Abstract
Introduction and Hypothesis:
Intradetrusor onabotulinumtoxinA (BTX) and sacral neuromodulation (SNM) are effective treatments for refractory urgency urinary incontinence/overactive bladder (UUI/OAB). BTX has a risk of urinary tract infection (UTI), concerning for development of multidrug resistant (MDR) UTI. We hypothesized that 1) BTX has higher risk of UTI and MDR UTI compared to SNM and 2) UTI and MDR UTI risk increases after repeat BTX injection.
Methods:
This retrospective cohort study included women undergoing BTX or SNM for refractory UUI/OAB in 2012–2016. UTI and MDR UTI were assessed up to 1 year post-treatment or until repeat treatment and compared between initial BTX and SNM and between repeat BTX injections. Univariate analyses included chi-square and Fisher’s exact tests and generalized linear models (GLM) with logit link function. Multivariate analyses used GLM to assess the best predictor variables for any UTI.
Results:
101 patients were included (28 BTX, 73 SNM). Rates of UTI (39.3% (95%CI 21.5, 59.4) BTX vs. 37.0% (95% CI 26.0, 49.1) SNM) were similar in both groups at all time intervals. One MDR UTI occurred after SNM. Risk of UTI did not increase with repeat BTX (11/28 (39.3%), 6/17 (35.3%) and 4/7 (57.1%) after 1, 2 and ≥3 treatments, respectively (p=0.62)). Multivariate analysis found history of recurrent UTI (OR 2.5, 95%CI 0.98–6.39) and prolapse repair (OR 4.6, 95%CI 1.23–17.07) had increased odds of UTI.
Conclusions:
UTI rates were similar in patients undergoing BTX and SNM. MDR UTI was rare. Patients with prior prolapse repair or recurrent UTI may have higher risk of UTI after either procedure.
Keywords: Drug Resistance, Onabotulinumtoxin, Sacral Neuromodulation, Urgency Urinary Incontinence, Urinary Tract Infection
Brief summary
We compared urinary tract infection (UTI) and multidrug resistant UTI rates in patients undergoing intradetrusor onabotulinumtoxinA injection versus sacral neuromodulation for overactive bladder/urgency urinary incontinence.
Introduction
Overactive bladder (OAB) and urgency urinary incontinence (UUI) affect between 17% and 19% of adults in the United States, respectively [1]. Intradetrusor onabotulinumtoxinA injections (BTX) and sacral neuromodulation (SNM) are recommended as third-line treatments for OAB in the 2015 guideline amendment by the American Urological Association and Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction [2]. The US Food and Drug Administration approved BTX for neurogenic bladder in 2011 (200 U) and for OAB in 2013 (100 U) [3–4]. BTX has been established as significantly more effective than anticholinergic medications in treating OAB and UUI in the ABC trial [5], and this was confirmed more recently in a phase 3b multicenter randomized controlled trial [6]. A host of studies confirm its efficacy in treatment of OAB and urgency urinary incontinence [5–13].
Despite the high efficacy rates of BTX, it also has a well-known increased risk of post-procedure urinary tract infection (UTI). One randomized trial comparing different doses of BTX found the rate of post-procedural UTI to be 34–48%, with the highest being in the 200 U dose group [8]. Other studies report similar UTI rates of 33–39%, compared to 11–13% in patients who received other modes of treatment (including anticholinergics and SNM) [5, 7]. More recent studies suggest UTI rates of 15–19% in the first 12 weeks after BTX 100 U [6, 13], and up to 25.5% any time after the first injection of BTX 100 U [6]. Long-term follow up data from the ROSETTA trial showed UTI rates after BTX 200 U of 36% in the first 6 months after surgery and 22% in months 7–12 post-procedure, compared to UTI rates after SNM of 15% during months 1–6 and 12% during months 7–12 [14].
Higher UTI rates and resulting increased antibiotic exposure is often associated with a greater risk of infection by drug-resistant organisms. One large US-based retrospective study reported that rates of multidrug resistant E. coli in community-acquired UTI increased from 9% in 2001 to 17% in 2010 [15]. In the most recent summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, catheter-associated UTIs constitute 38% of healthcare-associated infections. Of these, the most common uropathogen was E. coli (23.9%). Of these infections, E. coli resistance rates notably increased between 2011 and 2014. Resistance to extended-spectrum cephalosporins increased from 12.9 to 16.1%, resistance to fluoroquinolones increased from 32.7 to 34.8%, and overall MDR rates (resistance to at least 1 drug per category in at least 3 drug categories) increased from 5.5 to 8% [16].
As healthcare institutions and payers become increasingly concerned about quality and value-based care, rising antimicrobial drug resistance and its effect on society is an important topic for consideration in determining best practices. Thus, we aimed to determine whether BTX is associated with a higher proportion of women with UTI and MDR UTI compared to SNM, and to determine whether the UTI and MDR UTI rates increase after repeat compared to first-time BTX injections.
Materials and Methods
We conducted a single-center retrospective cohort study at a tertiary care hospital. Patients within our electronic medical record (EMR) were identified using procedure codes for SNM (combined stage I & II or stage II) or BTX (100–200 U) (CPT Codes 64581, 64590, and 52287, respectively). All female patients 18 years old and above identified as having undergone these procedures between January 1, 2012 and December 31, 2016 were screened for inclusion. Patients were excluded if they were undergoing SNM for urinary retention, had received BTX within one year prior to the index procedure, had prior pelvic radiation or renal transplant, were receiving treatment for bladder cancer at the time of the procedure, or required bladder drainage (intermittent self-catheterization or suprapubic or transurethral indwelling catheter) at the time of the index procedure. IRB approval was obtained prior to data collection.
Charts were reviewed, including all patient encounters related to the procedure as well as outside records accessible within our EMR. Details regarding patient baseline characteristics and medical history, procedure details, and follow up were recorded (Appendix 1). All patients were treated in the Urology or Urogynecology clinical programs. In our standard practice, patients are treated for OAB according to AUA guidelines [2]. Therefore patients do not receive third-line treatment options until they have failed first- and second-line treatments, including behavioral modifications, optional pelvic floor physical therapy, and pharmaceutical management.
Details on post-procedure UTI were also collected. UTI were defined as symptomatic if the patient reported increased urinary frequency, small volume voids, dysuria, urgency, suprapubic pain, new onset gross hematuria, or fevers [17]. A positive urine culture was defined as a single mid-stream clean catch specimen with colony count of ≥ 103 CFU/mL, or a single catheterized specimen with colony count of ≥100 CFU/mL [17–18]. If duration of treatment was not documented in the EMR, we assumed treatment length of 7 days (standard length of treatment for complicated UTI) [17].
Our first primary outcome was the proportion of patients with UTI and MDR UTI in both treatment groups between the date of the procedure up to 4 weeks, 4–12 weeks, 3–6 months and 6–12 months post-procedure (or until the patient underwent retreatment or change in treatment). Patients who received repeat BTX injections or switched from one treatment group to the other were analyzed for the timeframe from the initial treatment only. MDR UTI was defined as an isolate from a positive culture demonstrating resistance to at least one antibiotic per drug category in at least 3 categories (see Appendix 2 for defined drug categories), consistent with criteria most recently used by the CDC [16].
For our second primary outcome, we assessed whether increasing the number of BTX treatments was associated with increased rates of UTI and MDR UTI. For this, we separately analyzed patients undergoing multiple BTX treatments. Procedure data and post-procedure UTI details following each procedure were collected. The number of UTI and MDR UTI were recorded after each successive treatment. UTI details were also recorded for each UTI, excluding questions regarding healthcare utilization.
Secondary outcomes included patient risk factors for UTI and MDR UTI, as well as the number and type of healthcare resources utilized in treating UTI and MDR UTI in patients undergoing BTX and SNM. Resources assessed included outpatient visits, hospitalizations, nursing phone calls, and oral versus IV antibiotic requirement.
Rates were estimated with binomial exact 95% confidence intervals. Univariate analyses were conducted using Pearson’s Chi-Square and Fisher’s exact tests for categorical predictors and generalized linear models (GLMs) with a logit link function for continuous predictors. Multivariate analyses also employed GLMs in order to assess the most ideal collection of predictor variables to predict the dichotomous outcome. The selection criterion used to compare model fits was the Akaike information criterion (AIC) [19], an estimator of the relative quality of statistical models for a given set of data. Specifically, we used a corrected AIC (AICc), a variant of AIC more suitable in small sample settings [20]. Statistical analysis was performed using SAS (version 9.4, Cary, North Carolina).
Results
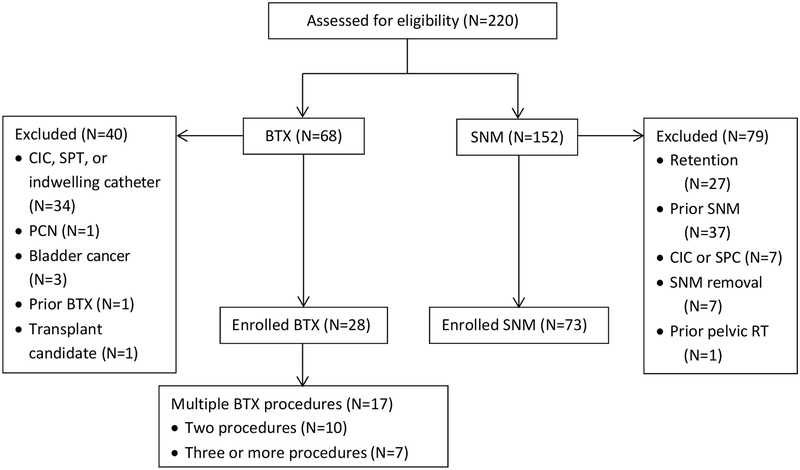
A total of 220 patients were identified by CPT codes. After exclusions, 101 patients were included in the study (28 BTX, 73 SNM) (Figure 1). Baseline characteristics are summarized in Table 1. Women in the BTX group were older than those in the SNM group (62.6±15.0 vs. 54.4±15.7, p=0.02). BTX patients were less likely to have interstitial cystitis, stress incontinence and psychiatric diagnoses compared to the SNM patients (3.6% vs 20.6%; 25% vs 50%; and 39.3% vs 65.8%, respectively, all p<0.05) and more likely to have neurogenic bladder (17.9% vs 1.4%, p<0.01). There were no statistically significant differences between the two treatment groups regarding BMI, medical comorbidities and history of recurrent UTI, hysterectomy, prolapse and anti-incontinence procedures.
Figure 1. Eligibility, exclusions, and enrollment.
CIC: clean-intermittent catheterization; SPC: suprapubic catheter; PCN: percutaneous nephrostomy; RT: radiation therapy.
Table 1.
Baseline Characteristics of Study Participants
| Health characteristics | |||
|---|---|---|---|
| BTX* N=28 | SNM* N=73 | p value | |
| Age [mean (sd)] | 62.6 (15.0) | 54.4 (15.7) | 0.017 |
| BMI (continuous variable) [mean (sd)] | 31.9 (7.9) | 33.6 (9.0) | 0.36 |
| Post-menopausal statusa (BTX: N=25; SNM: N=58) | 21 (84.0) | 41 (70.7) | 0.27 |
| Currently sexually activeb (BTX: N=25; SNM: N=61) | 16 (64.0) | 37 (60.7) | 0.77 |
| Never | 15 (53.6) | 36 (49.3) | 0.15 |
| Urogynecologic History | |||
| POP >hymen | 1 (3.6) | 1 (1.4) | 0.48 |
| Pessary use (current) | 0 (0) | 0 (0) | 1.00 |
| Recurrent UTI history | 8 (28.6) | 20 (27.4) | 0.91 |
| Kidney disease | 1 (3.6) | 4 (5.5) | 1.00 |
| IC/BPS | 1 (3.6) | 15 (20.6) | 0.037 |
| Neurogenic bladder | 5 (17.9) | 1 (1.4) | 0.006 |
| OAB | 19 (67.9) | 59 (80.8) | 0.16 |
| SUI | 7 (25.0) | 35 (50.0) | 0.036 |
| UUI | 23 (82.1) | 59 (80.8) | 0.88 |
| Both SUI/UUI (MUI) | 7 (25.0) | 34 (46.6) | 0.048 |
| Medical History | |||
| Diabetes | 3 (10.7) | 16 (21.9) | 0.20 |
| Hypertension | 17 (60.7) | 38 (52.0) | 0.43 |
| Cardiovascular disease | 7 (25.0) | 14 (19.2) | 0.52 |
| Otherc | 6 (21.4) | 18 (24.7) | 0.73 |
| Autoimmune disorderd | 11 (39.3) | 26 (35.6) | 0.73 |
| Psychiatric illness | 11 (39.3) | 48 (65.8) | 0.016 |
| Surgical History | |||
| Hysterectomy | 10 (35.7) | 32 (43.8) | 0.46 |
| Prolapse repair | 5 (17.9) | 8 (11.0) | 0.35 |
| Anti-incontinence procedures | 8 (28.6) | 17 (23.3) | 0.58 |
| Gynecologic cancer surgery | 0 (0) | 3 (4.1) | 0.56 |
| Spine surgery | 6 (21.4) | 12 (16.4) | 0.57 |
| Urologic surgery | 0 (0) | 2 (2.7) | 1.00 |
| Medications | |||
| Suppressive antibiotics for recurrent UTIe | 3 (10.7) | 11 (15.1) | 0.75 |
| Other antibiotics (any indication)e | 16 (57.1) | 41 (56.2) | 0.93 |
| Immunosuppressive medicationf | 4 (14.3) | 7 (9.6) | 0.49 |
Results reported as N (%) unless otherwise indicated.
3 (10.7%) of BTX patients and 12 (16.4%) of SNM patients had unknown menopausal status due to missing information in the medical record.
3 (10.7%) of BTX and 12 (16.4%) of SNM patients did not have sexual activity status documented.
Other neurologic diagnoses included migraines (16), restless leg syndrome (5), seizures (2), spinal stenosis (1), cerebral malaria (1), dementia (1), and HTLV-1 associated myelopathy-tropical spastic paraparesis (1)
Most patients included in the autoimmune disorder group had a diagnosis of hypothyroidism.
Exposure within 90 days prior to procedure
Exposure within 30 days prior to procedure
Abbreviations: BMI (body mass index); POP (pelvic organ prolapse); UTI (urinary tract infection); IC/BPS (interstitial cystitis/bladder pain syndrome); OAB (overactive bladder); SUI (stress urinary incontinence); UUI (urgency urinary incontinence); MUI (mixed urinary incontinence)
The proportions of patients with post-procedure UTI were similar in both BTX and SNM groups, ranging from 4–11% in the first month and 37–39% overall (39.3% (95%CI 21.5, 59.4) BTX and 37.0% (95% CI 26.0, 49.1) SNM) in the first year or until repeat injection (Table 2). This was true for all documented UTI at every time interval assessed (p>0.05 for all). The total number of UTI per patient ranged from 0 to 6 for the year after treatment.
Table 2:
Proportion of patients with MDR UTI and UTI at each post-procedure time-point and Total UTIs
| Proportion of patients with MDR UTI and UTI | Total UTI and MDR UTI** | ||||
|---|---|---|---|---|---|
| BTX* N=28 | SNM* N=73 | p-value | BTX | SNM | |
| MDR UTI | - | - | - | - | |
| 0–4 weeks | 0 (0) | 0 (0) | 1.00 | 0 | 0 |
| 4–12 weeks | 0 (0) | 0 (0) | 1.00 | 0 | 0 |
| 3–6 months | 0 (0) | 0 (0) | 1.00 | 0 | 0 |
| 6–12 months | 0 (0) | 1 (1.4) | 1.00 | 0 | 1 |
| Cumulative (0–12 mo) | 0 (0) | 1 (1.4) | 1.00 | 0 | 1 |
| All treated UTI | - | - | - | - | |
| 0–4 weeks | 3 (10.7) | 3 (4.1) | 0.34 | 3 | 3 |
| 4–12 weeks | 3 (10.7) | 4 (5.5) | 0.39 | 4 | 4 |
| 3–6 months | 5 (17.9) | 11 (15.1) | 0.76 | 6 | 15 |
| 6–12 months | 4 (14.3) | 19 (26.0) | 0.21 | 6 | 25 |
| Cumulative (0–12 mo) | 11 (39.3) | 27 (37.0) | 0.83 | 20 | 48 |
Results reported as N (%)
Results reported as N; each patient may have had >1 UTI during each time-point.
Abbreviations: UTI (urinary tract infection); MDR (multidrug resistant); BTX (intradetrusor onabotulinumtoxinA); SNM (sacral neuromodulation)
The proportion of patients with UTI did not increase after repeat BTX injections. We found that 11/28 39.3%)) had a UTI after the first treatment, 6/17 (35.3%) after the second treatment, and 4/7 patients (57.1%) after 3 or more treatments (p=0.62), though the number of patients receiving repeat BTX treatments was few (Table 3).
Table 3:
Cumulative BTX effect on MDR UTI and UTI
| Number and proportion of patients with infection* | ||||
|---|---|---|---|---|
| Treatment 1 N=28 | Treatment 2 N=10 | Treatment 3+ N=7 | p value | |
| MDR UTI | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Any UTI | 11 (39.3) | 6 (35.3) | 4 (57.1) | 0.62 |
| *Results reported as n (%) | ||||
| Total infections (among all patients) | ||||
| Treatment 1 | Treatment 2 | Treatment 3+ | ||
| MDR UTI | 0 | 0 | 0 | |
| Any UTI | 20 | 9 | 6 | |
| Abbreviations: UTI (urinary tract infection); MDR (multidrug resistant); BTX (intradetrusor onabotulinumtoxinA) | ||||
Only one MDR UTI was identified, occurring after SNM. The patient was a 61 year-old post-menopausal Caucasian female with a BMI of 26.8 kg/m2. She was a non-smoker, had a history of recurrent UTI, and was on antibiotic suppression (nitrofurantoin) at the time of the procedure. She also had a history of UUI and depression, as well as cervical spine fusion. Previous urodynamics confirmed detrusor overactivity. Two UTIs occurred during the 6 weeks prior to the procedure (K. pneumoniae resistant to ampicillin, nitrofurantoin, and trimethoprim-sulfamethoxazole, treated with ciprofloxacin; and E. coli resistant to beta-lactams, treatment not documented). She then underwent SNM, and the MDR UTI occurred just over 6 months after the procedure with E. coli demonstrating resistance to ciprofloxacin, nitrofurantoin, trimethoprim-sulfamethoxazole, as well as K. pneumoniae demonstrating resistance to ampicillin and nitrofurantoin. She was hospitalized and treated with IV piperacillin-tazobactam for four days and discharged home on oral cephalexin for three additional days.
When we considered potential UTI predictors as listed in Table 1, based on univariate analysis we found that the following variables met or approached statistical significance (p<0.05) and were considered for inclusion in the multivariable model: history of prolapse repair, hypertension, Vitamin C supplementation (often employed in patients with a history of recurrent UTI) (p<0.05 for all), and recurrent UTI (p<0.06). Multivariable analysis including procedure type as a covariate found increased odds of any UTI in patients with a history of recurrent UTI (OR 2.5, 95% CI 0.98–6.39) and history of prolapse repair (OR 4.6, 95% CI 1.23–17.07) after either BTX or SNM.
Table 4 summarizes utilized resources. Three patients were hospitalized for UTI treatment (1 after BTX and 2 after SNM) up to 1 year after treatment. One BTX patient and one SNM patient were documented as having been treated with IV antibiotics. The third patient (in the SNM group) reported in a follow up visit that they had been hospitalized for UTI, but details of antibiotic treatment type (i.e. IV versus oral) were not available. There were no significant differences in route of antibiotic administration, total courses of antibiotics, duration of treatment per UTI, hospitalizations, outpatient visits, or provider phone calls related to UTI between patients undergoing BTX versus SNM (p>0.1 for all).
Table 4.
Utilized Resources Related to UTI occurring after first treatment up to 12 months or until retreatment or change in treatment
| Per subject* | |||
|---|---|---|---|
| BTX** n=28 | SNM** n=73 | p value | |
| Antibiotics for UTI (any) | 11 (39.3) | 27 (37.0) | 0.83 |
| Bladder instillations | 0 (0) | 0 (0) | - |
| Continued from baseline | 2 (7.1) | 8 (11.0) | 0.72 |
| Hospitalizations | 1 (3.6) | 2 (2.7) | 0.83 |
| Outpatient visits [median (range)] | 0 (0–3) | 0 (0–5) | 0.22 |
| Provider phone calls [median (range)] | 0 (0–10) | 0 (0–14) | 0.99 |
| **Results reported as n (%) unless otherwise noted | |||
| Among total treated UTI | |||
| BTX* n=20 | SNM* n=47 | p value | |
| Bladder instillations | - | - | - |
| Unknown duration | 7 (35.0) | 3 (6.4) | 0.01 |
| Outpatient visits | 0.45 (0.51) | 0.79 (0.55) | 0.14 |
| Provider phone calls | 1.25 (1.77) | 1.38 (1.50) | 0.88 |
| Abbreviations: UTI (urinary tract infection); BTX (intradetrusor onabotulinumtoxinA); SNM (sacral neuromodulation); IV (intravenous) | |||
Discussion
In our population of patients with refractory UUI/OAB, UTI occurred in more than one third after both BTX and SNM procedures, results consistent with the BTX literature but higher than that reported after SNM in prior trials [10]. Patients with history of prolapse repair or history of recurrent UTI may be at higher risk of UTI after either procedure. These results may reflect our patient population: we included patients with recent UTI, history of recurrent UTI and neurogenic bladder who are often excluded from randomized trials.
There was a large proportion of patients with neurologic diagnoses, but these occurred in both treatment groups. On the other hand, there were more IC/BPS patients in the SNM treatment group. It is possible that IC/BPS patients may have been treated for more UTI due to overlapping symptoms, which might contribute toward the higher proportion of patients with UTI in the SNM group than has been reported in previous trials. However, there was also no difference in culture-confirmed UTI between BTX and SNM groups, suggesting these did represent bacterial cystitis, not simply empiric treatment for increased symptoms. Unlike past research suggesting higher rates of UTI after BTX compared to SNM treatment,[14] our study population may be more representative of typical patient selection for BTX and SNM for treatment of refractory UUI/OAB, and in real-life scenarios (as compared with randomized trials), these patients may have similar rates of post-procedure UTI.
Our results also suggest that, reassuringly, MDR UTI (using a stringent definition) is rare following these procedures. MDR UTIs are frequently defined as isolates with resistance to 1 or more drugs in at least 3 or more drug categories [15–16, 21], but meeting this definition depends on adequate susceptibility testing and reporting by microbiology laboratories. Other studies have used a less stringent definition of MDR as resistance to 1 drug in 2 or more categories [22]. We chose to use the more stringent definition of MDR UTI to be consistent with the more widely accepted definition in the literature. To our knowledge, this is the first study formally investigating drug resistance in patients undergoing SNM or BTX for OAB. Though MDR UTI was found to be rare overall, we did not have a large sample size in the BTX cohort, and therefore further studies are needed to verify our findings.
The ROSETTA trial demonstrated that, in the first 6 months after initial treatment, BTX 200 U is significantly more effective than SNM in reducing urgency urinary incontinence episodes, and BTX had significantly higher rates of complete resolution of UUI [7]. However, recently published 2-year follow up on these patients found that at 24 months both patients undergoing BTX and SNM had similar rates of reduction in symptoms and complete resolution of incontinence [14]. There are many reasons a surgeon may recommend one procedure over the other for their patients, though both are recommended as third-line treatment for refractory OAB/UUI [2]. Higher reported rates of UTI in patients undergoing BTX in previous studies may cause one to recommend SNM over BTX in patients with a history of recurrent UTI. However, based on our study results, these patients may ultimately have similar risk of UTI regardless of the procedure performed.
Strengths of our study include broad inclusion criteria allowing our population to be more representative of the typical patient being counseled on BTX vs SNM, as well as surveillance for outcomes over a long period of time (up to a year or multiple procedures), during which one would expect to see the bulk of the UTIs related to the procedure as the duration of BTX effect is typically up to 12 months [12]. Weaknesses of our study include its retrospective and non-randomized design, as well as its smaller sample size for BTX patients. Due to the non-randomized nature of the retrospective cohort design, there is the potential for selection bias based on perceived patient risk factors. It is certainly possible that patients who were seen as having a higher baseline risk of UTI could have been recommended SNM during counseling. In addition, we were unable to compare rates of MDR UTI in the BTX and SNM treatment groups given the very low rate of MDR UTI identified after BTX and SNM procedures (~1% overall) in our patient population. Lastly, we may not have captured all patients diagnosed with UTI if we did not have access to outside clinical information that was not accessible through our electronic medical record.
In conclusion, UTI occurred in more than one third of patients after both BTX and SNM procedures. MDR UTI was rare. Patients with history of prolapse repair or recurrent UTI may be at higher risk of UTI after BTX and SNM.
Supplementary Material
Footnotes
FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE
Contributor Information
Caroline G. Elmer-Lyon, Department of Obstetrics and Gynecology, Division of Urogynecology, University of Iowa Hospitals and Clinics, Iowa City, USA
Judy A. Streit, Department of Internal Medicine, Division of Infectious Disease, University of Iowa Hospitals and Clinics, Iowa City, USA
Elizabeth B. Takacs, Department of Urology, University of Iowa Hospitals and Clinics, Iowa City, USA
Patrick P. Ten Eyck, Institute for Clinical and Translational Research, University of Iowa Hospitals and Clinics, Iowa City, USA
Catherine S. Bradley, Department of Obstetrics and Gynecology, Division of Urogynecology, University of Iowa Hospitals and Clinics, Iowa City, USA
References
- 1.Stewart WF, Van Rooyen JB, Cundiff GW et al. (2003). Prevalence and burden of overactive bladder in the United States. World J Urol 20(6):327–36. doi: 10.1007/s00345-002-0301-4 [DOI] [PubMed] [Google Scholar]
- 2.Gormley EA, Lightner DJ, Faraday M et al. (2015) Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 193(5):1572–80. doi: 10.1016/j.juro.2015.01.087 [DOI] [PubMed] [Google Scholar]
- 3.American Urogynecologic Society and American College of Obstetricians and Gynecologists (2014) Committee opinion: onabotulinumtoxinA and the bladder. Female Pelvic Med Reconstr Surg 20(5):245–7. doi: 10.1097/SPV.0000000000000112 [DOI] [PubMed] [Google Scholar]
- 4.Weckx F, Tutolo M, De Ridder D, Van der Aa F (2016) The role of botulinum toxin A in treating neurogenic bladder. Transl Androl Urol 5(1):63–71. doi: 10.3978/j.issn.2223-4683.2016.01.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visco AG, Brubaker L, Richter HE et al. (2012). Anticholinergic therapy vs. onabotulinumtoxina for urgency urinary incontinence. N Engl J Med 367(19):1803–13. doi: 10.1056/NEJMoa1208872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herschorn S, Kohan A, Aliotta P et al. (2017). The Efficacy and Safety of OnabotulinumtoxinA or Solifenacin Compared with Placebo in Solifenacin Naïve Patients with Refractory Overactive Bladder: Results from a Multicenter, Randomized, Double-Blind Phase 3b Trial. J Urol 198(1):167–175. doi: 10.1016/j.juro.2017.01.069 [DOI] [PubMed] [Google Scholar]
- 7.Amundsen CL, Richter HE, Menefee SA et al. (2016). OnabotulinumtoxinA vs Sacral Neuromodulation on Refractory Urgency Urinary Incontinence in Women: A Randomized Clinical Trial. JAMA October 4;316(13):1366–1374. doi: 10.1001/jama.2016.14617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dmochowski R, Chapple C, Nitti VW et al. (2010). Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 184(6):2416–22. doi: 10.1016/j.juro.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 9.Avallone MA, Sack BS, El-Arabi A et al. (2016) Less Is More-A pilot study evaluating one to three intradetrusor sites for injection of OnabotulinumtoxinA for neurogenic and idiopathic detrusor overactivity. Neurourol Urodyn 36(4):1104–1107. doi: 10.1002/nau.23052 [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Luo D, Tang C et al. (2015) The safety and efficiency of onabotulinumtoxinA for the treatment of overactive bladder: a systematic review and meta-analysis. Int Urol Nephrol 47(11):1779–88. doi: 10.1007/s11255-015-1125-7 [DOI] [PubMed] [Google Scholar]
- 11.Chapple C, Sievert KD, MacDiarmid S et al. (2013) OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. Eur Urol 64(2):249–56. doi: 10.1016/j.eururo.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 12.Duthie JB, Vincent M, Herbison GP et al. (2011) Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev (12):CD005493. doi: 10.1002/14651858.CD005493.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitti VW, Dmochowski R, Herschorn S et al. (2017). OnabotulinumtoxinA for the Treatment of Patients with Overactive Bladder and Urinary Incontinence: Results of a Phase 3, Randomized, Placebo Controlled Trial. J Urol 197(2S):S216–S223. doi: 10.1016/j.juro.2016.10.109 [DOI] [PubMed] [Google Scholar]
- 14.Amundsen CL, Komesu YM, Chermansky C et al. (2018) Two-Year Outcomes of Sacral Neuromodulation Versus OnabotulinumtoxinA for Refractory Urgency Urinary Incontinence: A Randomized Trial. Eur Urol 74(1):66–73. doi: 10.1016/j.eururo.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez GV, Baird AM, Karlowsky JA et al. (2014) Nitrofurantoin retains antimicrobial activity against multidrug-resistant urinary Escherichia coli from US outpatients. J Antimicrob Chemother 69(12):3259–62. doi: 10.1093/jac/dku282 [DOI] [PubMed] [Google Scholar]
- 16.Weiner LM, Webb AK, Limbago B et al. (2016) Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol 37(11):1288–1301. doi: 10.1017/ice.2016.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naber KG, Scaeffer AJ, Heyns CF et al. (2010) Urogenital Infections. European Association of Urology – International Consultation on Urological Diseases. http://www.icud.info/urogenitalinfections.html. Accessed 10 January 2017 [Google Scholar]
- 18.Grabe M, Bartoletti R, Bjerklund Johansen TE et al. (2015) Guidelines on Urological Infections. European Association of Urology. https://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf. Accessed 10 January 2017 [Google Scholar]
- 19.Sugiura N (1978) Further analysts of the data by akaike’s information criterion and the finite corrections. Communications in Statistics – Theory and Methods 7:1, 13–26. doi: 10.1080/03610927808827599 [DOI] [Google Scholar]
- 20.Akaike H (1973) Information theory and an extension of the maximum likelihood principle, in: Petrov BN and Csaki F, (Eds.), 2nd International Symposium on Information Theory 267–281. Akademia Kiado, Budapest [Google Scholar]
- 21.Giancola SE, Mahoney MV, Hogan MD et al. (2017) Assessment of Fosfomycin for Complicated or Multidrug-Resistant Urinary Tract Infections: Patient Characteristics and Outcomes. Chemotherapy 62(2):100–104. doi: 10.1159/000449422 [DOI] [PubMed] [Google Scholar]
- 22.Mauldin PD, Salgado CD, Hansen IS et al. (2010) Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant gram-negative bacteria. Antimicrob Agents Chemother 54(1):109–15. doi: 10.1128/AAC.01041-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.