Abstract
Background and Objective:
To describe depth-resolved macular microvasculature abnormalities in patients with familial exudative vitreoretinopathy (FEVR) using optical coherence tomography angiography (OCT-A).
Study Design:
Twenty-two eyes (11 eyes of 6 patients with FEVR and 11 control eyes) were imaged with OCT-A. Graders qualitatively analyzed the OCT-A images of the superficial and deep vascular complexes for abnormal vascular features and compared to fluorescein angiography (FA).
Results:
Seven of 11 eyes with FEVR displayed abnormal macular vascular findings. Abnormalities in the SVC included dilation, disorganization, straightening, heterogeneous vessel density, and curls/loops. In the DVC abnormalities included areas of decreased density, disorganization, curls/loops, and “end-bulbs”. Except for dragging and straightening of the vessels, none of these macular features were visible on FA.
Conclusions:
OCT-A revealed marked macular abnormalities in eyes with FEVR that have not been previously observed with FA alone, suggesting this is more than a disease of the retinal periphery with macular and deep retinal vasculature abnormalities.
Introduction
Familial exudative vitreoretinopathy (FEVR) is a rare, inherited disorder of retinal vascular development leading to incomplete and anomalous vascularization of the peripheral retina1,2. The disease is thought to be caused by genetic mutations in the Wnt signaling pathway that is necessary for retinal angiogenesis3. Genetic mutations in Wnt pathway genes NDP4, FZD45,6, LRP57, TSPAN128, ZNF4089, CTNNB110, and KIF1111 have been implicated in the pathogenesis of FEVR; however, these genes account for only a fraction of patients with clinically-diagnosed disease12. Thus, clinical examination remains the gold standard for diagnosis. Patients with FEVR present with varying severity, possibly due to variable gene expressivity2,13,14, ranging from asymptomatic areas of nonperfusion in the retinal periphery to vitreoretinal adhesions, retinal folds, temporal macular dragging, neovascularization, subretinal exudation, and tractional retinal detachments that form secondary to the retinal ischemia14–16.
Prior imaging studies of the retinal vasculature in patients with FEVR using ultra-wide-field imaging and fluorescein angiography (FA) have described a range of associated retinal features, such as aberrant peripheral vessels, arterial tortuosity, telangiectasias, and capillary agenesis and have advanced the understanding of this disease16,17. However, those modalities do not allow for depth-resolved assessment of the retinal microvasculature. Optical coherence tomography angiography (OCT-A) is a non-invasive, high-resolution imaging modality that enables superior visualization of macular retinal microvasculature with differentiation of superficial, penetrating, and deep vascular complexes18–20. Based on previously reported findings in mutant mice with deficient Wnt signaling21,22, we hypothesized that patients with FEVR imaged using OCT-A would not only exhibit the gross vascular abnormalities observed on FA, but would also show abnormalities in the vertical penetration of the deeper retinal layers due to incomplete angiogenesis. In this study, we describe the macular superficial and deep retinal microvasculature changes observed in 11 eyes of 6 patients with FEVR compared to 11 age-appropriate control eyes.
Methods
A total of 22 eyes were imaged, including 11 eyes of 6 patients with clinically-diagnosed FEVR16 (mean age 17.5±7.5 years, median 20 years, range 2–25 years; 4 female, 2 male; 2 black, 2 white, 2 Hispanic; 5 born full-term, 1 born at 31 weeks’ gestation) and 11 control eyes of 11 patients without retinal disease per ophthalmic exam (age range 1.25 – 64 years, mean 18.3 ± 15.8 years, median 15 years; 5 female, 6 male; 3 black, 6 white, 2 Hispanic; all born full-term). Diagnosis of FEVR was determined by pediatric retinal specialists (L.V., C.A.T.) and based on clinical diagnostic criteria including fundus exam, history, and fluorescein angiography findings. Genetic testing was offered to all patients however due to insurance coverage, was only available to a few patients. The age-appropriate control patients were recruited from the existing patient population at the Duke University Eye Center who presenting for routine dilated ophthalmic exams or refractive error (2), strabismus surgery in the fellow eye (1), or unilateral pathology in the fellow eye (7). All patients were imaged using investigational Spectralis SD-OCT tabletop or Flex modules integrated with the OCT-A software (version 6.9, Heidelberg Engineering, Heidelberg, Germany). The Duke University Institutional Review Board approved this study and informed consent was prospectively obtained in all cases. The study followed the tenets of the Declaration of Helsinki. Two infants were imaged supine in the operating room using the Flex module and the remaining patients were imaged upright in clinic using the tabletop unit. One eye with FEVR was excluded due to significant media opacity that prevented acquisition of high quality images.
For all eyes imaged, a 10×10° image comprised of 512 A-scans per B-scan and 512 B-scans of the macula was captured. A trained grader (S.T.H.) reviewed the automated segmentation of the retinal layers, and manually corrected the segmentation if needed. OCT-A images were segmented and rendered by Spectralis software as follows: superficial vascular complex (SVC) from ILM to 17μm above the lower boundary of the internal plexiform layer (IPL), and deep vascular complex (DVC) from 17μm above IPL to the bottom boundary of the outer plexiform layer (OPL). The DVC images had the projection artifact removal feature of the software enabled.
The OCT-A images of the SVC and DVC of all 22 eyes were then randomly ordered for masked grading. Five experienced graders (A.P.F., X.C., R.H., C.A.T., L.V.) with experience in reviewing OCT-A images were masked to all clinical information including diagnosis, age, sex, and race/ethnicity. The graders were first trained on a set of OCT-A images of the SVC and DVC of 3 control eyes. They then graded the 22 sets of OCT-A images of the de-identified eyes, and analyzed the images qualitatively for each of these features: abnormal FAZ shape, heterogeneous areas of increased or decreased vessel density, disorganized vessel pattern, vessel dilation, stub-like vessel terminations, vascular curls and loops, and straightened vessels. These parameters are defined in Table 1 and examples of these findings are demonstrated in Figure 1. Qualitative features were considered present on OCT-A if the majority of readers (at least 3 of 5) agreed.
Table 1:
Definitions of Qualitative OCT-A Grading Characteristics in FEVR
| OCT-A Characteristic | Defining characteristics when compared to normals |
|---|---|
| Abnormal FAZ shape | Elongated, abnormally stretched FAZ or overall irregularity in shape |
| Increased or decreased vessel density | Areas of subjectively increased or decreased concentration of vessels |
| Disorganized vessel pattern | Deviation from the expected patterns of larger caliber vessels branching into finer vessels in the SVC and a regular and uniform lacy pattern in the DVC |
| Vessel dilation | Any vessels of larger than expected diameter |
| Stub-like terminations | Abnormally truncated vessels that exhibited bulbous ends |
| Vascular curls and loops | Evidence of vascular shunting, anastomoses, or curling patterns not seen in controls |
| Straightened vessels | Abnormally dragged or more linear appearing vessels than expected |
Figure 1.
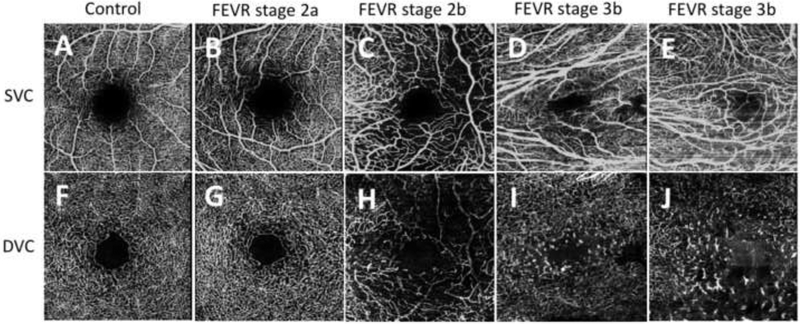
Optical coherence tomography angiography showing the superficial vascular complex (SVC) (top row A-E) and corresponding deep vascular complex (DVC) (bottom row F-J) for a (A,F) 16-year-old healthy control, (B,G) 22-year-old patient with familial exudative vitreoretinopathy (FEVR) stage 2a in the right eye, (C,H) 2-year-old with familial exudative vitreoretinopathy (FEVR) stage 2b in the left eye, (D,I) 16-year-old with FEVR stage 3b in the right eye, and (E,J) 21-year-old with FEVR stage 3b in the left eye. The SVC images show vessel dilation (C,D,E), disorganization (C,D,E), straightening (D,E), areas of increased and/or decreased density (C,D,E), and curls and loops (C,E). The DVC images show areas of decreased density (H,I,J), disorganization (H,I,J), and “end-bulbs,” or stub-like vessel terminations (H,I,J).
Clinical and demographic information including gestational age at birth, age, sex, race/ethnicity, ophthalmic examination findings, and any genetic testing performed for known FEVR mutations were reviewed for all patients. Eyes were classified into FEVR stages at initial presentation using the clinical staging criteria previously published by Pendergast and Trese.23 An ophthalmologist (A.P.F) also retrospectively analyzed FA images, all of which were obtained on a wide-field imaging system, Optos 200Tx or Retcam3, (Optos, Dunfermline, Scotland; Natus Medical Inc., Pleasanton, CA, USA) corresponding to the time of OCT-A imaging focusing on characterizing any macular abnormalities.
Results
The OCT-A and FA findings of this study are summarized in Table 2. FEVR staging15,23 based on examination at the time of presentation was as follows: stage 2a (n=2 eyes), stage 2b (n=5 eyes), stage 3b (n=3 eyes), and stage 5a (n=1 eye). Four of the 11 eyes (2/2 eyes with FEVR stage 2a, 2/5 eyes with stage 2b) of two patients who were siblings showed no vascular abnormalities on OCT-A in either the SVC or DVC. The remaining 7 eyes (3/5 eyes with FEVR stage 2b, 3/3 eyes with stage 3b, 1/1 eye with stage 5) imaged had abnormal FAZs, SVCs and DVCs with specific features described below, based on the masked reviewer grading.
Table 2.
Retinal vascular features on optical coherence tomography angiography (OCT-A) and fluorescein angiography of familial exudative vitreoretinopathy (FEVR).
| Patient, eye | FEVR Stage | Macular OCT-A findings | Fluorescein angiography findings | |||
|---|---|---|---|---|---|---|
| FAZ | SVC | DVC | Macula | Periphery | ||
| 1, OD | 2a | Normal | No abnormalities | No abnormalities | Mild vessel straightening | Temporal/inferotemporal nonperfusion, leakage at the border of perfused and nonperfused retina |
| 1, OS | 2b | Normal | No abnormalities | No abnormalities | No abnormalities | Temporal nonperfusion, 2 small peripheral vascular loops, staining of prior laser |
| 2, OD | 2b | Normal | No abnormalities | No abnormalities | No abnormalities | Staining of laser, leakage in inferotemporal periphery |
| 2, OS | 2a | Normal | No abnormalities | No abnormalities | No abnormalities | Temporal nonperfusion, no leakage |
| 3, OD | 2b | Abnormal | Decreased density, dilated vessels | Decreased density, disorganized, end bulbs, curls/loops | No abnormalities | Leakage in the temporal/superotemporal mid-periphery, mild peripheral nonperfusion, staining of prior laser |
| 3, OS | 2b | Abnormal | Decreased density, disorganized, dilated vessels, curls/loops | Decreased density, disorganized, end bulbs, curls/loops | Mild vessel straightening | Staining 360-degree laser treatment |
| 4, OD | 3b | Abnormal | Disorganized, dilated, straightened vessels | Decreased, density, disorganized, end bulbs | Macular dragging, vessel straightening, hyper-fluorescence of preretinal fibrosis | Nonperfusion in the temporal/nasal periphery, late leakage in inferotemporal periphery, hyperfluoresence of preretinal fibrosis |
| 4, OS | 2b | Abnormal | Disorganized, dilated, straightened vessels | Decreased density, disorganized, end bulbs, curls/loops | Significant macular dragging and straightening | Superotemporal/temporal/inferotemporal nonperfuson, temporal vascular shunting, leakage of vasculature in superotemporal/temporal periphery |
| 5, OD | 3b | Abnormal | Decreased density, disorganized, dilated, straightened vessels, curls/loops | Decreased density, disorganized, end bulbs, curls/loops | Mild staining of macular pigmentary changes | Leakage in temporal periphery, staining of prior laser |
| 5, OS | 5 | Not imaged due to bullous keratopathy | ||||
| 6, OD | 5 | Abnormal | Decreased density, disorganized, dilated, straightened vessels | Decreased density, disorganized, end bulbs | Macular dragging, vessel straightening | Staining of peripheral chorioretinal scarring |
| 6, OS | 3b | Abnormal | Decreased density, disorganized, dilated, straightened vessels, curls/loops | Decreased density, disorganized, end bulbs | Macular dragging, vessel straightening, telangiectatic vessels in temporal macula, leakage in temporal macula | Staining of peripheral chorioretinal scarring, late temporal/nasal/inferior leakage |
FAZ = foveal avascular zone; SVC = superficial vascular complex; DVC = deep vascular complex
SVC abnormalities in FEVR eyes were further characterized as having the following abnormal features: vessel dilation (7/7 eyes, 100%), disorganized vessel pattern (6/7 eyes, 86%), straightened vessels (5/7 eyes, 71%), areas of decreased vessel density (5/7 eyes, 71%), areas of increased vessel density (1/7 eyes, 14%), and vascular curls and loops (3/7 eyes, 43%) (Figure 1, Table 2). Straightening of the vessels and vascular dilatation were abnormalities marked in only the SVC and not the DVC. In contrast to these prominent abnormalities noted in the eyes with FEVR, none of 11 control eyes displayed these features in the SVC and no specific vascular abnormalities were noted.
The same 7 FEVR eyes that had abnormal SVCs also displayed abnormal DVCs. DVC abnormalities in FEVR eyes were further characterized as follows: areas of decreased density (7/7 eyes, 100%), disorganized vessel pattern (7/7 eyes, 100%), “end-bulbs” or stub-like vessel terminations (7/7 eyes, 100%), and vascular curls and loops (4/7 eyes, 57%) (Figure 1, Table 2). A prominent feature in the DVC of FEVR eyes were the end-bulbs (Figure 2), which presumably represented premature capillary endings without further arborization around the INL. They were associated with decreased vascular density but not noted in the SVC of any eye. In contrast to the FEVR eyes, 1 out of 11 control eyes had areas of increased vessel density and vascular curls and loops, and no other specific vascular abnormalities were noted.
Figure 2.
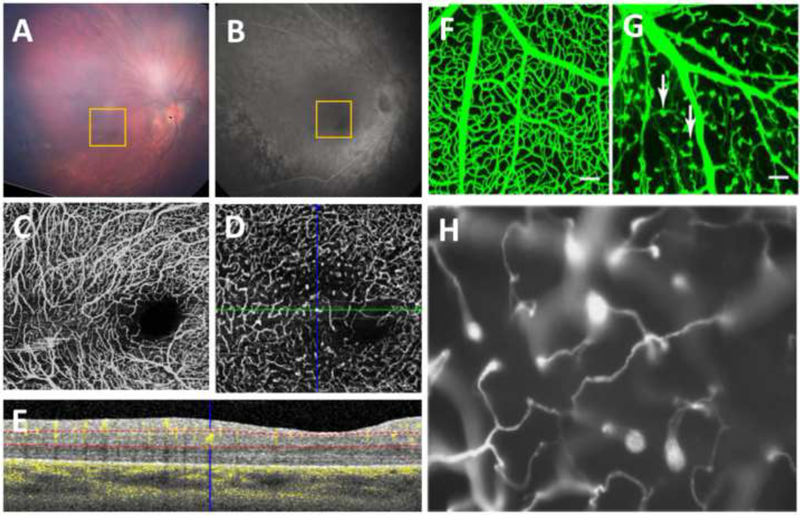
A 19-year-old white term-born male was diagnosed at 1.5 years old with familial exudative vitreoretinopathy (FEVR). Genetic testing showed a heterozygous mutation in the Wnt-pathway LRP5 gene. The right eye (A, fundus photo, and B, fluorescein angiography (FA)) underwent peripheral laser and cryotherapy. OCT-A of the macula showed vessel dilation, areas of non-uniform vessel density, vascular loops, and straightened vessels in the SVC (C). The DVC had a disorganized pattern, curls and loops, areas of decreased density, and characteristic end-bulbs (D). (E) An OCT/OCT-A B-scan of the location of the green line in (D) is shown, with the blue crosshair over one of the end-bulbs. The dotted red lines indicate the segmentation of the retinal layers used to form the en face OCT-A image of the DVC. The pattern seen in the patient resembles that of vasculature in mutant mice with defective Wnt signaling (F-H). (F,G) Image adapted from Ye et al.28 showing a wildtype (WT) mouse compared to a Frizzled4 knockout (FZ4−/−) mouse with white arrows pointing to clusters of endothelial cells only partially penetrating into the retina. (H) Image adapted from Xia et al.21 demonstrating incomplete vascularization with attenuated vessels in a homozygous r18 mutant mouse carrying a frameshift mutation in the LRP5 gene.
There were minimal FA changes in the macula of the 11 eyes with FEVR. Macular dragging and straightening of the vessels in the macula were the only features noted and were present in 6 of 11 eyes. One eye showed telangiectatic vessels in the temporal macula. All 11 eyes showed peripheral findings on fluorescein angiography including nonperfusion, leakage, and staining of prior laser treatment (five representative eyes in Figure 1 and a sixth eye in Figure 2).
Of the 6 patients with clinically-diagnosed FEVR, 2 patients underwent genetic testing. One patient was negative for any mutations in the known genes FZD4, LRP5, TSPAN12, and NDP; the other patient (retinal and OCT-A images in Figure 2) was confirmed as heterozygous for a mutation in LRP5.
Discussion
This is, to our knowledge, the first case series of depth-resolved macular imaging in FEVR and revealed abnormalities of both the superficial and deep vascular plexuses. Although there has been one report of OCT-A in a patient with FEVR24, these microvascular abnormalities were not otherwise evident on FA and not previously described (PubMed Search on June 11, 2018: “familial exudative vitreoretinopathy” AND “fluorescein angiography”; “familial exudative vitreoretinopathy” AND “optical coherence tomography angiography”). In the 7 eyes with vascular abnormalities, imaging of the SVC revealed increased vessel dilation, presence of vascular curls and loops, and straightening of the macular vasculature, and imaging of the DVC revealed disorganized vascular pattern with stub-like vessel terminations. Notably, these findings were present in eyes of patients ages 2 to 25 years. These unique macular microvascular changes provide new insights into the disease pathogenesis of FEVR.
The abnormally terminated vascular flow with “end-bulbs,” or stub-like, dilated vasculature in the DVC appears to be unique in FEVR. This was not visible in any of the control eyes nor has this been reported in OCT-A of other pediatric vascular diseases such as retinopathy of prematurity25,26. While OCT-A images work by capturing motion (blood flow) rather than structure (blood vessels), thus making histology necessary to confirm vessel termination, we believe these end-bulbs visualized on OCT-A could correlate with structural vessel termination, with suspended red blood cells in motion captured on OCT-A within these end-bulbs27. The prevalence of macular findings in 7 of 11 eyes suggests that FEVR is more than a disease of peripheral nonperfusion as previously thought, and suggests a more widespread and defective retinal angiogenesis particularly in the deeper retinal layers.
Interestingly, these DVC vascular end-bulbs visible on OCT-A in humans (including one with documented LRP5 mutation) parallel mouse models of FEVR with mutations in the Wnt signaling pathway (Figure 2). Mice with defective Norrin/Fzd4 signaling or LRP5 signaling, demonstrate retinal hypovascularization with both delayed radial migration of endothelial cells as well as defective arborization of deeper capillaries following the vertical endothelial innervation from the vitreal surface21,28. This similarity observed in patients with FEVR and mutant mice with defective Wnt signaling indicates a conservative retinal vascular growth pattern across species and suggests a potential role for OCT-A in the diagnosis of FEVR especially when a range of diagnoses are considered.
Limitations of this study include a small sample size and heterogeneity of FEVR disease stages, treatment history, and clinical findings. While the data presented here include eyes at different stages of disease and those that have undergone various vitreoretinal treatments, observations of vascular defects, particularly in the DVC, appear consistent and were generally distinguishable from normal controls by masked graders. Two of the patients imaged (the four eyes, two that were stage 2a and two that were stage 2b, without any macular abnormalities seen on OCT-A) were siblings, and thus may have had related genotypes that led to a less severe FEVR phenotype. Future studies with larger sample size will allow for quantification and robust statistical analysis of these OCT-A findings, particularly in regard to comparison to control eyes of the same race/ethnicity, age, and sex, and further correlation these findings with FEVR disease stages and clinical presentation. Additionally, this study aimed to investigate depth-resolved vascular abnormalities of the macular vasculature; future studies may explore imaging of the retinal periphery to investigate the changes in periphery retinal vasculature and their response to treatment.
OCT-A allows for depth-resolved visualization of striking vascular abnormalities in the macula that have not been previously described or imaged in FEVR patients. These findings suggest that FEVR, often defined as a disease of the retinal periphery, exhibits central microvascular changes and decreased vascularization of the deeper retina. Such findings may potentially assist in distinguishing FEVR from other diseases with similar clinical findings, such as retinopathy of prematurity29. As FEVR is a progressive disease that requires life-long monitoring, being able to accurately diagnose and potentially predict prognosis would greatly benefit patients. As we investigate these findings further in a larger number of patients and correlate with disease severity, the extent of these vascular abnormalities may aid not only in diagnosis, staging and prognosis of this retinal vascular disease but also in monitoring response to future treatment.
Funding acknowledgements:
Funding was provided by the following: International Association of Government Officials (iGO) Fund (LV), Research to Prevent Blindness Unrestricted Grant to Duke Eye Center (all), Research to Prevent Blindness Career Development Award and NEI K23EY028227 (XC), Lions Duke Pediatric Eye Research Endowment (RH), and NIH R01EY25009 (CAT). The above organizations had no role in study design, collection, analysis and interpretation of data, writing the report, and the decision to submit the report for publication.
Research equipment (Spectralis tabletop and Flex module) was provided by Heidelberg Engineering. Aside from agreement to submit the report for publication and corrections regarding terminology, they had no role in study design, collection, analysis and interpretation of data, and writing the report.
Other Acknowledgements
The authors would like to thank their pediatric ophthalmology colleagues, Sharon F. Freedman, MD and Nathan Cheung, DO, for their help in subject recruitment and our ophthalmic photographer, Michael P. Kelly, for assisting with image capturing.
Footnotes
Disclosures:
STH, APF, XC, RH, MPK – none
CAT - Alcon (Royalties)
LV - Knights Templar Eye Foundation (Recipient); Janssen Pharmaceutical (Recipient); Roche (Recipient); DORC (Recipient); Second Sight (Recipient); PDC’s ENABLE Award (Recipient); Alcon (Recipient); Genentech (Recipient)
This manuscript has not been presented at any meetings.
References
- 1.Criswick V, Schepens C. Familial Exudative Vitreoretinopathy. Am J Ophthalmol. 1969;68(4):578–594. [DOI] [PubMed] [Google Scholar]
- 2.Miyakubo H, Hashimoto K, Miyakubo S. Retinal Vascular Pattern in Familial Exudative Vitreoretinopathy. Ophthalmology. 1984;91(12):1524–1530. [DOI] [PubMed] [Google Scholar]
- 3.Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116(6):883–895. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZY, Battinelli EM, Fielder A, et al. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet. 1993;5(2):180–183. [DOI] [PubMed] [Google Scholar]
- 5.Robitaille J, MacDonald ML, Kaykas A, et al. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32(2):326–330. [DOI] [PubMed] [Google Scholar]
- 6.Kondo H, Hayashi H, Oshima K, Tahira T, Hayashi K. Frizzled 4 gene (FZD4) mutations in patients with familial exudative vitreoretinopathy with variable expressivity. Br J Ophthalmol. 2003;87(10):1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toomes C, Bottomley HM, Jackson RM, et al. Mutations in LRP5 or FZD4 Underlie the Common Familial Exudative Vitreoretinopathy Locus on Chromosome 11q. The American Journal of Human Genetics. 2004;74(4):721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulter JA, Ali M, Gilmour DF, et al. Mutations in TSPAN12 Cause Autosomal-Dominant Familial Exudative Vitreoretinopathy. The American Journal of Human Genetics. 2010;86(2):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collin RW, Nikopoulos K, Dona M, et al. ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature. Proc Natl Acad Sci U S A. 2013;110(24):9856–9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panagiotou ES, Sanjurjo Soriano C, Poulter JA, et al. Defects in the Cell Signaling Mediator beta-Catenin Cause the Retinal Vascular Condition FEVR. Am J Hum Genet. 2017;100(6):960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu H, Xiao X, Li S, Jia X, Guo X, Zhang Q. KIF11 mutations are a common cause of autosomal dominant familial exudative vitreoretinopathy. Br J Ophthalmol. 2016;100(2):278–283. [DOI] [PubMed] [Google Scholar]
- 12.Rao FQ, Cai XB, Cheng FF, et al. Mutations in LRP5,FZD4, TSPAN12, NDP, ZNF408, or KIF11 Genes Account for 38.7% of Chinese Patients With Familial Exudative Vitreoretinopathy. Invest Ophthalmol Vis Sci. 2017;58(5):2623–2629. [DOI] [PubMed] [Google Scholar]
- 13.Shastry B, Hartzer M, Treses M. Familial exudative vitreoretinopathy: multiple modes of inheritance Clin Genet. 1993;44:275–276. [DOI] [PubMed] [Google Scholar]
- 14.Benson WE. Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc.1995;93:473–521. [PMC free article] [PubMed] [Google Scholar]
- 15.Ranchod TM, Ho LY, Drenser KA, Capone A, Trese MT. Clinical Presentation of Familial Exudative Vitreoretinopathy. Ophthalmology. 2011;118(10):2070–2075. [DOI] [PubMed] [Google Scholar]
- 16.Kashani AH, Brown KT, Chang E, Drenser KA, Capone A, Trese MT. Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(11):2220–2227. [DOI] [PubMed] [Google Scholar]
- 17.Nijhuis FA, Deutman AF, Aan de Kerk AL. Flourescein angiography in mild stages of dominant exudative vitreoretinopathy. Mod Probl Ophthalmol. 1979;20:107–114. [PubMed] [Google Scholar]
- 18.Campbell JP, Zhang M, Hwang TS, et al. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci Rep. 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaide RF, Klancnik JM Jr., Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA ophthalmology. 2015;133(1):45–50. [DOI] [PubMed] [Google Scholar]
- 21.Xia C-H, Liu H, Cheung D, et al. A model for familial exudative vitreoretinopathy caused by LPR5 mutations. Hum Mol Genet. 2008;17(11):1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139(2):285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pendergast SD, Trese MT. Familial exudative vitreoretinopathy. Results of surgical management. Ophthalmology. 1998;105(6):1015–1023. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Viehland C, Carrasco-Zevallos OM, et al. Microscope-Integrated Optical Coherence Tomography Angiography in the Operating Room in Young Children With Retinal Vascular Disease. JAMA ophthalmology. 2017;135(5):483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nonobe N, Kaneko H, Ito Y, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY OF THE FOVEAL AVASCULAR ZONE IN CHILDREN WITH A HISTORY OF TREATMENT-REQUIRING RETINOPATHY OF PREMATURITY. Retina (Philadelphia, Pa). 2017. [DOI] [PubMed] [Google Scholar]
- 26.Vinekar A, Chidambara L, Jayadev C, Sivakumar M, Webers CA, Shetty B. Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS. 2016;20(3):271–274. [DOI] [PubMed] [Google Scholar]
- 27.Kashani AH, Green KM, Kwon J, et al. Suspended Scattering Particles in Motion: A Novel Feature of OCT Angiography in Exudative Maculopathies. Ophthalmology Retina. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med. 2010;16(9):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.John VJ, McClintic JI, Hess DJ, Berrocal AM. Retinopathy of Prematurity Versus Familial Exudative Vitreoretinopathy: Report on Clinical and Angiographic Findings. Ophthalmic surgery, lasers & imaging retina. 2016;47(1):14–19. [DOI] [PubMed] [Google Scholar]