Abstract
Macrophage-mediated inflammatory reaction to implant wear particles drives bone loss around total joint replacements (TJR). Although most TJR recipients are elderly, studies linking wear particle-activated macrophages and peri-implant osteolysis have not taken into account the multiple effects that aging has on the innate immune system and, in particular, on macrophages. To address this, we compared the wear particle responses of bone marrow macrophages obtained from young (2-month) and aged (18-month) mice. Macrophages were polarized to M0, M1, or M2 phenotypes in vitro, challenged with titanium particles, and their inflammatory response was characterized at multiple time points by qRT-PCR and ELISA. In addition, age-dependent changes in activation of transcription factor NF-κB were analyzed by a lentiviral vector-based luciferase reporter system. The particle stimulation experiment was further repeated using human primary macrophages isolated from blood donors of different ages. We found that the pro-inflammatory responses were generally higher in macrophages obtained from young mice, but differences between the age groups remained small and of uncertain biological significance. Noteworthily, M2 polarization effectively suppressed the particle-induced inflammation in both young and aged macrophages. These results suggest that aging of the innate immune system per se plays no significant role in the response of macrophages to titanium particles, whereas induction of M2 polarization appears a promising strategy to limit macrophage-mediated inflammation regardless of age.
Keywords: Aging, Macrophage, Wear particle, Polarization, Inflammation, Titanium
INTRODUCTION
Chronic foreign body reaction to implant wear particles remains the leading cause for peri-prosthetic osteolysis and aseptic loosening of total joint replacements (TJRs).1 This inflammatory host response is mediated by macrophages of the innate immune system as they recognize and phagocytose wear debris dispersed in peri-implant tissues.2 In response, particle-activated macrophages secrete various inflammatory cytokines and chemokines that perturb tissue homeostasis by stimulating other stromal cells and recruiting further circulating phagocytes to the site of insult.3 This persistent pro-inflammatory status at the bone-implant interface drives osteoclast activation and leads to progressive bone destruction impairing the stability and longevity of the implant.4
Wear particles together with inflammatory microenvironmental signals induce an M1-like macrophage polarization that exacerbates the production of pro-inflammatory mediators and favors osteoclastogenesis.5,6 In contrast, alternatively activated M2 macrophage phenotype is proposed to mitigate these adverse effects, and an M2 supporting immunomodulatory strategy has emerged as a promising means to counteract the particle induced inflammation and potentially increase the lifespan of the implant.7–9 Thus, M1 and M2 macrophages are seen as the opposite ends of the continuum of macrophage phenotypes where M0 represents a non-polarized activation state. With an ability to assume different phenotypes, macrophages are considered key players regulating the inflammatory response to wear particles and dictating the development of aseptic loosening.5
Accumulating evidence indicates that aging significantly alters the function of macrophages and affects many of their key characteristics including phagocytosis, toll-like receptor (TLR) signaling, and cytokine secretion.10–13 For instance, aged macrophages have been demonstrated to share increased resting levels of oxidative stress and ratios of pro- to anti-inflammatory markers.13–16 Furthermore, aged macrophages initiate a dysregulated inflammatory response followed by a prolonged resolution phase.17,18 Hence, a systemic low-grade, pro-inflammatory condition with elevated levels of circulating cytokines proposedly prevails among the aged population—a phenomenon referred to as “inflammaging”.19–21 Advanced age has also been found to impair macrophage polarization, but thus far the results have been inconclusive; both M1 and M2 responses have been reported as enhanced among macrophages isolated from aged mice.13,15,22,23
As aging seems to modify many aspects of the macrophage function, it might be an important factor determining how the inflammatory osteolysis progresses in TJR loosening. However, little is known about the effect of aging on the macrophage response to implant wear particles. Despite the fact that TJR failures mainly burden the elderly, most studies addressing particle-activated macrophages and subsequent peri-prosthetic bone loss have been conducted using macrophages from young donors or animals. In addition, the protective effect of M2 polarization against wear particle-induced inflammation remains unclear among aged macrophages.24,25
Given that a significant number of bone marrow derived macrophages infiltrate into peri-implant tissues during aseptic loosening, we compared the age-dependent responsiveness of these cells to titanium (Ti) particles in vitro. The production of inflammatory mediators from young and aged murine macrophages was assessed by qRT-PCR and ELISA, and the activity of NF-κB—an essential transcription factor regulating inflammatory responses—was measured using a novel lentiviral vector-based NF-κB responsive luciferase reporter system.26 We hypothesized that the pro-inflammatory responses would be exacerbated in aged macrophages due to their heightened inflammatory nature and/or dysregulated anti-inflammatory feedback mechanisms. We further studied how macrophage polarization modified these responses with the hypothesis that M2 polarization might be less effective in preventing the inflammation among aged macrophages. To examine if the results were applicable also to humans, we challenged primary macrophages obtained from the buffy coats of young and older blood donors with Ti particles and analyzed the cytokine secretion by ELISA.
MATERIALS AND METHODS
Mouse bone marrow macrophages
Following institutional guidelines for the care and use of laboratory animals, we obtained mouse bone marrow macrophages (mBMMs) from the femora and tibiae of five young (2-month) and five aged (18-month) male C57BL/6J mice (Jackson Laboratory). Whereas mice of this strain less than 3 months of age are considered young, mice aged 18 months or more meet the definition of old with senescent changes detected in almost all biomarkers, and are widely used to study the biology of aging. Treating both age groups separately, mBMMs were isolated using an established protocol.13 Bone marrow cells were then cultured in macrophage medium consisting of RPMI 1640 (Gibco, Life Technologies) supplemented with 30% L929 cell-conditioned medium, 10% heat inactivated fetal bovine serum (FBS, Gibco, Life Technologies), 1% antibiotic-antimycotic solution (Gibco, Life Technologies), and 10 ng/mL macrophage colony-stimulating factor (M-CSF, R&D Systems, Minneapolis, MN) for seven days, until lifted with 0.25% Trypsin-EDTA (Gibco, Life Technologies) and gentle scraping. Newly differentiated macrophages were divided for up to four passages, until harvested and stored in liquid nitrogen.
Titanium particles
Commercially available Ti particles (Alfa Aesar, Product No. 00681) were cleaned with five alternating treatments of 0.1 N NaOH in 95% ethanol and 25% nitric acid as previously described.27 Particles were tested endotoxin-free using Limulus amebocyte lysate assay kit (Pierce LAL Chromogenic Endotoxin Quantitation Kit). The supplier reported an average particle size of 2.8 μm in diameter according to a Blaine permeameter, but further analysis of 600 particles by transmission electron microscopy (TEM) revealed an average particle diameter of 1.6 μm (range 0.1–7.5 μm) with a median of 1.35 μm. Particles of this size range are typically used in similar in vitro experiments, and have been found in periprosthetic tissues around aseptically loosened total joint replacements.28
Polarization and particle stimulation of murine macrophages
For particle stimulation experiments, young and aged mBMMs were cultured in macrophage medium for seven days until divided to 24-well plates at a density of 1.0 × 105 cells/well. Cells were allowed to recover for one day, after which macrophage polarization was induced by supplementing the fresh culture medium with 20 ng/mL interferon gamma (IFN-γ, R&D systems) or 20 ng/mL interleukin-4 (IL-4, R&D systems) for M1 and M2 macrophage phenotypes respectively. Cells left untreated were considered non-polarized M0 macrophages. On the following day, M0, M1, and M2 macrophages were challenged with Ti particles at a concentration of 1.2 mg/mL for 6, 12, 24, or 48 hours. The experiment was accomplished in triplicates and in the presence of appropriate polarizing signals. At a given time point, cell culture media were collected and macrophages were disrupted by adding RLT buffer (RNeasy Mini kit, Qiagen, Valencia, CA). Supernatants and cell lysates were stored in - 80°C for later use.
To determine an optimal particle concentration for macrophage stimulation, preliminary experiments were performed with particle doses ranging from 0.4 to 2.4 mg/mL in cell culture medium. Based on dose-dependent cytotoxicity and inflammatory responses, the particle concentration of 1.2 mg/mL was chosen for final studies (Fig. S-1).
qRT-PCR
Cellular ribonucleic acid (RNA) was extracted from the lysates of murine macrophages using RNeasy Mini kit, and the amount of purified RNA was measured with a NanoDrop 1000 spectrophotometer (Thermo scientific, Waltham, MA). An equal amount of total RNA from each sample was directed into reverse transcription, and the complementary DNA (cDNA) was synthesized in a thermocycler using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). A reaction mix was prepared containing sample cDNA, a specific TaqMan primer-probe and TaqMan Gene Expression Master Mix (Applied Biosystems). The quantitative real-time PCR was performed with ABI 7900HT Sequencing Detection System (Applied Biosystems) for TNF, IL-1β, IL-6, IL-1Ra, iNOS, CCL2, CXCL9, MRC1, and TLR4 (all primer-probes from Applied Biosystems). The comparative Ct method was used to obtain the results with 18S ribosomal RNA serving as an internal control.
mBMMs transduction
The construction of lentiviral NF-κB reporter vector and transduction of mBMMs were conducted as previously described.26,29 Frozen macrophages were thawed and cultured for seven days, until exposed to mixture of macrophage medium and supernatant from transfected human embryonic kidney 293T cells at a ratio of 1:2 supplemented with 10 μM cyclosporine for 24 hours. After one day of recovery in fresh macrophage medium, the transduction was repeated for another 24 hours.
Three days after the second infection, transduced reporter macrophages were divided onto 24-well culture plates. Polarization of these reporter macrophages and initiation of particle stimulation were completed as described above in quadruplicate wells. Culture media were collected after 4 hours of particle exposure, and the firefly luciferase activity was measured with a luminometer (Turner BioSystems TD-20/20, Promega, Madison, WI) by using a Luciferase Assay System kit (Promega). Luciferase activity was normalized to the amount of total protein using a protein assay kit (Thermo Scientific).
Isolation and stimulation of human primary macrophages
Human peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy and voluntary blood donors, who had signed an informed consent document. Buffy coats were by-products of blood preparation intended for clinical use, and their allocation for scientific purposes was approved by the Finnish Red Cross Blood Service. For this study, we obtained blood samples from four young donors and four donors as old as possibly available. These blood donors comprised of one man and seven women with a mean age of 20 years for young donors (range 19–21) and a mean age of 55.8 years for older donors (range 52–63).
PBMCs were isolated by density gradient centrifugation and differentiated into macrophages as previously established.30 Briefly, mononuclear cells were plated onto 24-well culture plates at a concentration of 1.5 × 106 cells/well and allowed to adhere for one hour. Attached monocytes were differentiated into macrophages by culturing them for seven days in macrophage serum-free medium (Gibco) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF, Miltenyi Biotec, Bergisch Gladbach, Germany).
Mature macrophages from young and older donors were challenged with Ti particles at a concentration of 2.25 mg/mL in duplicate wells. Given the differing cell type, seeding concentration, and culture media, this particle dose was determined to be optimal for human macrophages. After eight hours, culture supernatants were collected and the cytotoxicity was affirmed non-significant by an LDH detection kit (Roche Diagnostics, Switzerland).
Enzyme-Linked Immunosorbent Assay (ELISA)
Supernatants from murine cell cultures were analyzed for TNF, IL-1β, IL-6, and IL-10 by Ready-Set-Go! mouse ELISA kits (Affymetrix eBioscience, San Diego, CA) following manufactureŕs instructions. For cytokine IL-1Ra, a Quantikine mouse ELISA kit (R&D Systems, Minneapolis, MN) was used. Concentrations of human TNF, IL-1β, IL-6, and IL-1Ra were investigated from the culture media by DuoSet ELISA assays (R&D Systems).
Statistical analyses
Statistical analyses were performed with GraphPad Prism version 7 (GraphPad Software, La Jolla, CA), and two-way analysis of variance (ANOVA) was applied to compare differences between experimental groups. Basal gene expression levels of murine macrophages were assessed for the effects of aging and polarization using standard two-way ANOVA with Tukeýs multiple comparison tests. This method was employed to compare the effects of aging and particle stimulation as well. Statistical comparison between young and aged particle stimulated human macrophages was completed using repeated measures two-way ANOVA followed by Holm-Šídákś post hoc tests. Criteria for statistical significance was set at p<0.05. The data are presented as mean ± standard error of mean (SEM).
RESULTS
Comparison of basal gene expression levels between young and aged macrophages
Regardless of the polarization status, young murine bone marrow macrophages expressed heightened basal levels of IL-6, whereas a similar trend was observed for IL-1β and IL-1Ra (Fig. 1a–c). Older cells, by contrast, expressed higher levels of iNOS, CCL2, TLR4, CXCL9, and MRC1, but only the differences in TLR4 expression reached statistical significance in all polarization states (Fig. 1d–h). The basal expression of TNF differed between the age groups only in M1 polarization, in which aged macrophages showed increased expression (Fig. 1i). IFN-γ stimulation amplified the expression level of M1 marker CXCL9, but also the expressions of IL-1β, IL-1Ra, iNOS, TLR4 and TNF in both age groups. Compared to M0 phenotype, IL-4 stimulus reduced the expression of TNF, but elevated the expressions of chemokine CCL2 and M2 marker MRC1.
Figure 1.
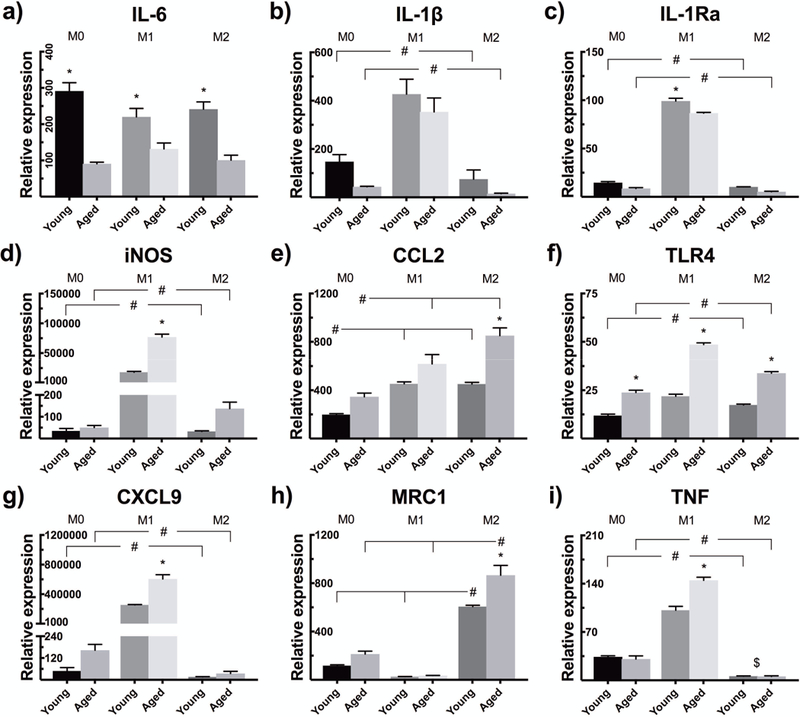
Bone marrow macrophages from young and aged mice were polarized to M1 or M2 phenotype with IFN-γ or IL-4, respectively, or left untreated as non-polarized M0 macrophages. The mRNA expressions of (a) IL-6, (b) IL-1β, (c) IL-1Ra, (d) iNOS, (e) CCL2, (f) TLR4, (g) CXCL9, (h) MRC1, and (i) TNF were determined 30 hours after the induction of polarization by qRT-PCR. * indicates statistical significance between similarly polarized young and aged macrophages, whereas # illustrates significant difference between polarizations within the same age group. $ stands for significant difference between M0 and M2 phenotype in both age groups.
Particle-induced TNF production lasts longer among young murine macrophages
Ti particle stimulation resulted in an increased mRNA expression of TNF at 6 and 12 h time points in all macrophage phenotypes (Fig. 2a–b). The expression had returned close to the level of unstimulated control cells after 24 hours (Fig. 2c). Compared to M0 polarization, M1 macrophages enhanced and M2 macrophages suppressed the TNF mRNA expression levels. Age-related differences were clearly observed only in suppressive M2 polarization; after particle stimulation, young M2 macrophages expressed TNF at a higher level compared to their aged counterparts. More responsive M0 and M1 phenotypes had similar expression profiles between young and senescent macrophages, albeit young particle-stimulated macrophages showed higher TNF expression still at the 24 h time point.
Figure 2.
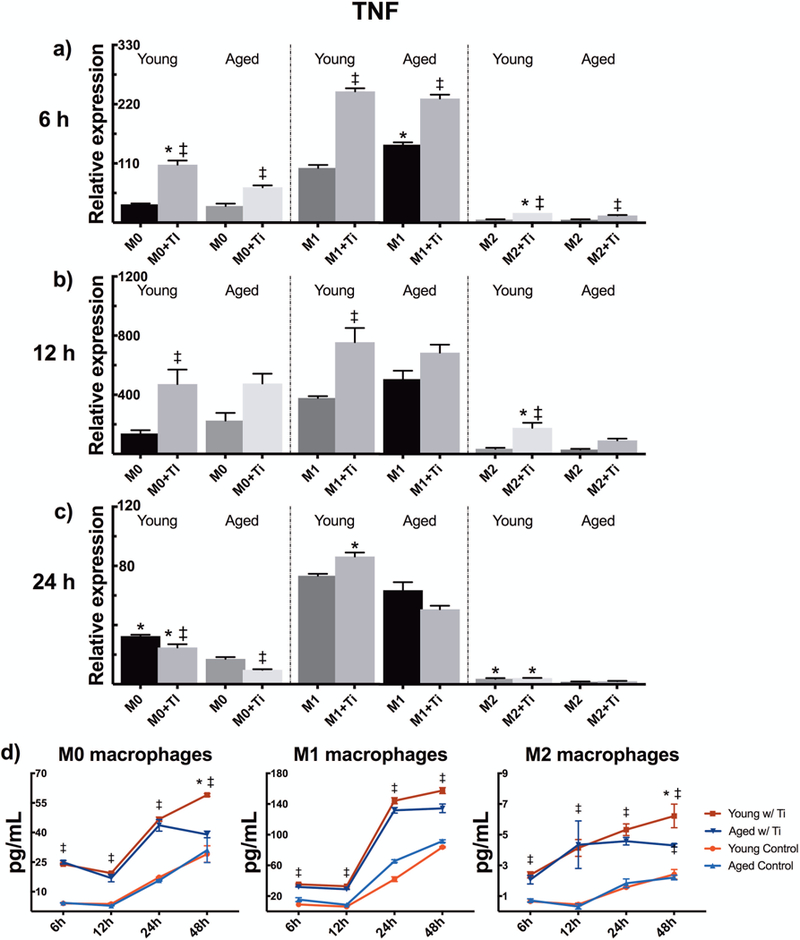
Young and aged mBMMs were polarized to M0, M1, or M2 phenotypes, and challenged with titanium particles. The relative expression of pro-inflammatory cytokine TNF was analyzed (a) 6 h, (b) 12 h, and (c) 24 h after the initiation of particle stimulation. (d) TNF secretion was measured from the culture supernatants until 48 h by ELISA. * indicates statistical significance between similarly treated young and aged macrophages, whereas ‡ demonstrates significant effect of particle stimulation.
Using ELISA, we found that Ti particle stimulation significantly increased TNF secretion, with young and aged macrophages secreting nearly an equal amount of TNF during the first 24 hours (Fig. 2d). However, as suggested by qPCR results, young macrophages produced more TNF at the later 48 h time point. This finding reached statistical significance in M0 and M2 polarizations. In line with the expression data, M1 polarization intensified, and M2 polarization diminished the magnitude of TNF produced when compared to M0 phenotype in both young and aged macrophages.
IL-1β and IL-6 expressions reach a higher peak in young particle-stimulated macrophages
Particle challenge increased the mRNA expressions of both IL-1β and IL-6 in all M0, M1, and M2 phenotypes (Fig. 3a–f). This trend occurred at a higher magnitude among young murine macrophages and reached statistical significance in multiple points of comparison. As compared to M0 phenotype, M1 polarization of particle challenged cells did not considerably promote these expressions. By contrast, M2 polarization suppressed the production of these pro-inflammatory cytokines and especially aged M2s were almost unresponsive in this respect. IL-1β expression was reduced to below the level of unstimulated control cells by 24 hours, while M0 and M1 macrophages still displayed an elevated IL-6 expression at this time.
Figure 3.
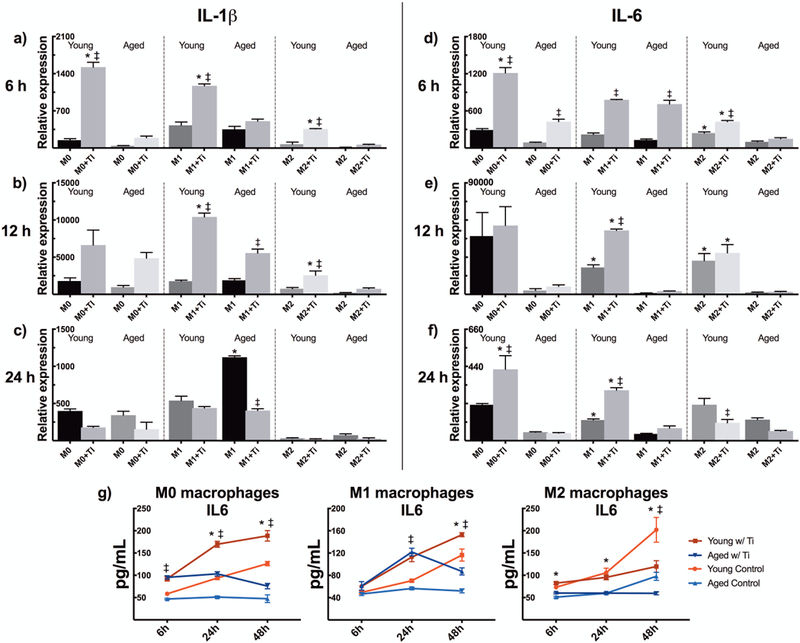
Young and aged mBMMs of M0, M1, and M2 phenotype were challenged with titanium particles in vitro. Using qRT-PCR, the inflammatory response to particles was characterized by studying the relative mRNA expressions of (a-c) IL-1β and (d-f) IL-6 at 6 h, 12 h, and 24 h. (g) The amount of secreted IL-6 was further assessed by ELISA. * represents statistical significance between young and aged macrophages, while ‡ identifies significant effect of particle stimulation.
When compared to aged macrophages young macrophages showed higher degree of IL-6 protein expression in all polarized phenotypes (Fig. 3g). Reflecting the mRNA expression data, particle stimulation increased the extent of IL-6 secreted by M0 and M1 phenotypes resulting in rather similar secretion patterns between these conditions. On the contrary, particle-challenged M2 macrophages produced no IL-6 and showed diminished IL-6 levels at 48 hours in comparison to untreated control cells. The amount of mature IL-1β secreted was below the detection limit of the ELISA kit used.
Aged murine macrophages display an increased secretion of IL-1Ra
Besides the production of pro-inflammatory cytokines, we detected a strong anti-inflammatory feedback response to particle stimulation as well: Both young and aged macrophages strengthened their IL-1Ra mRNA expression in all polarization states (Fig. 4a–c). Young macrophages expressed IL-1Ra mRNA at a significantly higher degree at 6 and 24 hours, while aged macrophages increased its expression at 12 h time point. This phenomenon was seen in all phenotypes with M1 macrophages intensifying and M2 polarization diminishing the anti-inflammatory responses.
Figure 4.
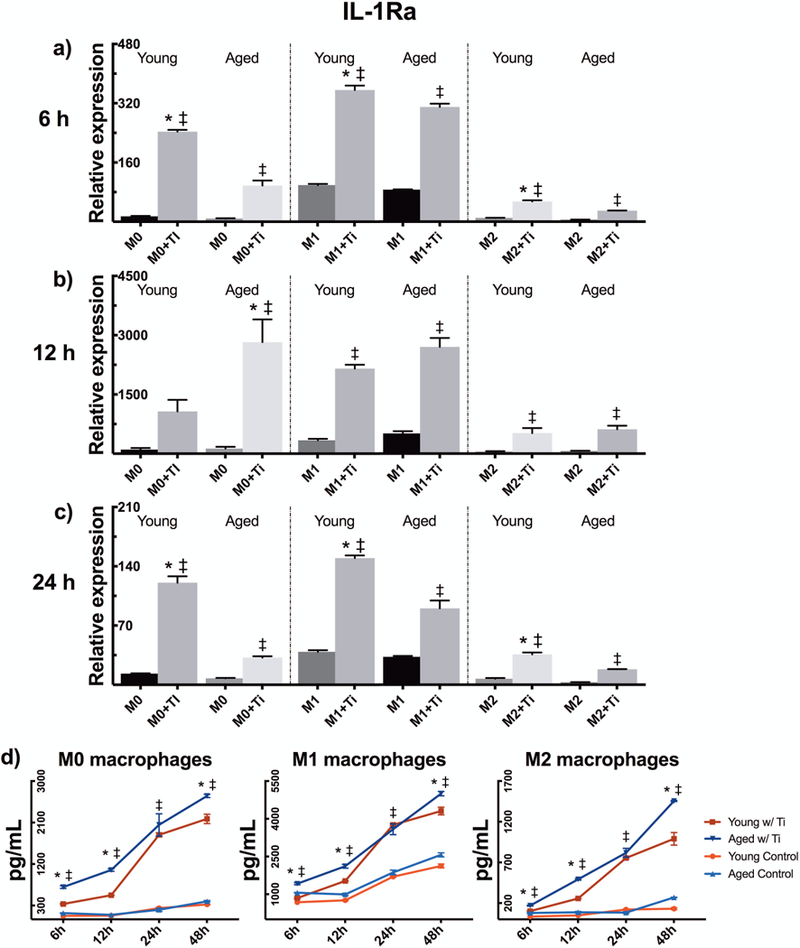
Young and aged mBMMs were polarized to M0, M1, and M2 phenotype, challenged with titanium particles, and their anti-inflammatory response was evaluated by measuring IL-1Ra production. The relative mRNA expression of IL-1Ra was determined at (a) 6 h, (b) 12 h, and (c) 24 h by qRT-PCR, and (d) the cytokine secretion was assessed from culture supernatants by ELISA. * indicates significant difference between young and aged particle-stimulated macrophages, whereas ‡ illustrates significance of particle stimulation.
As studied by ELISA, however, aged particle-stimulated macrophages secreted IL-1Ra at a higher level starting already at six hours (Fig. 4d). This heightened IL-1Ra secretion was observed among senescent cells for the most part of the experiment with 24 h time point being the only one without statistical significance. In line with qPCR data, M1 macrophages elevated the amount of secreted IL-1Ra, whereas the opposite was found with M2 polarization. Concentrations of another anti-inflammatory cytokine IL-10 remained undetectable in all experimental groups with the ELISA kit used.
Aged macrophages express more iNOS and CCL2 after particle stimulation
The basal mRNA expressions of iNOS and CCL2 were elevated among aged murine macrophages compared to younger macrophages in all M0, M1, and M2 polarizations (Fig. 1d–e). Addition of Ti particles further raised the expression of the above factors to significantly higher levels as compared to young cells at 6 h time point (Fig. 5a, d). M1 macrophages maintained a heightened iNOS expression during the whole experiment with particle-stimulated aged M1s being more reactive still at 12 hours (Fig. 5a–c). In comparison to the M1 phenotype, particle-induced changes in iNOS expression were virtually negligible in M0 and M2 cells.
Figure 5.
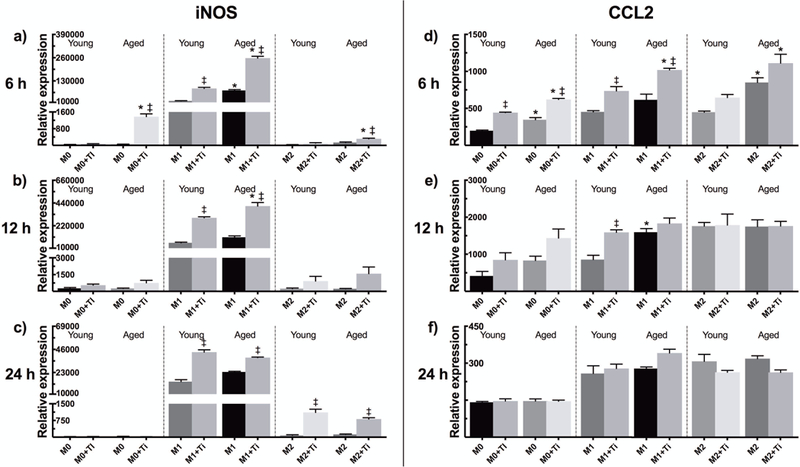
A qRT-PCR analysis was performed for young and aged particle-challenged mBMMs. The mRNA expressions of (a-c) iNOS and (d-f) CCL2 were assessed at 6 h, 12 h, and 24 h with M0, M1, and M2 macrophage phenotypes included. * represents significant difference between similarly treated young and aged macrophages, while ‡ indicates statistical significance of particle stimulation.
After an initially enhanced CCL2 expression, particle stimulation had little effect on CCL2 expression level at later time points (Fig. 5d–f). Compared to the M0 phenotype, expressions of this chemokine were elevated in both M1 and M2 macrophages.
Aging shows no difference in NF-κB activation
Using lentiviral transduced NF-κB reporter macrophages, we observed no considerable differences in NF-κB activation between young and aged cells (Fig. 6a–b). Surprisingly, stimulation with endotoxin-free titanium particles provoked no NF-κB activation in macrophages of any phenotype. Nevertheless, IFN-γ-induced M1 polarization markedly raised the base line activity of NF-κB in both age groups. M2 polarization showed no significant effect on NF-κB activation as compared to M0 activation state.
Figure 6.
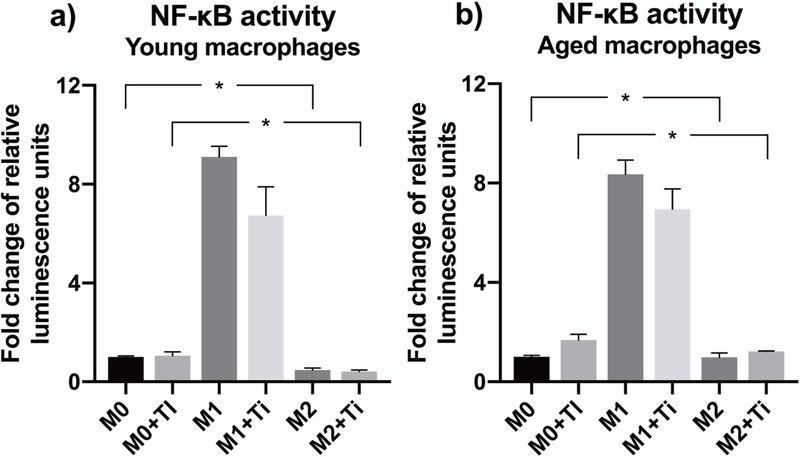
Using a lentiviral vector-based luciferase reporter system, the activity of transcription factor NF-κB was analyzed from (a) young and (b) aged particle-stimulated mBMMs of M0, M1, and M2 phenotype. The luciferase activity was measured 4 h after the initiation of particle challenge, and normalized to the amount of total protein. Data are presented as fold change compared to untreated M0 macrophages. * stands for statistically significant difference between indicated phenotypes.
Young human macrophages secrete more TNF but less IL-6
When repeated with human primary macrophages, Ti particle stimulation experiment resulted again in an increased secretion of TNF, IL-6, and IL-1Ra as studied by ELISA (Fig. 7a–c). Young cells released TNF to a slightly higher degree, whereas the opposite was found for IL-6. IL-1Ra was secreted in a similar manner from both young and older macrophages. Importantly, none of these differences reached statistical significance between the age groups. Secretion of mature IL-1β could not be detected in our ELISA assay from human macrophages either.
Figure 7.
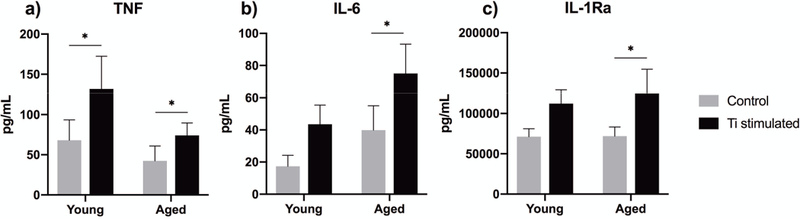
Human primary macrophages were isolated from young and older blood donors, and challenged with titanium particles for 8 h. The secretion of (a) TNF, (b) IL-6, and (c) IL-1Ra was determined from culture supernatants by ELISA. * indicates significant difference between particle-stimulated macrophages and untreated control cells. For each cytokine, the effect of aging was found statistically non-significant.
DISCUSSION
As macrophage response to implant wear particles largely dictates the development of aseptic loosening, factors modulating macrophage function will affect the progression of this condition. In recent years, aging has emerged as one factor that affects key macrophage characteristics.31 Previous studies investigating the interaction of macrophages and wear particles have not taken into account these potential aging-induced changes in macrophage function. Therefore, we decided to study how aging alters the inflammatory response of bone marrow macrophages to titanium particles. Given that macrophage polarization is an important determinant of cell responsiveness, we included M0, M1, and M2 phenotypes in the current investigation.32,33
We have previously thoroughly characterized the polarizing effects of IFN-γ and IL-4 in this specific macrophage cell culture model.13,34 In the current study, increased expressions of iNOS and CXCL9 for M1 macrophages and MRC1 for M2s demonstrated successful macrophage polarization. Interestingly, these M1 and M2 polarization markers were elevated significantly higher among aged macrophages, suggesting that these cells have maintained their plasticity and retain their ability to assume different functional phenotypes. In fact, two recent studies reached a similar conclusion.35,36 Markedly increased basal expressions of TNF, IL-1β, and TLR4, and activity of NF-κB in M1 macrophages further validate a more reactive phenotype. Conversely, M2 polarization mitigated the expression of these pro-inflammatory cytokines. There were no consistent differences in the basal expression of inflammatory markers between the young and aged cells with some of the cytokines (IL-1β, IL-6, IL-1Ra) being more highly expressed among young macrophages and other mediators (TNF, CCL2, iNOS, CXCL9) increased in their aged counterparts. While levels of NF-κB activation remained similar in both age groups, these findings suggest no clear difference in the underlying inflammatory status between the young and senescent cells.
As found in preliminary experiments, Ti particle stimulation resulted in an increased production of various inflammatory mediators from macrophages. In contrast to what was expected, the pro-inflammatory response to particles seemed somewhat heightened in young macrophages. These cells enhanced the production of classical inflammatory cytokines TNF, IL-1β, and IL-6 as compared to aged particle-challenged macrophages. For TNF, age-related differences were relatively small and became evident only later on during the experiment. Likewise, an increased IL-6 secretion from young macrophages was distinguishable at later time points of the study. In that regard, decreased TNF and IL-6 production from senescent macrophages does not support our hypothesis of their prolonged inflammatory response to wear particles. Considering IL-6 production in M2 macrophages, a strong negative feedback mechanism was observed after particle stimulation. At 48 hours, particles actually suppressed IL-6 secretion when compared to untreated control cells. This trend could also be due to the proposed ability of IL-4 to stimulate IL-6 secretion from macrophages.37,38
Despite some notable differences in IL-1β expression between the age groups, these changes occurred only at the level of mRNA production. We detected no secretion of this highly pro-inflammatory cytokine probably because endotoxin-free Ti particles failed to activate the inflammasome—a large intracellular protein complex regulating the release of mature IL-1β.39 As inflammasome activation is mediated by a two-checkpoint mechanism40, it appears that sterile particles alone were not sufficient to provide both of these activating signals. This finding is supported by the lack of particle-induced NF-κB activation, which is considered necessary for inflammasome activation.41 Had the NF-κB activation peaked at another time point in our experiment, particle-induced IL-1β secretion should still have been observed from the culture supernatants over a prolonged 48-hour incubation period. As Ti particles can activate a pre-primed inflammasome42, we believe that an NF-κB activating co-stimulatory signal—such as bacterial lipopolysaccharide (LPS)—would have been needed in order to license IL-1β secretion. However, we still observed that wear particles led to an elevated production of TNF and IL-6, whose secretion is regulated by activation of pathways including NF-κB.43,44 Consequently, these results suggest that endotoxin-free Ti particles trigger downstream signaling of other inflammation-related pathways instead.
Even though the qPCR data demonstrated a delayed and shortened anti-inflammatory response in particle-stimulated aged macrophages, these cells secreted an increased amount of IL-1Ra throughout the experiment. This outcome further diminishes the pro-inflammatory particle responses of aged macrophages and opposes our hypothesis of their dysregulated anti-inflammatory feedback. In lieu of pro-inflammatory cytokines, aged macrophages were found to intensify the production of iNOS and chemokine CCL2—factors that share increased inflammatory properties as well. Whereas chemokines are important in recruiting more monocyte/macrophage lineage cells to the site of inflammation, iNOS drives deleterious tissue reactions with elevated levels of reactive nitrogen and oxygen intermediates.45 These results are in line with previous studies demonstrating both chemokine secretion and oxidative stress enhanced in aged macrophages.14–16,36,46 Two of these studies in particular focused on age-related differences in macrophage response to implant materials and arrived at similar conclusions suggesting a more pro-inflammatory M1-like profile in aged macrophages.16,36
As compared to M0 macrophages, we observed that M1 polarization intensified and M2s reduced the particle-induced expression of several inflammatory mediators. Of note, M2 polarization effectively suppressed the inflammatory response in aged macrophages as well. This finding suggests the potential of M2 phenotype to protect against particle-related adverse tissue reactions also in the elderly. Although senescent macrophages seem to preserve expression of M2 markers in vitro, aged mice demonstrated a deficient M2 activation in response to implant material in vivo.36 With this in mind, an M2 restorative immunomodulation might be particularly effective in alleviating wear particle responses in aged individuals.
While we observed macrophages derived from young mice to have higher levels of pro-inflammatory cytokines in response to Ti particles, their aged counterparts magnified the synthesis of other inflammatory mediators. Given the multiple time points and markers studied, differences between the age groups remained relatively small and of somewhat uncertain biological significance. When repeated with human primary macrophages from young and older donors, particle stimulation experiment revealed no significant differences in their cytokine secretion profiles either. Based on these results, it seems that aging has no substantial contribution in exacerbating the macrophage-mediated inflammatory reaction to titanium particles. Rather than altering the innate immune response, advanced age could affect TJR loosening also by impairing normal bone formation.26,47,48 Thus, age effects on bone regeneration—already compromised by accumulating implant wear—might be of more importance in determining the progression of peri-prosthetic bone resorption among the elderly. In addition, the particle load from articulating prosthesis surfaces inevitably increases over time thereby prolonging and escalating the inflammatory exposure in peri-implant tissues. Hence, an amplified wear rate appears a self-explanatory causation to age-related implant failures.
Our results contrast with some of the previous studies characterizing the inflammatory response of aged macrophages. The reasons for this phenomenon remains speculative. However, the origin of macrophages used in these studies seems to vary greatly. Whereas peritoneal, alveolar, or splenic macrophages have all been included in previous age-associated studies15,16,49, we believe that the use of bone marrow macrophages is more relevant when approaching the pathogenesis of aseptic loosening. Besides the host species and tissue of origin, the nature of macrophage stimulant can also explain the differences between other related publications.50 We acknowledge small sample sizes, only one type of particle material, and a limited number of inflammatory markers included as shortcomings in our study. In addition, results obtained from human macrophages may have been affected not only by age-related factors, but also by other patient specific characteristics. Larger sample pools with appropriate multivariate analyses would shed further light on the subject.
The current investigation does not include other cell types and complex tissue signals that could potentially modulate macrophage function. The low-grade subclinical pro-inflammatory status found in the elderly likely exerts additional influence on the inflammatory responses of macrophages. Indeed, several studies suggest that aged macrophages produce higher cytokine levels in vivo yet reduced secretion in vitro.24,51 This paradox reflects our results and highlights the role of the aging microenvironment over cell-intrinsic defects in dictating the host response to implant material. Therefore, more studies for age-associated changes in wear particle responses are warranted in vivo. Our work also encourages future studies for clarification of alternative particle-activated intracellular pathways, and detection of possible inflammasome priming signals present at the aseptic interface.
Within the limitations of an in vitro model, our results suggest that aging of the innate immune system per se plays no considerable role in the response of macrophages to titanium particles. Nevertheless, other age-related factors such as compromised bone regeneration, increased load of wear particles, and native environmental signals may still contribute to TJR failures. Importantly, induction of M2 polarization appears a promising strategy to limit particle-induced inflammation and subsequent peri-implant bone loss regardless of age.
Supplementary Material
Bone marrow macrophages from 2-month-old mice were challenged with increasing concentrations of titanium particles for 6 hours. The dose-dependent mRNA expressions of cytokines (a) TNF, (b) IL-1β, (c) IL-6, and (d) IL-1Ra were assessed by qRT-PCR. (e) Viability of macrophages was measured from the culture supernatants after particle challenge using a lactate dehydrogenase (LDH) detection kit (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to manufacturer’s protocol. As a compromise between particle-induced inflammatory response and cytotoxicity, the particle concentration of 1.2 mg/mL (V3) was selected for further experiments.
ACKNOWLEDGEMENTS
This work was supported by grants from the Finnish Cultural Foundation, ORTON Orthopaedic Hospital of the ORTON Foundation, Jane and Aatos Erkko Foundation, Emil Aaltonen Foundation, Otto A. Malm Foundation, Alfred Kordelin Foundation, Finnish Medical Foundation, Finnish Research Foundation for Orthopaedics and Traumatology, and NIH grants 2R01 AR055650 and 1R01 AR063717, and the Ellenburg Chair in Surgery in Stanford.
REFERENCES
- 1.Ulrich SD, Seyler TM, Bennett D, et al. 2008. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 32(5):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo J, Goodman SB, Konttinen YT, Raska M. 2013. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 19(2):213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landgraeber S, Jager M, Jacobs JJ, Hallab NJ. 2014. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 185150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Amer Y, Darwech I, Clohisy JC. 2007. Aseptic loosening of total joint replacements: mechanisms underlying osteolysis and potential therapies. Arthritis Res Ther. 9(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nich C, Takakubo Y, Pajarinen J, et al. 2013. Macrophages – Key Cells in the Response to Wear Debris from Joint Replacements. J Biomed Mater Res A. 101(10):3033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahon OR, O’Hanlon S, Cunningham CC, et al. 2018. Orthopaedic implant materials drive M1 macrophage polarization in a spleen tyrosine kinase- and mitogen-activated protein kinase-dependent manner. Acta Biomater. 65:426–35. [DOI] [PubMed] [Google Scholar]
- 7.Rao AJ, Nich C, Dhulipala LS, et al. 2013. Local effect of IL-4 delivery on polyethylene particle induced osteolysis in the murine calvarium. J Biomed Mater Res A. 101(7):1926–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato T, Pajarinen J, Behn A, et al. 2016. The effect of local IL-4 delivery or CCL2 blockade on implant fixation and bone structural properties in a mouse model of wear particle induced osteolysis. J Biomed Mater Res A. 104(9):2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin T, Kohno Y, Huang JF, et al. 2018. NFkappaB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles. J Biomed Mater Res A. 106(10):2744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunston CR, Griffiths HR. 2010. The effect of ageing on macrophage Toll-like receptor-mediated responses in the fight against pathogens. Clin Exp Immunol. 161(3):407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linehan E, Fitzgerald DC. 2015. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol (Bp). 5(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stranks AJ, Hansen AL, Panse I, et al. 2015. Autophagy Controls Acquisition of Aging Features in Macrophages. J Innate Immun. 7(4):375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibon E, Loi F, Cordova LA, et al. 2016. Aging Affects Bone Marrow Macrophage Polarization: Relevance to Bone Healing. Regen Eng Transl Med. 2(2):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebastian C, Herrero C, Serra M, et al. 2009. Telomere shortening and oxidative stress in aged macrophages results in impaired STAT5a phosphorylation. J Immunol. 183(4):2356–64. [DOI] [PubMed] [Google Scholar]
- 15.Smallwood HS, Lopez-Ferrer D, Squier TC. 2011. Aging enhances the production of reactive oxygen species and bactericidal activity in peritoneal macrophages by upregulating classical activation pathways. Biochemistry. 50(45):9911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno ME, Sittner M, Cabrini RL, et al. 2015. In vitro age dependent response of macrophages to micro and nano titanium dioxide particles. J Biomed Mater Res A. 103(2):471–8. [DOI] [PubMed] [Google Scholar]
- 17.Barrett JP, Costello DA, O’Sullivan J, et al. 2015. Bone marrow-derived macrophages from aged rats are more responsive to inflammatory stimuli. J Neuroinflammation. 12:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albright JM, Dunn RC, Shults JA, et al. 2016. Advanced Age Alters Monocyte and Macrophage Responses. Antioxid Redox Signal. 25(15):805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschi C, Bonafe M, Valensin S, et al. 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 908:244–54. [DOI] [PubMed] [Google Scholar]
- 20.Chung HY, Lee EK, Choi YJ, et al. 2011. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 90(7):830–40. [DOI] [PubMed] [Google Scholar]
- 21.Oishi Y, Manabe I. 2016. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech Dis. 2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly J, Ali Khan A, Yin J, et al. 2007. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 117(11):3421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahbub S, Deburghgraeve CR, Kovacs EJ. 2012. Advanced age impairs macrophage polarization. J Interferon Cytokine Res. 32(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DC, Ruiz CR, Lebson L, et al. 2013. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol Aging. 34(6):1610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimitrijevic M, Stanojevic S, Blagojevic V, et al. 2016. Aging affects the responsiveness of rat peritoneal macrophages to GM-CSF and IL-4. Biogerontology. 17(2):359–71. [DOI] [PubMed] [Google Scholar]
- 26.Lin TH, Gibon E, Loi F, et al. 2017. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. J Orthop Res. 35(2):281–8. [DOI] [PubMed] [Google Scholar]
- 27.Ragab AA, Van De Motter R, Lavish SA, et al. 1999. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res. 17(6):803–9. [DOI] [PubMed] [Google Scholar]
- 28.Grosse S, Haugland HK, Lilleng P, et al. 2015. Wear particles and ions from cemented and uncemented titanium-based hip prostheses—A histological and chemical analysis of retrieval material. J Biomed Mater Res B Appl Biomater. 103(3):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajarinen J, Lin TH, Sato T, et al. 2015. Establishment of Green Fluorescent Protein and Firefly Luciferase Expressing Mouse Primary Macrophages for In Vivo Bioluminescence Imaging. PLoS One. 10(11):e0142736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurmi K, Kareinen I, Virkanen J, et al. 2017. Hemin and Cobalt Protoporphyrin Inhibit NLRP3 Inflammasome Activation by Enhancing Autophagy: A Novel Mechanism of Inflammasome Regulation. J Innate Immun. 9(1):65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibon E, Lu L, Goodman SB. 2016. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonios JK, Yao Z, Li C, et al. 2013. Macrophage polarization in response to wear particles in vitro. Cell Mol Immunol. 10(6):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pajarinen J, Kouri VP, Jämsen E, et al. 2013. The response of macrophages to titanium particles is determined by macrophage polarization. Acta Biomater. 9(11):9229–40. [DOI] [PubMed] [Google Scholar]
- 34.Rao AJ, Gibon E, Ma T, et al. 2012. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 8(7):2815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linehan E, Dombrowski Y, Snoddy R, et al. 2014. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 13(4):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hachim D, Wang N, Lopresti ST, et al. 2017. Effects of aging upon the host response to implants. J Biomed Mater Res A. 105(5):1281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casella G, Garzetti L, Gatta AT, et al. 2016. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation. 13(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Jain A, Syed SN, et al. 2018. IL-6 augments IL-4-induced polarization of primary human macrophages through synergy of STAT3, STAT6 and BATF transcription factors. Oncoimmunology. 7(10):e1494110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo H, Callaway JB, Ting JP. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 21(7):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo EK, Kim JK, Shin DM, Sasakawa C. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 13(2):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauernfeind FG, Horvath G, Stutz A, et al. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 183(2):787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.St Pierre CA, Chan M, Iwakura Y, et al. 2010. Periprosthetic osteolysis: characterizing the innate immune response to titanium wear-particles. J Orthop Res. 28(11):1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakashima Y, Sun DH, Trindade MC, et al. 1999. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 81(5):603–15. [DOI] [PubMed] [Google Scholar]
- 44.Beidelschies MA, Huang H, McMullen MR, et al. 2008. Stimulation of macrophage TNFalpha production by orthopaedic wear particles requires activation of the ERK1/2/Egr-1 and NF-kappaB pathways but is independent of p38 and JNK. J Cell Physiol. 217(3):652–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadtman ER. 2006. Protein oxidation and aging. Free Radic Res. 40(12):1250–8. [DOI] [PubMed] [Google Scholar]
- 46.Swift ME, Burns AL, Gray KL, DiPietro LA. 2001. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 117(5):1027–35. [DOI] [PubMed] [Google Scholar]
- 47.Kaar SG, Ragab AA, Kaye SJ, et al. 2001. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res. 19(2):171–8. [DOI] [PubMed] [Google Scholar]
- 48.Manolagas SC, Parfitt AM. 2010. What old means to bone. Trends Endocrinol Metab. 21(6):369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renshaw M, Rockwell J, Engleman C, et al. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 169(9):4697–701. [DOI] [PubMed] [Google Scholar]
- 50.Kohut ML, Senchina DS, Madden KS, et al. 2004. Age effects on macrophage function vary by tissue site, nature of stimulant, and exercise behavior. Exp Gerontol. 39(9):1347–60. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs EJ, Boe DM, Boule LA, Curtis BJ. 2017. Inflammaging and the Lung. Clin Geriatr Med. 33(4):459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bone marrow macrophages from 2-month-old mice were challenged with increasing concentrations of titanium particles for 6 hours. The dose-dependent mRNA expressions of cytokines (a) TNF, (b) IL-1β, (c) IL-6, and (d) IL-1Ra were assessed by qRT-PCR. (e) Viability of macrophages was measured from the culture supernatants after particle challenge using a lactate dehydrogenase (LDH) detection kit (CytoTox 96 Non-Radioactive Cytotoxicity Assay, Promega, Madison, WI) according to manufacturer’s protocol. As a compromise between particle-induced inflammatory response and cytotoxicity, the particle concentration of 1.2 mg/mL (V3) was selected for further experiments.


