Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an incredibly deadly disease with a 5-year survival rate of 9%. The presence of pancreatic cystic lesions (PCLs) confers an increased likelihood of future pancreatic cancer in patients placing them in a high-risk category. Discerning concurrent malignancy and risk of future PCL progression to cancer must be carefully and accurately determined to improve survival outcomes and avoid unnecessary morbidity of pancreatic resection. Unfortunately, current image-based guidelines are inadequate to distinguish benign from malignant lesions. There continues to be a need for accurate molecular and imaging biomarker(s) capable of identifying malignant PCLs and predicting the malignant potential of PCLs to enable risk stratification and effective intervention management. This review provides an update on the current status of biomarkers from pancreatic cystic fluid, pancreatic juice, and seromic molecular analyses and discusses the potential of radiomics for differentiating PCLs harboring cancer from those that do not.
Keywords: Pancreatic cancer, Pancreatic ductal adenocarcinoma, Pancreatic cystic lesions, IPMN, Biomarker, Radiomics, Early detection
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastatingly lethal disease with a five-year survival of 9% [1]. It is currently the third leading cause of cancer-related deaths and is projected to replace colon cancer as the second leading cause within the next decade. Surgery remains the only possible curative option for those with PDAC, as long as it is performed in the early stages or prior to precursor lesion progression [2]. The high lethality is due to the discovery of PDAC at later stages, with metastatic disease present at the time of initial diagnosis [3]. Screening those at high risk of developing pancreatic malignancy can facilitate earlier diagnosis and life-saving surgical intervention, which still remains the most effective curative modality. Among the determined high-risk groups are those with a current or previous diagnosis of a pancreatic cystic lesion (PCL) [4]. Patients harboring a cystic lesion are more likely to progress to cancer than even those with a family history of PDAC [5] making them a prime target population for screening and surveillance modalities. However, complexities arise because PCLs present a variable risk for malignant progression: while some PCLs carry up to a threefold increased risk of developing PDAC [6,7], others present with a marginal risk or low probability of developing into PDAC [8].
The overall prevalence of all PCL types in the general population has increased in recent years due to the ubiquitous use of cross-sectional imaging modalities. According to the American Gastroenterological Association, the proportion of incidental PCL discoveries in patients who undergo magnetic resonance imaging (MRI) for an unrelated reason is 15% [9]. It is estimated that 0.7–2.6% of the general asymptomatic population harbors some form of PCLs [10]. The prevalence of these lesions increases with age; autopsies of 70 to 79-year-old patients revealed that 25% harbor a pancreatic cyst, increasing to 37% for patients older than 80 [11–13]. These proportions extrapolate to an estimated 3.5 million people with cystic lesions in the U.S. alone. The transformation potential of all cysts is minimal and an investigation utilizing the SEER database found that 33.2 per 100,000 pancreatic cysts will progress to PDAC [14].
Though screening and intervention are warranted in this high-risk group, the pervasiveness of PCLs leads to thousands of unnecessary medical and surgical interventions per year. These high-risk procedures carry great comorbidity, especially for the elderly. Even if surgery is not required, immense amounts of time and resources are utilized to monitor patients for years following initial discovery [15]. This monitoring is multimodal and can involve expensive sequential imaging methods along with invasive procedures such as repeat Endoscopic Ultrasound-guided Fine Needle Aspiration (EUS-FNA), culminating in eventual excision. This leads to an increased economic burden on the health care system. Importantly, the anxiety and stress imparted on the patient after a PCL diagnosis cannot be understated, and the ability to ameliorate this burden would be immensely impactful [16].
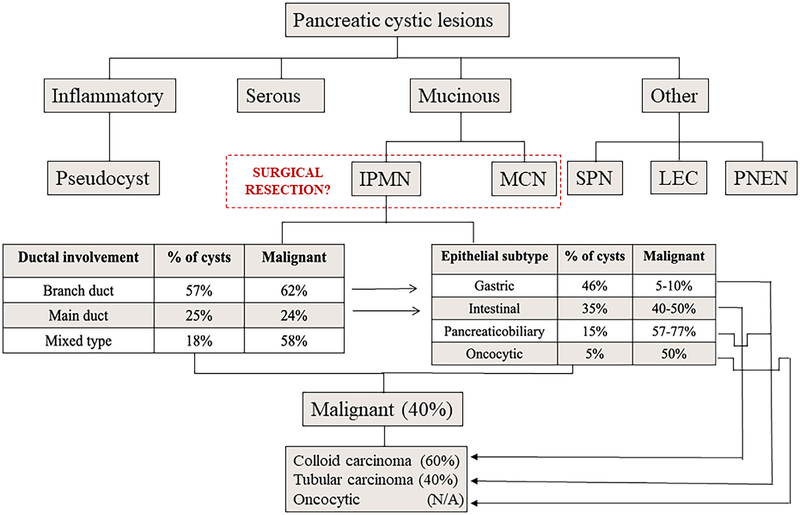
The decades-old challenge still remains to identify the high-risk patients harboring early malignancy or precursor lesions that will eventually progress to PDAC. The goal of early cyst detection, characterization, and fluid analysis is not only to identify the high-risk patients but also to determine which pre-neoplastic lesions have the highest chance of progressing to PDAC, thereby accurately determining the necessity of surgery (Fig. 1). In effect, the task remains to identify the most at risk within this high-risk population. The use of novel biomarkers in conjunction with the current imaging and international consensus guidelines governing cystic clinical strategy can lead to improved utilization of limited resources and, more importantly, improved patient surgical stratification.
Fig. 1.
Chart depiction of pancreatic cystic lesions (PCLs) types. All PCLs are divided into three main types, inflammatory, serous and mucinous. Those grouped under the “Other” heading are rare cyst types that include pseudopapillary tumors (SPT), lymphoendothelial cysts (LEC), and pancreatic neuroendocrine neoplasia (PNEN). The inflammatory type is predominately comprised of pseudocysts. Importantly, serous type cysts are rarely malignant. Mucinous cysts, however, divided into intraductal pancreatic mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN), harbor a greater potential for malignancy. Thus, this is the primary PCL population that undergoes surgical resection. The prevalence of IPMN types (branch, main, and mixed duct), as well as sub-classifications (gastric, intestinal, pancreaticobiliary, and oncocytic), are shown along with their respective proportions that harbor concurrent malignancy.
2. Classification of pancreatic cystic lesions
As per WHO classification (2000) [17–19] PCL’s can be divided into; A. Serous tumors - including serous cystadenoma and serous cystadenocarcinoma B. Mucinous tumors - including mucinous cystadenoma, mucinous cystadenocarcinoma, intraductal papillary-mucinous adenoma and intraductal papillary-mucinous adenocarcinoma C. Solid pseudopapillary tumors.
2.1. Serous cystic neoplasms of the pancreas
Serous cystadenomas/Serious cystic neoplasms (SCAs/SCNs) are benign tumors that can be sub-divided into serous microcystic adenoma (SMA) and serous oligocystic adenoma (SOA) [17]. SMAs have a proclivity towards body or tail in 50–75% of cases while the rest involve the head of the pancreas [20,21]. SMAs account for 1–2% of all exocrine pancreatic tumors. Females (70%) are affected more than males with a mean age of 66 years (range 34–91 years) at presentation. SOAs are far less common than SMA with no sex predilection and are located mainly in the head and body of the pancreas [22,23]. On cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI), SCAs are often multilocular with a honeycomb-like appearance and contain a central stellate scar [24] (Fig. 2A). This characteristic appearance can often lead to a definitive diagnosis via imaging. Histologically, the cysts are lined with glycogen-rich simple cuboidal epithelium, which is positive on periodic acid-Schiff (PAS) stain without diastase digestion [25]. If imaging results are inconclusive, EUS/FNA of cystic fluid can also be done but has low sensitivity though the addition of cytobrushing can improve the sensitivity of EUS-FNA [26,27]. SCNs have relatively lower carcinoembryonic antigen (CEA) levels than other PCL types with a higher risk of malignant progression [24]. However, there is no concrete evidence to support a direct correlation between CEA levels and risk of malignant progression [28].
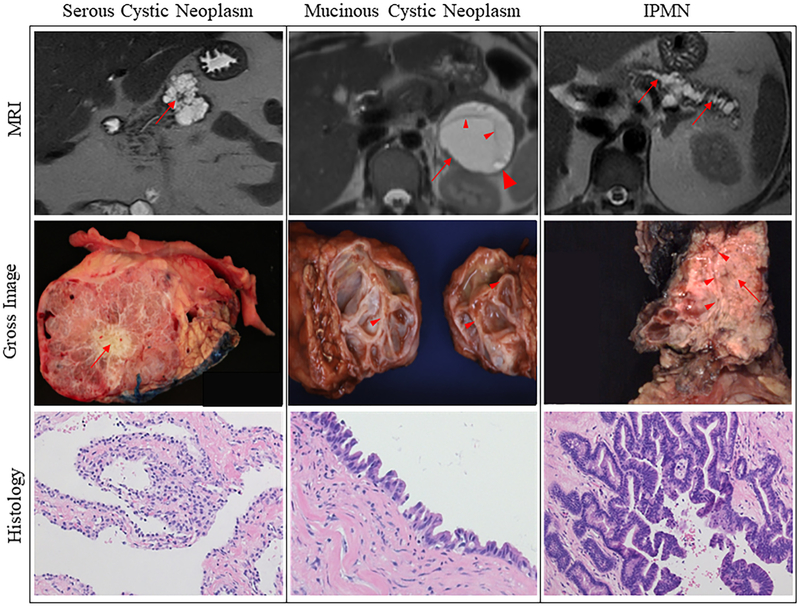
Fig. 2.
MRIs, gross images, and histology of various cystic lesion types resected from patients. The left column has serous cystic neoplasm (SCN) examples. A characteristic stellate scar is appreciated on the MRI and the gross specimen demarcated by red arrows. SCN histology is characterized by the presence of simple cuboidal epithelial cells with clear cytoplasm. The middle column has mucinous cystic neoplasm (MCN) examples. The MRI of an MCN is demarcated by a red arrow that is septated (small arrowheads) and contains an enhanced mural nodule (large arrowhead). The gross specimen contains a large septated lesion with a thick-walled pseudocapsule. Typical MCN histologic presentation with dense ovarian type stroma and epithelial cells lining the cyst that form papillae. The right column has examples of intraductal papillary mucinous neoplasms (IPMNs). Dilation of the main pancreatic duct is appreciated on MRI as demarcated by red arrows. The image of a gross IPMN specimen shows communication between the main pancreatic duct (arrowheads) and the solid/papillary lesion (arrow). Histologic presentation is typical of IPMNs with large papillary structures lined by epithelial cells harboring various degrees of atypia. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
SCAs are considered benign lesions that do not communicate with the pancreatic ducts [29]. Their progression to serous cystadenocarcinoma is exceedingly rare, with an incidence rate of < 1%, including the largest series that reported three cases of cystadenocarcinoma out of 2622 patients [30]. For this reason, the clinical recommendation is to observe serous cystadenomas, with or without serial imaging, to check on the growth rate of the tumor. Resection is warranted only if mass effect symptoms are present such as abdominal pain, nausea, jaundice, or rapid cystic growth. Palliative resection can also be considered if the lesion transforms into a serous cystadenocarcinoma and becomes malignant [31].
2.2. Mucinous cystic lesions
2.2.1. Mucinous cystic neoplasms
Mucinous cystic neoplasms (MCNs) represent 2–5% of all exocrine pancreatic tumors [22]. The mean age at diagnosis is 49 years (20–82 years) and these lesions are predominantly found in women (F: M > 20:1) [22,32]. MCNs are mainly located in the body and tail of the pancreas and if present in the head of the pancreas, they are highly suspicious for mucinous adenocarcinoma [33,34]. MCN’s are typically a single lesion, that can be unilocular or multilocular, which does not communicate with the pancreatic duct [35] (Fig. 2B). Morphologically, MCNs are characterized by a large, solitary, septated, thick-walled cyst with a pseudo-capsule containing either mucin or mixture of mucin and hemorrhagic material [36]. Histological analysis can reveal ovarian-like stroma in addition to columnar cells with abundant mucin production [37]. As per international consensus in 2004, the histological presence of unique ovarian-type stroma was necessary to confirm the diagnosis of MCNs [38]. In contrast to the aforementioned serous cystic lesions, MCNs have an increased propensity to be malignant or progress to a malignant state. Because of this, several studies have tried to identify a marker that can differentiate between the two (Table 1). The incidence rate of mucinous adenocarcinoma varies between 6 and 36% [39]. If the lesion is multilocular, contains papillary projections, and/or contains mural nodules the risk of malignancy drastically increases [40]. The spectrum of differentiation in terms of histology ranges from normal-appearing columnar epithelium to the atypical epithelium. Tumors can be classified as MCN with low/intermediate grade dysplasia, MCN with high-grade dysplasia, or MCN with an associated invasive carcinoma [41].
Table 1.
Cystic fluids markers that identify mucinous and non-mucinous cysts.
| Marker | Cyst type | Cut-off | Sn/Sp (%) | Reference |
|---|---|---|---|---|
| CEA | Mucinous | > 192ng/mL | 73/84 | [26] |
| Amylase | Pseudocyst | > 250 U/mL | 44/98 | [98] |
| CA19–9 | Mucinous | > 50,000 U/mL | 75/90 | [104] |
| MUC5AC + endorepellina | Mucinous | NA | 92/94 | [130] |
| MUC5AC + CA19–9 | Mucinous | NA | 87/86 | [132] |
| VEGF-A | Serous | > 8500 pg/mL | 100/97 | [141] |
| VEGF-C | Serous | > 200 pg/mL | 100/90 | [141] |
| SPINK1b | IPMN | 25,000 ng/mL | 48/98 | [145] |
| GNAS/KRAS mutation | Mucinous | NA | 65/100 | [168] |
| Glucose | Non-Mucinous | 66 mg/mL | 94/64 | [207] |
| Kynurenine | Non-Mucinous | 185,650c | 90/100 | [207] |
Sn - sensitivity, Sp - specificity, NA - not available, CA19–9 - cancer antigen 19–9, CEA - carcinoembryonic antigen, MUC - mucin, VEGF - vascular endothelial growth factor.
Glycan variant of MUC5AC that reacts with a wheat germ lectin.
From pancreatic juice, not cystic fluid.
LC/MS determined abundance cutoff.
In an analysis of 163 patients with resected MCNs, the prevalence of adenocarcinoma was reported to be 17.5% by Crippa et al. [42]. The older patients with invasive adenocarcinoma in this cohort suggested a progression from adenoma to carcinoma. Thus, this group stated that resection should be considered in patients with high-risk MCNs and patients with low-risk MCNs, defined as size < 4 cm and no nodules, can be considered for non-radical resections [42].
2.2.2. Intraductal papillary-mucinous neoplasms
Intraductal papillary-mucinous neoplasms (IPMNs) are mucin-producing tumors arising from the main pancreatic duct or its branches [43]. These lesions are characterized by a dilation of the pancreatic duct resulting from immense mucus production and papillary growth of ductal epithelium (Fig. 2C, key differences between SCNs, MCNs, and IPMNs are presented in Table 2). IPMNs comprise 1–3% of exocrine pancreatic neoplasms, with an incidence rate of 1 per 100,000 per year [12,44,45]. and their frequency is higher in males than in females with a median age of diagnosis in the 6–7th decade [35,46]. IPMNs can be divided into low-risk and high-risk, with the latter defined as dilated main pancreatic duct > 5 mm or the presence of a mural nodule. The pooled cumulative incidence of high-grade dysplasia or pancreatic cancer for low-risk IPMNs is 0.02%, 3.12% and 7.77% at 1 year, 5, and 10 years, respectively. While that for high-risk IPMNs is 1.95%, 9.77% and 24.68% at 1 year, 5 and 10 years, respectively [47]. IPMNs can involve the main duct (MD-IPMN) or side branches (BD-IPMN) or both known as mixed IPMN. BD-IPMNs are not only the most common IPMNs but also the most common pancreatic cyst. MD-IPMNs carry a higher risk of malignancy than BD-IPMN, with 38–68% of the resected specimens of MD-IPMNs showing high-grade dysplasia or cancer [48]. The relative risk of malignant transformation for multifocal IPMNs is not at higher risk as compared to a single cystic lesion [49]. Mixed-type IPMNs contain features of both, yet behave most similarly to MD-IPMNs in terms of progression and malignant potential and are clinically treated as such. Differentiating IPMNs that are malignant/invasive from those that are benign is a persistent and important problem to address. Recent studies that have identified molecular markers in various body fluids capable of distinguishing between invasive and non-invasive IPMNs are summarized in Table 3.
Table 2.
Key differences between serious cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), and intraductal papillary mucinous neoplasms (IPMNs).
| Cyst type | SCN | MCN | IPMN |
|---|---|---|---|
| Decade of presentation | Seventh | Fifth | Sixth to seventh |
| Gender ratio | F > M | F > M | F < M |
| Location | Body/tail | Body/tail | Head |
| Imaging features | Microcystic | Macrocystic | Macrocystic |
| Honeycomb-like | Unilocular/multilocular | Ductal involvement with accompanying duct dilation | |
| Stellate scar | No duct communication | Thickened/irregular walls | |
| Central calcification | Peripheral calcifications | ||
| Viscositya | Low | High | High |
| Amylasea | Low | Low | High |
| CEAa | Low | High | High |
| Epithelial lining | Simple cuboidal | Ovarian-like columnar with mucus production | Papillary structures with large quantity of atypia |
| Malignancy potential | Very low | High | Branch duct IPMN-low |
| Main duct IPMN-high | |||
| Treatment | Observation unless symptomatic, then resection | Resection | Fukuoka criteria guided observation or resection |
Cystic fluid characteristics.
Table 3.
Markers for malignant/invasive cystic lesions.
| Marker | Sn/Sp (%) | Fluid source | Reference | Features |
|---|---|---|---|---|
| Cytology | ||||
| Cell morphology | 55–65/91 | Cystic fluid | [99] | Cells from EUS/FNA with nuclear atypia, dysplasia, and abnormal morphology are suggestive of PDAC |
| Protein | ||||
| CA19–9 | 40/89 | Serum | [113] | Present on the surface of cells and glycoproteins. Only clinically used serum marker for monitoring PDAC |
| 90/43 | Pancreatic juice | [111] | ||
| 81/69 | Cystic fluid | [112] | ||
| CEA | 18/93 | Serum | [113] | Normally found in embryonic tissue. Increased levels are associated with MCNs and PDAC |
| 94/85a | Pancreatic juice | [110] | ||
| 92/64 | Cystic fluid | [112] | ||
| MUC1 (mRNA) | 89/71 | Pancreatic juice | [122] | Mucins are a family of high-molecular-weight glycoproteins that are aberrantly expressed in the setting of PDAC |
| MUC2 | NA | Cystic fluid | [60] | |
| MUC4 | NA | Cystic fluid | [60] | |
| MUC5AC | NA | Serum | [60] | |
| IL-1 | 79/95 | Cystic fluid | [139] | Cytokine with a role in the regulation of immune and inflammatory response |
| Ubiquitinb | NA | Cystic fluid | [150] | Regulatory molecule involved in protein degradation |
| Plectin-1 | NA | Cystic fluid | [151] | Links microfilaments, microtubules, and intermediate filaments |
| mAb Das-1 | 89/100 | Cystic fluid | [152] | Monoclonal antibody against colonic epithelium and reactive to premalignant upper gastrointestinal conditions |
| PGE2 | NA | Cystic fluid | [155] | Mediates inflammation and is a product of COX2 conversion of arachidonic acid |
| DNA | ||||
| KRAS | 45/96 | Cystic fluid | [163] | Known driver mutation in PDAC |
| GNAS | NA | Cystic fluid | [166] | Encodes the a subunit of G Protein |
| MOCA | 75/92 | Cystic fluid | [170] | Diagnostic combinaton of patient characteristics and genomic data |
| PathFinderTG® | 83/91 | Cystic fluid | [171] | Integrated Molecular Pathology Assay |
| Non-coding RNA | ||||
| miRs-210, 223, 221, 155, 187 | NA | Cystic fluid | [176] | miRs are small (19–24 nucleotides) non-coding, single-stranded RNA. These molecules regulate gene expression and have been implicated in various cancers including PDAC. |
| miRs-24, 30a-3p, 18a, 92a, 342–3p, 106b, 142–3p, 532–3p | 89/100 | [181] | ||
| miR-155 | NA | Cystic fluid | [185] | |
| lncs- ADARB2-AS1, ANRIL, GLIS3-AS1, LINC00472, MEG3, PANDA, PVT1, UCA1 | 79/76 | Plasma | [188] | lncRNAs are non-coding RNAs larger than 200 nucleotides in length. They resemble coding transcripts but lack open reading frames. |
| 71/100c | ||||
Sn - sensitivity, Sp - specificity, NA - statistically significant but Sn/Sp not reported, CA19–9 - cancer antigen 19–9, CEA - carcinoembryonic antigen, MUC - mucin, IL - interleukin, PGE2 - prostaglandin E2, KRAS - Kirsten rat sarcoma viral oncogene homolog, GNAS - guanine nucleotide-binding protein, MOCA - multivariate organization of combinatorial alterations, miR - microRNA, lnc - long-noncoding RNA.
Malignancy in BD-IPMNs only.
Sn/Sp of FNA samples was combined with tissue data.
SN/SP value of eight lncRNA panel when combined with miRNA and imaging features.
Based on the cytoarchitectural features and mucin immunohistochemistry (i.e. MUC1, MUC2, and MUC5AC), IPMNs have been classified into four histopathological types; gastric (49–63%), intestinal (18–36%), pancreaticobiliary (7–18%), and oncocytic (1–8%) [49–51]. Recent investigations showed benign EUS findings (cyst size < 5 mm and the absence of a mural nodule) are associated with gastric type IPMN [52]. Corroborating this finding, Furukawa et al. found prognostic relevance to IPMN classification, where patients with gastric type had a better prognosis than patients with intestinal-type IPMNs [53]. Gastric type is associated with the more indolent BD-IPMN, whereas intestinal type is often associated with MD-IPMN [54]. The pancreaticobiliary type has been regarded by some as a high-grade version of the gastric type. These lesions are uncommon, not well characterized, and the invasive carcinoma associated with this type is more aggressive [55,56]. Oncocytic type is relatively uncommon, tends to be large lesions with obscure intraductal appearance, and is less invasive [57]. A retrospective study of patients with cystic lesion type verified on pathology found that even though gastric type IPMN had a better prognosis than intestinal type, those with gastric type who developed invasive disease had worse outcomes when compared to those with progression arising from intestinal-type [53]. The reason for this phenomenon was that gastric IPMNs had the potential to develop a more aggressive tubular (ductal) carcinoma as opposed to the colloid (mucinous) carcinoma arising from intestinal IPMNs [53].
Pancreatic/cystic fluid has been investigated extensively for identifying biomarkers to classify IPMNs into histologic categories that dictate the need for resection. A study by Hara et al. consisting of 36 patients with surgically resected IPMNs found that mucin histology of pancreatic fluid cytology could differentiate the four subtypes of IPMNs with an accuracy of 89% [58]. The same study reported that a combination of cytology and MUC positivity had a sensitivity and specificity of 77% and 86% respectively, for distinguishing high-grade dysplasia/invasion from benign lesions [58]. Table 4 lists histological classifications of IPMN along with their respective mucin expression levels. This table is comprised of the World Health Organization (WHO) recommendations for mucin profiling to classify IPMN subtypes [41] along with the findings of various groups investigating specific mucin expression in the various subtypes (and low-grade vs high-grade dysplasia) including MUC1 [59], MUC2 [59,60], MUC5AC [59], MUC6 [59], MUC4 [61], and MUC13 [62]. EUS-FNA can preoperatively differentiate between the four subtypes of IPMNs, provided biopsy is adequately taken from the epithelial lining of the cyst [63].
Table 4.
Expressional status of mucins across various histology based subtypes of IPMN.
| Mucin | Gastric | Intestinal | Pancreaticobiliary | Oncocytic | Reference |
|---|---|---|---|---|---|
| MUC1a | − | − | + | + | [41]/[59] |
| MUC2a | − | + | − | − | [41]/[60] |
| MUC5ACa | + | + | + | + | [41]/[59] |
| MUC6a,b | + | ± | ± | + | [59] |
| MUC4b | + + | + + + | − | ND | [61] |
| MUC13b | − | + + | + + | ND | [62] |
ND - not determined.
MUCs recommended by the WHO for classification of histological subtypes of IPMN in combination with morphology of papillae, cell differentiation pattern and staining for CDX2.
Differential expression was observed in high-grade dysplastic lesions.
2.3. Other pancreatic cystic lesions
Other cystic lesions of the pancreas include solid pseudopapillary tumors (SPT), lymphoendothelial cysts (LEC), and neuroendocrine neoplasms (PNENs). SPT is a rare tumor seen most frequently in young women in their 20’s. < 10% of SPT’s have aggressive tumor behavior pathologically with a 5-year disease-specific survival of over 98% [64]. The tumor is considered indolent given the high survival rates of patients even when metastases are present [65]. The diagnostic accuracy of preoperative imaging for SPT is high with a sensitivity of 95% [66]. LECs are extremely rare complex lesions that are often round and exophytic that predominately occur in males [67]. They are often anechoic or hypoechoic on EUS and can present with elevated CEA and amylase levels in the cyst fluid aspirate [67]. While these lesions can harbor malignancy, their rarity has prevented adequate pathological characterization [68]. PNENs are also rare and may be solid, cystic, or mixed in morphology. They are usually non-functioning and may occur sporadically or in individuals with multiple endocrine neoplasia type 1 (MEN1) and/or Von Hippel-Lindau (VHL) [69]. They usually present in the sixth decade and have equal gender predisposition. Unlike SPTs, the diagnostic accuracy of preoperative imaging for PNEN is low (sensitivity of 53.3%) [66]. However, EUS-FNA of PNENs has a 90% diagnostic accuracy [70].
Inflammatory pseudocysts are non-malignant fluid-filled sacs often filled with necrotic and hemorrhagic material along with pancreatic enzymes [71]. These are not true cysts as they do not have an epithelial lining. In the absence of any clinical symptoms, they can be monitored with imaging while symptomatic lesions can be effectively treated with steroids and/or surgical drainage [72]. Notably, pseudocysts are often associated with a history of chronic and/or acute pancreatitis [73], alcoholic pancreatitis [74], or autoimmune pancreatitis [75]. These studies found that 42–56% of pancreatitis patients harbor pseudocysts [73,74]. Unfortunately, some cystic neoplasms, including those with malignant potential, can initially present with pancreatitis or even cause recurrent bouts of pancreatitis [16,76]. Along with this, serum amylase and lipase levels, the standard metrics by which pancreatitis is assessed in clinics, are unable to differentiate between MCNs and pancreatitis without supplemental imaging and invasive procedures [77]. Up to 15% of IPMN patients present with pancreatitis [78] as well as 9% of those with MCNs [42] and some IPMNs can elicit an immune response thus inducing autoimmune pancreatitis [79]. BD-IPMNs, in particular, can be difficult to differentiate from pseudocysts in the setting of pancreatitis and thus, many pseudocysts are often mismanaged as IPMNs [16]. It has been reported that 10–15% of cystic lesions discovered with a background of pancreatitis can be malignant [75,80]. Large cyst size and poor response to steroids increase the likelihood of the presence of malignancy [75,80].
3. Imaging and diagnostic algorithms
Multiple imaging modalities have been utilized to differentiate between malignant and benign pancreatic cystic lesions. These include computerized tomography (CT), positron emission tomography (PET), endoscopic ultrasound (EUS), magnetic resonance imaging (MRI), and magnetic resonance cholangiopancreatography (MRCP). A systematic review including 19 studies showed that the accuracy of CT and MRI or MRCP to differentiate benign from malignant cysts was 71–80% and 55–76%, respectively [81]. MRI or MRCP has a higher sensitivity of 96% as compared to CT, which has a sensitivity of 80% in diagnosing IPMN. The meta-analysis suggested the use of CT as an initial diagnostic modality followed by MRI or MRCP for further confirmation [81]. EUS imaging can differentiate between benign and malignant cysts in 65–96% of the cases, which is similar to the accuracy of MRI or MRCP, and CT scans [81]. However, EUS identifies high-risk features such as mural nodules, which could be missed on MRI and MRCP. Although, EUS is not recommended as a first-line modality for small pancreatic cysts with a clear diagnosis, as it is invasive and has comparable accuracy as CT and MRI or MRCP. De Jong et al. showed increased sensitivity with a combination of MRI and EUS as compared with either modality alone in identifying IPMNs, MCNs, pancreatic cancer, and cysts with high-grade dysplasia [82,83]. Combination PET-CT was found to be superior to CT alone in distinguishing benign and malignant lesions [81]. Kauhanen et al. in a prospective study reported the diagnostic accuracy of PET-CT to be 94% compared with 77% for MDCT and 87% for MRI/MRCP in identifying the pancreatic cysts [84]. However, PET-CT is currently not included in the standard diagnostic algorithm for PCL due to insufficient data.
The decision to operate or observe an IPMN is guided by the 2017 revised Fukuoka International Consensus Guidelines [15]. These guidelines elucidate specific radiological features, particularly worrisome and high-risk stigmata, which dictate clinical decision making. The presence of worrisome features requires continual sequential surveillance, while the presence of high-risk stigmata necessitates resection. Imaging high-risk stigmata include main pancreatic duct (MPD) dilation greater than or equal to 10 mm, an enhancing solid component within a BD-IPMN, cysts > 3 cm in size, thickened/enhancing walls, non-enhancing mural nodules, and/or abrupt change in the caliber of the MPD with distal pancreatic atrophy [15]. However, multiple analyses of IPMNs found that size may not accurately predict malignancy, and thus, the importance of size was downgraded in the most recent consensus guidelines [85–87]. An investigation of IPMNs resected according to the Fukuoka criteria found the overall prevalence of PDAC to be only 15.2% [88]. However, this study did conclude that these criteria, in comparison to the 2015 AGA guidelines, were far more sensitive for resection of IPMNs with pathology confirmed high-grade dysplasia [88]. A study with MCN patients found that the sensitivity and specificity of CT scan were 100% and 98% for malignancy if mural nodules were present [89]. Another study analyzing tumor markers and cyst size found that a size > 3.0 cm had greater than four times the risk of malignancy compared to smaller cysts but tumor markers (serum CEA, CA19.9, and cyst fluid CEA) had no predictive value for these circumstances [90].
Prior to the advent of the Fukuoka guidelines, a retrospective study was conducted on surgically resected PCLs that were discovered incidentally. It showed that while the majority of resections contained premalignant or malignant pathological changes, only the malignant group (20.4%) had diminished overall survival [91]. Thus, almost 80% of surgical interventions were unnecessary. After the implementation of the Sendai criteria and then Fukuoka guidelines, the stratification of PCLs into low-risk and high-risk groups lead to improved triaging of patients and refinement of surgical candidates [92]. Congruently, PCL patients with benign features are far less likely to be recommended for surgery when comparing Fukuoka guidelines to Sendai Criteria [92].
While these studies prove that Fukuoka guidelines are an improvement upon previous criteria, the overall sensitivity and specificity for lesions containing PDAC and for those which may progress to PDAC remain low. The increase in sensitivity for detecting malignancy, paired with the limited increase in specificity offered by the Fukuoka guidelines, has resulted in many unnecessary surgical interventions [93]. These criteria have been continually explored in retrospective studies and have been found to improve the association of PCL resection with malignancy when compared to non-adherence [94–96]. However, other studies trying to validate the consensus guidelines have yielded inconsistent results. A meta-analysis of 7 such studies found a positive predictive value of only 36% and a negative predictive value of 90% [87]. Other studies analyzing the cyst-malignancy correlation found that operative resection is undertaken in 30–32% of cystic cases with only 20–38% of those resected lesions found to be malignant on pathology [26,91]. Yet another study showed only correlation with malignancy in 41% of resected cases [97]. The variation among different studies calls into question the inherent validity of utilizing only Fukuoka guidelines to determine PCL malignancy potential and, by extension, clinical strategy.
4. Predictive biomarkers
4.1. Cellular and molecular profiling of cystic fluid
Historically, cystic fluid has been collected and assessed for myriad molecular markers for two primary reasons; discerning between mucinous and serous cysts (Table 1), and differentiating benign from malignant lesions (Table 3). Coupled with EUS imaging, fine-needle aspiration (EUS-FNA) is a procedure used to obtain cystic fluid for analysis. This fluid can be assessed for the presence of a myriad of diagnostically useful species such as cells, proteins, and nucleic acids.
4.2. Cytology
Patients harboring cysts with worrisome features, or those > 3 cm, are recommended to undergo EUS. Each cyst subtype has discreet characteristic cellular typology, morphology, and immunohistochemical staining. Pseudocysts contain inflammatory cells while serous mucinous neoplasms demonstrate glycogen-containing cells [29]. Mucinous cystadenocarcinomas demonstrate cells with malignant features (increased nuclear/cytoplasmic ratio, pleomorphism, anaplasia, hyperchromatism, prominent nucleoli, and mitoses) nearly 50% of the time, but the yield is usually low [98]. MUC2 and CDX2 are indicative of intestinal differentiation and can be used as immunohistochemical markers to identify intestinal-type IPMN [56,59]. A meta-analysis by Suzuki et al. showed a sensitivity and specificity of EUS-FNA cytology to be 65% and 91%, respectively, for distinguishing malignant and benign IPMNs [99]. There has always been a concern with peritoneal seeding secondary to EUS/FNA; however, a study found no significant increase in metastatic disease secondary to the procedure in patients with IPMNs who underwent EUS/FNA [100].
While cystic fluid cytology can help differentiate between various cyst types and can be highly specific, the cellular yield is usually too low to be diagnostically useful thereby greatly diminishing the sensitivity. In the Cooperative Pancreatic Cyst study (CPC study) that involved 341 patients with PCLs, the sensitivity of cyst fluid cytology for diagnosing mucinous cysts was only 34% because of the low number of cells found in cystic fluid [101]. In conjunction, a multiloculated cystic lesion leads to the compartmentalization of fluid species, thus, FNA may not be representative of the lesion’s gestalt.
4.3. Protein markers
4.3.1. CA19.9 and CEA
CA19.9 is a Sialyl Lewis A glycan present on multiple glycoproteins and is currently used in the clinic to monitor patients for PDAC progression and/or evaluation after surgical resection. Carcinoembryonic antigen (CEA) is a secreted glycoprotein involved in cell adhesion and has been used as a biomarker for various gastrointestinal malignancies [102,103]. These proteins are often aberrantly expressed in cystic fluid and much research has been conducted to discern their respective diagnostic and prognostic significance (Tables 1 and 3).
Cyst fluid CA19.9 levels have proven useful in diagnosing MCNs [104] as well as IPMNs [10]. CA19.9, however, can yield confounding results since it is often elevated in PDAC[66]. CEA is one of the most clinically useful cystic fluid biomarkers for PCLs and the prediction of malignancy. A multicenter prospective trial found that a CEA cystic fluid level > 192 ng/mL could distinguish MCNs and IPMNs from the other types of cysts with a sensitivity and specificity of 73% and 84%, respectively [26,54]. Congruently, a study involving 112 patients with histologically confirmed PCLs found CEA to have a sensitivity of 91% for correctly diagnosing a mucinous cyst prior to resection. Unfortunately, the specificity was 31% with an AUC of 0.61 [26].
Cystic fluid CEA and CA19.9 levels have also been shown to be higher in malignant compared to benign cysts [105]. In this study, the sensitivity/specificity for CEA and CA19.9 for predicting malignancy were 92%/64% and 81%/69%, respectively [105]. Another study found CEA levels > 800 ng/mL are also suggestive of premalignant or malignant lesions [98], while a different group determined that cystic fluid CA19.9 has the potential to detect cancer in PCLs [106]. However, other studies have shown that cystic fluid levels for either of these markers are not sufficient alone to specifically differentiate malignant vs nonmalignant cysts [107–109]. Interestingly, there is increasing evidence that these biomarkers are efficacious when measured in pancreatic fluid [110,111]. Pancreatic fluid offers many advantages over cystic fluid including facile and less invasive collection using FNA, the increased representation of the pancreatic gestalt, and less of the complexity introduced by multiloculated lesions.
A combination of serum CA19.9 and CEA was used to determine the presence of malignancy in resected IPMNs [112]. CA19.9 alone had a sensitivity/specificity of 74%/86%, CEA had a sensitivity/specificity of 40%/92%, and a combination of CA19.9 (cutoff > 37 U/mL) and CEA (cutoff > 5 g/mL) had a sensitivity/specificity of 80%/82% [112]. Hirono et al. examined CEA (cutoff > 30 ng/mL) in pancreatic juice in BD-IPMNs and reported sensitivity/specificity of 94%/85% for malignancy [110]. A meta-analysis looking at the ability of serum CA19.9 and CEA to identify invasive and malignant IPMNs found CA19.9 had a pooled sensitivity of 52% and 40% and specificity of 88% and 89%, respectively while CEA has a pooled sensitivity of 18% for both invasive and malignant IPMNs and a specificity of 95% and 93%, respectively [113].
CA19.9 is limited in its diagnostic utility as Le(a−b−) patients do not have the necessary fucosyltransferase enzyme to produce it and also, this phenotype is highly prevalent among different ethnic groups: Asian (7%), European (8%), African (19%) [114]. CEA can be expressed in serum resulting from other diseases including lung fibrosis, Alzheimer’s disease, and a variety of other cancers [115]. With this, these studies demonstrate that clinically used cystic fluid, pancreas juice, and serum markers are variable in their diagnostic potential and that more accurate biomarkers are needed to determine malignancy in pancreatic cystic lesions.
4.3.2. Mucins
Mucins are large O-linked glycoproteins that are expressed on the cell membrane or secreted into ducts and function to protect the luminal surfaces in the liver, lungs, gastrointestinal tract, mammary ducts, and various other organs. In cancer cells, mucins vary in expression, glycosylation, localization, and splicing [116–118]. These mucin traits are exploited by tumor cells to interact with their microenvironment and proliferate and metastasize [116].
MUC1 is a transmembrane mucin that is expressed by most epithelial cells and plays a role in cell proliferation through its interaction with the growth factor receptor tyrosine kinase, ErbB [119]. Its expression is elevated in mucinous cystadenocarcinomas as well as malignant IPMNs [120,121]. MUC1 mRNA levels are increased in the pancreatic juice of malignant IPMNs with a sensitivity and specificity of 89% and 71%, respectively (Table 3) [122]. However, some studies found no correlation between cyst fluid MUC1 protein or RNA with the risk of malignancy in IPMNs or MCNs [60,123]. Further investigation into MUC1 expression in mucinous cysts is warranted based on the current conflicting data.
MUC2 is a secreted, gel-forming mucin that was found to be elevated in cystic fluid from noninvasive MCNs [120]. It is also found in intestinal-type IPMN as it is secreted by goblet cells in normal physiology. Multiple studies have found that MUC2 detected by IHC was associated with an increased risk of malignant IPMNs [56,124]. MUC2 has also been found to be predictive of malignancy specifically in mixed-type IPMNs [125]. The usefulness of MUC2 as a marker in mixed-type IPMNs can be attributed to their predominant grouping in the intestinal category and propensity for goblet cell formation. While MUC2 levels have been found to be significantly elevated in the fluid of highly dysplastic/malignant intestinal IPMNs [60], in other histological types of IPMNs MUC2 is either absent or at a low level, thus limiting its usefulness in diagnosing malignancy in other IPMN categories [41,125,126] (Table 4).
MUC4 is a transmembrane mucin that is not normally expressed in the pancreas. It has been discovered in early pancreatic intraepithelial neoplasms as well as adenocarcinoma and also mediates its functional effects through the growth factor family member ErbB [119]. Kitazono et al. conducted a tissue analysis on 142 IPMN samples comparing gastric vs. intestinal IPMNs and found a significantly higher expression of MUC4 in intestinal-type [61] (Table 4). In addition, this study found that MUC4 expression increased as the level of dysplasia advanced, regardless of IPMN type [61]. MUC4 is also increased in the cystic fluid of dysplastic and invasive IPMNs [60]. In an elegant study, Maker et al. utilized various target types including mRNA, miRNA, and DNA to develop a qPCR based assay to discern high-risk (high grade dysplasia to invasive cancer) from low-risk (low to moderate grade dysplasia) IPMNs [127] A combination of Interleukin 1β, MUC4, and prostaglandin E synthase 2 mRNAs proved to be the most accurate at discerning between low-risk and high-risk cysts with an AUC of 0.86 [127]. These studies did not verify their findings in external validation sets, thus further investigation is warranted.
MUC5AC is a secreted mucin that is a marker of gastric epithelium and has been found to be present in all MCNs based on immunohistochemistry of tissue specimens as well as in all four IPMN categories [128] (Table 4). MUC5AC was significantly increased in the serum of high-risk IPMN patients [60] and is also present in the tissue of pancreatic ductal adenocarcinoma, thus making it a malignancy marker [129]. A glycan variant of MUC5AC, when measured in cystic fluid along with CA19.9, provided a sensitivity and specificity of 87% and 86% respectively, for differentiating MCNs and IPMNs from SCNs [130]. In a clinical trial evaluating the effect of neoadjuvant erlotinib (a tyrosine kinase inhibitor) on IPMN growth, researchers hypothesized that MUC5AC production is dependent upon the EGFR pathway, and inhibition of the EGFR effector molecules may reduce growth. A decrease in growth and a reduction in MUC5AC expression was observed in the subsequent resected tissue [131]. Recently, variation in the glycoforms of MUC5AC with a concurrent assessment of endorepellin was proven to be a highly efficacious biomarker for mucinous cysts [132]. Another combinatorial panel of MUC5AC, MUC2, and CEA was tested and provided up to 99% accuracy to discriminate malignant from premalignant lesions in a cohort of 24 patients [133]. While this finding is quite encouraging, predictive efficacy is still in question as the patient number was limited.
MUC6 is expressed in IPMN lesions and has a slight proclivity towards the gastric subtype [59]. However, MUC6 has an intermittent expression in the other types, thus rendering it not the most effective for IPMN delineation alone, but still useful when assessed in combination with other mucins [59].
MUC13 has been shown to be moderately differentially expressed in the fluid of the various IPMN types [62]. In this same study, the MUC13 expression levels were greater in high-risk IPMNs as compared to low-risk [62]. Taken together, this suggests that while MUC13 may not be applicable to IPMN differentiation per se, it may offer valuable insight into the presence or absence of malignant progression.
MUC16 (CA125), is a membrane-bound mucin that is ubiquitously used as a biomarker in the surveillance of ovarian cancer [134] as well as advanced PDAC [135]. It has been found in the cystic fluid of pancreatic lesions, however, its levels were not useful in accurately distinguishing between the various types of cysts [26].
4.3.3. Other proteins
Proteomic analysis of cystic fluid using mass spectrometry has been employed for discovering biomarkers for differentiating mucinous cysts and defining the risk of malignancy with greater accuracy [128]. Ke et al. identified amylase isoenzymes, mucins (MUC1, MUC5AC, MUC5B, and MUC16), CEACAM family members (CEACAM 5, 6, and 7), and S100 homologs that provide valuable information on the invasive potential of a pancreatic cyst [136]. Another mass spectrometry analysis identified olfactomedin-4 as associated exclusively with MCN and IPMNs [137]. Mass to charge (m/z) ratio was found to be different between samples from malignant and benign IPMNs. Five protein peaks were identified that were highly accurate in discerning a malignant IPMN [138]. These studies are limited by their sample numbers; however, they are useful for identifying novel proteins for further individual analysis.
General inflammatory markers have been measured in cystic fluid to differentiate between the cystic subtypes and categories and determine malignant potential. Maker et al. analyzed cystic fluid from 40 patients consisting of predetermined high risk and low-risk cysts. They examined the fluid for IL-1, 2, 4, 5, 8, 10, 12, 13, INFα, and TNFα. Only IL-1 was predictive for high-risk IPMNs with a sensitivity and specificity of 79% and 95% [139]. Additionally, markers of specific pancreas damage/inflammation have also been utilized to determine the presence of cancer within a cystic lesion. For example, a significant correlation was found between increased serum pancreatic enzymes (amylase and lipase) and malignancy/invasiveness of IPMNs in patients without a history of pancreatitis [140]. However, these conclusions may be complicated by the fact that a cystic fluid amylase level > 250 U/mL correlates with a pseudocyst [98].
In a study consisting of 87 patients with pathology confirmed PCLs, vascular endothelial growth factors VEGF-A and VEGF-C were found to be significantly increased in fluid from benign SCNs compared to MCNs. Although these markers failed to distinguish between benign and malignant mucinous lesions, a diagnostic algorithm combining VEGF-A, VEGF-C, and CEA as biomarkers to distinguish serous from mucinous cysts may help increase overall diagnostic capabilities [141].
Tissue polypeptide antigen (TPA) is produced by rapidly proliferating tissue. Its levels in cystic fluid distinguished mucinous cystadenocarcinomas (MCAC) from benign lesions with a sensitivity and specificity of 75% and 97% at a cutoff value of 100,000 U/mL [142]. IPMNs and PDAC control cases were not included in this study, so further analysis of this protein will be required in fluid from IPMNs to observe any effects on its accuracy.
SPINK1, a protease inhibitor also known as pancreatic secretory trypsin inhibitor, has previously been utilized for the diagnosis and risk assessment of hereditary pancreatitis [143,144]. SPINK1 has also been used to identify IPMNs based on pancreatic juice analysis with a specificity of 98% [145]. Recently, it has been measured in cystic fluid and distinguished between benign and potentially malignant lesions in addition to differentiating MD-IPMN from BD-IPMN [146]. These studies highlight SPINK1 potential for cystic lesion stratification, but validation is required as the utility could be limited by the aforementioned association with pancreatitis [147].
Claudins are transmembrane proteins that are a component of tight junctions and have also been analyzed in the tissues of PCLs. Claudins 2, 4, and 18 have been found to be elevated in mucinous lesions. Their relative tissue profiles can also help distinguish between the different histologic subtypes of IPMNs [148]. Increased claudin 4 expression in pancreaticobiliary IPMNs and increased claudin 18 in gastric IPMNs was reported. They also found increased expression of claudins 4 and 18 and decreased expression of claudin 2 with tumor progression. Claudin 18 expression has been found to be increased in the tissues of other premalignant lesions such as Pan-IN (97%) and MCNs as well as PDAC [149]. There is a lack of studies assessing claudins in cystic fluid but should be encouraged based upon tissue data, as they may prove to be a useful marker of the malignant potential of pancreatic cystic lesions.
Ubiquitin and thymosin-4 (an actin sequestering protein) were found to be significantly over-expressed in FNA samples of IPMNs with high-grade dysplasia [150]. Plectin-1 was also found in 100% (4/4) of malignant cystic fluid samples from IPMNs and no measurable Plectin-1 was detected in the three benign IPMNs studied [151]. mAb Das-1 is a monoclonal antibody against colonic epithelium and reactive to premalignant upper gastrointestinal conditions. It was able to differentiate cystic fluid from high risk/malignant IPMNs vs. low-risk lesions with a sensitivity of 89% and specificity of 100% in a study consisting of 27 patients undergoing resection of their IPMNs [152]. The same group recently validated these results in a larger patient cohort and were able to detect high-risk IPMNs (i.e. with cancer, high-grade dysplasia, or intestinal-type histology with low-grade dysplasia) with 88% sensitivity and 99% specificity [153]. This finding is limited because it was a retrospective analysis but the group is currently beginning a prospective study.
Prostaglandin E2 (PGE2) has shown to have increased expression in the pancreatic cancer tissue as compared to the normal tissue [154]. PGE2 concentrations in the pancreatic cyst fluid can help in distinguishing between different types of mucinous cysts. Schmidt et al. did fluid analysis of 58 resected cystic lesions which showed higher PGE2 concentrations in IPMNs versus MCNs. However, the utility of this biomarker is limited in the clinical setting due to the overlap of PGE2 concentrations in benign MCNs and SCNs [155].
Sonic hedgehog (SHH) is a signaling peptide that plays a role in organogenesis. It is abnormally expressed in pancreatic intraepithelial neoplasia (PanINs) and is increased in pancreatic juice obtained from patients with IPMNs. Thus it may aid in the differentiation between IPMN and chronic pancreatitis, which is a distinction that is difficult to make based on imaging alone [156].
The analysis of proteins in cystic fluid and serum has expanded in recent years, which speaks to two primary notions. The first is that proteomic analysis of PCLs holds incredible potential for differentiating patients with malignant versus benign lesions as well as classifying those within the PCLs, which have the potential to progress to PDAC. The second is that the multitude and variation in proteins tested displays a lack of one definitive marker capable of accurate diagnoses. Further investigation is required to discover a possible multi-marker means of proteomic PDAC screening for PCL patients.
4.4. Genomic markers
4.4.1. DNA
Genomic data has also been studied and recognized as a highly valuable target in cystic fluid. It was recently demonstrated that cell-free supernatant of pancreatic FNA samples does contain DNA that can be used for analysis [157].
KRAS mutations have been well characterized in the pathogenesis of pancreatic cancer [158,159]. Alterations in KRAS are thought to be an early event in IPMN biogenesis as it is found in all types without significant differences [160,161]. Mutant KRAS in cystic fluid was found to be highly specific (92–96%) for mucinous cyst diagnosis but with low sensitivity (33–45%) [120]. It was observed in 4 out of 5 cystic fluid samples from malignant mucinous cysts [162] and these mutations have also been found in a majority of tubular carcinomas arising from intestinal-type IPMNs [160]. In conjunction, cysts without high-grade dysplasia were found to have fewer KRAS mutations as well as a lower risk of progression [160]. Thus, the potential of KRAS as a biomarker for poor prognosis is feasible yet not necessarily the best for lesion stratification and cancer detection.
A large and comprehensive investigation, termed the PANDA study, was a prospective multicenter study consisting of 113 patients that analyzed DNA in the cystic fluid. High amplitude KRAS mutations were able to detect malignancy with high specificity (96%) but low sensitivity (45%) [163]. This study most importantly concluded the use of DNA analysis in highly selected circumstances to detect the presence of malignancy when cyst cytology is negative. In addition to KRAS analysis, higher DNA quantity and allelic loss in amplitude over 82% in cystic fluid were found to have diagnostic efficacy for malignancy [120,163]. There was a confirmed association between the patient requirement for operative intervention and survival rates, however, there was selection bias as no DNA analysis data was provided on those who did not have surgery. Thus, further investigation of KRAS DNA analysis with long-term follow-up is needed before making it a routine testing method [164].
GNAS codes for the alpha subunit of a stimulatory G protein and a gain of function mutation results in uncontrolled cellular signaling via continuous cAMP production [165]. An activating GNAS mutation is detected in the tissues of 61% of IPMNs, but its individual presence does not correlate with clinical outcome [166]. When analyzed in conjunction with mutations in KRAS, 96% of IPMNs were positive for at least one of the oncogenes, and the GNAS mutation was not present in other types of cystic lesions or in PDAC [167]. Singhi et al. found that a mutation in either GNAS or KRAS in cystic fluid had a sensitivity and specificity of 65% and 100%, respectively, for mucinous differentiation [168]. This finding was further corroborated by next-generation sequencing, which confirmed that mutations in GNAS and KRAS supported an IPMN with non-mucinous CEA in 71% of the cysts. Furthermore, 19% of the non-malignant cysts with non-mucinous CEA were reclassified as mucinous by the presence of KRAS mutation [162]. Recently, Singhi et al. in another study showed that NGS detection of KRAS/GNAS mutations was 100% specific for IPMN and in combination with TP53/PIK3CA/PTEN alterations, was able to distinguish between IPMNs with low and high-grade dysplasia. However, KRAS/GNAS mutation was detected in < 50% of cyst patients [169]. GNAS mutations have recently been associated with colloid carcinoma, which is less aggressive than PDAC which can also arise from intestinal-type IPMN [160]. These findings highlight the potential GNAS has in the detection of early precancerous lesions. This could prove invaluable in the challenge of detecting PDAC at a resectable stage.
A recently developed technique called the multivariate organization of combinatorial alterations (MOCA) was utilized to determine the diagnostic efficacy of a combination of patient characteristics and cystic fluid genomic data [170]. Cystic fluid samples were analyzed for mutations in genes shown to be involved with pancreatic cystic lesions (BRAF, CDKN2A, CTNNB1, GNAS, KRAS, NRAS, PIK3CA, RNF43, SMAD4, TP53, and VHL), to indicate loss of heterozygosity at CDKN2A, RNF43, SMAD4, TP53, and VHL tumor suppressor loci, and to indicate aneuploidy in samples. A composite of these molecular markers distinguished cysts requiring resection (SPN, MCN, IPMN with high-grade dysplasia or cancer) with a sensitivity and specificity of 75% and 92%, respectively. However, when combined with clinical factors such as age, pain, communication with the main pancreatic duct (MPD), and dilation of the MPD, the sensitivity increased to 89%; however, it came with a substantial decrease in specificity to 69%.
Of note, integrated molecular pathology (IMP) testing (PancraGen/PathFinder TGR, Interspace Diagnostics) is a validated DNA mutational analysis platform for cystic fluid analysis. It measures various oncogene activation and loss of heterozygosity markers to determine the malignant potential of cystic lesions. This was utilized in a multicenter retrospective study that analyzed cystic fluid from 492 patients with IMP and then followed them for at least 23 months. IMP had similar sensitivity compared to the 2012 international consensus guidelines with imaging as the primary diagnostic modality (83% vs. 91%). However, IMP outperformed the guidelines with a specificity of 91% versus 46%, p < 0.0001 [171]. Epigenetic modification of DNA also holds promise as a novel biomarker for PCL characterization and progression. One such study investigated the methylation of various DNA markers found in cystic fluid and found the methylation patterns of four genes (SOX17, SLIT2, EYA4, SFRP1) were distinguished high-risk from low-risk cystic neoplasms with 88% overall accuracy [172]. Taken together, these data show novel DNA markers that can offer a method to individualize and target PCL therapy, in addition to imaging [173].
4.4.2. miRNA
MicroRNAs (miRNAs) have been increasingly investigated as potential biomarkers for pancreatic cancer in recent years [174]. Multiple studies have used resected tissue to determine miRNA profiles that are differently expressed between low and high-risk IPMNs [175–177]. A four-member miRNA panel (miR-21–5p, miR-483–3p, miR-708–5p, and miR-375) was observed to differentiate between IPMN and PDAC with a sensitivity and specificity of 95% and 85% respectively [178]. These findings in tissue have led to further analysis of cystic fluid. Cystic fluid miR-21 was able to differentiate between mucinous and non-mucinous cystic lesions with 80% sensitivity and 76% specificity [179]. Corroborating the value of miR-21, another group found a combination of miR-21 and miR-221 are indicative of malignancy in pancreatic cystic lesions [180].
Through logistic regression analysis of a set of 65 cystic fluid samples, Matthaei et al. found nine miRNAs were able to accurately identify cysts requiring resection versus observation, with a sensitivity/specificity of 89% and 100%, respectively [181]. In an interesting validation study, Utomo et al. utilized the Matthaei miR panel in an attempt to accurately stratify cystic lesions in a prospective manner [182]. They were unfortunately only able to detect lesions requiring resection with 10% sensitivity but 100% specificity and concluded that this panel was unlikely to improve clinical management of cystic lesion patients [182].
Next-generation sequencing in surgical specimens and EUS/FNA samples from PDAC and IPMN patients showed miRNA expression overlap between the two sets and specifically miR-93 was capable of identifying PDAC and IPMN patients from healthy controls with sensitivity/specificity of 100%/96% and 89%/88%, respectively [183]. Additionally, next-generation sequencing from cystic fluid from low-risk and high-risk IPMNs yielded a panel of 13 miRNAs that were differentially expressed and further, complexed with targets involved five canonical pathways that are implicated in cancer [184].
Of note, miRNA studies have also been conducted on various biofluids and with other non-coding RNAs. For example, MiR-155 expression specifically has been shown to be increased in the pancreatic juice from surgically resected IPMNs [120,185]. Plasma miR-4830–3p was able to discern PDAC from IPMN and miR-21 was significantly correlated with advanced disease [186]. Another study involving plasma miRNAs was able to determine the cancer status of IPMNs with 81%/53% sensitivity/specificity [187]. Permuth et al. investigated the diagnostic utility of a different type of seromic non-coding RNA, long non-coding RNAs (lncRNAs: RNA molecules > 200 nucleotides in length that resemble coding sequences but lack open reading frames), and combined these with imaging features and miRNA data for the early detection of PDAC [188]. They were able to show that eight lncRNAs were capable of differentiating malignant and non-malignant IPMNs (sensitivity/specificity, 79%/76%) and the specificity was dramatically improved when combined with imaging features and miRNA data (sensitivity/specificity, 71%/100%) [188] (Table 3).
These studies demonstrate that miRNA may be a powerful predictor of malignancy in EUS-FNA and serum/plasma samples. However, a limitation could be imposed by inadequate quantities of miRNA isolated from various biofluids. For example, only 58% of pancreatic fluid samples yielded enough miRNA to be analyses in one study [189]. Another caveat in the use of miRNA is the fact that so many studies have been undertaken and have subsequently elucidated hundreds of miRNA targets with few overlapping targets between groups.
4.4.3. Single-cell RNA sequencing
Single-cell RNA sequencing is a next-generation sequencing technology developed in recent years. It involves isolating single cells, extracting their transcripts, and mapping these transcripts to each individual cell. As a result, it provides a higher resolution of cellular differences as compared to conventional RNA sequencing methods, provides detailed insight into the existence and behavior of different cell types, and elucidates regulatory relationships between genes [190,191].
In a first reported single-cell transcriptomic study on precursor cystic lesions of pancreatic cancer, Bernard et al. performed RNA sequencing on 5403 cells from two low-grade IPMNs, two high-grade IPMNs and two PDAC cases, obtained from surgically resected specimens [192]. Their prime objective was to understand the intralesional epithelial heterogeneity and tumor microenvironment heterogeneity that could provide insight into the progression of IPMN to PDAC. They found that the epithelial component of IPMNs and PDAC had similar as well as different transcriptomic elements. Notably, KRT1 and MUC1 were found in all samples, CEACAM6 was found only in IPMNs with high-grade dysplasia and cancer, and MUC5AC was only present in IPMNs with low-grade dysplasia [192]. They also found tumor suppressor genes such as RAP1GAP to be expressed in lesions with low-grade dysplasia and silenced in those with high-grade dysplasia along with a concomitant increase in transcripts of oncogenes including S100P and S100A10 [192].
Their predominant finding described regarding the evolution of the tumor microenvironment during the progression of low-grade IPMNs to PDAC was alteration in the immune profile. Notably, pro-inflammatory immune cells, predominantly cytotoxic T-cells (measured by granzyme-and perforin-related transcripts), CD4+ T-cells (CD69 expression) and cDC2-type dendritic cells (antigen-presenting cells), were seen in low-grade IPMNs but depleted during neoplastic progression. PDAC exhibited infiltration of immunosuppressive pro-tumorigenic CD11b+, S100A9+, CCL3+, APOE+ myeloid-derived suppressor cells (MDSCs) reaching 51% of stromal cells profiled, while IPMN stroma, with both low-grade and high-grade dysplasia, was comprised of 2.3% and 3.5% MDSCs, respectively. Interestingly, this MDSC population has previously been shown to be associated with cancer progression [193]. Thus, they postulated that in the future, single-cell analyses would be able to establish a threshold which foretells the invasive nature of a cystic lesion even in the absence of radiologically detectable features [192]. While intriguing and a first of its kind, limitations of the present study were the extremely small sample size for each cohort (2 cases each) and the lack of wet-lab validation of the virtual dissection data.
In another report, Beatty et al. observed higher infiltration of pro-inflammatory cells (CD8+, CD4+) in IPMNs with high-grade dysplasia as compared to those with low-grade dysplasia and normal pancreas tissue [194]. Other studies have also observed that infiltrating and/or circulating neutrophil-to-lymphocyte ratio could be a better indicator of IPMN malignancy [195–197]. Thus, the immune microenvironment and effects on PCL progression garner further attention.
4.5. Metabolomics
Metabolic reprogramming has long been associated with the transformation and growth of cancer [198]. The assessment of metabolic alterations in the setting of cancer for use as early detection biomarkers or therapeutic target delineation has gained high attention in recent times [199–202]. A serum-based panel comprised of 10 metabolites was observed to differentiate patients with cancer from normal controls with AUC of 0.92 on a ROC curve [203]. Another study utilized six metabolites panel (five of which were involved in lipid metabolism) to classify pancreatic cancer patients with a sensitivity/specificity of 90%/85% [204]. Branch chain amino acids were found to be increased in the serum of cancer patients up to ten years prior to the time of detection in another study [205,206]. While these findings have immense potential for cystic lesion stratification and malignancy determination, these studies utilized chronic pancreatitis, type 2 diabetes mellitus, and healthy patients as controls and did not test their findings in PCL patients or used PCL patients as benign controls.
However, some groups have started implying metabolomics to predict cystic lesion stratification. The initial study conducted in 2015 found that glucose and kynurenine are significantly lower in MCNs as compared to non-MCNs [207]. Glucose sensitivity and specificity for differentiating between MCN and non-MCNs was 94% and 64%, respectively, at a cutoff level of 66 mg/mL. Kynurenine was likewise able to discern between MCNs and non-MCNs with a sensitivity/specificity of 90%/100% [207]. Further other studies have also highlighted the utility of glucose to distinguish between MCNs and non-MCNs [208–210]. Though promising for cystic lesions classification, neither glucose nor kynurenine was differentially expressed in malignant cysts.
In a comprehensive study by Gaiser et al., a combination of metabolomic and lipidomic profiling was applied to cystic fluid or serum samples to discriminate the malignancy status of various cystic lesions [211]. The cystic fluid IPMN metabolites represented many pathways associated with lipid metabolism (as compared to SCNs) including phosphatidylethanolamine synthesis, phosphatidylcholine synthesis, taurine metabolism, oxidation of branch-chain fatty acids, and sphingolipid metabolism [211]. Principal component analysis (PCA) was able to discriminate between IPMNs and SCNs with 100% accuracy utilizing cystic fluid or plasma. Interestingly, PCA of cystic fluid metabolites was able to differentiate between PCLs with high-grade dysplasia/cancer and all other cysts with a sensitivity and specificity of 89% and 92%, respectively. When plasma was used, the sensitivity dropped to 64% but the specificity increased to 100% [211].
These metabonomic data are supported by the D’Alessandro group’s recent work that elucidated a “metabolic timeline” of PDAC by PCA analysis of 215 metabolites in healthy, IPMN, local disease, and advanced disease patient plasma samples [212]. Relevant to cystic lesion stratification, they found that 10 metabolites were different between IPMN and local disease (early stage) patient plasma and PCA analysis was able to predict patient status. From this, they concluded that metabolomics may provide a means of assessing PCL patient progression along the metabolic timeline (i.e. IPMN vs local disease), but may not provide a benefit to healthy patient screening [212]. These findings were further corroborated in another study on a rat model of PDAC where serum metabolic differences were observed to higher across non-malignant precursor lesions (PanINs) and invasive disease in comparison to normal pancreata and PanINs [213].
4.6. Radiomics
Recent advancements in image acquisition and analysis have led to the development of a novel field in cancer research called radiomics. With this new technique, it has now become possible to convert images into mineable data through high-throughput extraction of quantitative features by computers. The extracted data can then be combined with patients’ clinical attributes to contrive a model that will improve the accuracy of diagnosis and prognosis for cancer as well as other diseases [214]. The core premise of radiomics is that the differences in size, shape, texture, and greyness of a tumor contoured from a radiological image can reflect the variations in histological phenotype and genotype of the tumor [215]. The workflow of radiomics involves the acquisition of images (i.e. USG, CT, MRI or PET), segmentation (defining boundaries) of the region of interest and the organ involved, extraction and analysis of features, and statistical modeling to predict outcome [216]. A primary benefit of radiomics is that the data can be calculated from already acquired images through freely available tools, so it bears no extra cost or burden on the patients. This method could provide us with a better, non-invasive tool for diagnosis and prognosis, as compared to biopsies which harbor inherent limitations including increased risk to patients, limited sample amounts, and fail to incorporate lesion heterogeneity leading to sampling errors.
In recent times, radiomics has been used for tumor diagnosis, prognosis, survival time prediction, guiding therapy selection, response to therapy, and potential areas for physical biopsy [214]. Texture analysis and machine learning, which are integral parts of radiomics, have been used in multiple studies to either predict the presence of a tumor or to accurately differentiate between benign or malignant tumors in various cancers including breast, lung, thyroid, bladder, prostate, liver and pancreas [217–223]. It’s only recently that radiomics, particularly texture analysis, has been used in PCLs and more specifically IPMNs [43].
Dmitriev et al. described an automatic classification algorithm to classify four common pancreatic cysts into their respective groups using CT images [224]. They utilized intensity, shape, and fine texture features to achieve 84% accuracy. This was the first CAD (computer-aided diagnosis) algorithm to classify common types of pancreatic cysts [224]. A similar study was performed by Chu et al. to classify PCLs into five types and segregate cystic lesions into IPMN and non-IPMN with approximately 78% and 82% accuracy, respectively [225]. Chakraborty et al. went a step further and developed a pre-operative model to differentiate between low risk and high-risk BD-IPMN by combining novel radiographically inspired features, standard texture features, and clinical variables [226]. They used pretreatment CT images of 103 patients with pathology-confirmed BD-IPMN to extract and combine features to predict malignant potential and achieved an accuracy of 81% [226].
In one of the initial studies employing radiomics on PCLs, Hanania et al. developed a model to predict the histopathological grade of IPMN in CT images of 53 patients [227]. Using a cross-validation design with logistic regression, their model yielded an impressive 96% accuracy. The study identified 14 imaging biomarkers to differentiate IPMNs with low-grade dysplasia from those with high-grade dysplasia [227]. During the same time, Permuth et al. utilized a unique plasma-based miRNA genomic classifier (MGC) with quantitative radiomics CT features of pathologically proven IPMN patients (n = 38) [228]. They concluded this noninvasive radio-genomic approach is more accurate in predicting IPMN pathology than using standard ‘high-risk’ or ‘worrisome’ radiologic features for malignancy considered in the Fukuoka consensus guidelines [15,228]. A recent study included 260 patients who underwent pancreatic resection for a PCL, with the aim to differentiate serous cystic neoplasms from other pancreatic cystic neoplasms and achieved a remarkable accuracy of 84%. Importantly, it was the first study to divide the patient cohort into two groups, a cross-validation cohort to extract features and an independent validation cohort to test the model [229].
These initial studies involving radiomics have shown promise but due to its recent genesis, the approach has its own challenges and difficulties. There is a lack of set protocols and guidelines for image acquisition and feature extraction, along with a lack of standards for validating results. Additionally, almost all of the studies are retrospective single institutional with limited patient cohorts [214,216]. A logical next step is to formulate reproducible, robust, and repeatable metrics capable of producing consistently high-quality results with different scanners, software, and time points [216]. The end goal should be to develop a model capable of automated identification of regions of interest with the ability to precisely characterize the lesion in question [230]. This burgeoning field is poised to prove invaluable in the future for the identification of cancer and its precursor lesions, including PCLs. The diagnostic and prognostic capabilities of this technology could be further enhanced via astute integration with molecular/genomic markers obtained from the cystic fluid, pancreatic juice, or serum in addition to the incorporation of clinical data. Heretofore only one group has done so, as described above [228]. This ability of radiomics data to be combined with other molecular and clinical data makes it a prime candidate for developing models to accurately predict the presence of PDAC within IPMNs and even defining the risk for malignant progression.
5. Conclusions and perspective
The ability to determine the need for surgical resection of premalignant and malignant pancreatic lesions depends upon an accurate diagnosis. Comprehensive research has been carried out to try and uncover biomarkers for identifying malignant cystic lesions using cystic fluid, pancreatic juice, imaging, and blood samples. However, all these approaches have been unable to attain high enough specificity and sensitivity, due to some inherent limitations associated with each method. While tissue-based cyst classification can be incredibly accurate and allow for true histological analysis and verification of disease by a pathologist, sample acquisition can carry significant comorbidities due to the invasive nature of the procedure. Further, single-site biopsies do not account for the entirety of the lesion, thus increasing the possibility of missing malignant disease. Conversely, the use of imaging for classification is non-invasive and can take into account the holistic state of the lesion. Unfortunately, current imaging modalities are expensive, are susceptible to observer variation, and the respective guidelines (i.e. Fukuoka) have limited accuracy in differentiating malignant vs non-malignant cysts. Cystic fluid offers a less invasive means (i.e. EUS/FNA) of cytological analysis than biopsy. It can also be used for molecular profiling including assessment of protein, RNA, DNA, and metabolites. Yet many FNA samples have scant cellularity leading to inconclusive results and multiloculated cysts can partition fluid, thereby preventing gestalt assessment. Also, many of the biomarker studies discussed herein have limitations including low patient sample numbers and the lack of blinded external validation sets. Further, although some markers have been able to determine PCL cancer status, the majority of these studies have focused on mucinous vs nonmucinous cyst differentiation and have not shown robust efficacy in elucidating malignancy. This has led to a lack of uniformly agreed-upon cystic fluid molecular markers in the field.
MD-IPMNs and MCNs have a higher risk of malignancy and are often resected upon diagnosis, thus there continues to be a need for a reliable biomarker for surveillance [97]. The current guidelines for predicting the risk of malignancy status of PCLs require expensive imaging as well as invasive tests. The goal of cyst fluid analysis is to supplement the information gained from imaging. An intense investigation into biomarkers for the noninvasive characterization of PCLs is required. These biomarkers must consider the diversity in the genetic origins of PDAC [231].
Biomarkers could be used in conjunction with the current consensus for imaging guidelines to determine the need for surgical resection. These include options such as the assessment of circulating endothelial cells [232], cell-free DNA measurements [233], improved imaging/biopsy modalities [101], and extracellular vesicle (EV) analysis [234,235]. Continued analysis of the EV contents and membranes may provide unique biomarkers that may also be found in serum.
Radiomics could offer a means of determining the malignancy status of pancreatic cysts in a noninvasive manner. Future improvements, including guideline standardization for imaging acquisition, discernment of repeatable analysis metrics, and integration with molecular markers will increase the diagnostic and prognostic efficacy of this new technology. Although radiomics has already begun to enhance the power of existing imaging modalities and helps identify diagnostic and differentiating features that are otherwise not discernable, algorithms need to be further optimized to realize its full potential for surgical stratification.
Basturk and colleagues have proposed a revised classification system for neoplastic precursor lesions of the pancreas that will utilize the subtle molecular differences that have been found between PCL types [236]. Such a revision could facilitate appropriate clinical decision making and continue to evolve with a deeper understanding of the machinery that transforms these precursor lesions into cancer. Continued individualization is necessary for improved prediction of the future of the disease for patients.
While today’s medicine lacks the noninvasive tools to accurately differentiate benign from malignant (or potentially malignant) PCLs [237], there is great potential for the development of novel biomarkers in the near future. If an inexpensive, noninvasive, and accurate screening modality can be discovered with high sensitivity and specificity, quality of life could be increased for the thousands of people who suffer from pancreatic cystic lesions and improve our ability to detect pancreatic cancer at a treatable stage.
Funding
The work/authors were supported, in parts, by the National Institutes of Health, United States [R01CA210637, R01CA206444, RO1CA183459, R44DK117472, U01CA200466, U01CA210240, P01CA217798, R43CA235984, and R44CA224619].
Footnotes
Declaration of competing interest
Dr. Surinder Batra is a co-founder of Sanguine Diagnostics and Therapeutics.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics 2019, Cancer statistics, 2019, Cancer J. Clin 69 (1) (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH, Therapeutic developments in pancreatic cancer: current and future perspectives, Nat Rev Gastroenterol Hepatol 15 (6) (2018) 333–348. [DOI] [PubMed] [Google Scholar]
- [3].Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. , Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline Update, J. Clin. Oncol 35 (20) (2017) 2324–2328. [DOI] [PubMed] [Google Scholar]
- [4].Decker GA, Batheja MJ, Collins JM, Silva AC, Mekeel KL, Moss AA, et al. , Risk factors for pancreatic adenocarcinoma and prospects for screening, Gastroenterol Hepatol (N Y) 6 (4) (2010) 246–254. [PMC free article] [PubMed] [Google Scholar]
- [5].Mukewar SS, Sharma A, Phillip N, Gupta R, Aryal-Khanal A, de Pretis N, et al. , Risk of pancreatic cancer in patients with pancreatic cysts and family history of pancreatic cancer, Clin. Gastroenterol. Hepatol 16 (7) (2018) 1123–1130. [DOI] [PubMed] [Google Scholar]
- [6].Esposito I, Konukiewitz B, Schlitter AM, Kloppel G, Pathology of pancreatic ductal adenocarcinoma: facts, challenges and future developments, World J. Gastroenterol 20 (38) (2014) 13833–13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E, Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology 274 (1) (2015) 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohno E, Hirooka Y, Kawashima H, Ishikawa T, Kanamori A, Ishikawa H, et al. , Natural history of pancreatic cystic lesions: a multicenter prospective observational study for evaluating the risk of pancreatic cancer, J. Gastroenterol. Hepatol 33 (1) (2018) 320–328. [DOI] [PubMed] [Google Scholar]
- [9].Vege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines C, American Gastroenterology A, American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts, Gastroenterology 148 (4) (2015) 819–822. [DOI] [PubMed] [Google Scholar]
- [10].Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, et al. , Clinical implication of serum carcinoembryonic antigen and carbohydrate antigen 19–9 for the prediction of malignancy in intraductal papillary mucinous neoplasm of pancreas, J Hepatobiliary Pancreat Sci 22 (9) (2015) 699–707. [DOI] [PubMed] [Google Scholar]
- [11].de Jong K, Bruno MJ, Fockens P, Epidemiology, diagnosis, and management of cystic lesions of the pancreas, Gastroenterol. Res. Pract 147465 (10) (2012) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brugge WR, Diagnosis and management of cystic lesions of the pancreas, J Gastrointest Oncol 6 (4) (2015) 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kimura W, Nagai H, Kuroda A, Muto T, Esaki Y, Analysis of small cystic lesions of the pancreas, Int. J. Pancreatol 18 (3) (1995) 197–206. [DOI] [PubMed] [Google Scholar]
- [14].Gardner TB, Glass LM, Smith KD, Ripple GH, Barth RJ, Klibansky DA, et al. , Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults, Am. J. Gastroenterol 108 (10) (2013) 1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tanaka M, Fernandez-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. , Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas, Pancreatology 17 (5) (2017) 738–753. [DOI] [PubMed] [Google Scholar]
- [16].Farrell JJ, Prevalence, diagnosis and management of pancreatic cystic neoplasms: current status and future directions, Gut Liver 9 (5) (2015) 571–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Capella CSE, Pape K.I. G, Hruban RH, Serous cystic neoplasms of the pancreas, in: SR Hamilton ALA (Ed.), World Health Organization Classification of Tumors, IARC Press, Lyon, France, 2000, pp. 234–236. [Google Scholar]
- [18].Zamboni GKG, Hruban RH, Longnecker DS, Adler G, Mucinous cystic neoplasms of the pancreas, in: SR Hamilton AL (Ed.), World Health Organization Classification of Tumors, IARC Press, Lyon, France, 2000, pp. 234–236. [Google Scholar]
- [19].Longnecker DSAG, Hruban RH, Intraductal papillarymucinous neoplasms of the pancreas, in: SR Hamilton AL (Ed.), World Health Organization Classification of Tumors, IARC Press, Lyon, France, 2000, pp. 237–241. [Google Scholar]
- [20].Compagno J, Oertel JE, Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases, Am. J. Clin. Pathol 69 (3) (1978) 289–298. [DOI] [PubMed] [Google Scholar]
- [21].Alpert LC, Truong LD, Bossart MI, Spjut HJ, Microcystic adenoma (serous cystadenoma) of the pancreas. A study of 14 cases with immunohistochemical and electron-microscopic correlation, Am. J. Surg. Pathol 12 (4) (1988) 251–263. [DOI] [PubMed] [Google Scholar]
- [22].Solcia ECC, Klöppel G, Tumors of the Pancreas. AFIP Atlas of Tumor Pathology, 3rd Series, Fasc 20 Washington, Armed Forces Institute of Pathology. Tumors of the Pancreas. AFIP Atlas of Tumor Pathology. Fascicle 20, Armed Forces Institutes of Pathology, Washington. D.C., 1997. [Google Scholar]
- [23].Egawa N, Maillet B, Schroder S, Mukai K, Kloppel G, Serous oligocystic and ill-demarcated adenoma of the pancreas: a variant of serous cystic adenoma, Virchows Arch. 424 (1) (1994) 13–17. [DOI] [PubMed] [Google Scholar]
- [24].Charville GW, Kao CS, Serous neoplasms of the pancreas: a comprehensive review, Arch Pathol Lab Med 142 (9) (2018) 1134–1140. [DOI] [PubMed] [Google Scholar]
- [25].Bogomoletz WV, Adnet JJ, Widgren S, Stavrou M, McLaughlin JE, Cystadenoma of the pancreas: a histological, histochemical and ultrastructural study of seven cases, Histopathology 4 (3) (1980) 309–320. [DOI] [PubMed] [Google Scholar]
- [26].Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. , Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study, Gastroenterology 126 (5) (2004) 1330–1336. [DOI] [PubMed] [Google Scholar]
- [27].Al-Haddad M, Gill KR, Raimondo M, Woodward TA, Krishna M, Crook JE, et al. , Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: a controlled study, Endoscopy 42 (2) (2010) 127–132. [DOI] [PubMed] [Google Scholar]
- [28].Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR, Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts, Pancreas 40 (7) (2011) 1024–1028. [DOI] [PubMed] [Google Scholar]
- [29].Robinson SM, Scott J, Oppong KW, White SA, What to do for the incidental pancreatic cystic lesion? Surg. Oncol 23 (3) (2014) 117–125. [DOI] [PubMed] [Google Scholar]
- [30].Jais B, Rebours V, Malleo G, Salvia R, Fontana M, Maggino L, et al. , Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas), Gut 65(2) (2016) 305–312. [DOI] [PubMed] [Google Scholar]
- [31].Yoshimi N, Sugie S, Tanaka T, Aijin W, Bunai Y, Tatematsu A, et al. , A rare case of serous cystadenocarcinoma of the pancreas, Cancer 69 (10) (1992) 2449–2453. [DOI] [PubMed] [Google Scholar]
- [32].Hruban RHPM, Klimstra DS, Tumors of the Pancreas. Atlas of Tumor Pathology, 6 ed., American Registry of Pathology and Armed Forces Institute of Pathology, Washington, D.C., 2007. [Google Scholar]
- [33].Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et al. , Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors, Am. J. Surg. Pathol 23 (4) (1999) 410–422. [DOI] [PubMed] [Google Scholar]
- [34].Sarr MG, Carpenter HA, Prabhakar LP, Orchard TF, Hughes S, van Heerden JA, et al. , Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann. Surg 231 (2) (2000) 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yamada M, Kozuka S, Yamao K, Nakazawa S, Naitoh Y, Tsukamoto Y, Mucin-producing tumor of the pancreas, Cancer 68 (1) (1991) 159–168. [DOI] [PubMed] [Google Scholar]
- [36].Campbell F, Azadeh B, Cystic neoplasms of the exocrine pancreas, Histopathology 52 (5) (2008) 539–551. [DOI] [PubMed] [Google Scholar]
- [37].Pittman ME, Rao R, Hruban RH, Classification, morphology, molecular pathogenesis, and outcome of premalignant lesions of the pancreas, Arch Pathol Lab Med 141 (12) (2017) 1606–1614. [DOI] [PubMed] [Google Scholar]
- [38].Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. , International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas, Pancreatology 6 (1–2) (2006) 17–32. [DOI] [PubMed] [Google Scholar]
- [39].Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, et al. , Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer, Clin. Gastroenterol. Hepatol 2 (11) (2004) 1026–1031. [DOI] [PubMed] [Google Scholar]
- [40].Li T, Chen ZQ, Meng ZX, Hong JG, Zhi XT, Calcified cystic lesion of the pancreas, J. Gastrointest. Surg 20 (6) (2016) 1272–1274. [DOI] [PubMed] [Google Scholar]
- [41].Bosman FWHO, Bosman F (Ed.), International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System, 4 ed., IARC Press, Lyon, France, 2010. [Google Scholar]
- [42].Crippa S, Salvia R, Warshaw AL, Dominguez I, Bassi C, Falconi M, et al. , Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients, Ann. Surg 247 (4) (2008) 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tanaka M, Intraductal papillary mucinous neoplasm of the pancreas as the main focus for early detection of pancreatic adenocarcinoma, Pancreas 47 (5) (2018) 544–550. [DOI] [PubMed] [Google Scholar]
- [44].Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, et al. , 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital, Surgery 152 (3 Suppl 1) (2012) S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morohoshi T, Held G, Kloppel G, Exocrine pancreatic tumours and their histological classification. A study based on 167 autopsy and 97 surgical cases, Histopathology 7 (5) (1983) 645–661. [DOI] [PubMed] [Google Scholar]
- [46].Madura JA, Wiebke EA, Howard TJ, Cummings OW, Hull MT, Sherman S, et al. , Mucin-hypersecreting intraductal neoplasms of the pancreas: a precursor to cystic pancreatic malignancies, Surgery 122 (4) (1997) 786–792 (discussion 92–3). [DOI] [PubMed] [Google Scholar]
- [47].Choi SH, Park SH, Kim KW, Lee JY, Lee SS, Progression of unresected intraductal papillary mucinous neoplasms of the pancreas to cancer: a systematic review and meta-analysis, Clin. Gastroenterol. Hepatol 15 (10) (2017) 1509–1520 (e4). [DOI] [PubMed] [Google Scholar]
- [48].Stark A, Donahue TR, Reber HA, Hines OJ, Pancreatic cyst disease: a review, JAMA 315 (17) (2016) 1882–1893. [DOI] [PubMed] [Google Scholar]
- [49].Rosenblatt R, Dorfman V, Epelboym I, Poneros JM, Sethi A, Lightdale C, et al. , Demographic features and natural history of intermediate-risk multifocal versus unifocal intraductal papillary mucinous neoplasms, Pancreas 44 (3) (2015) 478–483. [DOI] [PubMed] [Google Scholar]
- [50].Monzen M, Shimizu K, Hatori T, Furukawa T, Shiratori K, Usefulness of cell block cytology for preoperative grading and typing of intraductal papillary mucinous neoplasms, Pancreatology 13 (4) (2013) 369–378. [DOI] [PubMed] [Google Scholar]
- [51].Hisaka T, Horiuchi H, Uchida S, Ishikawa H, Kawahara R, Kawashima Y, et al. , Potential usefulness of mucin immunohistochemical staining of preoperative pancreatic biopsy or juice cytology specimens in the determination of treatment strategies for intraductal papillary mucinous neoplasm, Oncol. Rep 30 (5) (2013) 2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yoon WJ, Daglilar ES, Mino-Kenudson M, Morales-Oyarvide V, Pitman MB, Brugge WR, Characterization of epithelial subtypes of intraductal papillary mucinous neoplasm of the pancreas with endoscopic ultrasound and cyst fluid analysis, Endoscopy 46 (12) (2014) 1071–1077. [DOI] [PubMed] [Google Scholar]
- [53].Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, et al. , Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas, Gut 60 (4) (2011) 509–516. [DOI] [PubMed] [Google Scholar]
- [54].Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang J-Y, et al. , International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas, Pancreatology 12 (3) (2012) 183–197. [DOI] [PubMed] [Google Scholar]
- [55].Luttges J, Zamboni G, Longnecker D, Kloppel G, The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma, Am. J. Surg. Pathol 25 (7) (2001) 942–948. [DOI] [PubMed] [Google Scholar]
- [56].Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, et al. , Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas, Am. J. Surg. Pathol 28 (7) (2004) 839–848. [DOI] [PubMed] [Google Scholar]
- [57].Adsay NV, Adair CF, Heffess CS, Klimstra DS, Intraductal oncocytic papillary neoplasms of the pancreas, Am. J. Surg. Pathol 20 (8) (1996) 980–994. [DOI] [PubMed] [Google Scholar]
- [58].Hara T, Ikebe D, Odaka A, Sudo K, Nakamura K, Yamamoto H, et al. , Preoperative histological subtype classification of intraductal papillary mucinous neoplasms (IPMN) by pancreatic juice cytology with MUC stain, Ann. Surg 257 (6) (2013) 1103–1111. [DOI] [PubMed] [Google Scholar]
- [59].Castellano-Megias VM, Andres CI, Lopez-Alonso G, Colina-Ruizdelgado F, Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas, World J Gastrointest Oncol 6 (9) (2014) 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Maker AV, Katabi N, Gonen M, DeMatteo RP, D’Angelica MI, Fong Y, et al. , Pancreatic cyst fluid and serum mucin levels predict dysplasia in intraductal papillary mucinous neoplasms of the pancreas, Ann. Surg. Oncol 18 (1) (2011) 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kitazono I, Higashi M, Kitamoto S, Yokoyama S, Horinouchi M, Osako M, et al. , Expression of MUC4 mucin is observed mainly in the intestinal type of intraductal papillary mucinous neoplasm of the pancreas, Pancreas 42 (7) (2013) 1120–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Stiles ZE, Khan S, Patton KT, Jaggi M, Behrman SW, Chauhan SC, Transmembrane mucin MUC13 distinguishes intraductal papillary mucinous neoplasms from non-mucinous cysts and is associated with high-risk lesions, HPB (Oxford) 21 (1) (2019) 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hibi Y, Fukushima N, Tsuchida A, Sofuni A, Itoi T, Moriyasu F, et al. , Pancreatic juice cytology and subclassification of intraductal papillary mucinous neoplasms of the pancreas, Pancreas 34 (2) (2007) 197–204. [DOI] [PubMed] [Google Scholar]
- [64].Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, et al. , A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas 43 (3) (2014) 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yu PF, Hu ZH, Wang XB, Guo JM, Cheng XD, Zhang YL, et al. , Solid pseudopapillary tumor of the pancreas: a review of 553 cases in Chinese literature, World J. Gastroenterol 16 (10) (2010) 1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Salvia R, Malleo G, Marchegiani G, Pennacchio S, Paiella S, Paini M, et al. , Pancreatic resections for cystic neoplasms: from the surgeon’s presumption to the pathologist’s reality, Surgery 152 (3 Suppl 1) (2012) S135–S142. [DOI] [PubMed] [Google Scholar]
- [67].Borhani AA, Fasanella KE, Iranpour N, Zureikat AH, Singhi AD, Furlan A, et al. , Lymphoepithelial cyst of pancreas: spectrum of radiological findings with pathologic correlation, Abdom Radiol (NY) 42 (3) (2017) 877–883. [DOI] [PubMed] [Google Scholar]
- [68].Arumugam P, Fletcher N, Kyriakides C, Mears L, Kocher HM, Lymphoepithelial cyst of the pancreas, Case Rep Gastroenterol 10 (1) (2016) 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Moore PS, Missiaglia E, Antonello D, Zamo A, Zamboni G, Corleto V, et al. , Role of disease-causing genes in sporadic pancreatic endocrine tumors: MEN1 and VHL, Genes Chromosomes Cancer 32 (2) (2001) 177–181. [DOI] [PubMed] [Google Scholar]
- [70].Atiq M, Bhutani MS, Bektas M, Lee JE, Gong Y, Tamm EP, et al. , EUS-FNA for pancreatic neuroendocrine tumors: a tertiary cancer center experience, Dig. Dis. Sci 57 (3) (2012) 791–800. [DOI] [PubMed] [Google Scholar]
- [71].Habashi S, Draganov PV, Pancreatic pseudocyst, World J. Gastroenterol 15 (1) (2009) 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Braha J, Tenner S, Fluid collections and pseudocysts as a complication of acute pancreatitis, Gastrointest. Endosc. Clin. N. Am 28 (2) (2018) 123–130. [DOI] [PubMed] [Google Scholar]
- [73].Kloppel G, Maillet B, Pseudocysts in chronic pancreatitis: a morphological analysis of 57 resection specimens and 9 autopsy pancreata, Pancreas 6 (3) (1991) 266–274. [PubMed] [Google Scholar]
- [74].Ammann RW, Heitz PU, Kloppel G, Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study, Gastroenterology 111 (1) (1996) 224–231. [DOI] [PubMed] [Google Scholar]
- [75].Matsubayashi H, Iwai T, Matsui T, Wada T, Kawata N, Ito H, et al. , Pancreatic cystic lesions with atypical steroid response should be carefully managed in cases of autoimmune pancreatitis, J. Gastroenterol. Hepatol 31 (1) (2016) 270–276. [DOI] [PubMed] [Google Scholar]
- [76].Brugge WR, Diagnosis and management of relapsing pancreatitis associated with cystic neoplasms of the pancreas, World J. Gastroenterol 14 (7) (2008) 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pezzilli R, Melzi d’Eril G, Barassi A, Can serum pancreatic amylase and lipase levels be used as diagnostic markers to distinguish between patients with mucinous cystic lesions of the pancreas, chronic pancreatitis, and pancreatic ductal adenocarcinoma? Pancreas 45 (9) (2016) 1272–1275. [DOI] [PubMed] [Google Scholar]
- [78].Crippa S, Fernandez-Del Castillo C, Salvia R, Finkelstein D, Bassi C, Dominguez I, et al. , Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics, Clin. Gastroenterol. Hepatol 8 (2) (2010) 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hedayat AA, Lisovsky M, Suriawinata AA, Longnecker DS, Association of IgG4 response and autoimmune pancreatitis with intraductal papillary-mucinous neoplasms, Pancreatology 17 (2) (2017) 263–266. [DOI] [PubMed] [Google Scholar]
- [80].Gomez D, Rahman SH, Won LF, Verbeke CS, McMahon MJ, Menon KV, Characterization of malignant pancreatic cystic lesions in the background of chronic pancreatitis, JOP 7 (5) (2006) 465–472. [PubMed] [Google Scholar]
- [81].Jones MJ, Buchanan AS, Neal CP, Dennison AR, Metcalfe MS, Garcea G, Imaging of indeterminate pancreatic cystic lesions: a systematic review, Pancreatology 13 (4) (2013) 436–442. [DOI] [PubMed] [Google Scholar]
- [82].de Jong K, van Hooft JE, Nio CY, Gouma DJ, Dijkgraaf MG, Bruno MJ, et al. , Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions, Scand. J. Gastroenterol 47 (8–9) (2012) 1056–1063. [DOI] [PubMed] [Google Scholar]
- [83].Khashab MA, Kim K, Lennon AM, Shin EJ, Tignor AS, Amateau SK, et al. , Should we do EUS/FNA on patients with pancreatic cysts? The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms, Pancreas 42 (4) (2013) 717–721. [DOI] [PubMed] [Google Scholar]
- [84].Kauhanen S, Rinta-Kiikka I, Kemppainen J, Gronroos J, Kajander S, Seppanen M, et al. , Accuracy of 18F-FDG PET/CT, multidetector CT, and MR imaging in the diagnosis of pancreatic cysts: a prospective single-center study, J. Nucl. Med 56 (8) (2015) 1163–1168. [DOI] [PubMed] [Google Scholar]
- [85].Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, et al. , Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report, J. Gastrointest. Surg 12 (2) (2008) 234–242. [DOI] [PubMed] [Google Scholar]
- [86].Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. , Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology, Ann. Surg 246 (4) (2007) 644–654. [DOI] [PubMed] [Google Scholar]
- [87].Goh BK, Lin Z, Tan DM, Thng CH, Khor CJ, Lim TK, et al. , Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: results from a systematic review of 1,382 surgically resected patients, Surgery (May 29 2015), 10.1016/j.surg.2015.03.021 (pii: S0039–6060(15)00214–7). [DOI] [PubMed] [Google Scholar]
- [88].Xu MM, Yin S, Siddiqui AA, Salem RR, Schrope B, Sethi A, et al. , Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms, Medicine (Baltimore) 96 (35) (2017) e7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Le Baleur Y, Couvelard A, Vullierme MP, Sauvanet A, Hammel P, Rebours V, et al. , Mucinous cystic neoplasms of the pancreas: definition of preoperative imaging criteria for high-risk lesions, Pancreatology 11 (5) (2011) 495–499. [DOI] [PubMed] [Google Scholar]
- [90].Hoffman RL, Gates JL, Kochman ML, Ginsberg GG, Ahmad NA, Chandrasekhara V, et al. , Analysis of cyst size and tumor markers in the management of pancreatic cysts: support for the original Sendai criteria, J. Am. Coll. Surg 220 (6) (2015) 1087–1095. [DOI] [PubMed] [Google Scholar]
- [91].Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, et al. , Cystic pancreatic neoplasms: observe or operate, Ann Surg 239 (5) (2004) 651–657 (discussion 7–9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goh BK, Tan DM, Thng CH, Lee SY, Low AS, Chan CY, et al. , Are the Sendai and Fukuoka consensus guidelines for cystic mucinous neoplasms of the pancreas useful in the initial triage of all suspected pancreatic cystic neoplasms? A single-institution experience with 317 surgically-treated patients, Ann. Surg. Oncol 21(6) (2014) 1919–1926. [DOI] [PubMed] [Google Scholar]
- [93].Heckler M, Michalski CW, Schaefle S, Kaiser J, Buchler MW, Hackert T, The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN - a meta-analysis on their accuracy, Pancreatology 17 (2) (2017) 255–262. [DOI] [PubMed] [Google Scholar]
- [94].Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T, et al. , Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection, World J. Surg 32 (2) (2008) 271–278. [DOI] [PubMed] [Google Scholar]
- [95].Schmidt D, Textor B, Pein OT, Licht AH, Andrecht S, Sator-Schmitt M, et al. , Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis, EMBO J. 26 (3) (2007) 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hipp J, S.M, Pott J, Sick O, Makowiec F, Hopt UT, Fichtner-Feigl S, Wittel UA, Management and outcomes of intraductal papillary mucinous neoplasms, BJS Open 2 (4) (2019) 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tanaka M, Intraductal papillary mucinous neoplasm of the pancreas as the main focus for early detection of pancreatic adenocarcinoma, 47 (5) (2018) 544–550. [DOI] [PubMed] [Google Scholar]
- [98].van der Waaij LA, van Dullemen HM, Porte RJ, Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis, Gastrointest. Endosc 62 (3) (2005) 383–389. [DOI] [PubMed] [Google Scholar]
- [99].Suzuki R, Thosani N, Annangi S, Guha S, Bhutani MS, Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: a systematic review and meta-analysis, Pancreatology 14 (5) (2014) 380–384. [DOI] [PubMed] [Google Scholar]
- [100].Yoon WJ, Daglilar ES, Fernandez-del Castillo C, Mino-Kenudson M, Pitman MB, Brugge WR, Peritoneal seeding in intraductal papillary mucinous neoplasm of the pancreas patients who underwent endoscopic ultrasound-guided fine-needle aspiration: the PIPE study, Endoscopy 46 (5) (2014) 382–387. [DOI] [PubMed] [Google Scholar]
- [101].Vilas-Boas F, Macedo G, Pancreatic cystic lesions: new endoscopic trends in diagnosis, J. Clin. Gastroenterol 52 (1) (2018) 13–19. [DOI] [PubMed] [Google Scholar]
- [102].Zhao Q, Zhan T, Deng Z, Li Q, Liu Y, Yang S, et al. , Glycan analysis of colorectal cancer samples reveals stage-dependent changes in CEA glycosylation patterns, Clin. Proteomics 15 (2018) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Yoshikawa M, Morine Y, Ikemoto T, Imura S, Higashijima J, Iwahashi S, et al. , Elevated preoperative serum CEA level is associated with poor prognosis in patients with hepatocellular carcinoma through the epithelial-mesenchymal transition, Anticancer Res. 37 (3) (2017) 1169–1175. [DOI] [PubMed] [Google Scholar]
- [104].Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, et al. , Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas, Gastroenterology 108 (4) (1995) 1230–1235. [DOI] [PubMed] [Google Scholar]
- [105].Talar-Wojnarowska R, Pazurek M, Durko L, Degowska M, Rydzewska G, Smigielski J, et al. , Pancreatic cyst fluid analysis for differential diagnosis between benign and malignant lesions, Oncol. Lett 5 (2) (2013) 613–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Aljebreen AM, Romagnuolo J, Perini R, Sutherland F, Utility of endoscopic ultrasound, cytology and fluid carcinoembryonic antigen and CA 19–9 levels in pancreatic cystic lesions, World J. Gastroenterol 13 (29) (2007) 3962–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ngamruengphong S, Bartel MJ, Raimondo M, Cyst carcinoembryonic antigen in differentiating pancreatic cysts: a meta-analysis, Dig. Liver Dis. 45 (11) (2013) 920–926. [DOI] [PubMed] [Google Scholar]
- [108].Al-Haddad M, DeWitt J, Sherman S, Schmidt CM, LeBlanc JK, McHenry L, et al. , Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts, Gastrointest. Endosc 79 (1) (2014) 79–87. [DOI] [PubMed] [Google Scholar]
- [109].Kurita Y, Kuwahara T, Hara K, Mizuno N, Okuno N, Matsumoto S, et al. , Diagnostic ability of artificial intelligence using deep learning analysis of cyst fluid in differentiating malignant from benign pancreatic cystic lesions, Sci. Rep 9 (1) (2019) 6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, et al. , The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas, Ann. Surg 255 (3) (2012) 517–522. [DOI] [PubMed] [Google Scholar]
- [111].Maire F, Voitot H, Aubert A, Palazzo L, O’Toole D, Couvelard A, et al. ,Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy, Am. J. Gastroenterol 103 (11) (2008) 2871–2877. [DOI] [PubMed] [Google Scholar]
- [112].Fritz S, Hackert T, Hinz U, Hartwig W, Buchler MW, Werner J, Role of serum carbohydrate antigen 19–9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas, Br. J. Surg 98 (1) (2011) 104–110. [DOI] [PubMed] [Google Scholar]
- [113].Wang W, Zhang L, Chen L, Wei J, Sun Q, Xie Q, et al. , Serum carcinoembryonic antigen and carbohydrate antigen 19–9 for prediction of malignancy and invasiveness in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis, Biomed Rep 3 (1) (2015) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Corvelo TC, de Loiola Rdo S, Aguiar DC, de Matos Gde C, de Brito DC, The Lewis histo-blood group system: molecular analysis of the 59T > G, 508G > A, and 1067T > A polymorphisms in an Amazonian population, PLoS One 8 (7) (2013) e69908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Hao C, Zhang G, Zhang L, Serum CEA levels in 49 different types of cancer and noncancer diseases, Prog. Mol. Biol. Transl. Sci 162 (2019) 213–227. [DOI] [PubMed] [Google Scholar]
- [116].Kaur S, Kumar S, Momi N, Sasson AR, Batra SK, Mucins in pancreatic cancer and its microenvironment, Nat Rev Gastroenterol Hepatol 10 (10) (2013) 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, Kaur S, et al. , Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications,J. Thorac. Oncol 10 (1) (2015) 19–27. [DOI] [PubMed] [Google Scholar]
- [118].Pai P, Rachagani S, Dhawan P, Sheinin YM, Macha MA, Qazi AK, et al. , MUC4 is negatively regulated through the Wnt/beta-catenin pathway via the Notch effector Hath1 in colorectal cancer, Genes Cancer 7 (5–6) (2016) 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Bafna S, Kaur S, Batra SK, Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells, Oncogene 29 (20) (2010) 2893–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Rockacy M, Khalid A, Update on pancreatic cyst fluid analysis, Annals of Gastroenterology : Quarterly Publication of the Hellenic Society of Gastroenterology 26 (2) (2013) 122–127. [PMC free article] [PubMed] [Google Scholar]
- [121].Yonezawa S, Taira M, Osako M, Kubo M, Tanaka S, Sakoda K, et al. , MUC-1 mucin expression in invasive areas of intraductal papillary mucinous tumors of the pancreas, Pathol. Int 48 (4) (April 1998) 319–322. [DOI] [PubMed] [Google Scholar]
- [122].Shimamoto T, Tani M, Kawai M, Hirono S, Ina S, Miyazawa M, et al. , MUC1 is a useful molecular marker for malignant intraductal papillary mucinous neoplasms in pancreatic juice obtained from endoscopic retrograde pancreatography, Pancreas 39 (6) (2010) 879–883. [DOI] [PubMed] [Google Scholar]
- [123].Carrara S, Cangi MG, Arcidiacono PG, Perri F, Petrone MC, Mezzi G, et al. , Mucin expression pattern in pancreatic diseases: findings from EUS-guided fine-needle aspiration biopsies, Am. J. Gastroenterol 106 (7) (2011) 1359–1363. [DOI] [PubMed] [Google Scholar]
- [124].Nissim S, Idos GE, Wu B, Genetic markers of malignant transformation in Intraductal papillary Mucinous neoplasm of the pancreas: a meta-analysis, Pancreas 41 (8) (2012) 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Masuda A, Arisaka Y, Hara S, Matsumoto I, Takenaka M, Sakai A, et al. , MUC2 expression and prevalence of high-grade dysplasia and invasive carcinoma in mixed-type intraductal papillary mucinous neoplasm of the pancreas, Pancreatology 13 (6) (2013) 583–588. [DOI] [PubMed] [Google Scholar]
- [126].Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, et al. , Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study, Virchows Arch. 447 (5) (2005) 794–799. [DOI] [PubMed] [Google Scholar]
- [127].Maker AV, Hu V, Kadkol SS, Hong L, Brugge W, Winter J, et al. , Cyst fluid biosignature to predict intraductal papillary mucinous neoplasms of the pancreas with high malignant potential, J. Am. Coll. Surg 228 (5) (2019) 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Kwon RS, Simeone DM, The use of protein-based biomarkers for the diagnosis of cystic tumors of the pancreas, International Journal of Proteomics 2011 (2011) 413646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Luttges J, Feyerabend B, Buchelt T, Pacena M, Kloppel G, The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas, Am. J. Surg. Pathol 26 (4) (2002) 466–471. [DOI] [PubMed] [Google Scholar]
- [130].Cao Z, Maupin K, Curnutte B, Fallon B, Feasley CL, Brouhard E, et al. , Specific glycoforms of MUC5AC and endorepellin accurately distinguish mucinous from nonmucinous pancreatic cysts, Molecular & Cellular Proteomics : MCP 12 (10) (2013) 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Lipkin S, Lee J, Imagawa D, Hewitt SM, Tucker C, Zell JA, et al. , Phase IIA trial testing erlotinib as an intervention against intraductal pancreatic mucinous neoplasms, Cancer Prev. Res. (Phila.) 4 (4) (2011) 512–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sinha J, Cao Z, Dai J, Tang H, Partyka K, Hostetter G, et al. , A gastric glycoform of MUC5AC is a biomarker of mucinous cysts of the pancreas, PLoS One 11 (12) (2016) e0167070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jabbar KS, Arike L, Verbeke CS, Sadik R, Hansson GC, Highly accurate identification of cystic precursor lesions of pancreatic cancer through targeted mass spectrometry: a phase IIc diagnostic study, J. Clin. Oncol 36 (4) (2018) 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Fortner RT, Schock H, Le Cornet C, Husing A, Vitonis AF, Johnson TS, et al. , Ovarian cancer early detection by circulating CA125 in the context of anti-CA125 autoantibody levels: results from the EPIC cohort, Int. J. Cancer 142 (7) (2018) 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Muniyan S, Haridas D, Chugh S, Rachagani S, Lakshmanan I, Gupta S, et al. , MUC16 contributes to the metastasis of pancreatic ductal adenocarcinoma through focal adhesion mediated signaling mechanism, Genes Cancer 7 (3–4) (2016) 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Ke E, Patel BB, Liu T, Li XM, Haluszka O, Hoffman JP, et al. , Proteomic analyses of pancreatic cyst fluids, Pancreas 38 (2) (2009) e33–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Cuoghi A, Farina A, Z’Graggen K, Dumonceau JM, Tomasi A, Hochstrasser DF, et al. , Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts, J. Proteome Res. 10 (5) (2011) 2664–2670. [DOI] [PubMed] [Google Scholar]
- [138].Corcos O, Couvelard A, Dargere D, Sauvanet A, Hammel P, Paradis V, et al. , Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas, Pancreas 41 (2) (2012) 169–174. [DOI] [PubMed] [Google Scholar]
- [139].Maker AV, Katabi N, Qin LX, Klimstra DS, Schattner M, Brennan MF, et al. , Cyst fluid interleukin-1b (IL1b) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas, Clin. Cancer Res. 17 (6) (March 15 2011) 1502–1508 (Epub 2011 Jan 25), 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Roch AM, DeWitt JM, Al-Haddad MA, Schmidt CM 2nd, Ceppa EP, House MG, et al. , Nonoperative management of main pancreatic duct-involved intraductal papillary mucinous neoplasm might be indicated in select patients, J. Am. Coll. Surg 219 (1) (2014) 122–129. [DOI] [PubMed] [Google Scholar]
- [141].Yip-Schneider MT, Wu H, Dumas RP, Hancock BA, Agaram N, Radovich M, et al. , Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts, J. Am. Coll. Surg 218 (4) (2014) 608–617. [DOI] [PubMed] [Google Scholar]
- [142].Yang JM, Southern JF, Warshaw AL, Lewandrowski KB, Proliferation tissue polypeptide antigen distinguishes malignant mucinous cystadenocarcinomas from benign cystic tumors and pseudocysts, Am. J. Surg 171 (1) (1996) 126–129 (discussion 9–30). [DOI] [PubMed] [Google Scholar]
- [143].Kiraly O, Boulling A, Witt H, Le Marechal C, Chen JM, Rosendahl J, et al. , Signal peptide variants that impair secretion of pancreatic secretory trypsin inhibitor (SPINK1) cause autosomal dominant hereditary pancreatitis, Hum. Mutat 28 (5) (2007) 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Patel MR, Eppolito AL, Willingham FF, Hereditary pancreatitis for the endoscopist, Ther. Adv. Gastroenterol 6 (2) (2013) 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Raty S, Sand J, Laukkarinen J, Vasama K, Bassi C, Salvia R, et al. , Cyst fluid SPINK1 may help to differentiate benign and potentially malignant cystic pancreatic lesions, Pancreatology 13 (5) (2013) 530–533. [DOI] [PubMed] [Google Scholar]
- [146].Shirai Y, Sogawa K, Yamaguchi T, Sudo K, Nakagawa A, Sakai Y, et al. , Protein profiling in pancreatic juice for detection of intraductal papillary mucinous neoplasm of the pancreas, Hepato-Gastroenterology 55 (86–87) (2008) 1824–1829. [PubMed] [Google Scholar]
- [147].Frossard JL, Morris MA, Two SPINK1 mutations induce early-onset severe chronic pancreatitis, Case Rep Gastroenterol. 11 (1) (2017) 85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lee JH, Kim KS, Kim TJ, Hong SP, Song SY, Chung JB, et al. , Immunohistochemical analysis of claudin expression in pancreatic cystic tumors, Oncol. Rep 25 (4) (2011) 971–978. [DOI] [PubMed] [Google Scholar]
- [149].Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, et al. , Claudin-18 is an early-stage marker of pancreatic carcinogenesis, The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society. 59 (10) (2011) 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Rebours V, Le Faouder J, Laouirem S, Mebarki M, Albuquerque M, Camadro JM, et al. , In situ proteomic analysis by MALDI imaging identifies ubiquitin and thymosin-beta4 as markers of malignant intraductal pancreatic mucinous neoplasms, Pancreatology 14 (2) (2014) 117–124. [DOI] [PubMed] [Google Scholar]
- [151].Bausch D, Mino-Kenudson M, Fernandez-Del Castillo C, Warshaw AL, Kelly KA, Thayer SP, Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms, J. Gastrointest. Surg 13 (11) (2009) 1948–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Das KK, Xiao H, Geng X, Fernandez-Del-Castillo C, Morales-Oyarvide V, Daglilar E, et al. , mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN), Gut 63 (10) (2014) 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Das KK, Geng X, Brown JW, Morales-Oyarvide V, Huynh T, Pergolini I, et al. , Cross validation of the monoclonal antibody Das-1 in identification of high-risk mucinous pancreatic cystic lesions, Gastroenterology 157 (3) (2019) 720–730 (e2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Crowell PL, Schmidt CM, Yip-Schneider MT, Savage JJ, Hertzler DA 2nd, Cummings WO, Cyclooxygenase-2 expression in hamster and human pancreatic neoplasia, Neoplasia 8 (6) (2006) 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Schmidt CM, Yip-Schneider MT, Ralstin MC, Wentz S, DeWitt J, Sherman S, et al. , PGE(2) in pancreatic cyst fluid helps differentiate IPMN from MCN and predict IPMN dysplasia, J. Gastrointest. Surg 12 (2) (2008) 243–249. [DOI] [PubMed] [Google Scholar]
- [156].Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, et al. , Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice, J. Pathol 210 (1) (2006) 42–48. [DOI] [PubMed] [Google Scholar]
- [157].Deftereos G, Finkelstein SD, Jackson SA, Ellsworth EM, Krishnamurti U, Liu Y, et al. , The value of mutational profiling of the cytocentrifugation supernatant fluid from fine-needle aspiration of pancreatic solid mass lesions, Modern Pathology : An Official Journal of the United States and Canadian Academy of Pathology, Inc 27 (4) (2014) 594–601. [DOI] [PubMed] [Google Scholar]
- [158].Shiroma N, Arihiro K, Oda M, Orita M, KRAS fluorescence in situ hybridisation testing for the detection and diagnosis of pancreatic adenocarcinoma, J. Clin. Pathol 71 (10) (2018) 865–873. [DOI] [PubMed] [Google Scholar]
- [159].Chen PY, Muzumdar MD, Dorans KJ, Robbins R, Bhutkar A, Del Rosario A, et al. , Adaptive and reversible resistance to Kras inhibition in pancreatic cancer cells, Cancer Res. 78 (4) (2018) 985–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Tan MC, Basturk O, Brannon AR, Bhanot U, Scott SN, Bouvier N, et al. , GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma, J. Am. Coll. Surg 220 (5) (2015) 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Satoh K, Shimosegawa T, Moriizumi S, Koizumi M, Toyota T, K-ras mutation and p53 protein accumulation in intraductal mucin-hypersecreting neoplasms of the pancreas, Pancreas 12 (4) (1996) 362–368. [DOI] [PubMed] [Google Scholar]
- [162].Jones M, Zheng Z, Wang J, Dudley J, Albanese E, Kadayifci A, et al. , Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts, Gastrointest. Endosc 83 (1) (2016) 140–148. [DOI] [PubMed] [Google Scholar]
- [163].Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, et al. , Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study, Gastrointest. Endosc 69 (6) (2009) 1095–1102. [DOI] [PubMed] [Google Scholar]
- [164].Anderson MA, Kwon RS, Scheiman JM, PANDA cyst-fluid analysis: eats, shoots and leaves? Gastrointest. Endosc 69 (6) (2009) 1103–1105. [DOI] [PubMed] [Google Scholar]
- [165].Zauber P, Marotta SP, Sabbath-Solitare M GNAS gene mutation may be present only transiently during colorectal tumorigenesis, Int J Mol Epidemiol Genet 7 (1) (2016) 24–31. [PMC free article] [PubMed] [Google Scholar]
- [166].Dal Molin M, Matthaei H, Wu J, Blackford A, Debeljak M, Rezaee N, et al. , Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas, Ann. Surg. Oncol 20 (12) (2013) 3802–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. , Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development, Sci. Transl. Med 3 (92) (2011) 92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Singhi AD, Nikiforova MN, Fasanella KE, McGrath KM, Pai RK, Ohori NP, et al. , Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts, Clin. Cancer Res. 20 (16) (2014) 4381–4389. [DOI] [PubMed] [Google Scholar]
- [169].Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, et al. ,Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia, Gut 67 (12) (2018) 2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, et al. , A combination of molecular markers and clinical features improve the classification of pancreatic cysts, Gastroenterology 149 (6) (2015) 1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Al-Haddad MA, Kowalski T, Siddiqui A, Mertz HR, Mallat D, Haddad N, et al. , Integrated molecular pathology accurately determines the malignant potential of pancreatic cysts, Endoscopy 47 (2) (2015) 136–142. [DOI] [PubMed] [Google Scholar]
- [172].Hata T, Dal Molin M, Hong SM, Tamura K, Suenaga M, Yu J, et al. , Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid DNA methylation markers, Clin. Cancer Res. 23 (14) (2017) 3935–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Moris D, Damaskos C, Spartalis E, Papalampros A, Vernadakis S, Dimitroulis D, et al. , Updates and critical evaluation on novel biomarkers for the malignant progression of Intraductal papillary mucinous neoplasms of the pancreas, Anticancer Res. 37 (5) (2017) 2185–2194. [DOI] [PubMed] [Google Scholar]
- [174].Sun X, Zhou X, Zhang Y, Zhu X, Liu H, Systematic review and meta-analysis of diagnostic accuracy of miRNAs in patients with pancreatic cancer, Dis. Markers 2018 (2018) 6292396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hernandez YG, Lucas AL, MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions, World J Gastrointest Oncol. 8 (1) (2016) 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Wang L, Zheng J, Sun C, Wang L, Jin G, Xin L, et al. , MicroRNA expression levels as diagnostic biomarkers for intraductal papillary mucinous neoplasm, Oncotarget 8 (35) (2017) 58765–58770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Permuth-Wey J, Chen YA, Fisher K, McCarthy S, Qu X, Lloyd MC, et al. , A genome-wide investigation of microRNA expression identifies biologically-meaningful microRNAs that distinguish between high-risk and low-risk intraductal papillary mucinous neoplasms of the pancreas, PLoS One 10 (1) (2015) e0116869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Lee LS, Szafranska-Schwarzbach AE, Wylie D, Doyle LA, Bellizzi AM, Kadiyala V, et al. , Investigating microRNA expression profiles in pancreatic cystic neoplasms, Clin. Transl. Gastroenterol 5 (2014) e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Ryu JK, Matthaei H, Dal Molin M, Hong SM, Canto MI, Schulick RD, et al. , Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma, Pancreatology 11 (3) (2011) 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Farrell JJ, Toste P, Wu N, Li L, Wong J, Malkhassian D, et al. , Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer, Am. J. Gastroenterol 108 (8) (2013) 1352–1359. [DOI] [PubMed] [Google Scholar]
- [181].Matthaei H, Wylie D, Lloyd MB, Dal Molin M, Kemppainen J, Mayo SC, et al. , miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts, Clin. Cancer Res. 18 (17) (2012) 4713–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Utomo WK, Looijenga LH, Bruno MJ, Hansen BE, Gillis A, Biermann K, et al. , A microRNA panel in pancreatic cyst fluid for the risk stratification of pancreatic cysts in a prospective cohort, Mol Ther Nucleic Acids 5 (2016) e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Vila-Navarro E, Vila-Casadesus M, Moreira L, Duran-Sanchon S, Sinha R, Gines A, et al. , MicroRNAs for detection of pancreatic neoplasia: biomarker discovery by next-generation sequencing and validation in 2 independent cohorts, Ann. Surg 265 (6) (2017) 1226–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wang J, Paris PL, Chen J, Ngo V, Yao H, Frazier ML, et al. , Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions, Cancer Lett. 356 (2 Pt B) (2015) 404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Habbe N, Koorstra JB, Mendell JT, Offerhaus GJ, Ryu JK, Feldmann G, et al. , MicroRNA miR-155 is a biomarker of early pancreatic neoplasia, Cancer Biology & Therapy 8 (4) (2009) 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Abue M, Yokoyama M, Shibuya R, Tamai K, Yamaguchi K, Sato I, et al. ,Circulating miR-483–3p and miR-21 is highly expressed in plasma of pancreatic cancer, Int. J. Oncol 46 (2) (2015) 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Permuth-Wey J, Chen DT, Fulp WJ, Yoder SJ, Zhang Y, Georgeades C, et al. , Plasma microRNAs as novel biomarkers for patients with intraductal papillary mucinous neoplasms of the pancreas, Cancer Prev. Res. (Phila.) 8 (9) (2015) 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Permuth JB, Chen DT, Yoder SJ, Li J, Smith AT, Choi JW, et al. , Linc-ing circulating long non-coding RNAs to the diagnosis and malignant prediction of intraductal papillary mucinous neoplasms of the pancreas, Sci. Rep 7 (1) (2017) 10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Henry JC, Bassi C, Giovinazzo F, Bloomston M, MicroRNA from pancreatic duct aspirate differentiates cystic lesions of the pancreas, Ann. Surg. Oncol 20 (Suppl.3) (2013) S661–S666. [DOI] [PubMed] [Google Scholar]
- [190].Olsen TK, Baryawno N, Introduction to single-cell RNA sequencing, Curr Protoc Mol Biol 122 (1) (2018) e57. [DOI] [PubMed] [Google Scholar]
- [191].Hwang B, Lee JH, Bang D, Single-cell RNA sequencing technologies and bioinformatics pipelines, Exp. Mol. Med 50 (8) (2018) 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Bernard V, Semaan A, Huang J, San Lucas FA, Mulu FC, Stephens BM, et al. , Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression, Clin. Cancer Res. 25 (7) (2019) 2194–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, et al. , Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade, Nat. Commun 8 (2017) 14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Beatty PL, van der Geest R, Hashash JG, Kimura T, Gutkin D, Brand RE, et al. , Immunobiology and immunosurveillance in patients with intraductal papillary mucinous neoplasms (IPMNs), premalignant precursors of pancreatic adenocarcinomas, Cancer Immunol. Immunother 65 (7) (2016) 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Gemenetzis G, Bagante F, Griffin JF, Rezaee N, Javed AA, Manos LL, et al. , Neutrophil-to-lymphocyte ratio is a predictive marker for invasive malignancy in intraductal papillary mucinous neoplasms of the pancreas, Ann. Surg 266 (2) (2017) 339–345. [DOI] [PubMed] [Google Scholar]
- [196].Ohno R, Kawamoto R, Kanamoto M, Watanabe J, Fujii M, Ohtani H, et al. , Neutrophil to lymphocyte ratio is a predictive factor of malignant potential for intraductal papillary mucinous neoplasms of the pancreas, Biomark. Insights 14 (2019) (1177271919851505). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Arima K, Okabe H, Hashimoto D, Chikamoto A, Kuroki H, Taki K, et al. , The neutrophil-to-lymphocyte ratio predicts malignant potential in intraductal papillary mucinous neoplasms, J. Gastrointest. Surg 19 (12) (2015) 2171–2177. [DOI] [PubMed] [Google Scholar]
- [198].Boroughs LK, DeBerardinis RJ, Metabolic pathways promoting cancer cell survival and growth, Nat. Cell Biol. 17 (4) (2015) 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Kobayashi T, Nishiumi S, Ikeda A, Yoshie T, Sakai A, Matsubara A, et al. , A novel serum metabolomics-based diagnostic approach to pancreatic cancer, Cancer Epidemiol. Biomark. Prev 22 (4) (2013) 571–579. [DOI] [PubMed] [Google Scholar]
- [200].Hirata Y, Kobayashi T, Nishiumi S, Yamanaka K, Nakagawa T, Fujigaki S, et al. , Identification of highly sensitive biomarkers that can aid the early detection of pancreatic cancer using GC/MS/MS-based targeted metabolomics, Clin. Chim. Acta 468 (2017) 98–104. [DOI] [PubMed] [Google Scholar]
- [201].Mayerle J, Kalthoff H, Reszka R, Kamlage B, Peter E, Schniewind B, et al. , Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis, Gut 67 (1) (2018) 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Fahrmann JF, Bantis LE, Capello M, Scelo G, Dennison JB, Patel N, et al. , A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer, J. Natl. Cancer Inst. 111 (4) (2019) 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Mehta KY, Wu HJ, Menon SS, Fallah Y, Zhong X, Rizk N, et al. , Metabolomic biomarkers of pancreatic cancer: a meta-analysis study, Oncotarget 8 (40) (2017) 68899–68915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Unger K, Mehta KY, Kaur P, Wang Y, Menon SS, Jain SK, et al. , Metabolomics based predictive classifier for early detection of pancreatic ductal adenocarcinoma, Oncotarget 9 (33) (2018) 23078–23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Katagiri R, Goto A, Nakagawa T, Nishiumi S, Kobayashi T, Hidaka A, et al. , Increased levels of branched-chain amino acid associated with increased risk of pancreatic cancer in a prospective case-control study of a large cohort, Gastroenterology 155 (5) (2018) 1474–1482 (e1). [DOI] [PubMed] [Google Scholar]
- [206].Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. , Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development, Nat. Med 20 (10) (2014) 1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Park WG, Wu M, Bowen R, Zheng M, Fitch WL, Pai RK, et al. , Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine, Gastrointestinal Endoscopy 78 (2) (2013) 295–302 (e2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Lopes CV, Cyst fluid glucose: an alternative to carcinoembryonic antigen for pancreatic mucinous cysts, World J. Gastroenterol 25 (19) (2019) 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Carr RA, Yip-Schneider MT, Simpson RE, Dolejs S, Schneider JG, Wu H, et al. , Pancreatic cyst fluid glucose: rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts, Surgery 163 (3) (2018) 600–605. [DOI] [PubMed] [Google Scholar]
- [210].Zikos T, Pham K, Bowen R, Chen AM, Banerjee S, Friedland S, et al. , Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts, Am. J. Gastroenterol 110 (6) (2015) 909–914. [DOI] [PubMed] [Google Scholar]
- [211].Gaiser RA, Pessia A, Ateeb Z, Davanian H, Fernandez Moro C, Alkharaan H, et al. , Integrated targeted metabolomic and lipidomic analysis: a novel approach to classifying early cystic precursors to invasive pancreatic cancer, Sci. Rep 9 (1) (2019) 10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Moore HB, Culp-Hill R, Reisz JA, Lawson PJ, Sauaia A, Schulick RD, et al. , The metabolic time line of pancreatic cancer: opportunities to improve early detection of adenocarcinoma, Am. J. Surg (2019), 10.1016/j.amjsurg.2019.08.015. [DOI] [PubMed] [Google Scholar]
- [213].Lin X, Zhan B, Wen S, Li Z, Huang H, Feng J, Metabonomic alterations from pancreatic intraepithelial neoplasia to pancreatic ductal adenocarcinoma facilitate the identification of biomarkers in serum for early diagnosis of pancreatic cancer, Mol. BioSyst 12 (9) (2016) 2883–2892. [DOI] [PubMed] [Google Scholar]
- [214].Gillies RJ, Kinahan PE, Hricak H, Radiomics: images are more than pictures, they are data, Radiology 278 (2) (2016) 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. , Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach, Nat. Commun 5 (2014) 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Varghese BA, Cen SY, Hwang DH, Duddalwar VA, Texture analysis of imaging: what radiologists need to know, AJR Am. J. Roentgenol 212 (3) (2019) 520–528. [DOI] [PubMed] [Google Scholar]
- [217].Li Z, Yu L, Wang X, Yu H, Gao Y, Ren Y, et al. , Diagnostic performance of mammographic texture analysis in the differential diagnosis of benign and malignant breast tumors, Clin Breast Cancer 18 (4) (2018) (e621-e7). [DOI] [PubMed] [Google Scholar]
- [218].Yap FY, Hwang DH, Cen SY, Varghese BA, Desai B, Quinn BD, et al. , Quantitative contour analysis as an image-based discriminator between benign and malignant renal tumors, Urology 114 (2018) 121–127. [DOI] [PubMed] [Google Scholar]
- [219].Digumarthy SR, Padole AM, Lo Gullo R, Singh R, Shepard JO, Kalra MK, CT texture analysis of histologically proven benign and malignant lung lesions, Medicine (Baltimore) 97 (26) (2018) e11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Fan TW, Malhi H, Varghese B, Cen S, Hwang D, Aron M, et al. , Computed tomography-based texture analysis of bladder cancer: differentiating urothelial carcinoma from micropapillary carcinoma, Abdom Radiol (NY) 44 (1) (2019) 201–208. [DOI] [PubMed] [Google Scholar]
- [221].Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, et al. , Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores, Eur. Radiol 25 (10) (2015) 2840–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Lewis S, Peti S, Hectors SJ, King M, Rosen A, Kamath A, et al. , Volumetric quantitative histogram analysis using diffusion-weighted magnetic resonance imaging to differentiate HCC from other primary liver cancers, Abdom Radiol (NY) 44 (3) (2019) 912–922. [DOI] [PubMed] [Google Scholar]
- [223].Chu LC, Goggins MG, Fishman EK, Diagnosis and detection of pancreatic cancer, Cancer J 23 (6) (2017) 333–342. [DOI] [PubMed] [Google Scholar]
- [224].Dmitriev K, Kaufman AE, Javed AA, Hruban RH, Fishman EK, Lennon AM, et al. , Classification of pancreatic cysts in computed tomography images using a random forest and convolutional neural network ensemble, Med Image Comput Comput Assist Interv 10435 (2017) 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Chu L, Park S, Fishman E, AB032. P002. Radiomics based classification of pancreatic cystic neoplasms, Annals of Pancreatic Cancer 1 (2018) AB032, 10.21037/apc.2018.AB032. [DOI] [Google Scholar]
- [226].Chakraborty J, Midya A, Gazit L, Attiyeh M, Langdon-Embry L, Allen PJ, et al. , CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas, Med. Phys 45 (11) (2018) 5019–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Hanania AN, Bantis LE, Feng Z, Wang H, Tamm EP, Katz MH, et al. , Quantitative imaging to evaluate malignant potential of IPMNs, Oncotarget 7 (52) (2016) 85776–85784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Permuth JB, Choi J, Balarunathan Y, Kim J, Chen DT, Chen L, et al. , Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms, Oncotarget 7 (52) (2016) 85785–85797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Wei R, Lin K, Yan W, Guo Y, Wang Y, Li J, et al. , Computer-aided diagnosis of pancreas serous cystic neoplasms: a radiomics method on preoperative MDCT images, Technol Cancer Res Treat 18 (2019) (1533033818824339). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. , Radiomics: the process and the challenges, Magn. Reson. Imaging 30 (9) (2012) 1234–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Patra KC, Bardeesy N, Mizukami Y, Diversity of precursor lesions for pancreatic cancer: the genetics and biology of intraductal papillary mucinous neoplasm, Clin. Transl. Gastroenterol 8 (4) (2017) e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Poruk KE, Valero V 3rd, He J, Ahuja N, Cameron JL, Weiss MJ, et al. , Circulating epithelial cells in intraductal papillary mucinous neoplasms and cystic pancreatic lesions, Pancreas 46 (7) (2017) 943–947. [DOI] [PubMed] [Google Scholar]
- [233].Berger AW, Schwerdel D, Costa IG, Hackert T, Strobel O, Lam S, et al. , Detection of hot-spot mutations in circulating cell-free DNA from patients with intraductal papillary mucinous neoplasms of the pancreas, Gastroenterology 151 (2) (2016) 267–270. [DOI] [PubMed] [Google Scholar]
- [234].Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. , Glypican-1 identifies cancer exosomes and detects early pancreatic cancer, Nature 523 (7559) (2015) 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Goto T, Fujiya M, Konishi H, Sasajima J, Fujibayashi S, Hayashi A, et al. , An elevated expression of serum exosomal microRNA-191, −21, −451a of pancreatic neoplasm is considered to be efficient diagnostic marker, BMC Cancer 18 (1) (2018) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, et al. , A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas, Am. J. Surg. Pathol 39 (12) (2015) 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Hardacre JM, McGee MF, Stellato TA, Schulak JA, An aggressive surgical approach is warranted in the management of cystic pancreatic neoplasms, Am. J. Surg 193 (3) (2007) 374–378 (discussion 8–9). [DOI] [PubMed] [Google Scholar]