Abstract
Glucocorticoid receptors (GRs) are ubiquitous transcription factors widely studied for their role in controlling events related to inflammation, stress and homeostasis. Recently, GRs have reemerged as crucial targets of investigation in neurological disorders, with a focus on pharmacological strategies to direct complex mechanistic GR regulation and improve therapy. In the brain, GRs control functions necessary for neurovascular integrity, including responses to stress, neurological changes mediated by the hypothalamic-pituitary- adrenal axis and brain-specific responses to corticosteroids. Therefore, this review will examine GR regulation at the neurovascular interface in normal and pathological conditions, pharmacological GR modulation and glucocorticoid insensitivity in neurological disorders.
Introduction
The glucocorticoid receptor (GR) is a member of a class of ligand-binding nuclear receptors, which induce transcription of target genes in response to the secretion of endocrine hormones and steroids. Activity of GRs governs multiple pathways in the human body under normal and pathological conditions, affecting brain homeostasis. However, the role of expression and activation of GRs in neurological disorders has only recently regained attention owing to the increasing relevance of GR in pathological conditions. The interaction of activated GR with downstream proteins or response elements, activity influenced by other transcription factors and differential inherent activity can mediate the intracellular effects of the GR [1,2]. Although these interactions are necessary for normal function in the brain, GR isoforms and polymorphisms have recently been linked to the development of neurological disorders and disease, indicating a role for GR function in controlling aspects of disease pathogenesis [3–5]. Thus, the GR could prove a valuable and challenging clinical target for intervention in neurological disorders.
Mechanisms of action and modes of GR regulation
Classical GR interactions and regulatory mechanism
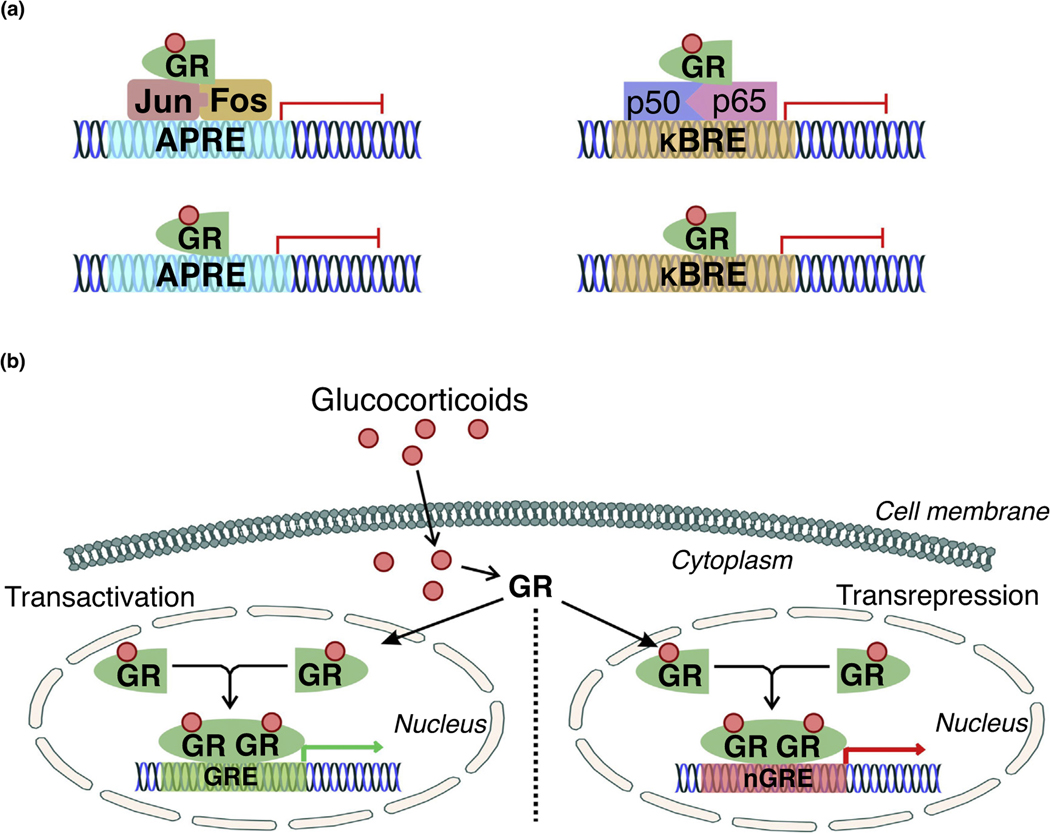
In general, the classical mechanism of GR-mediated transrepression (Fig. 1) follows a tethering model involving normal formation of either the activator protein 1 (AP-1) or nuclear factor (NF)-kB complexes, followed by the attachment of a GR monomer that prevents activity of the assembled transcription machinery. As in other tissues, this tethering is a primary mechanism of GR anti-inflammatory effects in the brain. Interestingly, mutations that prevent interactions between GR and NF-κB limit expression of genes downstream of the negative glucocorticoid response element (GRE), although this effect has not been demonstrated with AP-1 in the brain or other tissues [6]. Thus, dysfunction in nongenomic mechanisms of GR-mediated repression could be indicative of transcription-related alteration in genomic GR transrepression signaling. Further, this might indicate a role of the GR DNA-binding region in mediating nongenomic interactions in the repression of proinflammatory signaling in the brain and other tissues.
FIGURE 1.
Classical activity of glucocorticoid receptor (GR) in transactivation and transrepression. (a) Classical GR transrepression activity involves association with the activator protein-1 (AP-1, composed of the immediate early response genes Jun and Fos, which respond to cellular stimulation before protein synthesis) or nuclear factor (NF)-κB (composed of the proinflammatory p50 and p65 subunits) complexes. The GR binds to AP-1 or NF-κB to inhibit transcription by limiting their association with their respective binding regions in gene promoters (AP-1 response element, APRE; κB response element, κBRE). The GR can further bind directly to GR-binding elements in the APRE or κBRE to physically prevent the interactions of AP-1 and NF-κB with their respective response elements. (b) GR transactivation and transrepression is primarily mediated by glucocorticoids. Endogenous or exogenous glucocorticoids bind to the ligand-binding site of GR, initiating translocation to the nucleus. Dimerization between activated, ligand-bound GR monomers occurs in the nucleus, subsequently followed by binding of the dimer to glucocorticoid response elements (GREs) or negative glucocorticoid response elements (nGREs) to promote the transcription of GRE-associated genes.
Recent reports also indicate that the transrepression effect of the GR is not mediated by these protein–protein interactions alone, as several groups have found GR-binding sites contained in response elements for AP-1 and NF-κB [6,7]. GR monomers can compete with either NF-κB or AP-1 complexes for these sites, spatially limiting the ability of either transcription factor to induce their corresponding target genes (Fig. 1). Because GRs can repress activity through tethering and interaction with binding sites in the genome, there is plausibly a role for endogenous glucocorticoid stimulation in mediating GR downregulation of inflammatory signals in several brain regions. This further suggests abnormal regulation of such repression contributes to the neuropathological state.
In the brain, the GR also interfaces with brain-derived neurotropic factor (BDNF), which belongs to the family of neurotrophic growth factors controlling neurogenesis, neuronal survival and long-term potentiation. Modulation of the GR has been reported to selectively alter expression of BDNF in the brain, with increased GR activation by endogenous glucocorticoids during cognition or exercise corresponding to increased BDNF and decreased GR or stimulation by exogenous glucocorticoids corresponding to lowered levels of BDNF [8]. Such coordinated expression of the GR and BDNF has been shown to be preserved in certain human brain disorders and models of these conditions [8,9]. Knockout of the GR in a rat model of depression caused a corresponding reduction of BDNF expression [8]; conversely, overexpression of GR (characteristic of epilepsy) accompanied an increased level of BDNF in human subjects [9]. The GR has further been shown to down- regulate BDNF exon 4 transcript, which is overexpressed in epilepsy [9,10]. Further, GR activity has been reported to increase movement of vesicles containing BDNF, including those trafficking BDNF away from the cell membrane [9,11]. Thus, GR regulation of BDNF in the brain could have a neuroprotective effect in certain neurological disorders.
Regulation of GR activity
Negative feedback
Within the brain, negative feedback to GR stimulation by endogenous or exogenous glucocorticoids involves activation of two distinct steroid receptor types in the brain: the high-affinity mineralocorticoid receptors (MRs), which are stimulated by basal levels of circulating cortisol, and the low-affinity GRs, which remain predominantly unstimulated by cortisol until a threshold for circulation is met owing to stress or a pathophysiological condition, primarily serving to determine a ‘ceiling’ for cortisol secretion during stress or peaks in the circadian rhythm [12]. Stimulation of these receptors with cortisol results in a rapid drop in corticotrophin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) secretion, followed by a slow genomic response to decrease the expression of CRH in the paraventricular nucleus and the ACTH precursor proopiomelanocortin in the anterior pituitary [13]. Combined stimulation of the MR and GR determines the threshold for stress-influenced stimulation, and controls aspects of learning, cognition and mood [14,15]. Dysfunction between these two systems appears to functionally influence cognitive and neurological functions; however, persistent GR stimulation appears to determine the neurological state under stress and to primarily contribute to the development of neurological and brain disorders [15–17].
Ligand dependency and co-regulators
GR regulation is known to arise from ligand dependency, by which activation of the GR and its subsequent intracellular effects require initial binding of an activator such as cortisol or other glucocorticoids (Fig. 1). The GR possesses a basal affinity for ligands, which, in the presence of co-regulators activating or repressing GR function, can accordingly increase or decrease. Activated GR exists in a complex which is composed of co-factors, including heat-shock protein (Hsp) 90 and Hsp70, which work in a coordinated manner to regulate the activity of the GR [18]. Binding ofHsp70andHsp90, along with other co-chaperones, has been reported to be a requirement for conformational changes favoring high affinity of the GR for ligands as studied in human and hamster cell lines [2]. Although the GR is capable of binding glucocorticoids in complex with cofactors, this ability is impaired in their absence, increasing the effect of co-regulators on the ligand-binding ability of the GR [19]. Ligand-dependent GR activation is minimal in the absence of Hsp90, whereas binding of Hsp70 can directly inhibit ligand-stimulated GR activity although such regulation needs better understanding in disease pathologies.
Circadian rhythm
The circadian rhythm, or biological clock, has also been reported to influence GR activation through multiple methods, including activity of proteins that convert precursors into glucocorticoids that are critical for brain function. Steroidogenic acute regulatory protein, which is active in the adrenal cortex, responds to activity of the circadian-rhythm-associated proteins circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL-1) to modulate available cortisol in circulation [20]. Activity of the protein 11 beta-hydroxysteroid dehydrogenase type 1 (11β-HDS1), which locally performs the conversion of cortisone to cortisol, can additionally contribute to modulation in the amount of circulating cortisol, including that which is available in the brain for GR activation [21]. Further, GR expression in the brain follows a diurnal pattern, as observed in rats [21], contributing to circadian rhythm control of glucocorticoid activation. Studies also suggest that the action of the endogenous glucocorticoids cortisol and corticosterone is exerted via binding to MRs and GRs, which are co-expressed in brain [22]. There is evidence that MRs and GRs also interact in the control of the circadian rise in hypothalamic-pituitary-adrenal (HPA) axis [12]. Thus, the possible connection of these receptors to HPA axis activation, as well as the secretion of cortisol that is linked to circadian rhythm, is not unlikely [23,24]. It has also been reported that aldosterone circulates in a 10–100-fold lower concentration than cortisol or corticosterone. An assessment of immunoreactive steroid in purified cell nuclei of the rat hippocampus revealed a tenfold higher amount of corticosterone than aldosterone under basal conditions. The nuclear ratio of corticosterone over aldosterone further increases toward the circadian peak, when corticosterone starts to occupy GRs. During stress, the exposure of hippocampal MRs to corticosterone relative to aldosterone is even further increased toward 100 over 1 [25]. Accordingly, the brain MR is exposed to corticosterone, which binds with a tenfold higher affinity to the MR than to the GR [26,27]. These reports together suggest that these receptors are differentially activated by the glucocorticoids during the circadian-rhythmic period and after stress.
Evidence also suggests that circadian-rhythm-mediated down-regulation of GR expression can decrease long-term potentiation and impair long-term memory. Disruption of the circadian rhythm through knockdown of BMAL-1 has been shown to reduce the expression of BDNF and the GR in the hippocampus of transgenic mice [28]. This led to subsequent reductions in short-term aversion memory development and novel object recognition, although the authors noted that these functional changes were reversible by physical activity [28]. Notably, the circadian rhythm also influences the association between Hsp90 and the GR in different regions of the brain, because expression of and associations between both proteins cycle with time. Increased GR expression in the cytosol of hippocampal cells and translocation to the nucleus at night have been reported in rats with a simultaneous increase in GR–Hsp90 associations, which decrease in the morning [29]. Interestingly, this pattern is reversed in the hypothalamus, where GR–Hsp90 associations and GR nuclear translocation increase in the morning rather than at night [29]. This suggests that the sensitization of the GR to cortisol by Hsp90 directs GR activation by cortisol stimulation to different regions of the brain at different times based on the biological clock.
Genome access
Another reported mechanism of GR regulation occurs through restriction of GR access to response elements in the genome. Accessibility of open-state chromatin to the GR is mandatory for transcription of downstream genes and is mediated by proteins that control the winding of chromatin within the nucleus [30]. Thus, proteins that can control the physical interactions between the GR and glucocorticoid response elements in the genome probably serve as regulatory factors for the GR.
As an example, histone deacetylases (HDACs), which decrease gene expression, have been reported to be primary modulators of this association by directly controlling the ability of the GR and other nuclear receptors to bind to DNA and induce transcription. Indeed, GR downregulation, despite upregulation of the androgen hormone receptor at the same binding site in target genes of mouse cell lines, was reported to be reliant on HDAC1 and HDAC2 [31]. Interestingly, reports suggest that excessive ligand binding to the GR can trigger a functional deactivation of the GR at negative response elements through association with nuclear receptor corepressor 1 and HDAC3 [32]. This interaction between the GR, coregulators and HDACs has previously been shown in the rat brain, with pharmacological inhibition of HDAC6 increasing expression and acetylation of Hsp90 decreasing GR activation in rats vulnerable to stress [33]. Therefore, access of the GR to DNA and cellular changes that affect this association could be an understudied mechanism of cellular GR regulation in the human brain.
Epigenetic and transgenerational regulation
In addition to the above mechanisms of GR regulation, reports also indicate that GR expression in the brain can be influenced by paternal and maternal stress states, as well as by stress and parental connection experienced by individuals early in development. It has been reported that neonatal exposure to maternal grooming increases hippocampal expression of the GR and reduces the magnitude of stress responses in adult rats, by increasing the effect of negative feedback during stress [34,35]. Conversely, human and rat subjects exposed to stress early in development have been reported to express low hippocampal levels of GR and increased HPA responses, as adults, to stress [36,37]. Similarly, chronic stress experienced in adulthood has the potential to cause physiological changes in germ cells of the body, leading to alterations in HPA circuitry of progeny. Notably, these effects are often sex-specific. One group reported that male and female offspring of male mice exposed to chronic stress in adolescence or adulthood show increased GR expression in the paraventricular nucleus (PVN) but decreased cortisone secretion in response to stress, indicating tighter control of the HPA axis activity in the brain [38]. Conversely, it has been reported that maternal stress during gestation in rats causes increased corticosterone secretion and lower hypothalamic GR following acute stress [39].
Implication of GR isoforms
The relevance of GR isoforms and their distribution in the brain is widely unknown, particularly in neurological disorders. Expression of GR isoforms is dependent upon alternative splicing of the GR mRNA transcript, as identified in studies regarding physiological or pharmacological induction of GR beta (GRβ) and similar to studies performed previously in other tissues [40,41]. This understanding might be helpful in exploring the GR-related mechanistic alteration of neurological disease development and progression, as well as assisting in directing treatment approaches involving synthetic glucocorticoids.
GR-associated activity is primarily performed by the GR alpha (GRα) isoform, which binds glucocorticoids to mediate downstream activity (Fig. 1). Owing to the presence of the ligand-binding domain, GRα is translocated to the nucleus following administration of glucocorticoids, and directs neurovascular-specific changes in the brain as previously studied in mice [42]. Omission of the ligand-binding domain in the GR transcript results in production of GRβ, which binds the same molecular chaperones and contains the same sequence of amino acids as GRα [43]. As a result, GRβ retains the same DNA-binding ability and dimerization capability as GRα but is unable to initiate transcription in a similar manner. Further, expression of GRβ is either absent from many healthy cell lines and tissues or magnitudes lower than the expression of GRα [44].
Regulation of GRβ expression has been previously studied in various somatic cell lines, which have indicated GRβ function in negatively regulating the activity of GRα [45,46] and in reducing transactivation of the GRα isoform following stimulation by certain synthetic glucocorticoids [47]. However, some debate exists as to the role of GRβ within different tissues, including the brain. GRβ is normally expressed at low levels in the brain, particularly in comparison with the GRα isoform [44,45], and it has been reported that the presence of GRβ alone does not significantly decrease GRα activity [48,49]. Nevertheless, higher expression of GRβ is present in the nucleus, and the GRβ isoform is capable of dimerization with GRα, supporting the notion of its role in negative regulation of GRα activity [45,50]. Therefore, further study of the activity of GRβ in the neurovasculature would be valuable in understanding its neurological regulation during disease progression.
Role of the GR in neurovascular physiology and function
The brain possesses a unique vasculature that restricts exchanges of nutrients and xenobiotics through paracellular and transcellular pathways between the brain parenchyma and the blood, referred to as the blood–brain barrier (BBB). The BBB is considered an active, dynamic and selective interface between the blood and brain, and is made up of endothelial cells, astrocytes and pericytes, and a few microns away from neuronal termini. Further, the BBB, because of its selectivity properties, is one of the major barriers to the successful development of active CNS compounds. This physiological barrier is formed by endothelial cells covering the inner lining of the brain microvessels and expressing tight-junction proteins. Astrocytes are in close contact with these brain endothelial cells and assist in maintenance of the barrier properties and in communication of signals related to physiological changes [51]. These brain vascular cells, along with the neurons, form the neurovascular unit. Compromised BBB function is often a hallmark of neurological disorders that can contribute to and be caused by disease states in the brain, including those with a pathogenesis related to the GR.
GR activity contributes to the formation and maintenance of the BBB (Fig. 2) by upregulating tight-junction proteins in endothelial cells of the brain vasculature [52]. As a result, pharmacological compounds are often used to exploit the effect of GR activation or inhibition on cells of the neurovascular unit, as summarized in Table 1. For example, steroid-induced GR activation increases the expression of angiopoetin-1, which prevents edema by stabilizing tight-junction formation in human brain endothelial cells [53]. In addition, studies have shown that inhibition of proteasomes that degrade the GR restores the ability of a GR ligand: dexamethasone, to improve tight-junction formation between cerebral endothelial cells in ischemia [54]. However, although it is known that the GR maintains vital components of the BBB, reports also suggest that the GR reduces the expression of vascular endothelial growth factor (VEGF), which induces vascular remodeling in the brain [53], as well as suppressing endothelial cell proliferation and enhancing pericyte coverage of microvessels in the brain [55]. As a result, promotion of vasodilation by the GR involves pathways exclusive of VEGF, which has been shown to cause edema in brain tissue [55,56]. It has also been reported that GR activation owing to signaling of neuronal activity instead induces vasodilation by the activation of endothelial nitric oxide synthase or by inhibition of endothelin receptors in human brain vasculature [57,58].
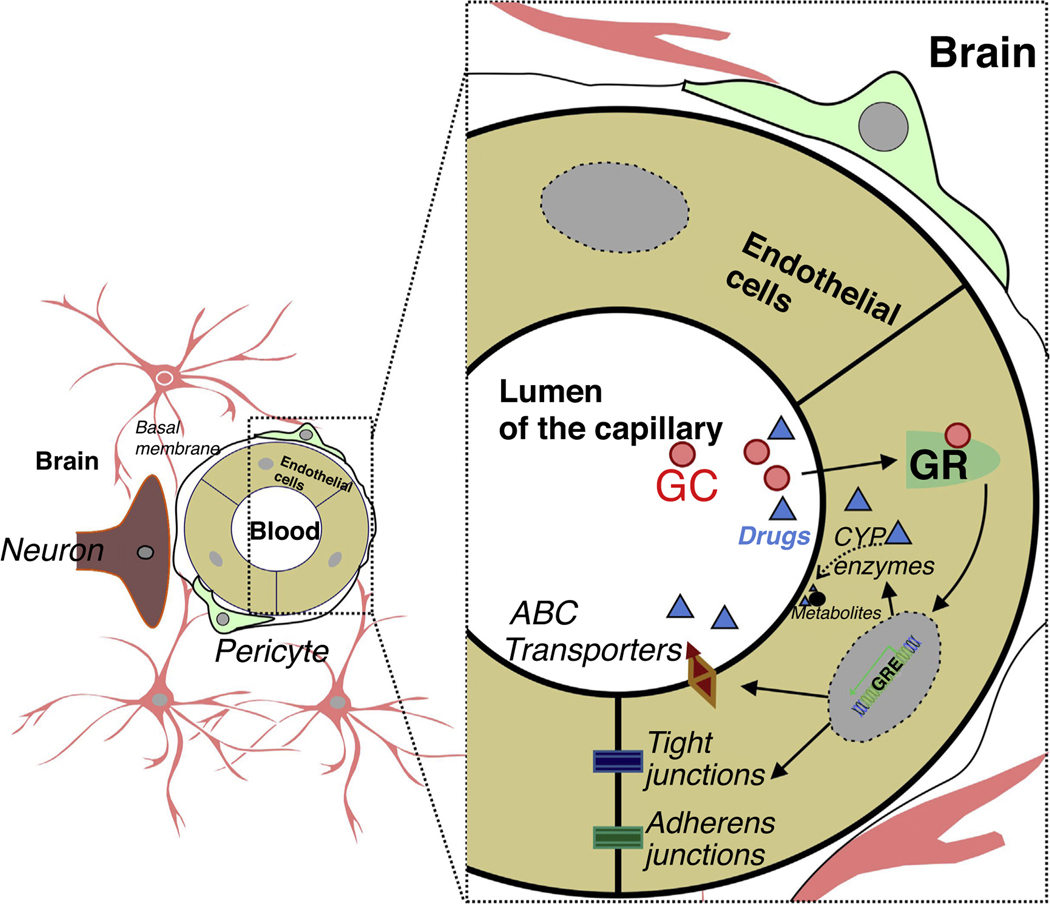
FIGURE 2.
Endothelium-specific glucocorticoid receptor (GR) function at the neurovascular unit. Glucocorticoids (GCs), or any drug as a GR ligand in circulation, diffuse across the cell membrane, bind to the GR in the cytoplasm and initiate translocation to the nucleus. Subsequent to GR modulation, the downstream events include regulation of tight-junction proteins (claudins, occluding, etc.) and adherens junction protein. Endothelial cytochrome P450 (P450 s) drug-metabolizing enzymes and ATP-binding cassette transporters/efflux drug transporters (ABC transporters), translated downstream of ligand-dependent GR activation, metabolize and/or remove xenobiotics at the blood–brain barrier, which is enhanced in pathological conditions.
TABLE 1.
Effects of selective GR agonists, modulators and inhibitors on brain cells
| Cell type | Influence of agonist (dexamethasone) |
Influence of agonist (methyl-prednisolone) |
Influence of modulator (ginsenoside Rg1) |
Influence of modulator (icariin) |
Influence of antagonist (mifepristone) |
Refs |
|---|---|---|---|---|---|---|
| Glutamatergic neurons | ↓ Glutamate release | ↑ Glutamate signaling | ↓ NLRP inflammasome induced neuronal damage | ↓ Depolarization | [63,65,157,169,170] | |
| ↓ Purkinje cell apoptosis | ||||||
| GABAergic neurons | ↑ GABA release | ↓ GABA signaling | ↓ NLRP inflammasome induced neuronal damage | ↓ GABA response | [64,65,169,171] | |
| ↑ Sensitizes GABA receptors | ↓ Purkinje cell apoptosis | |||||
| Dopaminergic neurons | ↓ Inflammation-induced neuronal degeneration | ↓ LPS-induced neurotoxicity and cell death | [172,173] | |||
| Astrocytes | ↓ Proliferation | ↓ Activation | ↑ Association with vasculature in the ischemic brain | ↑ Proliferation | [69,174,175] | |
| ↓ GR expression | ↓ GFAP and CSPG in activated astrocytes | ↑ GR expression (to normal level) | ||||
| Oligodendrocytes | ↑ Progenitor cell apoptosis | ↓ AMPA-induced cell death | [67,176] | |||
| ↑ MHC II molecules | ||||||
| Microglia | ↓ Microglial activation | ↓ Hypothermic cytotoxicity | ↓ LPS-induced microglial activation | ↓ LPS-induced microglial activation | ↓ Progesterone suppression of immune response involving microglia | [68,69,160,173,174,177,178] |
| ↓ MHC expression | ↓ Interleukin-6 secretion | ↓ LPS-induced prostaglandin secretion | ||||
| Endothelial cells | ↑ Tight-junction proteins | ↓ Adhesion molecule expression | ↑ Vascular endothelial growth factor | ↓ P-glycoprotein expression | [42,51,59,127,146,147] | |
| ↑ P-glycoprotein expression | ↓ Cytochrome P450 enzymes expression | |||||
| Pericytes | ↑ Vascular coverage in neonates | ↓ Glucocorticoid-induced apoptosis | [179] | |||
| ↑ Markers of apoptosis | ||||||
Abbreviations: NLRP nucleotide-binding oligomerization domain-like receptor protein; GABA gamma-aminobutyric acid; LPS lipopolysaccharide; GR glucocorticoid receptor; GFAP glial fibrillary acidic protein; CSPG chondroitin sulfate proteoglycans; MHC major histocompatibility complex; AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid.
In addition to the maintenance of physical BBB properties, the GR assists in the establishment of characteristics of the metabolic barrier to xenobiotics within the brain microvasculature. GR modulation in the brain endothelial cells in epilepsy has been recently reported to modulate drug biotransformation and drug penetration across the BBB (Fig. 3) [59,60]. Expression of cytochrome P450 (CYP) drug-metabolizing enzymes and multidrug efflux transporters is controlled by upstream molecular regulators, including the GR, in human drug-resistant epilepsy [61]. It has also been reported that GR is responsible for regulating the effect of the HPA axis [62] and penetration of synthetic glucocorticoids to the brain parenchyma [63] (Figs.1 and 2) modulating downstream proteins and molecules responsible for altered brain physiology and metabolic properties.
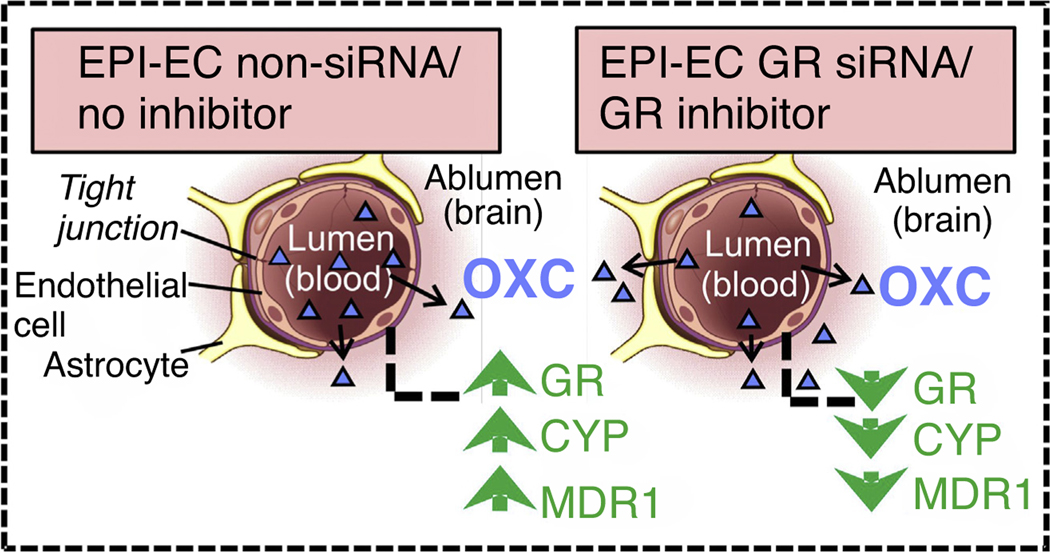
FIGURE 3.
Schematic representation of improved oxcarbazepine (OXC) penetration across the blood–brain barrier (BBB). The nonsilenced or noninhibited epileptic brain endothelial cells (EPI-EC) demonstrated increased drug metabolic potency at the BBB. Conversely, silencing the GR in EPI-ECs resulted in a reduction of OXC metabolism compared with nonsilenced conditions in EPI-ECs. In this manner, the penetration of OXC across the BBB EPI-ECs was improved upon GR silencing or inhibition [59].
Interestingly, the effects of GRs appear to vary widely among brain-cell types and are further based upon the degree of excitatory and inhibitory activity in the brain post-glucocorticoid stimulation (Table 1). GRs likewise govern the characteristics and function of secretory neurons at the neurovascular interface, because GR stimulation by dexamethasone in the amygdala has been reported to increase the secretion of GABA and the sensitivity of GABA receptors, while downregulating secretion of glutamate [64] creating a neurotransmitter imbalance. Inhibition of GR activity in the same neurons has a neuroprotective effect on neurons of the amygdala by reducing GABA activity, which has the potential to damage these cells but has also been reported to increase anxiety-related behavior and cause overall HPA axis hyperactivation in a rodent model [65,66].
The glial cells are similarly modulated by GR stimulation, which can dictate the health or disease state [67–70]. Evidence indicates that the anti-inflammatory effects of steroids are likewise governed by GR activity and rely on glial cell responses. Under normal conditions, oligodendrocytes express major histocompatibility complex (MHC) molecules I and II; this expression is reported to increase in the presence of dexamethasone and interferon (IFN)γ [71]. Further, exposure of oligodendrocytes to dexamethasone in the rat brain has been found to cause hypomyelination [67]. However, it is yet unknown whether basal levels of circulating cortisol oppose oligodendrocyte generation of myelin. Dexamethasone stimulation in microglia has likewise been reported to decrease expression of MHC molecules and inflammatory cytokines, including IFNγ, and to restore astrocytic function compromised by lipopolysaccharide exposure [68,69]. However, despite the astrocyte activation following glucocorticoid stimulation, negative feedback to glucocorticoids reduces GR expression and long-term astrocyte proliferation [70]. Thus, glucocorticoids mediate complex interactions occurring in these cell types through either GR stimulation or inhibition.
GR in neurological diseases
The GR controls a variety of functions in the brain related to memory, aging and behavior, summarized in part in Fig. 4. Primary GR regulation governs mood and memory formation [28,72–75], the HPA axis and negative feedback to glucocorticoid [65,76,77] and apoptosis [78,79]. Further, the GR has been reported to govern suppression of amygdala function in response to glucocorticoid [80] and the response cascade to serotonin in the hippocampus [81]. Therefore, GR dysregulation or hyperactivity can have a notable influence on HPA dysfunction [65,82–84] and contribute to mood disorders [3,76,85,86] or abnormal responses to chemical or somatic stimulation [83,87,88]. For the above reasons, we reviewed in-depth the effect of GR in common neurological diseases and its comorbidities.
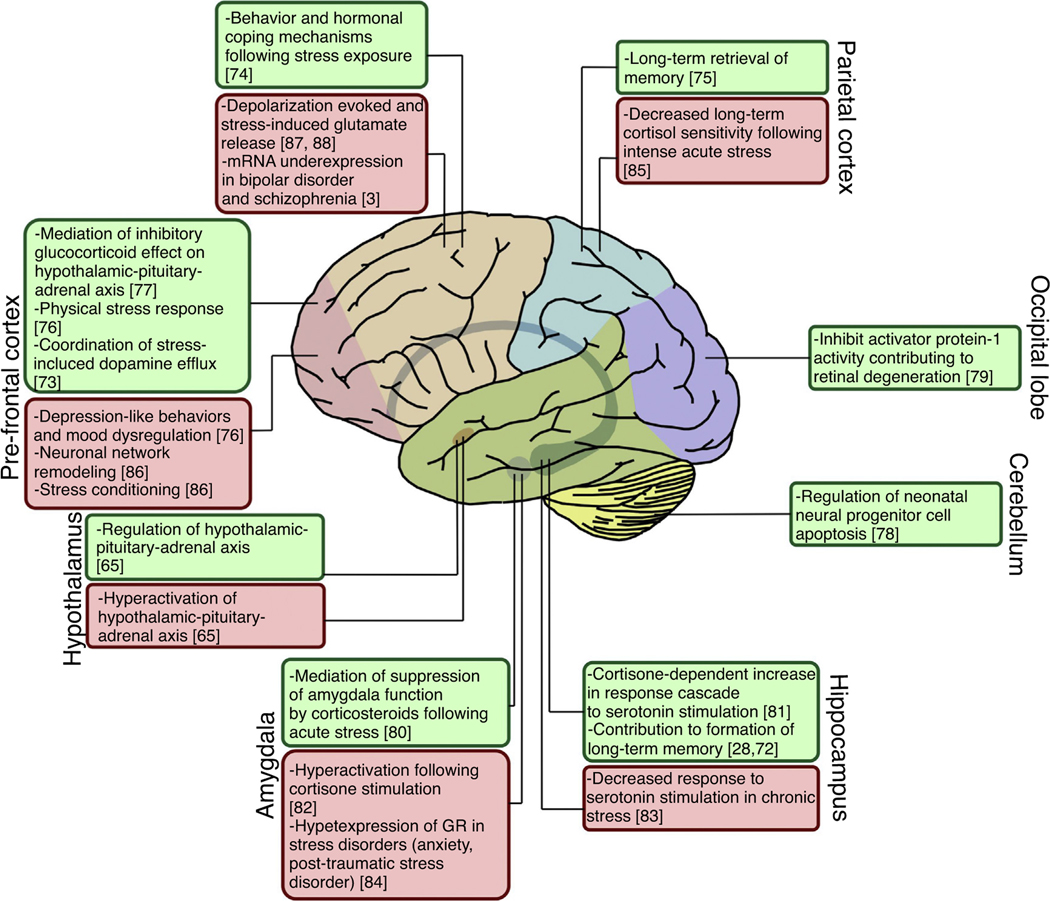
FIGURE 4.
Regional glucocorticoid receptor (GR) dysregulation in brain regions. Dysregulation or abnormal expression of the GR, as well as associated hypothalamic-pituitary-adrenal axis abnormalities, can cause disease pathogenesis in the brain. However, certain diseases are associated with GR dysregulation localized to specific regions of the brain. Green boxes indicate the role of the GR in normal conditions. Red boxes indicate abnormal GR expression, activity or response to pathophysiological conditions.
Epilepsy
In epilepsy, scattered reports are available indicating the role of glucocorticoids in contributing to the pathogenesis of epilepsy through stimulation of the GR. Bao et al. identified decreased expression of GR mRNA in the hippocampus in an amygdala-kindling epileptogenic rat model [89]. However, Kumar et al. determined that chronic stimulation of the glucocorticoid and mineralocorticoid receptors (with high and low affinity for cortisol, respectively) is involved in the acceleration of epileptogenesis in the same rodent model [90], and high levels of GR expression have been reported in human subjects with temporal lobe epilepsy [9,60]. Pharmacological inhibition of GR activity has also been shown to reduce markers of hippocampal pathology, such as hilar cell proliferation and mossy cell loss in a pilocarpine rodent model of seizure and epilepsy [91].
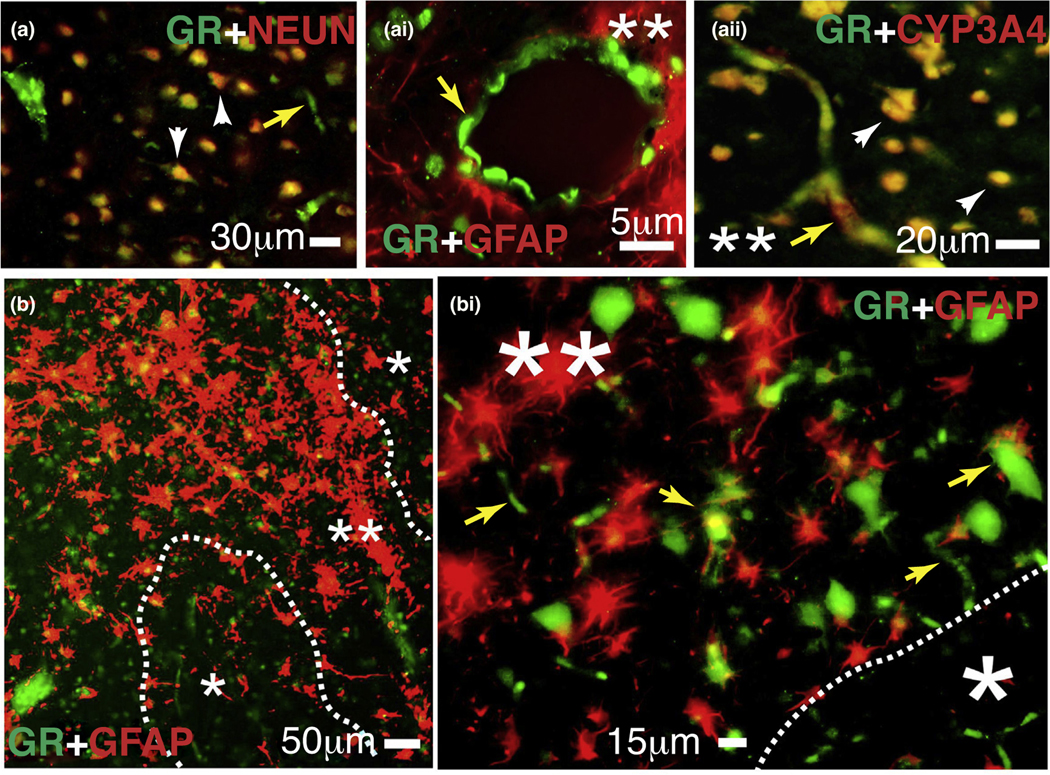
In epilepsy, evidence indicates that GR overexpression can contribute to alterations to the normal physiology of the brain and vasculature, contributing to seizures in humans [9,60,92]. Abnormally increased expression of the GR has been previously identified in human temporal lobe epilepsy [9,92]. The GR and pregnane-x receptor (PXR) regulatory mechanism is intertwined, as reports found for human hepatocytes suggest [93,94]. This notion is similar to recent findings obtained using human epileptic brain endothelial cells isolated from temporal lobe epilepsy (EPI-ECs) [60] and the discovery that PXR and GR are overexpressed in regions of the epileptic brain with reactive gliosis (Fig. 5) [60]. In addition, studies have shown that co-treatment of PXR ligands with dexamethasone resulted in enhanced basal- and ligand-dependent CYP3A4 promoter activity [95], which is classically regulated by GR expression. This induction was attenuated by treatment with a GR antagonist and by introduction of GR siRNA [95].
FIGURE 5.
Expression of the glucocorticoid receptor (GR) and cytochrome P450 (CYP)3A4 in human epileptic brains. (a–bi) The GR is found at the blood–brain barrier interface and in neurons in epileptic brains. Magnification of gliotic regions (**) and GFAP + staining on a resected human epileptic brain showed increased expression of GR and CYP3A4. This staining pattern was less evident in nongliotic regions (*). Note: the indicators for neurons are depicted as arrowheads and those for capillaries as yellow arrows. Colocalization was evaluated with neuronal markers (NEUN) and glial markers (GFAP). (a-ai) GR expression was observed at the vascular interface (a–ai, yellow arrows) and in neurons (a and aii). (aii) Colocalization of GR staining with CYP3A4 was found in regions with reactive gliosis (**). (b–bi) Representative images of brain samples from patients with temporal lobe epilepsy show GR staining in regions with reactive gliosis (**).
Additionally, treatment of hepatocytes with ketoconazole and miconazole, known antagonists of the GR, downregulated the expression of PXR and PXR-target genes [96]. In addition, studies in the hepatocytes have shown that activated GR is involved synergistically in the xenobiotic-responsive regulation of PXR-target genes, including CYPs [93,94,97]. Indeed, Ghosh et al. described elevated GR identified at the BBB in human temporal lobe epilepsy and a simultaneous increase in CYP enzymes CYP3A4 and CYP2C9 at the blood–brain interface [60]. Modulation of GR, either by pharmacological means or by GR silencing, improved penetration of antiepileptic drugs, such as phenytoin or oxcarbazepine, across the human BBB, while decreasing expression of PXR and CYP enzymes and drug efflux transporters [59]. Therefore, the GR at the brain endothelium appears to be an important regulator of the drug biotransformation machinery in human drug-resistant epilepsy.
Cancer
Activation of the GR has previously been linked to the development of malignant gliomas and to changes in the vasculature associated with tumor formation in the brain. Notably, GR expression in glioma is highest in the periphery of the tumor as opposed to the central portion and surrounding tissue [98], suggesting that sensitivity to the effects of endogenous or synthetic glucocorticoids is highest at the boundary of human gliomas. Stimulation of GR in glioma has been previously shown to reduce cell dispersion and proliferation [99] as well as to reduce edema associated with vascular leakage from angiogenesis in tumor formation [100]. Thus, treatment with dexamethasone or another glucocorticoid for tumor-associated edema in the brain has become standard in clinical practice (Table 2). However, recent reports indicate that stimulation in rat glioma cells increases the ratio of β2 to β1 adrenergic receptor expression, which contributes to cell proliferation in the tumor state [101]. This divergence could potentially be explained by differential expression of GR isoforms, which might be independently responsible for tumorigenesis and inhibition. GRβ, normally upregulated in response to injury, has been shown to associate with β-catenin or T-cell factor 4 and promote glial cell activation and proliferation, as well as the formation of gliomas [5,102]. Further, GRβ appears to competitively oppose the action of GRα in regulating β-catenin/T-cell factor/lymphoid-enhancer factor-driven pathogenesis in glioma. Thus, GRβ could be a potential target of study or modulation to treat the disease state. By contrast, the activity of GRα can oppose other GRβ-driven mechanisms of pathogenesis in glioma, through suppression of tumor formation and stabilization of the BBB. GRα activation suppresses activity of the oncogene-activated histone demethylase JMJD3, decreasing matrix-met alloprotease-induced tumor metastasis [103,104]. The GR further mediates vessel integrity in the disease state by inhibiting the expression of vascular permeability factor, thus preventing vascular remodeling and edema associated with tumor development in a rat model of glioma [105].
TABLE 2.
Glucocorticoid-receptor-modifying drugs in brain disorders
| Drug | Neurological effects | Refs | |
|---|---|---|---|
| Steroidal agonist | Dexamethasone | • Prevents tissue edema in meningitis cases | [100,137–140,142, 171,180,192] |
| • Reduces peritumoral edema in glioma and meningioma cases | |||
| • Inhibits apoptotic protein expression, inflammation and blood–brain barrier breakdown in eosinophilic meningitis | |||
| • Reduces meningitis-associated mortality and cerebrospinal fluid pressure | |||
| • Reduces risk of cerebral swelling during subdural grid electroencephalography | |||
| • Treats epileptic encephalopathy with continuous spike-and-wave during sleep | |||
| Methylprednisolone | • Acute treatment for relapses of multiple sclerosis (MS) | [145–149,181,182] | |
| • Reduces edema in brain tumors and surgical procedures | |||
| • Treats children with epileptic encephalopathy without underlying lesions | |||
| Hydrocortisone | • Reduces blood–brain barrier breakdown in inflammation | [183,184] | |
| • Reduces levels of serotonin in the brain | |||
| Betamethasone | • Reduces vascular density and suppresses expression of vascular endothelial growth factor | [55] | |
| • Increases pericyte coverage of neurovasculature | |||
| Nonsteroidal agonist | Ginsenosides | • Improve outcome in rat stroke model | [153,185] |
| • Reduce blood–brain barrier damage in experimental autoimmune encephalitis MS model | |||
| Antagonist | Mifepristone | • Neuroprotection in traumatic brain injury in rats | [157–162,186] |
| • Treatment of glioma and selective induction of tumor cell death | |||
| • Improvement in mood disorders | |||
| • Improves working memory and reduces depressive symptoms in bipolar disorder | |||
| Relicorilant (CORT125134) | • Treats Cushing disease and tumor pathology (Phase II/III clinical trial) | [164–166] | |
| Ketoconazole | • Treatment of refractory depression and major depressive disorder | [187] | |
| • Treatment of depressive symptoms in bipolar disorder | |||
| Aminogluthetimide | • Protection from ischemic injury and NMDA excitotoxicity | [188] | |
| Selective antagonist | LGD5552 | • Prevents incidence of disease in experimental autoimmune encephalitis MS rat model | [171] |
| CORT118335 | • Reduction of stress response | [163] | |
| CORT108297 | • Reduction of stress response, with delayed recovery from stress | [164] | |
| Modulator | Melatonin | • Reverses glucocorticoid-induced enhancement of cytotoxicity and reduction of hippocampal cell proliferation | [189] |
| Icariin | • Ameliorates disease pathology and improves clinical score in experimental autoimmune encephalitis rat model | [190,191] | |
Neurodegenerative diseases
Alzheimer’s disease
Within the brain, activation of the GR in astrocytes and neurons of the hippocampus contributes to long-term memory formation and recall [28,72] (Fig. 4). Because of this, GR abnormalities have been reported in human subjects and in rodent models of Alzheimer’s disease (AD). Consistent overexpression of the GR has been previously identified in the human hippocampus with AD compared with a control brain region [106]. Abnormal regulation of the HPA axis, related to improper GR function in the hypothalamus and decreased negative GR feedback to cortisol [65], has likewise been identified in human patients with AD and associates with decreased episodic memory retrieval [107]. Therefore, a mechanism for the development of AD probably involves cortisone dysregulation rather than abnormal GR function alone. This is strengthened by the findings that tau sensitizes cells to amyloid peptides and glucocorticoids, and that the generation of amyloid-β and tau pathology are reduced with the GR inhibitor mifepristone [108,109]. Similarly, inhibition of the GR in a mouse model of AD has previously been shown to improve episodic memory recall [110], suggesting the possible association of GR function and memory loss in AD.
Multiple sclerosis
In contrast to AD, the literature suggests that alteration to the GR protein has little influence on the progression or severity of symptoms in multiple sclerosis (MS). Despite this, reports suggest that, although hyperactivation of the HPA axis contributes to the development of MS, as is found in AD, such dysfunction, even when related to the GR, does not exercise a direct influence on the disease pathology [111]. Expression of the BclI or N363S polymorphisms, both associated with increased glucocorticoid sensitivity in brain tissue, increases risk to higher mortality and rapid development of disability [4,112]. However, even if such polymorphism is frequently expressed in patients with MS, the expression of high-sensitivity forms of the GR does not seem to improve negative feedback responses to glucocorticoids [113]. An increased number of corticotrophin-releasing hormone secretory neurons is also found in the hypothalamus of patients with MS [114]. Additionally, these findings are in line with reports that suggest the hypothesis of compensatory upregulation of GR-responsive genes in tissues with decreased GR expression [115]. Therefore, it is most likely that, despite compensatory mechanisms by the brain to restore response to glucocorticoids, GR insensitivity to natural or synthetic glucocorticoids contributes to the progression of MS.
A unique aspect of the progression of MS is the role of the immune system in destroying myelin-generating cells, which can be partially controlled by the GR. The GR is expressed in oligodendrocytes, and dexamethasone stimulation increases the expression of MHC II molecules in the presence of IFNγ [71]. This enables interactions between cytotoxic T cells, which can infiltrate brain tissue following disruption of the BBB, and oligodendrocytes, resulting in T-cell-mediated oligodendrocyte death and demyelination in the brain [71]. Given that dexamethasone stimulation is necessary for this interaction, this could also implicate HPA axis dysfunction, leading to hypercortisolemia in oligodendrocyte-dependent demyelination in the brain with MS.
Huntington’s disease
As described in other neurodegenerative disorders, pathology-related GR abnormalities can underlie the onset or pathogenesis of Huntington’s disease (HD) and the physiological changes common to HD and other disorders of the HPA axis, including weight loss, increased cortisol secretion and muscular atrophy as identified in human subjects and rodent models [116–118]. Pathological changes to certain areas of the brain such as the hypothalamus and pituitary have further been reported to accompany decreased GR expression in regions normally enriched in GR expression [116,119]. Additionally, restoration of cortisol to normal levels in adrenalectomized mice given high-dose cortisol was reported to alleviate certain symptoms of HD previously identified in other rodent models and human subjects [120]. Thus, a prevailing theory for the pathogenesis of HD involves the failure of negative feedback mechanisms in the brain and a subsequent hyperactivation of the HPA axis. This hypothesis is supported by reports that indicate that the elevation of cortisol levels in the blood does not occur until later stages of the disease [117], although to our knowledge no studies have been performed similarly assessing the expression of GR during progression of HD.
Stroke
Ischemic disorders such as stroke cause a sustained increase in circulating glucocorticoids owing to activation of the HPA axis. Although acute glucocorticoid and subsequent GR activity can be beneficial in some disease states, persistent abnormal elevation of GR activity is often harmful and can contribute to neuronal death following ischemia [121]. In addition to ischemia, stress is reported to cause atrophy of the dendrites in hippocampal neurons following ischemia in rats, which can be blocked by peripheral administration of the steroid synthesis inhibitor cyanoketone [122].
In addition to mechanisms of neuronal damage, the GR has been reported to influence a certain degree of physical recovery after ischemic stroke and infarct size during ischemia. As an example, one group reported increased activity of the GR during stress and reduced recovery of motor ability in a rat model of stroke [123]. Despite lower GR expression in the brains of stressed animals than in controls, higher GR activation in the lesioned hemisphere corresponded to decreased motor control in these animals [123]. Further, the size of the ischemic lesions in animals under stress increased, as reported by multiple groups [123,124], and this expansion was reversed upon administration of the GR inhibitor mifepristone [124]. Despite the contribution of GR to stroke pathology, restoration of cerebral blood flow during a stroke can be improved by dexamethasone GR stimulation, leading to nitric oxide synthase activity and subsequent vasodilation [58]. Additionally, other groups have shown that repression of the GR increases inflammatory signaling and infarct size during ischemia while decreasing neuroprotective BDNF signaling [125]. Thus, there can be a balance struck between GR stimulation and repression in the contribution to infarct size and recovery of cognitive and neuromuscular function. However, the conflicting results seen in these reports could also be partly the result of variations in the stimulation method of natural cortisone versus a synthetic glucocorticoid, or chronic versus acute high-dose glucocorticoid stimulation used.
Psychiatric disorders
A hallmark for psychiatric diseases involves elevated HPA activity and high circulating cortisol with negligible GR response. Indeed, this phenomenon could result from the insensitivity to glucocorticoids or GR resistance subsequent to chronic usage of medications that block GR activity, which in turn causes compensatory upregulation of the HPA axis [126]. A report suggests that GR also regulates expression of P-glycoprotein in the brain, which selectively regulates access of cortisol studied in humans and animal models [127]. Therefore, resistance to negative feedback of glucocorticoids could be a function of hyperactive GR activity in combination with abnormal GR regulation in the brain.
Depression
Unlike neurodegenerative diseases, development of psychiatric disorders related to the GR is equally likely to involve HPA axis and GR dysfunction. Notably, dysfunctions in or an absence of GR in the prefrontal cortex and hippocampus have been reported to result in depression-like behavior in mice and rats [8,76,128] (Fig. 4). Upregulation of the GR in the rat model has been reported to result in an increased GR response to glucocorticoids [8]. Interestingly, the same report indicated no noticeable difference in basal GR response to glucocorticoids; instead, stress (resulting in hypersecretion of glucocorticoids) caused a manifestation of depression-like behavior in mice with reduced hippocampal GR [8]. This increased response to glucocorticoids is absent in rats with a reduction in GR activity of the prefrontal cortex, implicating overexpression of the GR as a primary factor in the development of depression [76].
Interestingly, this dichotomy can arise from the conditions under which the condition of depression was developed and from a dysfunction of HPA axis circuitry, as demonstrated in humans. In human subjects, the presence of depression or depression-like behavior has been reported to arise from dysregulation between the MR and GR systems, because increases in either GR or MR activity and concurrent desensitization of the opposite receptor have been reported in subjects with depression [17,129]. Further, stressors in early childhood have been shown to cause an imbalance favoring low cortisol circulation and hyperactivation of the MR, whereas melancholic depression can arise from imbalances of the HPA axis, causing high circulation of cortisol and hyperactivation of the GR [17,129,130].
Anxiety and stress
Stress has been shown to cause neuronal network remodeling, dendritic retraction and altered neuronal function mediated by the glucocorticoid and dopamine receptors [86]. This further extends to the serotonin response, normally governed by the GR in the hippocampus [81], which is attenuated in chronic stress and is reported to be restored through administration of the GR antagonist mifepristone [86]. As with depression, mood disorders related to anxiety can arise from dysregulation of the GR in the prefrontal cortex. Indeed, activation of the GR probably mediates anxiety-like behavior, because reports indicate that mice with disrupted forebrain GR show decreased anxiety-associated actions [131]. However, rather than being isolated to a single region of the brain, abnormal GR arising from disease comorbidity can cause anxiety-like symptoms in multiple areas of the brain (Fig. 4). Similarly, imbalances in MR and/or GR activation have been reported to contribute to anxiety-like behavior, whereas inhibition of the same circuits reduces anxiety [132,133]. Thus, much like depression, stress that occurs early in development, contributing to reduced activity of the GR or hyperactivity of the GR owing to high levels of circulating cortisol, can contribute to the development of anxiety and related disorders.
Neurological effects of pharmacological GR stimulation
Exogenous GR regulators are employed in a variety of neuropathological states, primarily to alleviate symptoms associated with vascular dysfunction or chronic stress. Pharmacological GR activation is useful or in certain disease conditions and has been shown to have positive influence in several neurological conditions (Table 2). However, given recent information on the mechanisms of GR-related disease progression and glucocorticoid insensitivity with chronic administration of exogenous regulators, studies in the future might need to revisit the effects of GR agonism and the potential benefits of more-selective GR antagonism on neuropathological states. Further, cessation or discontinuation of synthetic glucocorticoids in chronic treatment has been noted to suppress the HPA axis, leading to adrenal atrophy and potentially creating a life-threatening situation.
Steroidal inducers
Dexamethasone
Dexamethasone, although not a prototype glucocorticoid, has been shown to have a notable effect on the process of GR regulation within the brain. Stimulation of the GR by dexamethasone has been previously reported to decrease expression and activity of AP-1 and NF-κB in the brains of rats treated with kainic acid [134,135]. Although kainic acid treatment increased the hippocampal and cortical formation of the AP-1 complex, this formation was attenuated by dexamethasone through reduction of AP-1–DNA binding [135]. Similarly, GR stimulation by dexamethasone was reported to reduce acute DNA-binding activity of NF-κB through direct interaction with the p65 subunit, despite decreasing expression of NF-κB inhibitor alpha protein (Fig. 1) [134]. This could potentially be an indication that primary inhibition of inflammation by the GR occurs at the level of transcription in the brain.
In clinical practice, dexamethasone is used to treat glioma and edema associated with neurological disorders and infection. Dexamethasone has been shown to ameliorate edema in an experimental rabbit model and to upregulate the expression of tight-junction proteins at the BBB via stimulation of the GR in a rat model of traumatic brain injury [52]. Notably, dexamethasone for this purpose is frequently employed in cases of bacterial infection to prevent cerebrovascular leakage and neuronal death as demonstrated in rats [136], and has previously been reported to reduce fatality in hospitalized human meningitis patients [137,138]. These results have been replicated in a rabbit model of infection with dexamethasone treatment and caused a corresponding decrease in cerebrospinal fluid lactate levels and intracranial pressure, in addition to resolving the edema [139]. However, in addition to reducing edema and upregulating tight junctions, dexamethasone has also been reported to increase the expression and activity of multidrug-resistance efflux transporters at the BBB in rats [61]. This in turn prevents the penetration of further dexamethasone, a situation that is reversed in multidrug-resistance protein-knockout mice [63,127]; this corresponds to reports of decreased macromolecular uptake by vesicles in brain endothelial cells in normal mice [140]. Thus, employment of dexamethasone as a treatment can render the brain environment resistant to further application of glucocorticoids, mandating the use of high-dose synthetics to achieve a sufficient therapeutic concentration.
Because systemic dexamethasone administration can activate the GR throughout the body, several groups have also studied alternate mechanisms of dexamethasone treatment to alleviate adverse effects typically associated with dexamethasone use. Meneses et al. found that intranasal administration of dexamethasone reduced astrocytic gliosis more rapidly than intravenous administration, with higher concentrations of dexamethasone found in the brain vasculature than with systemic dosing; additionally, fewer neutrophils, the activity of which is associated with several neuropathological states, entered brain tissue given intranasally compared with systemic administration [141]. Likewise, Ong et al. previously used depot devices to administer dexamethasone in a rat glioma model, with a successful reduction in edema-absent weight loss and with a lower dose than given systemically [142].
Interestingly, dexamethasone could also be used for genome-based therapeutics. Conjugation of dexamethasone with polyamidoamine generation 2 resulted in an improved cellular delivery and anti-inflammatory effect than occurred with dexamethasone alone; further, delivery of the heme-oxygenase 1 plasmid, which protected brain tissue from damage in the ischemic condition, predominated with this complex over other conjugate systems [143]. Although these results have yet to be replicated in human subjects, they are nevertheless promising indicators that targeted local therapy, rather than cessation of glucocorticoid use, could provide acute benefits to patients while alleviating adverse effects associated with systemic administration.
Methylprednisolone
Much like dexamethasone, methylprednisolone is employed in clinical use to reduce edema in the brain [144]. However, the effects of methylprednisolone have further been assessed in suppression of neuroinflammation following cardiac surgery, which has the potential to cause associated BBB dysfunction [145]. It has been reported that administration of methylprednisolone before such surgeries prevents cytokine release in response to surgical trauma, and subsequent systemic inflammation, but does not attenuate either BBB dysfunction or neuroinflammation in these patients undergoing surgery [145].
Methylprednisolone is most commonly administered during relapses of MS, and reduces expression of adhesion molecules in the brain vasculature to prevent movement of activated T cells into brain tissue [146]. Acute administration of high-dose methylprednisolone has been further shown to reduce gadolinium-enhanced lesions as viewed by imaging (e.g., MRI), indicating a decrease in cerebrovascular leakage, although several groups have also noted that this is a short-term effect [147,148]. Nevertheless, long-term administration improved overall patient outcome, with a 5-year course of pulsatile i.v. methylprednisolone administration reducing brain atrophy and MS progression [149].
Interestingly, a balance seems to have been struck with methylprednisolone stimulation of the GR between motor ability and memory formation. Administration of methylprednisolone appears to benefit memory formation and long-term potentiation between neurons, although this effect occurs only at low doses [150]. Rats exposed to an aversive stimulus were reported to develop longer aversion memory if given low-dose rather than high-dose methylprednisolone. Conversely, high-dose methylprednisolone has been shown to reduce motor deficits in a mouse experimental autoimmune encephalomyelitis model of MS but does not restore associated cognition deficits; in fact, memory deficits as reported in the study were reversed by inhibition of GR activity [151].
Nonsteroidal inducers
Ginseng
Recently, ginseng and derived ginsenosides have been analyzed for their therapeutic potential in reducing inflammation and preventing apoptosis in a variety of disease states, including stroke [152]. Varied studies indicate the pro-survival and anti-inflammatory effect of ginseng-derived compounds, which, in part, arise from their role in mediating by GR transactivation and transrepression activity. Hu et al. [153] reported minimal influence of ginsenosides on the transactivation and transrepression activity of the GR, with even the most efficacious ginsenosides (Rg1, Rh2 and PPD-type) binding minimally to the ligand-binding domain and nonsignificant expression of target genes of GR activation or repression. By contrast, GR-mediated angiogenesis is upregulated upon treatment of vascular cells with Korean ginseng extract, through induction of VEGF expression, upregulation of its corresponding receptor and the increased expression of nitric oxide synthase [154]. Similarly, it has been reported that ginsenosides can oppose dexamethasone-dependent downregulation of GR, while increasing transcription reliant on glucocorticoid stimulation [155].
Inhibitors
Mifepristone
Mifepristone is a GR inhibitor that has been demonstrably efficacious in mood disorders, neurodegenerative disorders and trauma, because it has a notable neuroprotective effects [66,156]. The benefit of its use has already been demonstrated in an Alzheimer’s mouse model [108], in human subjects with major depressive disorders [157] and in a rat model of traumatic brain injury [158]. Several groups have also begun to test the efficacy of mifepristone in mood disorders such as psychotic depression and in post-traumatic stress disorder.
A recent case report confirmed the ability of mifepristone to cross the BBB and treat advanced glioma reliant on progesterone receptors [159,160]. The ability of mifepristone to induce tumor cell death while sparing differentiated cells has also been previously demonstrated in mice [161]. Further, there has been demonstrated improvement reported in human subjects with benign, inoperable meningiomas subsequent to mifepristone treatment. Therefore, the use of mifepristone in the future could provide some benefit in treating brain tumors.
Relacorilant
With the variety of nonselective, GR-regulating drugs on the market, the focus of pharmacological modulation of the GR has begun to evolve and include xenobiotics with more-specific effects on GR activity [162,163]. Relacorilant (CORT125134) is one such drug. It is not yet on the market but has entered Phase II and Phase III clinical trials for use in treating prostate cancer and Cushing syndrome [164,165]. Relacorilant has been found to oppose the effects of prednisone and dexamethasone in human subjects, with an efficacy comparable to that of mifepristone [166,167]. Although it had no effect on weight gain, cardiac intervals or hematology and urinalysis results, Hunt et al. reported pain, nausea and vomiting as common treatment-associated adverse effects in human subjects given a single dose, and severe pain or gastrointestinal system disorders in patients given repeated doses [166]. The authors speculated that these adverse effects were caused by blockade of the GR and indicated that relacorilant might be better tolerated in patients with Cushing syndrome owing to the high level of circulating cortisol.
A major benefit in the use of relacorilant over mifepristone is its specificity for the GR over the progesterone receptor [167]. Although the action of mifepristone as an anti-progestin can be exploited in certain clinical cases, the nonspecific action of mifepristone compared with relacorilant can cause unwanted side effects, such as irregular bleeding and thickening of the uterine lining in women of child-bearing age. Thus, relacorilant might prove a safer alternative to mifepristone for treating Cushing syndrome, although it remains to be seen whether it displays effects comparable to those of mifepristone in treating neurological disorders.
Glucocorticoid resistance in brain disorders
Owing to strong anti-inflammatory and immunosuppressive effects, glucocorticoids are recommended as an adjunctive treatment in a variety of disorders, despite having some deleterious effects in the brain if used at length. Frequent use of glucocorticoids has been reported to desensitize the GR, and acute glucocorticoid use is known to downregulate GR expression. Further, certain physiological conditions associated with disease states in the brain are associated with high levels of circulating cortisol, which can reduce GR response to glucocorticoids, limiting their neuroprotective effect [54]. These conditions can contribute to or cause disorders in the brain normally related to GR dysfunction (as identified in Fig. 4), a phenomenon that has recently become the impetus for developing methods of glucocorticoid administration with reduced systemic side effects yet equivalent efficacy.
Glucocorticoid resistance in neurological disorders appears to be primarily regulated by mechanisms of negative feedback driven by hypercortisolemia and HPA axis dysfunction. Therefore, it seems logical to speculate that resistance to glucocorticoids appears to link to neurological disorders in which preferred treatments include glucocorticoids and direct pharmacological intervention. However, such a statement might be a slight generalization, because few of the discussed conditions have medications capable of effectively treating or slowing the progression of disease, and therefore cannot be analyzed for paradigms of pharmacoresistance. Nevertheless, in certain disorders such as depression, glucocorticoid resistance seems to have a notable association with resistance to antidepressant therapy. Dysregulation of the HPA axis is associated with glucocorticoid insensitivity and the development of depression as a disorder in these instances. Patients with a resistance to antidepressant therapy have been previously reported to express certain polymorphisms of FK506-binding protein 51, a co-chaperone of the GR that regulates sensitivity and is associated with cortisol resistance [168]. Thus, patients with refractory, drug-resistant brain disorders could likewise have resistance to cortisol and glucocorticoids used in therapy. This needs to be further investigated.
Concluding remarks
Myriad evidence indicates that the GR plays a notable part in maintaining health and homeostasis in the body, whereas the contribution of the GR to the same states in the brain, specifically at the neurovasculature, requires further investigation. GR dysfunction is a shared characteristic in a variety of neurological disorders. Further, hyperactivation of the improperly mediated HPA axis is a hallmark and a cause of the same diseases that progress in the presence of atypical GR. Therefore, further investigation of the GR as a target in neurological diseases, and of its activity related to the use of medications in such conditions, could prove to aid in treating such disorders with better therapy and minimal side effects.
Acknowledgments
This work is supported in part by the National Institute of Neurological Disorders and Stroke/National Institute of Health GRαnts (R01NS095825 and R01NS078307) awarded to CG.
Biography

Sherice Williams received her BS in biology from Cleveland State University (USA). She performs her research work in the Brain Physiology Laboratory in the Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute (USA), under the supervision of Dr Chaitali Ghosh. She has spent the past 4 years studying the role of the blood–brain barrier mechanism and function in human drug-resistant epilepsy and seizure models.

Chaitali Ghosh received her PhD in toxicology from the Indian Institute of Toxicology Research, Lucknow, (India) and Hamdard University, New Delhi (India), with subsequent postdoctoral training in vascular biology and epilepsy at Cleveland Clinic (USA). She is currently an Associate Professor of Molecular Medicine and Biomedical Engineering at Cleveland Clinic Lerner College of Medicine of Case Western Reserve University (USA) and an elected fellow of the American Heart Association Stroke Council. She leads the Brain Physiology Laboratory in the Department of Biomedical Engineering, Cleveland Clinic Lerner Research Institute (USA), with her experience in neurobiology, blood–brain barrier, drug-resistant epilepsy, neuropharmacology and toxicology. Her research, past and present, has been funded by several GRαnts from: National Institutes of Health (NIH); the American Heart Association Scientist Development GRαnt (AHA-SDG); Alternative Research and Development Foundation (ARDF); and Brain and Behavior Research Foundation (NARSAD).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Chatterjee S and Sikdar SK (2014) Corticosterone targets distinct steps of synaptic transmission via concentration specific activation of mineralocorticoid and glucocorticoid receptors. J. Neurochem. 128, 476–490 [DOI] [PubMed] [Google Scholar]
- 2.Rajapandi T. et al. (2000) The molecular chaperones Hsp90 and Hsc70 are both necessary and sufficient to activate hormone binding by glucocorticoid receptor. J. Biol. Chem. 275, 22597–22604 [DOI] [PubMed] [Google Scholar]
- 3.Sinclair D. et al. (2012) Glucocorticoid receptor mRNA and protein isoform alterations in the orbitofrontal cortex in schizophrenia and bipolar disorder. BMC Psychiatry 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Winsen LM et al. (2009) A glucocorticoid receptor gene haplotype (TthIII1/ ER22/23EK/9beta) is associated with a more aggressive disease course in multiple sclerosis. J. Clin. Endocrinol. Metab. 94, 2110–2114 [DOI] [PubMed] [Google Scholar]
- 5.Wang Q. et al. (2015) Glucocorticoid receptor beta acts as a co-activator of T-cell factor 4 and enhances glioma cell proliferation. Mol. Neurobiol 52, 1106–1118 [DOI] [PubMed] [Google Scholar]
- 6.Hudson WH et al. (2018) Cryptic glucocorticoid receptor-binding sites pervade genomic NF-kappaB response elements. Nat. Commun. 9, 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weikum ER et al. (2017) Tethering not required: the glucocorticoid receptor binds directly to activator protein-1 recognition motifs to repress inflammatory genes. Nucleic Acids Res. 45, 8596–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ridder S et al. (2005) Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 25, 6243–6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Levy GA et al. (2018) Increased expression of brain-derived neurotrophic factor transcripts I and VI, cAMP response element binding, and glucocorticoid receptor in the cortex of patients with temporal lobe epilepsy. Mol. Neurobiol. 55, 3698–3708 [DOI] [PubMed] [Google Scholar]
- 10.Chen H. et al. (2017) Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol. Brain 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adachi N. et al. (2015) Glucocorticoid affects dendritic transport of BDNF- containing vesicles. Sci. Rep. 5, 12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer RL et al. (1998) Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology 139, 2718–2726 [DOI] [PubMed] [Google Scholar]
- 13.Gjerstad JK et al. (2018) Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 21, 403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ter Heegde F. et al. (2015) The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology 52, 92–110 [DOI] [PubMed] [Google Scholar]
- 15.Brinks V. et al. (2007) Differential MR/GR activation in mice results in emotional states beneficial or impairing for cognition. Neural Plast. 2007, 90163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouanes S and Popp J (2019) High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front. Aging Neurosci.. 10.3389/fnagi.2019.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baes C et al. (2014) Early life stress in depressive patients: HPA axis response to GR and MR agonist. Front. Psychiatry 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirschke E et al. (2014) Glucocorticoid receptor function regulated by coordinated action of the Hsp90andHsp70chaperone cycles. Cell 157,1685–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaff SJ and Fletterick RJ (2010) Hormone binding and co-regulator binding to the glucocorticoid receptor are allosterically coupled. J. Biol. Chem. 285, 15256–15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Son GH et al. (2008) Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc. Natl. Acad. Sci. U. S. A. 105, 20970–20975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buren J et al. (2007) Hippocampal 11beta-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid expression has a diurnal variability that is lost in the obese Zucker rat. Endocrinology 148, 2716–2722 [DOI] [PubMed] [Google Scholar]
- 22.de Kloet ER et al. (2018) Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 49, 124–145 [DOI] [PubMed] [Google Scholar]
- 23.Chung S et al. (2011) Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim. Biophys. Acta 1812, 581–591 [DOI] [PubMed] [Google Scholar]
- 24.Garabedian MJ et al. (2017) Glucocorticoid receptor action in metabolic and neuronal function. F1000Res 6, 1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yongue BG and Roy EJ (1987) Endogenous aldosterone and corticosterone in brain cell nuclei of adrenal-intact rats: regional distribution and effects of physiological variations in serum steroids. Brain Res. 436, 49–61 [DOI] [PubMed] [Google Scholar]
- 26.Reul JM and de Kloet ER (1985) Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511 [DOI] [PubMed] [Google Scholar]
- 27.De Kloet ER and Reul JM (1987) Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology 12, 83–105 [DOI] [PubMed] [Google Scholar]
- 28.Kim SE et al. (2019) Treadmill exercise alleviates circadian rhythm disruption- induced memory deficits by activation of glucocorticoid receptor and brain-derived neurotrophic factor-dependent pathway. Int. Neurourol. J. 23 (suppl. 1), 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furay AR et al. (2006) Region-specific regulation of glucocorticoid receptor/HSP90 expression and interaction in brain. J. Neurochem. 98, 1176–1184 [DOI] [PubMed] [Google Scholar]
- 30.Biddie SC et al. (2011) Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol. Cell 43, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesikova M et al. (2016) Divergent binding and transactivation by two related steroid receptors at the same response element. J. Biol. Chem. 291, 11899–11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramamoorthy S and Cidlowski JA (2013) Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol. Cell Biol. 33, 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jochems J et al. (2015) Enhancement of stress resilience through Hdac6-mediated regulation of glucocorticoid receptor chaperone dynamics. Biol. Psychiatry 77, 345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weaver IC et al. (2004) Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 [DOI] [PubMed] [Google Scholar]
- 35.Liu D et al. (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662 [DOI] [PubMed] [Google Scholar]
- 36.Plotsky PM et al. (2005) Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30, 2192–2204 [DOI] [PubMed] [Google Scholar]
- 37.McGowan PO et al. (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci 12, 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodgers AB et al. (2013) Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33, 9003–9012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mueller BR and Bale TL (2008) Sex-specific programming of offspring emotionality following stress early in pregnancy. J. Neurosci. 28, 9055–9065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buoso E et al. (2017) Role of spliceosome proteins in the regulation of glucocorticoid receptor isoforms by cortisol and dehydroepiandrosterone. Pharmacol. Res. 120, 180–187 [DOI] [PubMed] [Google Scholar]
- 41.Goecke IA et al. (2007) Methotrexate regulates the expression of glucocorticoid receptor alpha and beta isoforms in normal human peripheral mononuclear cells and human lymphocyte cell lines in vitro. Mol. Immunol. 44, 2115–2123 [DOI] [PubMed] [Google Scholar]
- 42.Forster C et al. (2006) Glucocorticoid effects on mouse microvascular endothelial barrier permeability are brain specific. J. Physiol. 573, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Encio IJ and Detera-Wadleigh SD (1991) The genomic structure of the human glucocorticoid receptor. J. Biol. Chem. 266, 7182–7188 [PubMed] [Google Scholar]
- 44.Pujols L et al. (2002) Expression of glucocorticoid receptor alpha- and beta-isoforms in human cells and tissues. Am. J. Physiol. Cell Physiol. 283, C1324–C1331 [DOI] [PubMed] [Google Scholar]
- 45.Oakley RH et al. (1996) The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 271, 9550–9559 [DOI] [PubMed] [Google Scholar]
- 46.Bamberger CM et al. (1995) Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Invest. 95, 2435–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fruchter O et al. (2005) The human glucocorticoid receptor (GR) isoform {beta} differentially suppresses GR{alpha}-induced transactivation stimulated by synthetic glucocorticoids. J. Clin. Endocrinol. Metab. 90, 3505–3509 [DOI] [PubMed] [Google Scholar]
- 48.de Lange P et al. (1999) Natural variants of the beta isoform of the human glucocorticoid receptor do not alter sensitivity to glucocorticoids. Mol. Cell. Endocrinol. 153, 163–168 [DOI] [PubMed] [Google Scholar]
- 49.Hecht K et al. (1997) Evidence that the beta-isoform of the human glucocorticoid receptor does not act as a physiologically significant repressor. J. Biol. Chem. 272, 26659–26664 [DOI] [PubMed] [Google Scholar]
- 50.de Castro M et al. (1996) The non-ligand binding beta-isoform of the human glucocorticoid receptor (hGR beta): tissue levels, mechanism of action, and potential physiologic role. Mol. Med. 2, 597–607 [PMC free article] [PubMed] [Google Scholar]
- 51.Abbott NJ (2002) Astrocyte-endothelial interactions and blood–brain barrier permeability*. J. Anat. 200, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hue CD et al. (2015) Dexamethasone potentiates in vitro blood–brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J. Cereb. Blood Flow Metab. 35, 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim H et al. (2008) Dexamethasone coordinately regulates angiopoietin-1 and VEGF: a mechanism of glucocorticoid-induced stabilization of blood–brain barrier. Biochem. Biophys. Res. Commun. 372, 243–248 [DOI] [PubMed] [Google Scholar]
- 54.Kleinschnitz C et al. (2011) Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 42,1081–1089 [DOI] [PubMed] [Google Scholar]
- 55.Vinukonda G et al. (2010) Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke 41, 1766–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou J et al. (2013) Peritumoral brain edema in intracranial meningiomas: the emergence of vascular endothelial growth factor-directed therapy. Neurosurg. Focus 35, E2 [DOI] [PubMed] [Google Scholar]
- 57.Stanimirovic DB et al. (1994) Dexamethasone down-regulates endothelin receptors in human cerebromicrovascular endothelial cells. Neuropeptides 26, 145–152 [DOI] [PubMed] [Google Scholar]
- 58.Limbourg FP et al. (2002) Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J. Clin. Invest. 110, 1729–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh C et al. (2018) Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood–brain barrier model. Epilepsia 59, 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh C et al. (2017) Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia 58, 576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narang VS et al. (2008) Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood–brain barrier. Am. J. Physiol. Cell Physiol. 295, C440–C450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller MB et al. (2003) ABCB1 (MDR1)-type P-glycoproteins at the blood–brain barrier modulate the activity of the hypothalamic-pituitary-adrenocortical system: implications for affective disorder. Neuropsychopharmacology 28, 1991–1999 [DOI] [PubMed] [Google Scholar]
- 63.Meijer OC et al. (1998) Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-glycoprotein knockout mice. Endocrinology 139, 1789–1793 [DOI] [PubMed] [Google Scholar]
- 64.Wang GY et al. (2016) Glucocorticoid induces incoordination between glutamatergic and GABAergic neurons in the amygdala. PLoS One 11, e0166535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartmann J et al. (2017) Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol. Psychiatry 22, 466–475 [DOI] [PubMed] [Google Scholar]
- 66.Rakotomamonjy J et al. (2011) Novel protective effect of mifepristone on detrimental GABAA receptor activity to immature Purkinje neurons. FASEB J. 25, 3999–4010 [DOI] [PubMed] [Google Scholar]
- 67.Kim JW et al. (2013) Administration of dexamethasone to neonatal rats induces hypomyelination and changes in the morphology of oligodendrocyte precursors. Comp. Med. 63, 48–54 [PMC free article] [PubMed] [Google Scholar]
- 68.Hinkerohe D et al. (2010) Dexamethasone prevents LPS-induced microglial activation and astroglial impairment in an experimental bacterial meningitis coculture model. Brain Res. 1329, 45–54 [DOI] [PubMed] [Google Scholar]
- 69.Kiefer R and Kreutzberg GW (1991) Effects of dexamethasone on microglial activation in vivo: selective downregulation of major histocompatibility complex class II expression in regenerating facial nucleus. J. Neuroimmunol. 34, 99–108 [DOI] [PubMed] [Google Scholar]
- 70.Unemura K et al. (2012) Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J. Pharmacol. Sci. 119, 30–39 [DOI] [PubMed] [Google Scholar]
- 71.Bergsteindottir K et al. (1992) In the presence of dexamethasone, gamma interferon induces rat oligodendrocytes to express major histocompatibility complex class II molecules. Proc. Natl. Acad. Sci. U. S. A. 89, 9054–9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tertil M et al. (2018) Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl. Psychiatry 8, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butts KA et al. (2011) Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc. Natl. Acad. Sci. U. S. A. 108, 18459–18464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee AG et al. (2014) Coping and glucocorticoid receptor regulation by stress inoculation. Psychoneuroendocrinology 49, 272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izquierdo I et al. (1997) Sequential role of hippocampus and amygdala, entorhinal cortex and pariet al cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur. J. Neurosci. 9, 786–793 [DOI] [PubMed] [Google Scholar]
- 76.McKlveen JM et al. (2013) Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol. Psychiatry 74, 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diorio D et al. (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 13, 3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Noguchi KK et al. (2011) Glucocorticoid receptor stimulation and the regulation of neonatal cerebellar neural progenitor cell apoptosis. Neurobiol. Dis. 43, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wenzel A et al. (2001) Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest. Ophthalmol. Vis. Sci. 42, 1653–1659 [PubMed] [Google Scholar]
- 80.Henckens MJ et al. (2010) Time-dependent effects of corticosteroids on human amygdala processing. J. Neurosci. 30, 12725–12732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karst H et al. (2000) Corticosteroid actions in hippocampus require DNA binding of glucocorticoid receptor homodimers. Nat. Neurosci. 3, 977–978 [DOI] [PubMed] [Google Scholar]
- 82.Jakovcevski M et al. (2011) Susceptibility to the long-term anxiogenic effects of an acute stressor is mediated by the activation of the glucocorticoid receptors. Neuropharmacology 61, 1297–1305 [DOI] [PubMed] [Google Scholar]
- 83.Joels M et al. (2004) Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress 7, 221–231 [DOI] [PubMed] [Google Scholar]
- 84.Geuze E et al. (2012) Glucocorticoid receptor number predicts increase in amygdala activity after severe stress. Psychoneuroendocrinology 37, 1837–1844 [DOI] [PubMed] [Google Scholar]
- 85.Buwalda B et al. (2001) Temporal and spatial dynamics of corticosteroid receptor down-regulation in rat brain following social defeat. Physiol. Behav. 72, 349–354 [DOI] [PubMed] [Google Scholar]
- 86.Goldwater DS et al. (2009) Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164, 798–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonanno G et al. (2005) Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J. Neurosci. 25, 3270–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Musazzi L et al. (2010) Acute stress increases depolarization-evoked glutamate release in the rat prefrontal/frontal cortex: the dampening action of antidepressants. PLoS One 5, e8566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bao GS et al. (2011) Changes of glucocorticoid receptor mRNA expression in basolateral amygdale-kindled rats. Chin. Med. J. 124, 2622–2627 [PubMed] [Google Scholar]
- 90.Kumar G et al. (2007) The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology 32, 834–842 [DOI] [PubMed] [Google Scholar]
- 91.Wulsin AC et al. (2016) RU486 mitigates hippocampal pathology following status epilepticus. Front. Neurol. 7, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watzka M et al. (2000) Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids 65, 895–901 [DOI] [PubMed] [Google Scholar]
- 93.Pascussi JM et al. (2003) The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim. Biophys. Acta 1619, 243–253 [DOI] [PubMed] [Google Scholar]
- 94.Pascussi JM et al. (2008) The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu. Rev. Pharmacol. Toxicol. 48, 1–32 [DOI] [PubMed] [Google Scholar]
- 95.Cooper BW et al. (2008) Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol. Sci 103, 268–277 [DOI] [PubMed] [Google Scholar]
- 96.Duret C et al. (2006) Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol. Pharmacol. 70, 329–339 [DOI] [PubMed] [Google Scholar]
- 97.Ferguson SS et al. (2005) Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol. Pharmacol. 68, 747757 [DOI] [PubMed] [Google Scholar]
- 98.Ellemann K et al. (1988) Glucocorticoid receptors in glioblastoma multiforme: a new approach to antineoplastic glucocorticoid therapy. Acta Neurochir. 93, 6–9 [DOI] [PubMed] [Google Scholar]
- 99.Freshney RI et al. (1980) Control of cell proliferation in human glioma by glucocorticoids. Br. J. Cancer 41, 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sinha S et al. (2004) Effects of dexamethasone on peritumoural oedematous brain: a DT–MRI study. J. Neurol. Neurosurg. Psychiatry 75, 1632–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kiely J et al. (1994) Glucocorticoids down-regulate beta 1-adrenergic-receptor expression by suppressing transcription of the receptor gene. Biochem. J. 302, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin Y et al. (2013) Glucocorticoid receptor beta regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of beta-catenin/TCF transcriptional activity. Neurobiol. Dis. 59, 165–176 [DOI] [PubMed] [Google Scholar]
- 103.Na W et al. (2017) Dexamethasone suppresses JMJD3 gene activation via a putative negative glucocorticoid response element and maintains integrity of tight junctions in brain microvascular endothelial cells. J. Cereb. Blood Flow Metab. 37, 3695–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sherry-Lynes MM et al. (2017) Regulation of the JMJD3 (KDM6B) histone demethylase in glioblastoma stem cells by STAT3. PLoS One 12, e0174775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heiss JD et al. (1996) Mechanism of dexamethasone suppression of brain tumor-associated vascular permeability in rats. Involvement of the glucocorticoid receptor and vascular permeability factor. J. Clin. Invest. 98, 1400–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wetzel DM et al. (1995) Glucocorticoid receptor mRNA in Alzheimer’s diseased hippocampus. Brain Res. 679, 72–81 [DOI] [PubMed] [Google Scholar]
- 107.Elgh E et al. (2006) Cognitive dysfunction, hippocampal atrophy and glucocorticoid feedback in Alzheimer’s disease. Biol. Psychiatry 59, 155–161 [DOI] [PubMed] [Google Scholar]
- 108.Baglietto-Vargas D et al. (2013) Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol. Psychiatry 74, 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sotiropoulos I et al. (2008) Glucocorticoids trigger Alzheimer disease-like pathobiochemistry in rat neuronal cells expressing human tau. J. Neurochem. 107, 385–397 [DOI] [PubMed] [Google Scholar]
- 110.Lante F et al. (2015) Subchronic glucocorticoid receptor inhibition rescues early episodic memory and synaptic plasticity deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 40, 1772–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Michelson D et al. (1994) Multiple sclerosis is associated with alterations in hypothalamic-pituitary-adrenal axis function. J. Clin. Endocrinol. Metab. 79, 848–853 [DOI] [PubMed] [Google Scholar]
- 112.Melief J et al. (2016) Glucocorticoid receptor haplotypes conferring increased sensitivity (BclI and N363S) are associated with faster progression of multiple sclerosis. J. Neuroimmunol. 299, 84–89 [DOI] [PubMed] [Google Scholar]
- 113.Reder AT et al. (1987) Dexamethasone suppression test abnormalities in multiple sclerosis: relation to ACTH therapy. Neurology 37, 849–853 [DOI] [PubMed] [Google Scholar]
- 114.Erkut ZA et al. (1995) Increased activity of hypothalamic corticotropin-releasing hormone neurons in multiple sclerosis. J. Neuroimmunol. 62, 27–33 [DOI] [PubMed] [Google Scholar]
- 115.Bechmann L et al. (2014) Corticosteroid receptor expression and in vivo glucocorticoid sensitivity in multiple sclerosis. J. Neuroimmunol. 276, 159–165 [DOI] [PubMed] [Google Scholar]
- 116.Petersen A and Bjorkqvist M (2006) Hypothalamic-endocrine aspects in Huntington’s disease. Eur. J. Neurosci. 24, 961–967 [DOI] [PubMed] [Google Scholar]
- 117.Bjorkqvist M et al. (2006) Progressive alterations in the hypothalamic-pituitary-adrenal axis in the R6/2 transgenic mouse model of Huntington’s disease. Hum. Mol. Genet. 15, 1713–1721 [DOI] [PubMed] [Google Scholar]
- 118.Aziz NA et al. (2009) Increased hypothalamic-pituitary-adrenal axis activity in Huntington’s disease. J. Clin. Endocrinol. Metab. 94, 1223–1228 [DOI] [PubMed] [Google Scholar]
- 119.Petersen A et al. (2005) Orexin loss in Huntington’s disease. Hum. Mol. Genet. 14, 39–47 [DOI] [PubMed] [Google Scholar]
- 120.Dufour BD and McBride JL (2019) Normalizing glucocorticoid levels attenuates metabolic and neuropathological symptoms in the R6/2 mouse model of Huntington’s disease. Neurobiol. Dis. 121, 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sapolsky RM and Pulsinelli WA (1985) Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science 229, 1397–1400 [DOI] [PubMed] [Google Scholar]
- 122.Magarinos AM and McEwen BS (1995) Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 69, 89–98 [DOI] [PubMed] [Google Scholar]
- 123.Zucchi FC et al. (2010) Stress-induced glucocorticoid receptor activation determines functional recovery following ischemic stroke. Exp. Transl. Stroke Med. 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balkaya M et al. (2011) Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke 42, 3258–3264 [DOI] [PubMed] [Google Scholar]
- 125.Li Y et al. (2018) Repression of the glucocorticoid receptor aggravates acute ischemic brain injuries in adult mice. Int. J. Mol. Sci. 19, 2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Haarst AD et al. (1996) Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology 137, 4935–4943 [DOI] [PubMed] [Google Scholar]
- 127.Karssen AM et al. (2001) Multidrug resistance P-glycoprotein hampers the access of cortisol but not of corticosterone to mouse and human brain. Endocrinology 142, 2686–2694 [DOI] [PubMed] [Google Scholar]
- 128.Boyle MP et al. (2005) Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc. Natl. Acad. Sci. U. S. A. 102, 473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Juruena MF et al. (2006) Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology 189, 225–235 [DOI] [PubMed] [Google Scholar]
- 130.Paslakis G et al. (2011) Discrimination between patients with melancholic depression and healthy controls: comparison between 24-h cortisol profiles, the DST and the Dex/CRH test. Psychoneuroendocrinology 36, 691–698 [DOI] [PubMed] [Google Scholar]
- 131.Boyle MP et al. (2006) Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J. Neurosci. 26, 1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calvo N and Volosin M (2001) Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology 73, 261–271 [DOI] [PubMed] [Google Scholar]
- 133.Wei Q et al. (2012) Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biol. Psychiatry 71, 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Unlap MT and Jope RS (1997) Dexamethasone attenuates NF-kappa B DNA binding activity without inducing I kappa B levels in rat brain in vivo. Brain Res. Mol. Brain Res. 45, 83–89 [DOI] [PubMed] [Google Scholar]
- 135.Unlap T and Jope RS (1994) Dexamethasone attenuates kainate-induced AP–1 activation in rat brain. Brain Res. Mol. Brain Res. 24, 275–282 [DOI] [PubMed] [Google Scholar]
- 136.Tsai HC et al. (2015) Dexamethasone inhibits brain apoptosis in mice with eosinophilic meningitis caused by Angiostrongylus cantonensis infection. Parasit. Vectors 8, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Girgis NI et al. (1989) Dexamethasone treatment for bacterial meningitis in children and adults. Pediatr. Infect. Dis. J. 8, 848–851 [DOI] [PubMed] [Google Scholar]
- 138.de Gans J et al. (2002) Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347, 1549–1556 [DOI] [PubMed] [Google Scholar]
- 139.Tauber MG et al. (1985) Effects of ampicillin and corticosteroids on brain water content, cerebrospinal fluid pressure, and cerebrospinal fluid lactate levels in experimental pneumococcal meningitis. J. Infect. Dis. 151, 528–534 [DOI] [PubMed] [Google Scholar]
- 140.Hedley-Whyte ET and Hsu DW (1986) Effect of dexamethasone on blood- brain barrier in the normal mouse. Ann. Neurol. 19, 373–377 [DOI] [PubMed] [Google Scholar]
- 141.Meneses G et al. (2017) Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clin. Exp. Immunol. 190, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ong Q et al. (2015) Depot delivery of dexamethasone and cediranib for the treatment of brain tumor associated edema in an intracranial rat glioma model. J. Control. Release 217, 183–190 [DOI] [PubMed] [Google Scholar]
- 143.Jeon P et al. (2015) Dexamethasone-conjugated polyamidoamine dendrimer for delivery of the heme oxygenase-1 gene into the ischemic brain. Macromol. Biosci. 15, 1021–1028 [DOI] [PubMed] [Google Scholar]
- 144.Yamada K et al. (1983) Effects of methylprednisolone on peritumoral brain edema. A quantitative autoradiographic study. J. Neurosurg. 59, 612–619 [DOI] [PubMed] [Google Scholar]
- 145.Danielson M et al. (2018) Effects of methylprednisolone on blood–brain barrier and cerebral inflammation in cardiac surgery-a randomized trial. J. Neuroinflammation 15, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Martinez-Caceres EM et al. (2002) Treatment with methylprednisolone in relapses of multiple sclerosis patients: immunological evidence of immediate and short-term but not long-lasting effects. Clin. Exp. Immunol. 127, 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Richert ND et al. (2001) Interferonbeta-1b and intravenous methylprednisolone promote lesion recovery in multiple sclerosis. Mult. Scler. 7, 49–58 [DOI] [PubMed] [Google Scholar]
- 148.Barkhof F et al. (1994) Limited duration of the effect of methylprednisolone on changes on MRI in multiple sclerosis. Neuroradiology 36, 382–387 [DOI] [PubMed] [Google Scholar]
- 149.Zivadinov R et al. (2001) Effects of IV methylprednisolone on brain atrophy in relapsing-remitting MS. Neurology 57, 1239–1247 [DOI] [PubMed] [Google Scholar]
- 150.de Vargas LDS et al. (2017) Methylprednisolone as a memory enhancer in rats: effects on aversive memory, long-term potentiation and calcium influx. Brain Res. 1670, 44–51 [DOI] [PubMed] [Google Scholar]
- 151.Dos Santos N et al. (2019) High dose of dexamethasone protects against EAE-induced motor deficits but impairs learning/memory in C57BL/6 mice. Sci. Rep. 9, 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ye R et al. (2011) Ginsenoside Rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics 8, 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu C et al. (2017) Comparative analysis of ginsenosides in human glucocorticoid receptor binding, transactivation, and transrepression. Eur. J. Pharmacol. 815, 501–511 [DOI] [PubMed] [Google Scholar]
- 154.Sung WN et al. (2017) Korean Red Ginseng extract induces angiogenesis through activation of glucocorticoid receptor. J. Ginseng Res. 41, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ling C et al. (2005) Ginsenosides may reverse the dexamethasone-induced down-regulation of glucocorticoid receptor. Gen. Comp. Endocrinol. 140, 203–209 [DOI] [PubMed] [Google Scholar]
- 156.Ghoumari AM et al. (2006) Neuroprotective effect of mifepristone involves neuron depolarization. FASEB J. 20, 1377–1386 [DOI] [PubMed] [Google Scholar]
- 157.Belanoff JK et al. (2002) An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol. Psychiatry 52, 386–392 [DOI] [PubMed] [Google Scholar]
- 158.McCullers DL et al. (2002) Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience 109, 219–230 [DOI] [PubMed] [Google Scholar]
- 159.Check JH et al. (2014) Evidence that mifepristone, a progesterone receptor antagonist, can cross the blood brain barrier and provide palliative benefits for glioblastoma multiforme GRαde IV. Anticancer Res. 34, 2385–2388 [PubMed] [Google Scholar]
- 160.Lei B et al. (2014) Anti-inflammatory effects of progesterone in lipopolysaccharide-stimulated BV-2 microglia. PLoS One 9, e103969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y et al. (2012) Mifepristone-inducible caspase-1 expression in mouse embryonic stem cells eliminates tumor formation but spares differentiated cells in vitro and in vivo. Stem Cells 30, 169–179 [DOI] [PubMed] [Google Scholar]
- 162.Nguyen ET et al. (2017) A mixed glucocorticoid/mineralocorticoid receptor modulator dampens endocrine and hippocampal stress responsivity in male rats. Physiol. Behav. 178, 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Solomon MB et al. (2014) The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Norm. Behav. 65, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Szmulewitz R (2019) Study of drug 1 (enzalutamide) plus drug 2 (relacorilant) for patients with prostate cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT036748142019
- 165.Moraitis A (2019) A study of the efficacy and safety of relacorilant in patients with endogenous cushing syndrome. Available at: https://clinicaltrials.gov/ct2/show/NCT036971092019
- 166.Hunt H et al. (2018) Assessment of safety, tolerability, pharmacokinetics, and pharmacological effect of orally administered CORT125134: an adaptive, double-blind, randomized, placebo-controlled Phase 1 clinical study. Clin. Pharmacol. Drug Dev. 7, 408–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hunt HJ et al. (2017) Identification of the clinical candidate (R)-(1-(4- fluorophenyl)-6-((1-methyl-1H-pyrazol-4-yl)sulfonyl)-4,4a,5,6,7,8-hexahydro-1H-pyrazolo3,4-gisoquinolin-4a-yl)(4-(trifluoromethyl)pyridin-2-yl)methano ne (CORT125134): a selective glucocorticoid receptor (GR) antagonist. J. Med. Chem. 60, 3405–3421 [DOI] [PubMed] [Google Scholar]
- 168.Fabbri C et al. (2018) Pleiotropic genes in psychiatry: calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81, 203–210 [DOI] [PubMed] [Google Scholar]
- 169.Xu TZ et al. (2019) Ginsenoside Rg1 protects against H2O2induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int. J. Mol. Med. 43, 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Foroutan A et al. (1996) Effects of methylprednisolone on the GABA- and glutamate-induced currents: relevance to glucocorticoid-induced neurotoxicity and brain aging. Steroids 61, 354–366 [DOI] [PubMed] [Google Scholar]
- 171.Lopez FJ et al. (2008) LGD-5552, an anti-inflammatory glucocorticoid receptor ligand with reduced side effects, in vivo. Endocrinology 149, 2080–2089 [DOI] [PubMed] [Google Scholar]
- 172.Sun XC et al. (2016) Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation-induced dopaminergic neuronal degeneration in substantia nigra. J. Steroid Biochem. Mol. Biol. 155, 94–103 [DOI] [PubMed] [Google Scholar]
- 173.Wang GQ et al. (2017) Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 10, 441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 174.Chen J et al. (2019) GinsenosideRg1 promotes cerebral angiogenesis via the PI3K/Akt/mTOR signaling pathway in ischemic mice. Eur. J. Pharmacol. 856, 172418 [DOI] [PubMed] [Google Scholar]
- 175.Liu WL et al. (2008) Methylprednisolone inhibits the expression of glial fibrillary acidic protein and chondroitin sulfate proteoglycans in reactivated astrocytes. Glia 56, 1390–1400 [DOI] [PubMed] [Google Scholar]
- 176.Lee JM et al. (2008) Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J. Neurosci. 28, 3141–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zong Y et al. (2012) Ginsenoside Rg1 attenuates lipopolysaccharide-induced inflammatory responses via the phospholipase C-gamma1 signaling pathway in murine BV-2 microglial cells. Curr. Med. Chem. 19, 770–779 [DOI] [PubMed] [Google Scholar]
- 178.Schmitt KR et al. (2006) Methylprednisolone attenuates hypothermia- and rewarming-induced cytotoxicity and IL-6 release in isolated primary astrocytes, neurons and BV-2 microglia cells. Neurosci. Lett. 404, 309–314 [DOI] [PubMed] [Google Scholar]
- 179.Katychev A et al. (2003) Glucocorticoid-induced apoptosis in CNS microvascular pericytes. Dev. Neurosci. 25, 436–446 [DOI] [PubMed] [Google Scholar]
- 180.Araki T et al. (2006) Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia 47, 176–180 [DOI] [PubMed] [Google Scholar]
- 181.Almaabdi KH et al. (2014) Intravenous methylprednisolone for intractable childhood epilepsy. Pediatr. Neurol. 50, 334–336 [DOI] [PubMed] [Google Scholar]
- 182.Pera MC et al. (2015) Intravenous methylprednisolone pulse therapy for children with epileptic encephalopathy. Funct. Neurol. 30, 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Forster C et al. (2008) Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood–brain barrier. J. Physiol. 586, 1937–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Green AR and Curzon G (1968) Decrease of 5-hydroxytryptamine in the brain provoked by hydrocortisone and its prevention by allopurinol. Nature 220, 1095–1097 [DOI] [PubMed] [Google Scholar]
- 185.Lee MJ et al. (2016) Korean Red Ginseng and ginsenoside-Rb1/-Rg1 alleviate experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating regulatory T cells. Mol Neurobiol. 53, 1977–2002 [DOI] [PubMed] [Google Scholar]
- 186.Young AH et al. (2004) Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology 29, 1538–1545 [DOI] [PubMed] [Google Scholar]
- 187.Brown ES et al. (2001) Ketoconazole in bipolar patients with depressive symptoms: a case series and literature review. Bipolar Disord. 3, 23–29 [DOI] [PubMed] [Google Scholar]
- 188.Shirakawa H et al. (2006) Aminoglutethimide prevents excitotoxic and ischemic injuries in cortical neurons. Br. J. Pharmacol. 147, 729–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Quiros I et al. (2008) Melatonin prevents glucocorticoid inhibition of cell proliferation and toxicity in hippocampal cells by reducing glucocorticoid receptor nuclear translocation. J. Steroid Biochem. Mol. Biol. 110, 116–124 [DOI] [PubMed] [Google Scholar]
- 190.Wei Z et al. (2016) Icariin exerts estrogen-like activity in ameliorating EAE via mediating estrogen receptor β, modulating HPA function and glucocorticoid receptor expression. Am. J. Transl Res. 8, 1910–1918 [PMC free article] [PubMed] [Google Scholar]
- 191.Zeng KW et al. (2010) Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int. Immunopharmacol. 10, 668–678 [DOI] [PubMed] [Google Scholar]
- 192.Chen J et al. (2016) A prospective study of dexamethasone therapy in refractory epileptic encephalopathy with continuous spike-and-wave during sleep. Epilepsy Behav. 55, 1–5 [DOI] [PubMed] [Google Scholar]