Abstract
Objective:
A cerebrospinal fluid leak is one of the most serious complications in Otolaryngology. It may occur as a result of injury to the skull base, typically traumatic or iatrogenic. While the presence of a leak is often discerned in the emergent setting, it can be difficult to distinguish normal secretions from those containing cerebrospinal fluid during postoperative visits in the clinic. As most current laboratory-based assays are labor-intensive and require several days to result, we aim to develop a more user-friendly and rapid point-of-care cerebrospinal fluid detection device.
Study Design:
A barcode-style, lateral-flow immunoassay utilizing antibodies for beta-trace protein, a protein abundant in and specific for cerebrospinal fluid, has been developed by our laboratory using a concentration of 1.3 mg/L of beta-trace protein to delineate a positive result.
Setting:
Tertiary medical center
Subjects and Methods:
Tests using known concentrations of resuspended beta-trace protein and the contents of discarded lumbar drains (presumed to contain cerebrospinal fluid) were performed to validate our novel device.
Results:
Our results demonstrate the ability of our device to semi-quantitatively identify concentrations of beta-trace protein from 0.3–90 mg/L, which is within the required range to diagnose a leak, thus making beta-trace an excellent target for rapid clinical detection.
Conclusion:
Herein we detail the creation and initial validation of the first point-of-care cerebrospinal fluid detection device. This device is a feasible method to more efficiently and cost-effectively identify cerebrospinal fluid leaks, minimize costs, and improve patient outcomes.
Keywords: Cerebrospinal fluid leak, Semi-quantitative, Lateral-flow immunoassay, Diagnostic, Point-of-care, Otolaryngology, Beta-trace protein
Introduction
A communication between the fluid surrounding the brain and the outside world, also known as a cerebrospinal fluid or CSF leak, is a known complication of numerous procedures in Otolaryngology. The risk in endoscopic skull base surgery has been estimated at 13.8%, in endoscopic sinus surgery at 0.17%, and in cochlear implantation at 0.4%.1–3 Almost all procedures involving the sinuses, skull base, or ear have some risk of a leak. Individuals who have not undergone procedures are at risk as well. Trauma patients may have leaks from facial and skull base fractures, while some individuals are even unfortunate enough to present with rare spontaneous conditions such as middle cranial fossa CSF otorrhea.4
In the acute setting, imaging modalities such as magnetic resonance imaging or computed tomography are used for assessment. If there is high enough clinical suspicion (obvious facial or skeletal deformities or copious clear rhinorrhea or otorrhea), patients may be taken directly to the operating room for management. This involves identifying the site of the leak and using either native tissue or biocompatible materials to patch the affected site.
Identifying a CSF leak in the outpatient or postoperative hospital setting is often more difficult. It is not unusual for postoperative patients to have secretions, and therefore, distinguishing normal secretions from those containing CSF may be a challenging task. When physicians are concerned about a CSF leak, no proven diagnostic modalities exist that allow them to rapidly and non-invasively rule out the presence of a leak. Physicians must then turn to the same imaging techniques that are used in more acute settings, the cost of which may be difficult to justify in a patient who seems otherwise well.
Alternative methods of identifying CSF have been developed. The current laboratory gold standard for CSF detection involves identification of beta-2 transferrin through either electrophoresis or the enzyme-linked immunosorbent assay. These tests are 94–100% sensitive and 98–100% specific in detecting CSF; however, they typically require samples to be sent off to a central laboratory where days to weeks pass before results return.5 Despite the promising role of beta-2 transferrin testing in published guidelines, this test is often not useful to guide clinical decision making due to the time it takes for results to return. Other methods of CSF detection have been explored. These include glucose testing, cisternography, and spotting the CSF to look for a “halo” or “ring” sign. Unfortunately, literature has not found these methods to be sensitive or specific.6 As an alternative, researchers have looked at beta-trace protein (PTP) also known as prostaglandin D2 synthase lipocalin-type. βTP is a ubiquitous protein present at various concentrations in different body compartments, but notably one to two orders of magnitude higher in CSF than in nasal secretions and serum (Table 1). As a result, studies have demonstrated that nasal secretions with βTP concentrations measured above a certain threshold are suggestive of a CSF leak and need for immediate operative intervention.7–11 In a recent study by Bernasconi and coworkers using the nephelometric assay, it was determined that concentrations of βTP ≥1.3 mg/L indicate the presence of a CSF leak, whereas concentrations of βTP <0.7 mg/L indicate the absence of a CSF leak. βTP concentrations between 0.7 and 1.29 mg/L (denoted the “gray zone”) required further analysis and comparison with the patient’s βTP serum level to confirm the presence or absence of a CSF leak. This approach showed a sensitivity and specificity of 98.3% and 96%, respectively.13 While the nephelometric assay is more rapid than electrophoresis, it still requires centralized laboratory equipment that may not be available in every clinical setting. To address this issue, here we describe the development of a semi-quantitative, barcode-style lateral-flow immunoassay (LFA) for the detection of βTP. By classifying a sample as having a concentration of either <0.7, between 0.7 and 1.29, or ≥1.3 mg/L βTP, we predict that our equipment-free and disposable device, will allow clinicians to more rapidly and affordably identify the presence of a CSF leak.
Table 1:
Mean concentrations of βTP found in various bodily fluids as reported in the literature
| Nasal Secretion (mg/L) | Serum (mg/L) | Lumbar CSF (mg/L) | Proposed Cut-off (mg/L) | |
|---|---|---|---|---|
| Arrer et al.8 | 0.39 | 0.59 | 19.6 | 1.31 |
| Reiber et al.9 | 0.016 | 0.59 | 18.4 | 0.35a |
| Schabel et al.10 | < 0.25 | 16.3 | 1 |
If nasal secretion sample contains blood, use 1 mg/L instead of 0.35 mg/L.
Methods
Preparation of the barcode-style lateral-flow immunoassay (LFA)
All reagents and materials were purchased from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. First, to form antibody functionalized gold nanoprobes (GNPs), 35 μL of a 0.1 M sodium borate (pH 9) solution was added to 1 mL of a 40 nm citrate-capped gold nanoparticle suspension (Nanocomposix, San Diego, CA). Subsequently, 8 μg of anti-βTP antibodies were added and the mixture was allowed to react for 30 min. To prevent nonspecific binding of other proteins to the gold nanoparticles, 100 μL of a 10% w/v bovine serum albumin (BSA) solution was added to the mixture and allowed to react for 10 min. To purify unbound antibodies from the nanoparticles, the mixture was centrifuged and the pellet was resuspended in 100 μL of a 0.1 M sodium borate (pH 9) solution.
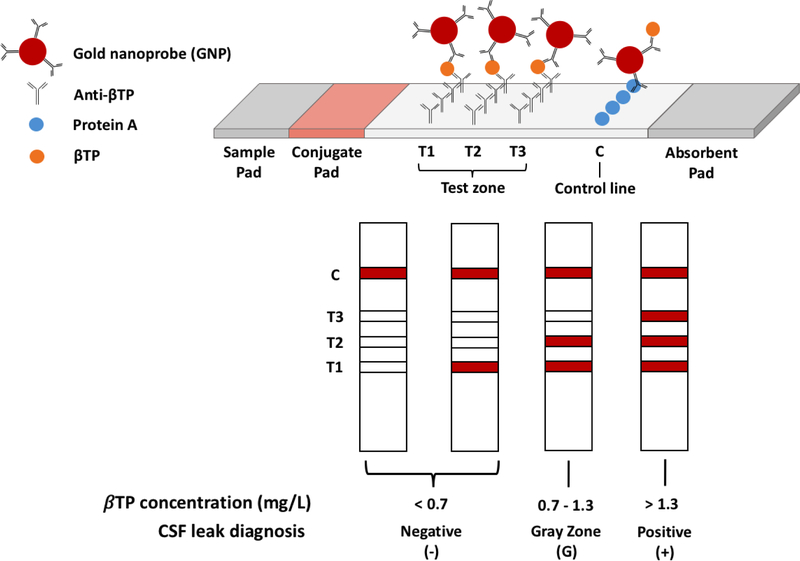
The LFA test strip is composed of overlapping pads secured to an adhesive backing (Fig. 1). These pads include a sample pad, a conjugate pad, a nitrocellulose membrane, and an absorbent pad. The sample pad consists of a 3 × 10 mm fiberglass paper treated with a 0.1 M Tris (pH 9) solution containing 1% BSA. GNPs were dehydrated onto a 3 × 10 mm fiberglass paper along with 1% BSA in diH2O to form the conjugate pad. Both the sample and conjugate pads were dehydrated under very low pressure using a Labconco FreeZone 4.5 lyophilizer (Fisher Scientific, Hampton, NH) for 2 hours.
Figure 1:
Schematic representation of our barcode-style LFA for the detection of βTP and CSF leaks
Anti-βTP antibodies were printed and immobilized on a nitrocellulose membrane at three different test line locations (T1, T2, and T3) to form the test zone. The antibodies at the T1, T2, and T3 locations were immobilized at 2, 0.5, and 0.35 mg/mL, respectively. These concentrations were experimentally determined to give the desired detection cutoffs. Protein A was immobilized at a concentration of 0.2 mg/mL on the nitrocellulose membrane downstream of the test zone to form the control line. Test and control line printing was performed using an Automated Lateral Flow Reagent Dispenser (Claremont BioSolutions, Upland, CA) and a Fusion 200 syringe pump (Chemyx, Stafford, TX) with a flow rate of 250 μL/min. The printed membrane was left in a vacuum-sealed desiccation chamber overnight. After protein immobilization, membranes were immersed in a 0.1 M Tris (pH 9) solution containing 1% BSA for 1 hour and dehydrated under very low pressure overnight using a lyophilizer.
To assemble the test strip, the absorbent pad was first adhered at the far end of the test strip, downstream of the control line, overlapping the nitrocellulose membrane. The conjugate pad was placed at the opposite end of the strip, upstream of the test zone, overlapping the nitrocellulose membrane. Lastly, the sample pad was placed on the test strip overlapping the conjugate pad. After assembly, the LFA strips were placed in bags containing drierite desiccant (Fisher Scientific, Hampton, NH), which were then placed inside an auto-desiccant chamber (Fisher Scientific, Hampton, NH) for storage.
Detection of βTP with the barcode-style LFA
To demonstrate the ability of the barcode-style LFA to detect and quantify βTP, we tested it with a variety of samples including recombinant βTP (Mybiosource, San Diego, CA), human serum, and human CSF mixed with human serum. These samples were first diluted in various amounts in phosphate-buffered saline (PBS) in order to adjust the βTP concentration to find the optimal detection range of the barcode-style LFA. Briefly, samples containing known concentrations of recombinant βTP in PBS (0.3–90 mg/L) were diluted 150-fold in PBS. Pooled human serum (Sigma-Aldrich, St. Louis, MO), which served as human βTP-containing samples that were negative for CSF, were diluted by 50-, 150-, and 500-fold in PBS. Lastly, varying dilutions of CSF in human serum (2-fold, 5-fold, 10-fold), which served to simulate nasal drip samples that contain varying amounts of CSF, were diluted by 150-fold in PBS.
Prior to running the assay, the above PBS-diluted samples were further diluted by 2-fold by mixing 25 μL of the samples with 25 μL of running buffer (0.4% BSA, 0.6% Tween 20, 0.2% polyethylene glycol, 0.1 M Tris buffer, pH 9) in a test tube. The LFA test strip was dipped vertically into the tube with the sample pad submerged. After 20 min, the test strips were imaged by a Canon EOS 1000D camera (Canon U.S.A., Inc., Lake Success, NY) in a controlled lighting environment. Three different CSF samples were tested with our assay. Representative images from one of these samples are shown.
Results
In our barcode-style LFA, the presence of the target biomarker would produce one to three visible test lines as the GNPs would first bind to the βTP in the sample and then be captured at the test lines (Fig. 1). Each test line has a cut-off, which is the minimum concentration of βTP in the sample that is necessary for that test line to become visible, and these cut-offs can be adjusted by varying the density of the capture antibody immobilized. Regardless of the presence of βTP in the sample, Protein A at the control line would bind to the antibodies on the GNPs resulting in the formation of a visible red control line, indicating successful sample flow through the strip and thus valid test results.
We aimed to design our barcode-style LFA to have test line cut-off values that correspond to the threshold values established by Bernasconi and coworkers.13 At βTP concentrations of <0.7 mg/L, zero or one test line should appear indicating no CSF leak. βTP concentrations between 0.7 and 1.3 mg/L, which are in the “gray zone”, should result in two test lines appearing. Samples classified within the gray zone cannot confidently be diagnosed and should undergo further testing before a clinical decision is made. Samples containing concentrations greater than 1.3 mg/L should result in the formation of three test lines, which would indicate the presence of a CSF leak.
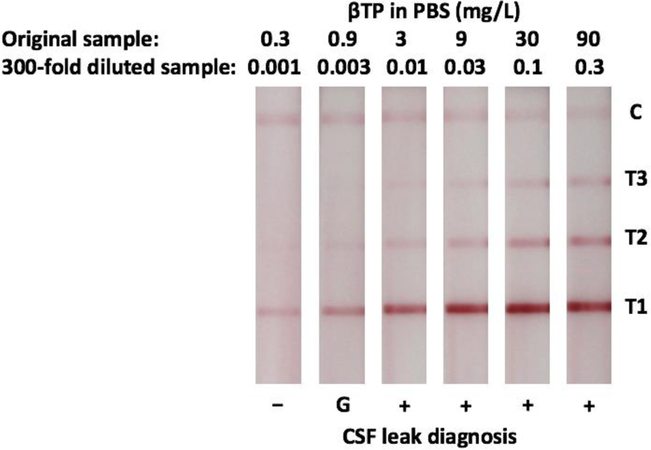
Initial range of detection and cut-off values of the LFA were determined using known concentrations of recombinant βTP in PBS. In all cases, pink control lines were visible indicating valid test results. βTP was accurately detected at all concentrations tested indicated by the formation of visible pink test lines on all strips. More specifically, when the original sample contained 0.3 mg/L of βTP, one visible test line at the T1 position was present, at 0.9 mg/L two visible test lines at the T1 and T2 position were present, and at 3 mg/L three visible test lines at the T1, T2, and T3 position were present (Fig. 2). These results correctly correspond with the desired cut-off values.
Figure 2:
Barcode-style LFA results for the detection of recombinant βTP in PBS. Negative (−), Gray Zone (G), and Positive (+) results are indicated.
Next, the LFA was tested with samples of pooled human serum. It was able to detect native βTP for all dilutions tested, indicated by the appearance of a visible line at the T1 location on all strips (Fig. 3). For the 300-fold and 1000-fold preanalytical sample dilutions of pooled human serum, T1 was the only visible line. These samples were thus classified as negative for the presence of CSF using our assay. In contrast, when the serum was diluted by only 100-fold, there were visible test lines at the T1 and T2 locations, which corresponds to the gray zone.
Figure 3:
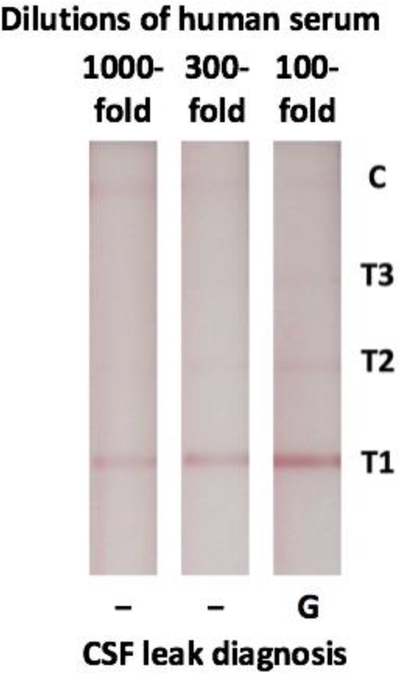
Barcode-style LFA results for the detection of native βTP from human serum. Negative (−) and Gray Zone (G) results are indicated.
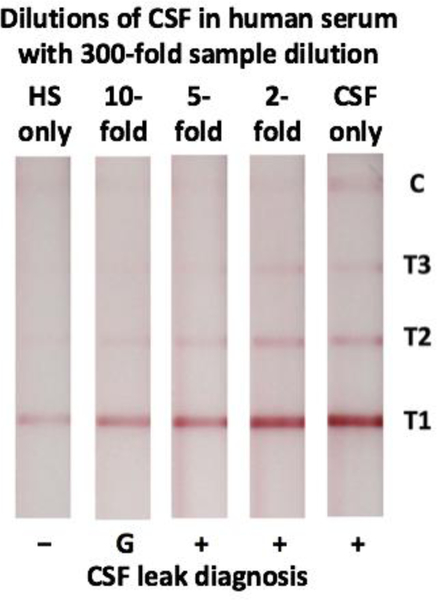
Finally, multiple samples of CSF obtained from de-identified lumbar drains were mixed with human serum to simulate patient nasal drip samples containing varying amounts of CSF. These samples were then diluted by 300-fold prior to application to the LFA (Fig. 4). When tested on the LFA, CSF that was not mixed in serum (CSF only) produced visible lines at all three test line locations. Three visible test lines were also visible down to the 5-fold dilution of CSF in human serum, correctly indicating these samples were positive for CSF. The 10-fold dilution of CSF in human serum produced two visible test lines, classifying this sample as in the gray zone where further testing is recommended. Lastly, human serum without any CSF produced only one test line, correctly indicating the sample is negative for CSF.
Figure 4:
Detection of CSF diluted in human serum (HS) with our barcode-style LFA. Negative (−), Gray Zone (G), and Positive (+) results are indicated.
Discussion
There is a need for a point-of-care test that would allow for rapid detection of skull base violation at the bedside and in clinics. The implications of a device capable of detecting CSF also extend beyond the field of Otolaryngology. Such a device could be used to identify injuries to the spinal cord or globe and may have a role in ruling out CSF leaks in postoperative neurosurgical patients with a low pretest probability of having a leak. One biomarker for detecting CSF leaks is βTP which has shown excellent sensitivity and specificity in a research setting. Unfortunately, it has not been widely accepted as a target in clinical detection, partly due to the current assays requiring expensive and centralized equipment that is not readily available in many clinical settings. This makes it an excellent candidate for novel translational advances. Therefore, we have created a barcode-style LFA that quantifies the βTP concentration in a sample for the rapid detection of CSF leaks.
Initial testing demonstrated that our LFA was able to detect concentrations of recombinant βTP much lower than the desired cut-offs, so it was determined that a 300-fold preanalytical sample dilution would be required to adjust the range of detection and reduce the instance of false positives (Fig. 2). When moving to patient samples, this preanalytical sample dilution also had the added benefit of reducing viscosity to enable better flow. Next, dilutions of human serum were tested with the LFA to confirm its ability to detect native human βTP. The βTP concentration of the serum is expected to be close to the previously reported average of 0.59 mg/L10,11. This is below the 0.7 mg/L cut-off for a negative result and so we expect one test line to be visible on the LFA strip with the appropriate dilution. The results shown in Fig. 3 support the use of the previously determined 300-fold preanalytical sample dilution to ensure this true negative result (one test line). While we acknowledge that the βTP concentration in serum is different (and greater) than that in nasal secretions, this was an important negative control as serum is a possible contaminant in nasal secretions.
Lastly, when a sample of CSF from lumbar drains (CSF only) was diluted by 300-fold and tested with the LFA, it was correctly identified as positive (Fig. 4). In addition to being able to detect pure CSF, it is important that our assay can correctly detect CSF that has been diluted in serum, as it is common for CSF to be diluted in nasal or otologic secretions prior to collection. When the sample to be tested containing CSF was diluted in the human serum, positive detection was observed down to the 5-fold dilution of CSF in serum, while the 10-fold dilution was classified as in the gray zone. While the gray zone is not a confirmatory result and future laboratory testing should be performed in a clinical setting, it does suggest that our device has the ability to identify leaks that have been diluted up to 10-fold by nasal or otologic secretions. This is comparable to previously reported values for beta-2 transferrin electrophoresis.14,15
Note that the absence of a visible control line on all tests containing human serum (Figs. 3 and 4) was not due to inadequate flow of the sample solution through the test, as all of the sample was wicked out of the test tube. We attributed this weak control line to endogenous antibodies in the human serum outcompeting the antibodies on the GNPs for Protein A on the control line. In the future version of our test strip, we will adjust this by replacing Protein A with a species specific secondary antibody.
Limitations of this device may include the detection of very dilute (<10%) samples and detection of intermittent leaks. These limitations, however, both similarly apply to samples analyzed electrophoretically for beta-2 transferrin.14,15 Also, while the stratified barcode readout makes interpretation simple, further testing and evaluation may still be required when samples test within the “gray zone”, limiting its use as a standalone test.
Other important considerations include contamination of samples with other body fluids known to contain βTP. It has been shown that individuals with end-stage renal disease, cardiovascular disease, and bacterial meningitis may have serum concentrations of βTP in the range of positive detection by our device.16,17 In the instance of cardiovascular disease, this has been cited as 1.33 +/− 0.63 mg/L, well within the range of positive detection by our device.16 These findings emphasize the importance of ensuring that samples do not have blood contamination and may cause clinicians to more carefully consider patient co-morbidities when a positive result is obtained.18,19 Furthermore, concentrations of βTP in nasal secretions are much lower than those in serum, and in the absence of serum contamination, should be one to two orders of magnitude lower than the βTP concentrations in CSF.11 An uncontaminated nasal secretion sample is thus unlikely to be incorrectly diagnosed as containing CSF. These findings highlight the importance of pre-test clinician suspicion for a CSF leak and suggest that this device may have a powerful role in excluding CSF leaks in appropriately selected patient populations.
Future work will involve the testing of healthy human nasal secretions to ensure true negative test results in this population. Additionally, equivocal samples from patients with suspected CSF leaks will be tested to evaluate the sensitivity and specificity of our device.
Conclusion
Cerebrospinal fluid leaks are an excellent target for point-of-care testing in Otolaryngology. The ability of a simple device to accurately detect cerebrospinal fluid will help diagnose these devastating leaks more rapidly and rule them out less expensively. Here we describe the creation of a barcode-style, lateral-flow immunoassay that detects beta-trace protein and classifies the concentration of detected protein in a semi-quantitative fashion into three ranges which allow for ease of clinical interpretation. In initial studies, this device has been able to detect resuspended beta-trace protein as well as cerebrospinal fluid samples diluted in serum up to 10%. This device shows that bedside cerebrospinal fluid identification is feasible and has the potential to influence decision-making without additional expensive diagnostic workup.
Acknowledgments
This work was supported by a 2016 Academy Core Resident Research Award and by the National Institutes of Health (Grant NS099800).
Footnotes
Conflict of Interest
D.T. Kamei is a co-founder of the company Phase Diagnostics that has interest in commercializing this technology.
This paper was presented at the 2016 Academy of Otolaryngology Annual Meeting in San Diego, CA in a Talk Titled “Point of Care Cerebrospinal Fluid Detection”.
The development of this device also received a 2016 Academy Core Resident Research Award.
References:
- 1.Naunheimer MR, Sedaghat AR, Lin DT, et al. Immediate and delayed complications following endoscopic skull base surgery. J Neurol Surg B Skull Base. 2015;76:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramakrishnan VR, Kingdom TT, Nayak JV, et al. Nationwide incidence of major complications in endoscopic sinus surgery. Int Forum Allergy Rhinol. 2012;2:34–39. [DOI] [PubMed] [Google Scholar]
- 3.Daneshi A, Ajalloueyan M, Ghassemi MM, et al. Complications in a series of 4400 paediatric cochlear implantation. 2015;79:1401–1403. [DOI] [PubMed] [Google Scholar]
- 4.Rao N, Redleaf M. Spontaneous middle cranial fossa cerebrospinal fluid otorrhea in adults. Laryngoscope. 2016;126:464–468 [DOI] [PubMed] [Google Scholar]
- 5.Marshall AH, Jones NS, Robertson IJ. An algorithm for the management of CSF rhinorrhea illustrated by 36 cases. Rhinology. 1999;37:184–185 [PubMed] [Google Scholar]
- 6.Oakley GM, Alt JA, Schlosser RJ, et al. Diagnosis of cerebrospinal fluid rhinorrhea: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:8–16 [DOI] [PubMed] [Google Scholar]
- 7.Bachmann-Harildstad G Diagnostic values of beta-2 transferrin and beta-trace protein as markers for cerebrospinal fluid fistula. Rhinology. 2008. 46: 82–85. [PubMed] [Google Scholar]
- 8.Arrer E, Meco C, Oberascher G, et al. p-Trace protein as a marker for cerebrospinal fluid rhinorrhea. Clin Chem. 2002;48:939–941. [PubMed] [Google Scholar]
- 9.Reiber H, Walther K, Althaus H. Beta-trace protein as sensitive marker for CSF rhinorhea and CSF otorhea. Acta Neurol Scand. 2003;108:359–362. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel C, Di Martino E, Gilsbach JM, et al. Comparison of β2-Transferrin and β-Trace Protein for Detection of Cerebrospinal Fluid in Nasal and Ear Fluids. Clin Chem. 2004;50:661–663. [DOI] [PubMed] [Google Scholar]
- 11.Morell-Garcia D, Bauca JM, Sastre MP, et al. Sample-dependent diagnostic accuracy of prostaglandin D synthase in cerebrospinal fluid leak. Clin Biochem. 2017;50:27–31. [DOI] [PubMed] [Google Scholar]
- 12.Sampaio MH, de Barros-Mazon S, Sakano E. Predictability of quantification of beta-trace protein for diagnosis of cerebrospinal fluid leak: cutoff determination in nasal fluids with two control groups. Am. J. Rhinol. Allergy 2009;23:585–590. [DOI] [PubMed] [Google Scholar]
- 13.Bernasconi L, Potzl T, Steuer C, et al. Retrospective validation of a p-trace protein interpretation algorithm for the diagnosis of cerebrospinal fluid leakage. Clin Chem Lab Med. 2017;55:554–560. [DOI] [PubMed] [Google Scholar]
- 14.Oberascher G, Arrer E. Efficiency of Various Methods of Identifying Cerebrospinal Fluid in Oto- and Rhinorrhea. ORL 1986;48(6):320–5 [DOI] [PubMed] [Google Scholar]
- 15.Normansell DE, Stacy EK, Booker CF, et al. Detection of beta-2 Transferrin in Otorrhea ad Rhinorrhea in a Routine Clinical Laboratory Setting. Clin Diagn Lab Immunol. 1994;1(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster MC, Coresh J, Chi-yuan H, et al. Serum B-Trace Protein and B2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults with CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 2016;68:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melegos DN, Grass A, Pierratos A, et al. Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology. 1999;53:32–37 [DOI] [PubMed] [Google Scholar]
- 18.Melegos DN, Diamdnis EP, Oda H, et al. Immunofluorometric assay of prostaglandin D synthase in human tissue extracts and fluids. Clin. Chem 1996;42:1984–1991. [PubMed] [Google Scholar]
- 19.Risch L, Lisec I, Jutzi M, et al. Rapid, accurate and non-invasive detection of cerebrospinal fluid leakage using combined determination of beta-trace protein in secretion and serum. Clin Chim Acta. 2005;351(1–2):169–176. [DOI] [PubMed] [Google Scholar]