth factor (EGF) signaling through induction of EGF receptor ligand production. EGF serves an important role in cellular differentiation, prevention of apoptosis, and protection against cellular injury during acute inflammation.26–29 Whether the observation of greater villus height and muscular layer thickness offers a protective role or improved intestinal physiology in this case will need to be evaluated in future studies.
Supplementation of the critical downstream LCPUFAs in the CLS1 and CLS2 groups not only maintained but significantly increased levels of DHA in plasma, distal ileum, and brain. The distal ileum also mirrored the plasma results for AA and EPA. In contrast, AA levels were maintained in the brain equally across the three groups and did not show an increase in EPA with the n-3 supplemented lipid emulsions as shown in the plasma. This control of AA and EPA levels suggests that the brain has a tightly regulated mechanism compared to the ileum in maintaining specific fatty acid levels during this critical time of neurologic development. This indicates that specific transport mechanisms reflecting tissue specificity and compartmentalization must exist and is supported in the literature.30,31
Unique to the two experimental lipid emulsions was the provision of other lipophilic nutrients in the composition, specifically tocopherol, lutein, and vitamin D3. Similar to many other nutrients, preterm delivery results in a lack of body fat and thus low body stores of these lipophilic molecules with subsequent decline due to inadequate replacement.6–8 The recommended requirements and levels of deficiency of most of these micronutrients with the exception of Vitamin D3 are unknown, however, their importance in eye, brain, bone, and immune development, and their role as antioxidants has been postulated in infant development.32–37 The concentrated, highly emulsified lipid supplement provided in this study resulted in adequate absorption of these important lipophilic nutrients.
Conclusions
The data in this preterm piglet study indicate that a rational approach can be taken to prevent the fatty acid deficits that accrue in preterm infants. The inclusion of both n-3 and n-6 LCPUFAs in specific ratios can be administered by the enteral route and mitigate the changes in critical fatty acid levels that have been linked with neonatal morbidities. From both the human preterm infant studies and the preterm piglet studies, we know the target LCPUFA levels (birth levels) which could serve as endpoints in a clinical trial with examination of the impact of fatty acid supplementation on specific neonatal morbidities.
Supplementary Material
Clinical Relevancy Statement.
Deficits in critical long chain fatty acids are linked to morbidities such as bronchopulmonary dysplasia, sepsis, and retinopathy of prematurity in the preterm infant. Preterm infants also fail to accrue lipophilic nutrients that are essential to health. We demonstrate the safety and efficacy of early administration of a novel enteral complex lipid supplement to augment systemic and tissue levels of arachidonic acid, docosahexaenoic acid, and other lipophilic nutrients in a preterm piglet model. We also define the arachidonic acid to docosahexaenoic acid ratio that successfully maintains birth levels of arachidonic acid, a result not achieved with docosahexaenoic acid dominant strategies often utilized in this population.
Financial disclosure and conflicts of interest statement
Dr. Martin received grant funding from Abbott Nutrition for this study, has grant support from Alcresta Pharmaceuticals, and serves on the scientific advisory boards of Prolacta Biosciences and Fresenius Kabi. Dr. Freedman is a co-investigator on the grant from Abbott Nutrition for this study, has grant support from Alcresta Pharmaceuticals and the Cystic Fibrosis Foundation, serves on the scientific advisory board of Prolacta Biosciences, and is a paid consultant to Akcea Therapeutics and Takeda Pharmaceuticals. Dr. Vurma, Dr. Ehling, and D. Gordon are employees of Abbott Nutrition. Dr. DeMichele is a former employee of Abbott Nutrition.
Funding information
This project was funded by Abbott Nutrition, NIH R01 DK 104346, and the Charles H. and Judy Hood Family Infant Health Research Program.
Footnotes
Supplementary Material
Tables S1–S8 and Figures S1–S2 are available online at http://pen.sagepub.com.
References
- 1.Larque E In vivo investigation of the placental transfer of 13C-labeled fatty acids in humans. J Lipid Res 2002;44(1):49. [DOI] [PubMed] [Google Scholar]
- 2.Martin CR, Dasilva DA, Cluette-Brown JE, et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 2011;159(5):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leaf AA, Leighfield MJ, Costeloe KL, Crawford MA Factors affecting long-chain polyunsaturated fatty acid composition of plasma choline phosphoglycerides in preterm infants. J Pediatr Gastroenterol Nutr 1992;14(3):300–8. [DOI] [PubMed] [Google Scholar]
- 4.D’Ascenzo R, D’Egidio S, Angelini L, et al. Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: Effect on plasma lipids and long-chain polyunsaturated fatty acids. J Pediatr 2011;159(1):33. [DOI] [PubMed] [Google Scholar]
- 5.Löfqvist CA, Najm S, Hellgren G, et al. Association of Retinopathy of Prematurity With Low Levels of Arachidonic Acid: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol 2018;136(3):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ Lutein and preterm infants with decreased concentrations of brain carotenoids. J Pediatr Gastroenterol Nutr 2014;59(5):659–65. [DOI] [PubMed] [Google Scholar]
- 7.Kelly FJ, Rodgers W, Handel J, Smith S, Hall MA Time course of vitamin E repletion in the premature infant. Br J Nutr 1990;63(3):631–8. [DOI] [PubMed] [Google Scholar]
- 8.Burris HH, Van Marter LJ, McElrath TF, et al. Vitamin D status among preterm and full-term infants at birth. Pediatr Res 2014;75(1–1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin CR, Stoll B, Cluette-Brown J, et al. Use of a novel docosahexaenoic acid formulation vs control in a neonatal porcine model of short bowel syndrome leads to greater intestinal absorption and higher systemic levels of DHA. Nutr Res 2017;39:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kansagra K, Stoll B, Rognerud C, et al. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 2003;285(6):G1162–70. [DOI] [PubMed] [Google Scholar]
- 11.Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids 2015;99:1–6. [DOI] [PubMed] [Google Scholar]
- 12.Makrides M, Gibson RA Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr 2000;71(1 Suppl):307S – 11S. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226(1):497–509. [PubMed] [Google Scholar]
- 14.Lebenthal E, Lee PC Development of functional responses in human exocrine pancreas. Pediatrics 1980;66(4):556–60. [PubMed] [Google Scholar]
- 15.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A 1993;90(3):1073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016;8(4):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alshweki A, Muñuzuri AP, Baña AM, et al. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr J 2015;14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor R, Huang Y-S Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol 2006;7(6):531–4. [DOI] [PubMed] [Google Scholar]
- 19.Hojsak I, Colomb V, Braegger C, et al. ESPGHAN Committee on Nutrition Position Paper. Intravenous Lipid Emulsions and Risk of Hepatotoxicity in Infants and Children: A Systematic Review and Meta-analysis. J Pediatr Gastroenterol Nutr 2016;62(5):776. [DOI] [PubMed] [Google Scholar]
- 20.D’Ascenzo R, Savini S, Biagetti C, et al. Higher docosahexaenoic acid, lower arachidonic acid and reduced lipid tolerance with high doses of a lipid emulsion containing 15% fish oil: A randomized clinical trial. Clin Nutr 2014;33(6):1002. [DOI] [PubMed] [Google Scholar]
- 21.Collins CT, Makrides M, McPhee AJ, et al. Docosahexaenoic Acid and Bronchopulmonary Dysplasia in Preterm Infants. N Engl J Med 2017;376(13):1245. [DOI] [PubMed] [Google Scholar]
- 22.Martin CR, Zaman MM, Gilkey C, et al. Resolvin D1 and lipoxin A4 improve alveolarization and normalize septal wall thickness in a neonatal murine model of hyperoxia-induced lung injury. PLoS One 2014;9(6):2147483647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DC, Levin MS Mechanisms of intestinal adaptation. Best Pract Res Clin Gastroenterol 2016;30(2):237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner BW The Pathogenesis of Resection-Associated Intestinal Adaptation. Cell Mol Gastroenterol Hepatol 2016;2(4):429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tappenden KA Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr 2014;38(1 Suppl):23S – 31S. [DOI] [PubMed] [Google Scholar]
- 26.Cabral M, Martín-Venegas R, Moreno JJ Role of arachidonic acid metabolites on the control of non-differentiated intestinal epithelial cell growth. Int J Biochem Cell Biol 2013;45(8):1620–8. [DOI] [PubMed] [Google Scholar]
- 27.Krysan K, Lee JM, Dohadwala M, et al. Inflammation, epithelial to mesenchymal transition, and epidermal growth factor receptor tyrosine kinase inhibitor resistance. J Thorac Oncol 2008;3(2):107–10. [DOI] [PubMed] [Google Scholar]
- 28.McElroy SJ, Hobbs S, Kallen M, et al. Transactivation of EGFR by LPS induces COX-2 expression in enterocytes. PLoS One 2012;7(5):e38373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaoka T, Yan F, Cao H, et al. Transactivation of EGF receptor and ErbB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci U S A 2008;105(33):11772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amusquivar E, Sánchez M, Hyde MJ, Laws J, Clarke L, Herrera E Influence of fatty acid profile of total parenteral nutrition emulsions on the fatty acid composition of different tissues of piglets. Lipids 2008;43(8):713. [DOI] [PubMed] [Google Scholar]
- 31.Greiner RC, Winter J, Nathanielsz PW, Brenna JT Brain docosahexaenoate accretion in fetal baboons: bioequivalence of dietary alpha-linolenic and docosahexaenoic acids. Pediatr Res 1997;42(6):826–34. [DOI] [PubMed] [Google Scholar]
- 32.Kuchan MJ, Jensen SK, Johnson EJ, Lieblein-Boff JC The naturally occurring α-tocopherol stereoisomer RRR-α-tocopherol is predominant in the human infant brain. Br J Nutr 2016;116(1):126–31. [DOI] [PubMed] [Google Scholar]
- 33.Tei M, De Bernardo G, Bazzini F, et al. Antioxidant Effects of Lutein in Neonatal Period. J Pediatr Biochem 2016;6(02):110–3. [Google Scholar]
- 34.Perrone S, Tei M, Longini M, Buonocore G The Multiple Facets of Lutein: A Call for Further Investigation in the Perinatal Period. Oxid Med Cell Longev 2016;2016:5381540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu Z, Meng SS, Burnim SB, Smith LE, Lo AC Lutein facilitates physiological revascularization in a mouse model of retinopathy of prematurity. Clin Experiment Ophthalmol 2017;45(5):529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onwuneme C, Blanco A, O’Neill A, Watson B, Molloy EJ Vitamin D enhances reactive oxygen intermediates production in phagocytic cells in term and preterm infants. Pediatr Res 2016;79(4):654–61. [DOI] [PubMed] [Google Scholar]
- 37.Karpen HE Mineral Homeostasis and Effects on Bone Mineralization in the Preterm Neonate. Clin Perinatol 2018;45(1):129–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.