Abstract
The α-Gal syndrome has many novel features that are relevant to diagnosis and management. In most cases the diagnosis can be made on a history of delayed allergic reactions to red meat and the blood test for IgE to the oligosaccharide galactose-α−1,3-galactose (α-Gal). In general, the diagnosis also dictates the primary treatment, i.e. – avoiding mammalian meat and also dairy in some cases. In the USA the lone star tick is the primary cause of this disease but different ticks are responsible in other countries. Blood levels of IgE to α-Gal often drop in patients who avoid recurrent tick bites, but the rate of decline is variable. Similarly, the delay before reactions is variable and the severity of the allergic reactions is not predicted by the delay or the titer of specific IgE. Some mammalian-derived products are only relevant to select patient groups, such as heart valves, gelatin-based plasma expanders, and pancreatic enzymes. A minority of cases may benefit from avoiding a wide range of products that are prepared with mammalian–derived constituents, such as gelatin. This review focuses on the nature of the syndrome, common challenges in diagnosis and management, and also gaps in our current knowledge that would benefit from additional investigation.
Keywords: α-Gal, anaphylaxis, IgE to oligosaccharide, glycolipids, red meat allergy
INTRODUCTION
In 2006 the monoclonal antibody (mAb) cetuximab was approved for treatment of cancer of both head and neck and the colon1. It soon became clear that allergic reactions were occurring in major clinics in the southeast of the USA2. Significantly, Dr. Tina Merritt obtained serum from two severe cases which occurred in Fayetteville, AR, and in addition we had developed the technique of measuring specific IgE using biotinylated proteins on a streptavidin ImmunoCAP3. In collaboration with both Bristol-Myers Squibb (BMS) and ImClone we received pre-treatment sera from 76 patients who had received cetuximab, of which 41 came from BMS and 35 came from Dr. Christine Chung and colleagues in the medical oncology group at Vanderbilt. Those studies demonstrated that the patients who had reactions had pre-existing IgE specific for cetuximab and that the target of those antibodies was the oligosaccharide galactose-α−1, 3-galactose (α-Gal) on the Fab portion of the mAb4, 5.
Prior to 2006 we had seen at least five patients in the clinic who reported adult onset of allergic reactions related to eating meat. Those subjects described surprisingly long delays between eating meat and the reactions, and prick tests to beef and pork were very small, i.e. ≤ 3mm. Thus, like most other allergists, we couldn’t make sense of their histories. During 2007 we looked in our allergy clinic for patients who had IgE specific to α-Gal comparable to that found in cancer patients who had reactions to cetuximab. Those studies identified that it was the patients who reported delayed reactions to red meat who had IgE specific for α-Gal. Furthermore, when we presented the early results to a meeting in Kansas City, MO, Dr. Barrett Lewis came forward and said “I have similar cases in Springfield, MO.” He sent us sera on 12 cases, eleven of whom had IgE specific to α-Gal and were included in the original paper describing the syndrome6.
During 2007 it became clear that cases of delayed anaphylaxis to red meat were present in the same area as reactions to cetuximab. Internet search suggested that this area, i.e. VA, NC, TN, AR, southern MO, and eastern OK, was similar to the maximum area of Rocky Mountain spotted fever (RMSF) reported by the Centers for Disease Control and Prevention (CDC)7. Accepting this association and the implication that this form of sensitization might relate to tick bites we questioned dozens of patients about the number of tick bites they had received. Those results supported a strong association between bites from the lone star tick, Amblyomma americanum, and the syndrome8. In addition, a history of prolonged itching at the site of tick bites was a significant predictor of sensitization8. Finally, we followed rises in specific IgE (sIgE) after tick bites in three cases, two of whom developed delayed urticarial reactions to red meat9. Strikingly, none of the patients reported a severe febrile illness comparable to clinical RMSF which is actually rare in this area7, 10. It is now clear that it is unusual for A. americanum ticks to carry Rickettsia rickettsii, but by contrast the majority of lone star ticks in this area carry the related R. amblyommatis10. Thus it appears that the febrile illnesses with positive Rickettsial serology which are sometimes classified as RMSF are likely to be caused by R. amblyommatis, or other spotted fever group Rickettsia, which can cause positive rickettsial serology but only mild symptoms11, 12. The details that explain the connection between tick bites and α-Gal sensitization remain an open question, but it is increasingly clear that certain ticks can express α-Gal and that tick antigens can have potent IgE-inducing properties13–16. The known properties of α-Gal as a blood-group-like antigen of non-primate mammals fit with the reported specificity of the allergic reactions to red meat from mammals that carry α-Gal17.
At the AAAAI meeting in 2008 we presented the story about α-Gal and learned about earlier reports suggesting a connection between tick bites and the onset of allergy to meat. In 2006 Dr. Sheryl van Nunen reported meat allergy cases related to tick bites in Sydney, Australia which she published in 200918. Dr. Deutsch in Athens, GA had reported five very similar cases to the Georgia Allergy Society in 1989. The importance of these cases is that they clearly show that the syndrome had been in existence before we became aware of it. Dr. van Hage and her colleagues in Stockholm had also identified that sera from some cat allergic patients had IgE specific for an oligosaccharide on cat IgA19. That epitope was later shown to be α-Gal, and the group in Sweden have now established the relevance of bites from the tick Ixodes ricinus to α-Gal sensitization and red meat allergy in southern Sweden20–22.
THE CLINICAL SYNDROME AND DIAGNOSIS
Today we have extensive experience with classical cases of the α-Gal syndrome (see Table 1A).
Table 1.
Clinical cases which reflect examples of allergic reactions to mammalian antigens in adults
| A. Severe anaphylactic cases in patients with specific IgE to α-Gal | |
|---|---|
| Case 1 – Anaphylaxis to “the other white meat” | Female employee of the medical center, age 79. Mild episodes were suspicious for the syndrome, however she thought pork was a “white meat”. She ate pork for lunch, started itching 3 hours later and soon thereafter collapsed in the lobby. She required resuscitation with IV fluids, Solumedrol and repeated adrenaline and was admitted overnight. Her α-Gal sIgE was 82.7 IU/ml and tryptase was 36.5 ng/ml. |
| Case 2 – Abdominal pain | A 45 year old female had multiple episodes occurring many hours after eating: full GI workup including EGD and colonoscopy did not reveal a cause. History of multiple tick bites including “chiggers”. Several episodes started with abdominal pain and diarrhea. She was found collapsed on the bathroom floor mentally confused and was admitted to hospital overnight. Her sIgE to α-Gal was 69.7 IU/ml and she had a good response to a diet including dairy avoidance. |
| Case 3 – Anaphylaxis with transient loss of sight | A 63 year old policeman living in rural Virginia experienced 3 tick bites and after that he had 3 mild episodes of hives. Three hours after eating “London broil” he developed itchy hives and “had to sit down”. Found to have BP of 40/0 and reported to EMS “I can’t see”. Required full resuscitation in ED including adrenaline drip overnight. Sight returned after 3 hours. His IgE to α-Gal was 23 IU/ml, tryptase was 95.5 ng/ml and he had a full recovery on an avoidance diet. |
| B. Allergic reactions to other Mammalian products “Masquerading” as α-Gal | |
| Case 4 – Pork-cat syndrome | 19 year old female with chronic GI symptoms associated with eating meat. Known tick exposure and also allergic to cat. IgE to α-Gal was 0.13 IU/ml, total IgE of 1406 kU/L, cat dander >100 IU/ml, IgE to cat serum albumin 29.5 IU/ml, pork serum albumin 18.6 IU/ml. She responded to a diet avoiding pork. |
| Case 5 – Anaphylaxis to vaccine containing gelatin | Patient with reactions to barbecue and other meat for several years who experienced a severe reaction within 20 minutes of shingles vaccination (Zostavax). She collapsed and required full resuscitation and hospitalization. Her IgE to α-Gal was negative but her IgE to gelatin was 5 IU/ml. Additional assays prepared in our laboratory showed IgE to bovine gelatin of 26.8 IU/ml and pork gelatin of 62.9 IU/ml. |
| Case 6 - Recurrent allergic reaction to milk and red meat | Female aged 66 with history of “insect” bite, reported periodic facial itch and swelling at night. Initially regarded as mammalian meat allergy, with IgE to α-Gal level of 0.11 IU/ml. Her episodes continued despite avoiding meat, including more severe swelling of face and throat. Found to have IgE to cow’s milk 72.9 IU/ml and IgE to BSA (Bos d 6) of >100 IU/ml and responded to dairy-free diet. |
They have onset in adult life after eating meat without problems for many years.
Reactions range from localized hives or angioedema to severe anaphylaxis which requires emergency treatment and admission to hospital.
Reactions start 2–6 hours after eating meat of mammalian origin, and the severity of reactions is not predicted by the delay23, 24.
They have positive sIgE for α-Gal. If the antibodies are ≥2 IU/ml or >2% of the total IgE this makes the diagnosis very likely. We often measure total IgE because some cases are non-atopic and have low total IgE24. Recently we saw a case with a convincing history whose total serum IgE was 6.0 IU/ml and sIgE to α-Gal was 0.8 IU/ml. Thus, although the sIgE was < 1.0 IU/mL it was nonetheless > 10% of the total IgE.
The final element of diagnosis depends on how they respond clinically to a diet avoiding red meat25. From conversations with allergists working in areas with a large number of cases it appears that at least 80% of cases fit the classical description, where both diagnosis and primary treatment are relatively straightforward.
However, it is important to realize that many cases do not present classically. For example, there are a significant number of pediatric cases and the published data suggests that the condition in children has similar features26. In particular, the specificity and titer of sIgE, the delay between eating meat and reactions, the relationship to tick bites, and the response to diet are all similar24, 27, 28. Another exception to the rule relates to the delay in symptom onset. Most evidence is consistent with a delay of 2–6 hours, however a recent study found that 16% of subjects with the syndrome reported subjective symptom onset in less than 2 hours24. Moreover, faster reactions were reported in a recent cohort from South Africa28, and have been consistently observed with pork kidney, both in clinical practice and in challenge tests29–31.
In terms of diagnosis it became clear in early studies that prick tests with extracts of beef or pork were unreliable6. Sensitization can be investigated using intradermal skin tests to beef, pork, and milk as well as chicken, turkey and fish as negative controls6. However, few clinics carry out intradermal tests for food antigens and the majority of allergists rely on a blood test for IgE to α-Gal. There are no strict criteria for what sIgE levels equate with the diagnosis, though the preponderance of our patients have IgE specific for α-Gal that is at least 1% of the total IgE. Diagnostically there are two groups that often cause confusion: patients with an unclear history who are found to have IgE to α-Gal and secondly patients who give a history of reacting to mammalian products but whose blood test is negative for IgE to α-Gal.
What is the range of symptoms that can occur in relation to the syndrome?
In our practice the most important group of non-classical symptoms are those that involve the gastrointestinal (GI) tract. GI elements are not uncommon as part of reactions that include hives. However there are also patients who have episodes of abdominal pain without any skin involvement. Those cases are a problem because the possibility of food allergy is not obvious, and they can be severe. Indeed, two of the most severe cases we are aware of manifested with significant GI symptoms but little if any dermatologic involvement. Interestingly, there have been very few subjects who have reported itching in the mouth or swelling of the oral tissue. Other diagnoses that arise less commonly are arthritis and chronic pruritus24. Distinguishing α-Gal from chronic hives can be challenging and there are some cases where the two entities may overlap32. In any case where the history is atypical but the sIgE is positive, the question that has to be asked is whether they improve with avoidance of red meat or full avoidance of mammalian products. It is important to remember that random cohorts of subjects in TN, VA, and NC have found that 15% or more of the population have IgE to α-Gal4, 27, 33. Thus, there must be many subjects who do not experience allergic symptoms despite having the sensitization.
Seronegative subjects with allergic responses to one or more mammalian products.
Obviously there are plenty of children with traditional immediate allergy to mammalian foods, but these are primarily related to proteins also present in cow’s milk and have typical rapid onset34. The cases we are interested in here are those with onset later in childhood or as adults who don’t always react rapidly and have symptoms related to mammalian proteins (see Table 1B). The first group that became clear was the pork-cat syndrome. Although pork-cat was long recognized in Europe it had been ignored in the USA35, 36. Most of the early cases we saw had been referred as possible cases of α-Gal syndrome37 and in a recent investigation of 261 subjects reporting red meat allergy nearly 2% had an IgE signature suggestive of pork-cat (i.e. – positive sIgE to cat, serum albumin of cat [Fel d 2] and serum albumin of swine [Sus s 1])24. At present there is no clear explanation for why they present as adults. We have not carried out challenges on pork-cat syndrome and it is our judgment that none of the three cases reported in Table 1B would have agreed to a challenge. However, there are occasional cases where a challenge may be necessary to resolve confusion.
MANAGEMENT
A. The natural history of the syndrome and relevance of tick avoidance
Following patients in the clinic, including questions about symptoms, diet and further tick bites, some aspects of natural history are already clear. Firstly, all patients should be informed that further tick bites can maintain or lead to increases in the titer of IgE to α-Gal38, 39. By contrast, most subjects who successfully avoid tick bites will experience a decrease in their α-Gal sIgE39. Although the rate of decline is variable and it is not clear what degree of decrease is required to successfully regain tolerance, we have followed many patients whose titers became negative (i.e. <0.1 IU/mL) and who re-reintroduced meat in their diet on their own. Avoiding tick bites in rural or suburban areas is difficult, but normal avoidance measures, i.e. appropriate clothing and sprays, can be effective (see https://www.cdc.gov/ticks/avoid/on_people.html or https://tickencounter.org/prevention/protect_yourself)40. The strongest evidence for sensitization to α-Gal relates to ticks, but it is possible that other ecto-parasites such as “chiggers” could also be relevant41. A complex question is why some people who live and work in an area with ticks do not become sensitized. This question may have two elements: firstly, are all individuals equally “attractive” to ticks? Multiple anecdotal stories report that ticks favor some subjects. Secondly, there are patients who report tick bites that do not itch and they do not become sensitized. There is currently no solid information about the characteristics that predict either the tendency to get tick bites or the likelihood of becoming sensitized after being bitten. It is clear, however, that many subjects who are not traditionally atopic (i.e., do not produce IgE to aeroallergens) can nonetheless develop sensitization to α-Gal and acquire the syndrome after getting tick bites. Levels of α-Gal sIgE and also the clinical presentation are similar in non-atopic subjects and their atopic counterparts24.
A question that often arises is whether eating red meat is alone sufficient to induce an IgE response to α-Gal. Our own published data provides strong evidence that people living in an area without ticks, such as Northern Sweden or New York City, do not develop sensitization to α-Gal or the syndrome despite eating large quantities of beef or pork8, 33. The same data also argues against a role for other mammalian exposures, such as cat ownership, in developing IgE to α-Gal. We are unaware of any data that has looked at whether eating red meat can influence the levels of α-Gal sIgE in subjects who have the syndrome. An additional point is that some subjects with the syndrome may tolerate red meat on some occasions with few or no symptoms but have severe reactions on others. This intra-individual variability is often not explained by the amount of meat and may reflect differences in the quantity or form of α-Gal which is present in the meat or could reflect the importance of co-factors such as exercise, non-steroidal anti-inflammatory medications and alcohol in modifying the allergic response42. In regards to the natural history of subjects who are sensitized but asymptomatic, there are currently no population-based follow up studies and we are not in a position to recommend management of these cases except to avoid tick bites.
B. Avoidance of “Red” Meat
The primary advice for newly diagnosed cases of the syndrome is to completely avoid meat of mammals (see Fig 1). In most areas of the United States this means beef, pork and lamb6. However internal organs are equally or more able to induce reactions42. This includes, kidneys, liver, heart, and intestines (tripe), but the evidence is strongest for pork kidneys29, 31. In Europe and most other countries many other animals are eaten, including rabbit, horse, goat, etc29. In rural USA it is worth mentioning that venison and bear meat and also many other small animals that can become part of “Brunswick stew” are all mammals.
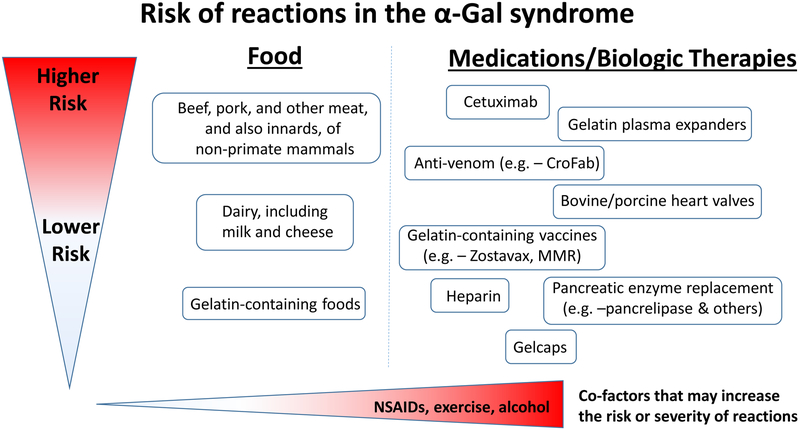
Fig 1.
The risk and also severity of reactions in the α-Gal syndrome relates to the amount of the oligosaccharide that is present in food, drugs or other therapeutics. The route of administration is relevant to the speed at which reactions occur, i.e. – intravenous administration is associated with rapid reactions whereas oral ingestion has delayed onset. Co-factors such as NSAIDS, exercise and alcohol can be additional risk modifiers. This schematic reflects clinical experience, as well as challenge studies and laboratory investigations.
C. The case for avoiding dairy products
We don’t include avoidance of dairy products as part of primary avoidance, because most of the patients do not react to milk or cheese (Fig 1). This is not because glycoproteins or glycolipids carrying α-Gal are absent from dairy products. Indeed it has been clear since 2009 that these patients have IgE antibodies that recognize α-Gal in cow’s milk extract, and give positive skin reactions to milk6. Until recently the primary reason for recommending full avoidance of dairy products has been inadequate control of symptoms in subjects avoiding red meat. Many of these patients have subsequently respond to a diet that also includes dairy avoidance. Avoiding products such as milk, yogurt, and ice cream is relatively easy because there are alternatives made from almond, oat, cashew or other mon-mammalian sources. The thing that patients miss most is English or French cheeses43.
The argument about a dairy-free diet changes completely if consumption of α-Gal containing products can contribute to inflammation without giving rise to allergic symptoms. Before the recognition of IgE to α-Gal, the cardiovascular group in Cambridge reported that patients with triple vessel coronary artery disease had a distinct pattern of IgG and IgD specific for α-Gal44. In 2018 we reported that there was significantly worse coronary artery disease in patients who had IgE to α-Gal45. That study needs confirmation, but it implies that the risk related to IgE to α-Gal is not restricted to subjects who have allergic reactions. If it is true that patients with IgE to α-Gal have an increased risk of atherosclerosis even if they don’t have allergic symptoms there are two groups of subjects who may need to be advised to go dairy free as well as avoiding red meat. Firstly patients whose symptoms are “controlled” by avoiding red meat and secondly, subjects who have IgE to α-Gal but haven’t recognized symptoms related to red meat. The evidence at present is not strong enough to make this as a recommendation but clearly it is an area that needs more research.
D. Additional considerations for managing the syndrome
There are “hundreds” of ways in which food and other products which are not obviously mammalian could nonetheless have products from mammals added during preparation or manufacture46. The evidence for the presence of α-Gal in these different food and medical products varies markedly, with some items clearly expressing the oligosaccharide (e.g.- cetuximab) and other products being labeled by some groups as “risky” seemingly only because there is some constituent that is derived from mammals (e.g. – magnesium stearate, glycerin). Although in many cases the data is insufficient to make informed judgments, we think it is worthwhile to attempt to create a framework for thinking about relative risk and by extension management of the syndrome (see Fig 1 and Table 2).
Table 2.
Categorization of food and medical products that are or may be problems in subjects with the α-Gal syndrome, stratified by relative risk.
| Management /Risk category |
Type | Source | Reference |
|---|---|---|---|
| Primary | Foods | • Red meat (beef, pork, lamb, deer) | 6, 23 |
| • Innards (pork kidney, liver, heart, intestines) | 29, 31 | ||
| • Pork gut casings for sausages | |||
| Meds | • Cetuximab | 4 | |
| • Gelatin-based colloid plasma substitute (Gelafundin) | 53, 75 | ||
| Secondary | Foods | • Dairy (milk, cheese, yogurt, butter) | 53 |
| • Gelatin (marshmallows, jelly babies, other sweets) | 47, 53 | ||
| • Collagen (e.g. -beef collagen casings) | 76 | ||
| • Lard | |||
| Meds | • Gelatin-containing vaccines (Zostavax, MMR, yellow fever) | 49–51 | |
| • Bovine and porcine heart valves | 58, 77 | ||
| • Pancreatic enzyme replacement | 55 | ||
| • Crotalidae polyvalent immune Fab (CroFab) | 61, 62 | ||
| Incomplete data | Foods | • Canned tuna (contaminated with dolphin or whale) | |
| • Chicken or fish cooked on a contaminated grill | |||
| Meds | • *Gelatin: e.g. – gel caps, Floseal hemostatic matrix, Surgifoam powder, Absorbable gelatin sponge, Gabapentin oral solution, Lidocaine patch | 66, 78, 79 | |
| • **Heparin | 63 | ||
| • **Stearic acid and/or magnesium stearate: e.g. -many tablets, including: Acetaminophen, Oxycodone, Lisinopril, Oxycontin | 66 | ||
| • **Glycerin: e.g. -many suspensions, including: Acetaminophen liquid, Methadone solution, Ibuprofen suspension | 66 | ||
| • **Lactose and derivatives: e.g. – Aspirin, Haloperidol injection, Hydromorphone injection | 66 |
Risk/management categories:
Primary: considered high risk and avoidance recommended for all subjects with the active syndrome.
Secondary: some subjects can tolerate without allergic reactions but should be used with caution (there is clear evidence these products can contain α-Gal).
Insufficient data:
limited evidence or case reports suggesting these products may contain alpha-gal and contribute to clinical reactions in some patients with the syndrome;
although heparin, stearic acid, glycerin and lactic acid are or can be derived from mammals there is no evidence these products intrinsically have α-Gal epitopes. It is possible that α-Gal is present as a “contaminant”, however there is little evidence that this would be sufficient to contribute to symptoms.
- Non-meat, non-dairy food products
- Pork gut casings for sausages: Many varieties of sausages use casings derived from pork gut. Hence, chicken and turkey sausages, which would otherwise be safe, can induce anaphylactic or other reactions. We have seen at least 5 patients who reacted clinically after eating chicken or turkey sausage which had a casing made of pork gut. Today most sausages are labelled correctly but caution is necessary.
- Mammalian fat in foods: Lard (rendered pork fat) is an important element of cooking, not only in pastry. Fat may also be added to mashed potatoes to add flavor, and pork fat is often added to venison either to add flavor or to make venison burgers. Thus, hunters who can normally tolerate venison may react if it has had pork fat added. Suet (un-rendered fat from sheep or cattle) is also used in cooking, for instance as part of mince-meat.
- Gelatin: Gelatin is a glycoprotein that is normally derived from collagen in the skin or hooves of cows or other large mammals. This protein can carry α-Gal epitopes but the quantity of the oligosaccharide varies according to the methods of extraction. In regards to food, gelatin is a main ingredient of jelly babies, marshmallows and jello. We have seen cases of severe reactions after eating marshmallows and a detailed report which including challenge tests has confirmed reactions to jelly babies47.
- Tuna and Dolphins: We have heard reports of apparent reactions to canned tuna where the possible explanation is contamination of the tuna with dolphin which is mammalian. This is a good example of a situation where it would be helpful to have an assay for α-Gal in foodstuff.
- Other medical uses of mammalian proteins or parts
- Monoclonal antibodies: Despite the fact that many mAb that are in clinical use are generated in non-primate mammalian cell systems, the evidence for α-Gal expression on most monoclonals other than cetuximab is minimal5. One exception is infliximab, which has been shown to express low amounts of the glycan and has been linked to reactions in a small number of subjects with the α-Gal syndrome48. The reason for why α-Gal glycosylation of the Fab occurs selectively on cetuximab as compared to other monoclonal is likely due to differences in the cell lines used in production. Cetuximab is produced in mouse SP2/0 cells whereas most other monoclonals are generated in other cell lines, such as Chinse Hamster Ovary cells. The specific amino acid sequence in the Fab of cetuximab could also play a role in favoring glycosylation.
- Gelatin-containing medications:
- Gelatin based capsules are used for many medicines and may give rise to symptoms in a small proportion of patients. Certainly they can add confusion in cases where a mammal-free diet has not successfully controlled symptoms (see Table 2).
- Enzyme replacement: The pancreatic enzymes which are used for replacement in some cases of cystic fibrosis or other causes of pancreatic failure are purified from the pancreas of large mammals. These proteins, and also other mammalian-derived enzymes, have been shown to express α-Gal and have functional activity in basophil activation tests55.
- Bovine or porcine heart valves: Soon after the description of α-Gal we heard reports of patients who experienced hives or even a mild episode of anaphylaxis after transplantation of mammalian heart valves56. We then became aware that Dr. Ankersmit and his colleagues in Vienna, Austria had reported that transplantation of mammalian valves could cause an increase in IgG to α-Gal57. Recently there have been reports that mammalian valves degenerate more rapidly in patients with IgE to α-Gal58. This is a problem that should be solved by using α-Gal knock-out pigs as a source of heart valves59, 60.
- Anti-venom: The presence of α-Gal epitopes in anti-venom formulations, which are purified Fab fragments derived from polyclonal IgG of venom-immunized non-primate mammals, was convincingly shown by Fischer et al61. Importantly, the anti-venom elicited basophil activation in an in vitro experiment with cells derived from a subject with the α-Gal syndrome. There has been at least one case report of an acute reaction to CroFab in an α-Gal sensitized subject which was attributed to the α-Gal syndrome62.
- Heparin: It is not obvious that the polysaccharide heparin, which itself does not appear to carry α-Gal, should be a problem in patients with the α-Gal syndrome. Nonetheless, there have been a small number of case reports that have raised the possibility and we are currently consulted on this question with some frequency63, 64. Heparin is a glycosaminoglycan derived from pig intestine and it is possible that some batches could be contaminated with glycosylated molecules derived from the pig. Alternatively it is possible that the IgE response to tick bites in some patients includes a wider range of specificities65. The fact that cases have only rarely been reported in UNC, UVA and/or Vanderbilt suggests that IgE-related reactions to heparin are uncommon, but this topic warrants additional investigation.
- Magnesium stearate, etc: There have been rare reports of subjects with α-Gal syndrome who experienced reactions that were attributed to oral medications, even those that did not contain gelatin. Magnesium stearate, which can be sourced from mammalian fat (i.e. – stearic acid), was described as a likely culprit in one such case66. Given that magnesium stearate is not structurally similar to α-Gal, the implication was that residual α-Gal could be present as a contaminant in some preparations.
E. Alternative medical approaches
We are aware that there are some alternative/complementary medical approaches being offered in the community for the treatment of the α-Gal syndrome, e.g. – auricular acupuncture, kinesiology and others. An interesting possibility is that the apparent efficacy observed in some of these cases actually reflects the natural history of the syndrome, i.e.- α-Gal sIgE levels can drop rapidly in some cases.
AREAS OF ONGOING RESEARCH
Why do ticks give rise to the IgE response to α-Gal?
Before the evidence focused on ticks many other possible causes of this form of sensitization were considered including local plants or fungi and even nematode worms. However, the experience in Virginia suggested that sensitization was primarily, if not exclusively, caused by bites of the lone star tick. Although the lone star is restricted to North America, sensitization to α-Gal in other countries has also been associated with the bites of different species of ticks [Table E1]. Certainly A. americanum, Ixodes ricinus and I. holocyclus are each the most common cause of bites in the USA, Sweden, and Australia respectively9, 22, 67. Several groups have now carried out investigations on ticks13, 14, 21, 68. None of those studies suggest that the production of α-Gal is a special property of the lone star tick. At present it seems likely that several species of ticks express α-Gal and have salivary constituents that can promote Th2-related immunity. In keeping with the general tick theory there is some evidence that other ticks in the USA can cause sensitization. In an area of northern Wisconsin and northern Minnesota where A. americanum is not established typical cases of the syndrome have been related to bites of “wood ticks”, i.e. Dermacentor variabillis.
Mouse and other animal models of the IgE response to tick bites
There is good evidence that either bites of live ticks or intradermal injections of tick extract will induce an increase in serum IgE in mice16, 68. However “wild type” mice express α-Gal on proteins and lipids and do not make IgE to α-Gal. To solve this problem strains of mice with the gene for α−1,3-galactosyltransferase knocked out (α-Gal KO mice) are used to study IgE production16, 68, 69. There are many questions that remain to be answered about IgE responses to oligosaccharides in humans however there is already significant data about the role of T cells in mice. A recent paper clearly demonstrates the relevance of T cells for the IgE response to tick extracts16. Interestingly, in earlier studies on the basis of production of IgG and IgM specific for α-Gal by human B cells, the evidence strongly implicated NKT cells as the source of T cell help70. The importance of this is that NKT cells can be activated by glycolipids.
What is the mechanism of the delay before onset of symptoms and can this mechanism also be relevant to understanding chronic inflammatory effects of α-Gal exposure?
The delay between eating red meat and the onset of symptoms has two elements: first the patients deny any early symptoms (either oral or systemic), and second that despite delays of 2–6 hours, symptoms can come on rapidly and episodes can be severe [Table 1]. Since in vitro activation of basophils will occur within 20 minutes of exposure to α-Gal and intradermal skin tests to cetuximab or beef develop within 15 minutes, it seems likely that the delay in symptoms reflects delayed arrival of the relevant form of antigen to mast cells that reside in peripheral tissues. Two things lead us to think about lipids; firstly many hunters said that venison, was less of a problem than meat from domestic mammals. Venison meat comes from true wild animals and has very little fat. Indeed, making “venison burgers” requires addition of fat to make it stick together. Secondly patients reported reacting after eating fat or pastry made with lard. The importance of this ‘glycolipid hypothesis’ comes because it links together the relevance of fat, the delay in symptoms and the possible relevance to inflammation in arteries45, 71, 72. In normal digestion fat is assembled into chylomicrons which enter the circulation after two hours. Over the next few hours the fat is converted into LDL and HDL which involves a change in particle size from 300 nanometers down to 20 or 10 nanometers. The important fact for the argument is that LDL or HDL can pass through the endothelial wall without inflammation. The hypothesis has to be that these particles derived from mammalian meat or fat can activate mast cells either in the skin or in arterial walls72, 73. This idea is supported by the recent report of Roman-Carrasco et al. who demonstrated that α-Gal carrying glycolipids from beef, but not glycoproteins from beef, could transit through a Caco-2 epithelial barrier model and be packaged into chylomicrons74.
CONCLUSION
In the ten years since the first description of delayed allergic reactions to mammalian meat, the α-Gal syndrome has become well recognized in many continents including Australia, Europe/Asia and North America. The features that distinguish this syndrome from other forms of food allergy include: i) onset as an adult or late in childhood, ii) a relationship to previous tick bite(s), iii) the epitope for the IgE response is an oligosaccharide, and iv) the characteristic delay between eating mammalian meat and onset of the symptoms. Many issues have arisen about the range of symptoms that develop in patients who are sensitized to α-Gal, but in the majority of cases (~ 80%) management by avoidance of red meat (and organs) is sufficient. A minority of cases (~5–20%) also need to avoid dairy and gelatin. A small minority of cases (~≤1%) needs to be aware of the large number of products that are derived from mammals. On the other hand, many sensitized subjects can continue to eat red meat or dairy without overt allergic symptoms. A big question that requires further research is whether all patients with the syndrome should avoid dairy because of the possible link with chronic inflammation of the arteries. We also need to better understand the risk of reactions to the wide variety of products that may include small amounts of material derived from mammals.
Supplementary Material
Grant Support:
This work was supported by funding from NIH grant R37-AI-20565 [TPM]
Abbreviations
- α-Gal
galactose-α−1,3-galactose
- BSA
Bovine serum albumin
- BMS
Bristol-Myers Squibb
- CDC
Center for Disease Control and Prevention
- CroFab
Crotalidae Polyvalent Immune Fab
- EGD
esophagogastroduodenoscopy
- Fab
Antigen binding fragment
- GI
gastrointestinal
- HDL
high-density lipoprotein
- IgE
immunoglobulin E
- LDL
low-density lipoprotein
- mAB
monoclonal antibody
- RMSF
Rocky Mountain spotted fever
Footnotes
Disclosures:
TPM has a patent on an IgE assay to α-Gal and has received assay support from Thermo-Fisher/Phadia. JMW has received research support from Thermo-Fisher/Phadia. The remaining authors declare that they have no conflicts.
REFERENCES
- 1.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78. [DOI] [PubMed] [Google Scholar]
- 2.O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(24):3644–8. [DOI] [PubMed] [Google Scholar]
- 3.Erwin EA, Custis NJ, Satinover SM, Perzanowski MS, Woodfolk JA, Crane J, et al. Quantitative measurement of IgE antibodies to purified allergens using streptavidin linked to a high-capacity solid phase. J Allergy Clin Immunol. 2005;115(5):1029–35. [DOI] [PubMed] [Google Scholar]
- 4.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammerts van Bueren JJ, Rispens T, Verploegen S, van der Palen-Merkus T, Stapel S, Workman LJ, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574–6. [DOI] [PubMed] [Google Scholar]
- 6.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, Behravesh CB. Expanding Range of Amblyomma americanum and Simultaneous Changes in the Epidemiology of Spotted Fever Group Rickettsiosis in the United States. Am J Trop Med Hyg. 2016;94(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Commins S, James H, Kelly E, Pochan S, Workman L, Perzanowski M, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α−1,3-galactose. JACI. 2011;127(5):1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, et al. Ehrlichia and spotted fever group Rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector Borne Zoonotic Dis. 2014;14(5):307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgdorfer W, Brinton LP. Mechanisms of transovarial infection of spotted fever Rickettsiae in ticks. Ann N Y Acad Sci. 1975;266:61–72. [DOI] [PubMed] [Google Scholar]
- 12.Biggs HM, Behravesh CB, Bradley KK, Dahlgren FS, Drexler NA, Dumler JS, et al. Diagnosis and Management of Tickborne Rickettsial Diseases: Rocky Mountain Spotted Fever and Other Spotted Fever Group Rickettsioses, Ehrlichioses, and Anaplasmosis - United States. MMWR Recomm Rep. 2016;65(2):1–44. [DOI] [PubMed] [Google Scholar]
- 13.Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of Alpha-Gal-Containing Antigens in North American Tick Species Believed to Induce Red Meat Allergy. Front Immunol. 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolovic D, Mihailovic J, Commins SP, Wijnveld M, Kazimirova M, Starkhammar M, et al. Allergenomics of the tick Ixodes ricinus reveal important alpha-Gal-carrying IgE-binding proteins in red meat allergy. Allergy. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabezas-Cruz A, Espinosa PJ, Alberdi P, Simo L, Valdes JJ, Mateos-Hernandez L, et al. Tick galactosyltransferases are involved in alpha-Gal synthesis and play a role during Anaplasma phagocytophilum infection and Ixodes scapularis tick vector development. Sci Rep. 2018;8(1):14224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous Exposure to Clinically Relevant Lone Star Ticks Promotes IgE Production and Hypersensitivity through CD4(+) T Cell- and MyD88-Dependent Pathways in Mice. J Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190(9):510–1. [DOI] [PubMed] [Google Scholar]
- 19.Adedoyin J, Gronlund H, Oman H, Johansson SG, van Hage M. Cat IgA, representative of new carbohydrate cross-reactive allergens. J Allergy Clin Immunol. 2007;119(3):640–5. [DOI] [PubMed] [Google Scholar]
- 20.Gronlund H, Adedoyin J, Commins SP, Platts-Mills TA, van Hage M. The carbohydrate galactose-alpha-1,3-galactose is a major IgE-binding epitope on cat IgA. J Allergy Clin Immunol. 2009;123(5):1189–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, et al. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68(4):549–52. [DOI] [PubMed] [Google Scholar]
- 22.Apostolovic D, Tran TA, Starkhammar M, Sanchez-Vidaurre S, Hamsten C, Van Hage M. The red meat allergy syndrome in Sweden. Allergo J Int. 2016;25(2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immun. 2014;134(1):108–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, et al. Investigation into the alpha-Gal syndrome: Characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinke JW, Platts-Mills TA, Commins SP. The alpha-gal story: lessons learned from connecting the dots. J Allergy Clin Immunol. 2015;135(3):589–96; quiz 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson JM, Platts-Mills TAE. Red meat allergy in children and adults. Curr Opin Allergy Clin Immunol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131(5):e1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabelane TBW, Botha M, Facey Thomas H, Ramjith, J, Levin, M. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018;29:841–9. [DOI] [PubMed] [Google Scholar]
- 29.Morisset M, Richard C, Astier C, Jacquenet S, Croizier A, Beaudouin E, et al. Anaphylaxis to pork kidney is related to IgE antibodies specific for galactose-alpha-1,3-galactose. Allergy. 2012;67(5):699–704. [DOI] [PubMed] [Google Scholar]
- 30.Hilger C, Fischer J, Swiontek K, Hentges F, Lehners C, Eberlein B, et al. Two galactose-alpha-1,3-galactose carrying peptidases from pork kidney mediate anaphylactogenic responses in delayed meat allergy. Allergy. 2016;71(5):711–9. [DOI] [PubMed] [Google Scholar]
- 31.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. The Journal of allergy and clinical immunology. 2014;134(3):755–9 e1. [DOI] [PubMed] [Google Scholar]
- 32.Pollack K, Zlotoff BJ, Borish LC, Commins SP, Platts-Mills TAE, Wilson JM. alpha-Gal Syndrome vs Chronic Urticaria. JAMA Dermatol. 2019;155(1):115–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Commins SP, Kelly LA, Ronmark E, James HR, Pochan SL, Peters EJ, et al. Galactose-alpha-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185(7):723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson JM, Platts-Mills TAE. Meat allergy and allergens. Mol Immunol. 2018;100:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouet M, Sabbah A, Le Sellin J, Bonneau JC, Gay G, Dubois-Gosnet C. [Fatal anaphylaxis after eating wild boar meat in a patient with pork-cat syndrome]. Allerg Immunol (Paris). 2001;33(4):163–5. [PubMed] [Google Scholar]
- 36.Hilger C, Kohnen M, Grigioni F, Lehners C, Hentges F. Allergic cross-reactions between cat and pig serum albumin. Study at the protein and DNA levels. Allergy. 1997;52(2):179–87. [DOI] [PubMed] [Google Scholar]
- 37.Posthumus J, James HR, Lane CJ, Matos LA, Platts-Mills TA, Commins SP. Initial description of pork-cat syndrome in the United States. J Allergy Clin Immunol. 2013;131(3):923–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-alpha-1,3-galactose carbohydrate IgE antibody levels: A retrospective cohort study in a single institution. J Am Acad Dermatol. 2018;78(6):1135–41 e3. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Straesser MD, Keshavarz B, Workman L, McGowan EC, Platts-Mills TAE, et al. IgE to Galactose-alpha-1, 3-Galactose Wanes Over Time in Patients Who Avoid Tick Bites. J Allergy Clin Immunol Pract. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prose R, Breuner NE, Johnson TL, Eisen RJ, Eisen L. Contact Irritancy and Toxicity of Permethrin-Treated Clothing for Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis Ticks (Acari: Ixodidae). J Med Entomol. 2018;55(5):1217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoltz LP, Cristiano LM, Dowling APG, Wilson JM, Platts-Mills TAE, Traister RS. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J Allergy Clin Immunol Pract. 2019;7(2):664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer J, Yazdi AS, Biedermann T. Clinical spectrum of alpha-Gal syndrome: from immediate-type to delayed immediate-type reactions to mammalian innards and meat. Allergo J Int. 2016;25:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drouet M, Sarre ME, Hoppe A, Bonneau JC, Leclere JM, le Sellin J, et al. Characteristics of a group of 21 patients allergic to meat by sensitization to alpha-Gal allergens. Rev Fr Allergol. 2016;56(7–8):533–8. [Google Scholar]
- 44.Mosedale DE, Chauhan A, Schofield PM, Grainger DJ. A pattern of anti-carbohydrate antibody responses present in patients with advanced atherosclerosis. J Immunol Methods. 2006;309(1–2):182–91. [DOI] [PubMed] [Google Scholar]
- 45.Wilson JM, Nguyen AT, Schuyler AJ, Commins SP, Taylor AM, Platts-Mills TAE, et al. IgE to the Mammalian Oligosaccharide Galactose-alpha-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38(7):1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ockerman HW, Hansen CL. Animal by-product processing & utilization. 1st ed. Lancaster, PA: Technomic Pub. Co., Inc.; 2000. xvi, 523 p. p. [Google Scholar]
- 47.Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2013;1(3):302–3. [DOI] [PubMed] [Google Scholar]
- 48.Chitnavis M, Stein DJ, Commins S, Schuyler AJ, Behm B. First-dose anaphylaxis to infliximab: a case of mammalian meat allergy. J Allergy Clin Immunol Pract. 2017. [DOI] [PubMed] [Google Scholar]
- 49.Stone CA Jr., Hemler JA, Commins SP, Schuyler AJ, Phillips EJ, Peebles RS Jr., et al. Anaphylaxis after zoster vaccine: Implicating alpha-gal allergy as a possible mechanism. J Allergy Clin Immunol. 2017;139(5):1710–3 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone CA Jr., Commins SP, Choudhary S, Vethody C, Heavrin JL, Wingerter J, et al. Anaphylaxis after vaccination in a pediatric patient: further implicating alpha-gal allergy. J Allergy Clin Immunol Pract. 2019;7(1):322–4 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Retterer MKC, Workman LJ, Bacon JR, Platts-Mills TAE. Specific IgE to gelatin as a cause of anaphylaxis to zoster vaccine. J Allergy Clin Immunol. 2018;141(5):1956–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farooque S, Kenny M, Marshall SD. Anaphylaxis to intravenous gelatin-based solutions: a case series examining clinical features and severity. Anaesthesia. 2019;74(2):174–9. [DOI] [PubMed] [Google Scholar]
- 53.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–42 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehlich J, Fischer J, Hilger C, Swiontek K, Morisset M, Codreanu-Morel F, et al. Basophil Activation Test Differentiates Between Patients with Alpha-Gal Syndrome and Asymptomatic Alpha-Gal Sensitization. J Allergy Clin Immunol. 2018. [DOI] [PubMed] [Google Scholar]
- 55.Swiontek K, Morisset M, Codreanu-Morel F, Fischer J, Mehlich J, Darsow U, et al. Drugs of porcine origin-A risk for patients with alpha-gal syndrome? J Allergy Clin Immunol Pract. 2019;7(5):1687–90 e3. [DOI] [PubMed] [Google Scholar]
- 56.Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TAE, Commins SP. Porcine or bovine valve replacement in 3 patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2014;2(5):637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangold A, Szerafin T, Hoetzenecker K, Hacker S, Lichtenauer M, Niederpold T, et al. Alpha-Gal specific IgG immune response after implantation of bioprostheses. Thorac Cardiovasc Surg. 2009;57(4):191–5. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins RB, Frischtak HL, Kron IL, Ghanta RK. Premature Bioprosthetic Aortic Valve Degeneration Associated with Allergy to Galactose-Alpha-1,3-Galactose. J Card Surg. 2016;31(7):446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGregor C, Byrne G, Rahmani B, Chisari E, Kyriakopoulou K, Burriesci G. Physical equivalency of wild type and galactose alpha 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta biomaterialia. 2016;41:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hawkins RB, Ghanta RK. Mammalian meat allergy and advances in bioprosthetic valve technology. J Thorac Cardiovasc Surg. 2017;154(4):1327–8. [DOI] [PubMed] [Google Scholar]
- 61.Fischer J, Eberlein B, Hilger C, Eyer F, Eyerich S, Ollert M, et al. Alpha-gal is a possible target of IgE-mediated reactivity to antivenom. Allergy. 2017;72(5):764–71. [DOI] [PubMed] [Google Scholar]
- 62.Rizer J, Brill K, Charlton N, King J. Acute hypersensitivity reaction to Crotalidae polyvalent immune Fab (CroFab) as initial presentation of galactose-alpha-1,3-galactose (alpha-gal) allergy. Clin Toxicol (Phila). 2017;55(7):668–9. [DOI] [PubMed] [Google Scholar]
- 63.Sell-Dottin K, Sola M, Caranasos T. Impact of Newly Emerging Alpha-Gal Allergies on Cardiac Surgery: A Case Series. Clinics in Surgery. 2017;2. [Google Scholar]
- 64.Kleiman AM, Littlewood KE, Groves DS. Delayed Anaphylaxis to Mammalian Meat Following Tick Exposure and Its Impact on Anesthetic Management for Cardiac Surgery: A Case Report. A A Case Rep. 2017;8(7):175–7. [DOI] [PubMed] [Google Scholar]
- 65.Mawhirt SBE. Successful intravenous heparin administration during coronary revascularization surgery in a patient with alpha-gal anaphylaxis history. . Ann Allergy Asthma Immunol. 2019;In Press. [DOI] [PubMed] [Google Scholar]
- 66.Muglia C, Kar I, Gong M, Hermes-DeSantis ER, Monteleone C. Anaphylaxis to medications containing meat byproducts in an alpha-gal sensitized individual. J Allergy Clin Immunol Pract. 2015;3(5):796–7. [DOI] [PubMed] [Google Scholar]
- 67.van Nunen SA. Tick-induced allergies: mammalian meat allergy and tick anaphylaxis. Med J Aust. 2018;208(7):316–21. [DOI] [PubMed] [Google Scholar]
- 68.Araujo RN, Franco PF, Rodrigues H, Santos LCB, McKay CS, Sanhueza CA, et al. Amblyomma sculptum tick saliva: alpha-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int J Parasitol. 2016;46(3):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koike C, Uddin M, Wildman DE, Gray EA, Trucco M, Starzl TE, et al. Functionally important glycosyltransferase gain and loss during catarrhine primate emergence. Proc Natl Acad Sci U S A. 2007;104(2):559–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu SKT, Tchervenkov J. CD1d-Mediated Interaction Between Activated T Cells and B Cells Is Essential to B-Cell Proliferation and Anti-α-Gal Antibody Production. Transplantation Proceedings. 2009(41):398–402. [DOI] [PubMed] [Google Scholar]
- 71.Wilson JM, Platts-Mills TAE. The oligosaccharide galactose-α−1,3-galactose and the α -Gal syndrome: insights from an epitope that is causal in IgE-mediated immediate and delayed anaphylaxis. EMJ Allergy Immunol. 2018;3(1):89–98. [Google Scholar]
- 72.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–12. [DOI] [PubMed] [Google Scholar]
- 73.Wilson JM, McNamara CA, Platts-Mills TAE. IgE, alpha-gal and atherosclerosis. Aging. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roman-Carrasco P, Lieder B, Somoza V, Ponce M, Szepfalusi Z, Martin D, et al. Only aGal bound to lipids, but not to proteins, is transported across enterocytes as an IgE reactive molecule that can induce effector cell activation. Allergy. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uyttebroek A, Sabato V, Bridts CH, De Clerck LS, Ebo DG. Anaphylaxis to succinylated gelatin in a patient with a meat allergy: galactose-alpha(1, 3)-galactose (alpha-gal) as antigenic determinant. J Clin Anesth. 2014;26(7):574–6. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi H, Chinuki Y, Tanaka A, Morita E. Laminin gamma-1 and collagen alpha-1 (VI) chain are galactose-alpha-1,3-galactose-bound allergens in beef. Allergy. 2014;69(2):199–207. [DOI] [PubMed] [Google Scholar]
- 77.Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TA, Commins SP. Porcine or bovine valve replacement in 3 patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2014;2(5):637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dunkman WJ, Rycek W, Manning MW. What Does a Red Meat Allergy Have to Do With Anesthesia? Perioperative Management of Alpha-Gal Syndrome. Anesth Analg. 2018. [DOI] [PubMed] [Google Scholar]
- 79.Vidal C, Mendez-Brea P, Lopez-Freire S, Gonzalez-Vidal T. Vaginal Capsules: An Unsuspected Probable Source of Exposure to alpha-Gal. J Investig Allergol Clin Immunol. 2016;26(6):388–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.