Abstract
Summary
Background
In the Denosumab and Teriparatide Administration (DATA) study, we showed that denosumab fully inhibits teriparatide-induced bone resorption while allowing for continued teriparatide-induced bone formation, resulting in larger increases in hip and spine bone mineral density (BMD) than with either drug alone. We aimed to assess whether administration of denosumab with high dose teriparatide would stimulate larger increases in bone mass than those observed in the DATA study.
Methods
DATA-HD was an open-label, randomised, controlled phase 4 trial done at Massachusetts General Hospital. Eligible women were postmenopausal women (at least 36 months since last menses or since hysterectomy with a follicle-stimulating hormone concentration of ≥40 U/L) with osteoporosis. Participants were randomly assigned (1:1) to receive teriparatide 20 μg (standard dose) or 40 μg (high dose) daily via subcutaneous injection for 9 months. At 3 months, both groups were started on denosumab 60 mg every 6 months via subcutaneous injection for 12 months. Areal BMD (aBMD) was measured at 0, 3, 9, and 15 months. Treatment was given open label, but outcome assessors were masked. The primary endpoint was percentage change from baseline in spine areal BMD (aBMD) at 15 months. Women who completed at least one study visit after baseline were included in the modified intention-to-treat analysis. Safety was assessed in all randomly assigned participants. This study is registered with ClinicalTrials.gov, number NCT02176382.
Findings
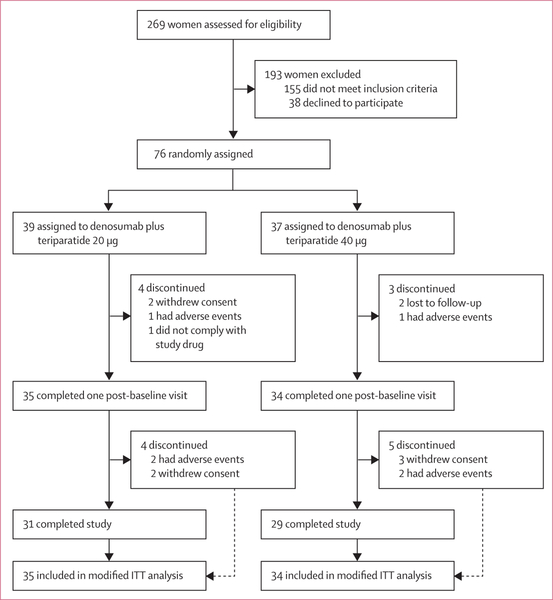
Between Oct 15, 2014, and June 10, 2016, 269 women were assessed for eligibility. 76 participants were randomly assigned to 20 μg teriparatide (n=39) or 40 μg teriparatide (n=37), of whom 69 completed at least one post-baseline visit. At 15 months, mean spine aBMD had increased to a significantly greater extent in the 40 μg group (17·5% [SD 6·0] increase) than the 20 μg group (9·5% [3·2]; difference 8·1%, 95% CI 5·5 to 10·6, p<0·0001). Mean femoral neck aBMD had also increased to a greater extent in the 40 μg group (6·8% [SD 4·1] increase) than the 20 μg group (4·3% [3·7]; difference 2·5%, 0·5 to 4·5, p=0·04), as did mean total hip aBMD (40 μg group, 6·1% [3·4] increase; 20 μg group, 3·9% [2·9] increase; difference 2·2%, 0·6 to 3·8, p<0·0001). 30 (77%) of 39 participants in the 20 μg group and 29 (78%) of 37 participants in the 40 μg group had an adverse event, and seven (18%) and two (5%) patients had serious adverse events. The most frequent adverse events were joint pain (15 [38%]), muscle cramp (15 [38%]), and fatigue (12 [31%]) in the 20 μg group group and fatigue (14 [38%]), nausea (16 [43%]), and joint pain (17 [46%]) in the 40 μg group. No deaths were reported.
Interpretation
Combined treatment with teriparatide 40 μg and denosumab increases spine and hip BMD more than standard combination therapy. This large and rapid increase in bone mass suggest that this high dose regimen might provide a method of restoring skeletal integrity in patients with osteoporosis.
Introduction
Although the number of osteoporosis therapies has expanded substantially over the past two decades, available antiresorptive and anabolic medications result in only modest increases in bone mineral density (BMD) and modest reductions in non-vertebral fracture rates.1–10 Since the global population is ageing, osteoporosis remains a considerable health-care burden and an urgent need exists to develop new therapeutic regimens that can fully restore skeletal integrity. By contrast with many other chronic, age-related diseases, osteoporosis remains a disease primarily treated with monotherapy.
Several studies11–14 have investigated the potential efficacy of combining teriparatide or related compounds with the most commonly used antiresorptive drugs—the nitrogen-containing bisphosphonates such as alendronate or zoledronic acid—but this approach has not been found to have any advantage compared with monotherapy. Conversely, in the Denosumab and Teriparatide Administration (DATA) trial,15–18 we showed that the combination of teriparatide and the receptor activator of nuclear factor κ-B ligand inhibitor, denosumab, increased BMD, improved skeletal microarchitecture, and increased estimated bone strength to a greater extent than either drug alone.15–18 In DATA, biochemical markers of bone resorption were identically suppressed in patients treated with denosumab alone and in those treated with denosumab plus teriparatide, whereas markers of bone formation were suppressed to a greater extent in those treated with denosumab alone than those treated with denosumab plus teriparatide, indicating that the efficacy of this combination is likely to be due to denosumab’s ability to fully block the resorptive effects of teriparatide while possibly allowing for teriparatide-induced stimulation of modelling-based bone formation (ie, bone formation that occurs over bone surfaces not undergoing concomitant bone resorption). On the basis of these findings, we aimed to investigate whether combining a more potent anabolic stimulus (high dose teriparatide) with denosumab over a short duration would result in larger increases in BMD than that with standard dose teriparatide.
Methods
Study design and participants
The Denosumab and High-dose Teriparatide Administration (DATA-HD) study was an open-label, phase 4, randomised controlled trial done at Massachusetts General Hospital (Boston, MA, USA).
Women aged 45 years or older were recruited through targeted mailings, advertisements, and physician referrals. Eligible women were postmenopausal (at least 36 months since last menses or since hysterectomy with a follicle-stimulating hormone concentration of ≥40 U/L) and had osteoporosis with a high risk of fracture (defined as a BMD T score of ≤−2·5 at the spine, hip, or femoral neck; a T score of ≤−2·0 with at least one BMD-independent risk factor [ fracture after age 50 years, parental hip fracture after age 50 years, previous hyperthyroidism, inability to rise from a chair with arms elevated, or current smoking];19 or a T score of ≤−1·0 at the spine, hip, or forearm with a history of ≥1 fragility fractures). Women were excluded if they had evidence of hyperparathyroidism, vitamin D deficiency (serum level <20 ng/mL), congenital or acquired bone disease, history of malignancy (with the exception of non-melanoma skin cancer), history of radiation therapy, significant cardiopulmonary, liver, or renal disease, major psychiatric disease, excessive alcohol intake, known sensitivity to denosumab or teriparatide, or were planning extensive dental work in the 2 months before or following enrolment. Women who had taken oral bisphosphonate or denosumab within 6 months before enrolment, oestrogens, selective oestrogen receptor modulators, or calcitonin within 3 months before enrolment, oral or parenteral glucocorticoids for more than 14 days within 6 months before enrolment, or who had ever received strontium or any parenteral bisphosphonate were also excluded. Full exclusion criteria are in the appendix (pp 5, 6).
The study protocol was approved by the Partners Healthcare Institutional Review Board. Written informed consent was received from all participants before inclusion in the study. The study protocol is in the appendix (p 2).
Randomisation and masking
Before randomisation, women were stratified on the basis of age (≥65 years vs or <65 years) and previous bisphosphonate use (yes or no). Participants were randomly assigned (1:1) to receive teriparatide 20 μg (standard dose) or 40 μg (high dose). Randomisation was done by computer-generated cards provided by our research pharmacy. Within each stratum, the randomisation block size varied randomly to minimise the predictability of treatment assignments. Participants were aware of treatment assignment, but outcome assessors were masked.
Procedures
Eligible participants received teriparatide 20 μg (standard dose) or 40 μg (high dose) daily via subcutaneous injection for 9 months (appendix p 1). At 3 months, both groups were started on denosumab 60 mg every 6 months via subcutaneous injection for 12 months. Thus, between 9 and 15 months participants received denosumab monotherapy. Participants received calcium carbonate and vitamin D supplements if needed to achieve a total daily intake of 1200 mg elemental calcium and to maintain serum 25-hydroxyvitamin D concentrations of at least 20 ng/mL. Adherence to teriparatide was assessed via participant diaries. At 15 months, all participants were given the opportunity to participate in an extension study that consisted of receiving one dose of intravenous zoledronic acid 5 mg, which is ongoing.
Areal BMD (aBMD) of the posterior-anterior lumbar spine, total hip, femoral neck, and the distal one-third of the radial shaft was assessed at 0, 3, 9, and 15 months by dual x-ray absorptiometry (DXA) using a Hologic QDR 4500A densitometer (Hologic, Waltham, MA, USA). All scans of an individual patient were done using the same densitometer. Daily quality control assessments were done with a Hologic anthropomorphic spine phantom. The SD values for in vivo reproducibility of measurements are 0·005 g/cm2 for the lumbar spine, 0·006 g/cm2 for the total hip, and 0·007 g/cm2 for the femoral neck. Vertebrae in which obvious deformities or focal sclerosis were detected were excluded. Forearm DXA of the non-dominant arm was done if there was no history of wrist fracture.
Volumetric BMD (vBMD) of the lumbar spine (L1-L2) and proximal femur was assessed using a 16-multidetector-row quantitative CT scanner (Revolution GSI; GE Healthcare, Waukesha, WI, USA). vBMD was assessed at 0 and 15 months only. Participants were placed supine in the quantitative CT scanner on a calibration phantom (Mindways Software, Austin, TX, USA), and helical scanning of the lumbar spine and from the proximal articular surface of the femoral head to 1 cm below the lessor trochanter, was done using the following parameters: 120 kV, 100 mA (lumbar spine), 120 kV, 200 mA (proximal femur), slice thickness 2·5 mm, field of view 500 mm, and table height of 144 mm (2 year coefficient of variation ≤2%). We used basic engineering principles to apply a finite element analysis to both the spine and hip using VirtuOst software (O.N. Diagnostics, Berkeley, CA, USA), as described elsewhere.20 Briefly, the image voxels were converted into cube-shaped finite elements and then a compressive force on the spine or a sideways fall at the hip was simulated on this finite element model. For the lumbar spine, a thin layer of plastic was virtually applied over each endplate through which the vertebral body was loaded to simulate failure for a uniform compressive overload. For the hip, a sideways fall was simulated with the diaphysis angled at 15° to the ground and 15° of internal rotation. For both sites, bone strength was calculated from the resulting non-linear force-deformation curves as the force at 1·9% and 4·0% overall deformation of the spine and hip, respectively. This technique enabled the estimation of differences in strength at 15 months compared with baseline for the whole bone at the spine and total hip. The software used for the finite element analysis of bone strength is approved by the US Food and Drug Administration for clinical diagnostic and monitoring purposes and has been validated in multiple clinical fracture outcome studies.21–24
Fasting morning blood samples were obtained at 0, 3, 9, and 15 months. All samples, including calcium serum concentrations, were assessed 24 h after the last teriparatide dose when applicable. Bone formation markers serum type I N-terminal propeptide of type 1 procollagen (P1NP) and osteocalcin and the bone resorption marker C-terminal telopeptide of type 1 collagen (CTX) were measured using the Roche Cobas e411 auto-analyser (Roche Diagnostics, Penzberg, Germany). The interassay coefficients of variation for P1NP, osteocalcin, and CTX were 4·5, 5·3, and 4·4%, respectively. For each marker, all blood samples from a participant were frozen at −80°C and analysed together in the same assay run after one freeze-thaw cycle. Biochemical markers of bone turnover were only measured in participants who completed all study visits.
Adverse events including bone fractures were documented at each study visit. All serious adverse events were reviewed by an independent data and safety monitoring board. Adverse events were considered serious if participants were admitted to hospital or there was a clinically significant change in medical condition.
Outcomes
The primary endpoint was percentage change from baseline in lumbar spine aBMD at 15 months, assessed by DXA. Secondary endpoints were the change in total hip, femoral neck, and distal radius aBMD, assessed by DXA; vBMD and estimated strength assessed by spine and hip quantitative CT; change in distal tibia and distal radius assessed by high resolution peripheral quantitative CT (HR-pQCT); and biochemical markers of bone turnover. Change in distal tibia and distal radius by HR-pQCT secondary outcomes will be reported elsewhere.
Statistical analysis
To detect a between-group difference in lumbar spine aBMD of 2·5% (ie, the lower limit of what is considered clinically important and greater than the least significant change based on spine DXA), with 80% power at a two-sided significance level of 5%, a sample size of 35 participants per group was required, assuming a dropout rate of 14%. Between-group differences were assessed using repeated measures ANOVA. The subject level random intercept, time, fixed treatment effect, and the interaction between group and time were included in the model. The inference of the regression coefficient associated with the interaction term (difference in slopes) determined differences between treatment groups. The model allowed for the inclusion of all available partial observations for the non-completers. The primary endpoint was assessed in the modified intention-to-treat analysis set, which included all participants who completed at least one study visit after baseline. Secondary endpoints were also assessed in the modified intention-to-treat analysis set, with exception of the biochemical markers, which was only assessed in patients who completed the study. Safety was assessed in all randomly assigned participants. Quantitative CT spine and hip results were omitted for two participants in the 20 μg group: one patient had a missing quantitative CT phantom at the time of the scan and one participant did not wish to have quantitative scans done. We did not impute missing data. Between-group differences in quantitative CT measures (two measures for each participant) were assessed by Wilcoxon-Mann-Whitney test after assessment for normality. A p value of 0·05 or less was considered to indicate statistical significance. Data are presented as mean (SD) for endpoints with a normal distribution and as median (IQR) for endpoints without a normal distribution. For the mean percentage change in aBMD by DXA, SEs have been presented in figures rather than SDs, as per the reporting conventions for this study area. Statistical analysis was done with SAS (version 9.2). This study is registered with ClinicalTrials.gov, number NCT02176382.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Oct 15, 2014, and June 10, 2016, 269 women were assessed for eligibility. 76 participants were randomly assigned to 20 μg teriparatide (n=39) or 40 μg teriparatide (n=37), of whom 69 completed at least one post-baseline visit and were included in this analysis (figure 1). Baseline characteristics are shown in table 1. 34 (97%) of 35 women in the 20 μg group and 34 (100%) of 34 women in the 40 μg group reported taking at least 85% of their daily teriparatide doses. Two women in the 40 μg group reported reducing their teriparatide dose to 20 μg daily after 5 months due to nausea. All women in both groups received all expected denosumab doses. The final 15 month follow-up visit was completed on Sept 27, 2017
Figure 1: Trial profile.
ITT=intention-to-treat.
Table 1:
Baseline characteristics of the modified intention-to-treat population
| Denosumab plus teriparatide 20 μg (n=35) |
Denosumab plus teriparatide 40 μg (n=34) |
|
|---|---|---|
| Age, years | 65·9 (7·0) | 67·0 (7·3) |
| Body-mass index, kg/m2 | 23·0 (2·9) | 22·8 (3·9) |
| White, non-Hispanic | 33 (94%) | 33 (97%) |
| History of adult fragility fracture* | 16 (46%) | 23 (68%) |
| Previous bisphosphonate use | 21 (60%) | 19 (56%) |
| Duration of use, months | 45·3 (30·8) | 64·5 (51·9) |
| Time since discontinuation,months | 72·3 (60·9) | 74·7 (57·7) |
| Serum osteocalcin, μg/L | 22·9 (5·7) | 25·2 (7·6) |
| Serum P1NP, μg/L | 49·1 (15·9) | 51·5 (16·0) |
| Serum CTX, ng/L | 426 (166) | 465 (165) |
| Areal bone mineral density, g/cm2 | ||
| Lumbar spine | 0·830 (0·104) | 0·794 (0·109) |
| Femoral neck | 0·649 (0·092) | 0·623 (0·073) |
| Total hip | 0·743 (0·102) | 0·737 (0·074) |
| Distal one-third of the radial shaft | 0·604 (0·071) | 0·594 (0·070) |
| Volumetric bone density, mg/cm2 | ||
| Lumbar spine | 96·4 (27·7) | 93·6 (21·6) |
| Femoral neck | 272·3 (39·3) | 264·3 (47·9) |
| Total hip | 240·0 (39·8) | 239·2 (30·4) |
| Bone strength, N† | ||
| Lumbar spine | 4862 (965) | 4796 (829) |
| Hip | 3382 (546) | 3335 (436) |
Data are mean (SD) or n (%). P1NP=N-propeptide of type 1 procollagen. CTX=C-terminal telopeptide of type 1 collagen. N=Newtons.
Adult fragility fracture defined as low trauma fracture after age 40 years.
Determined by finite element analysis.
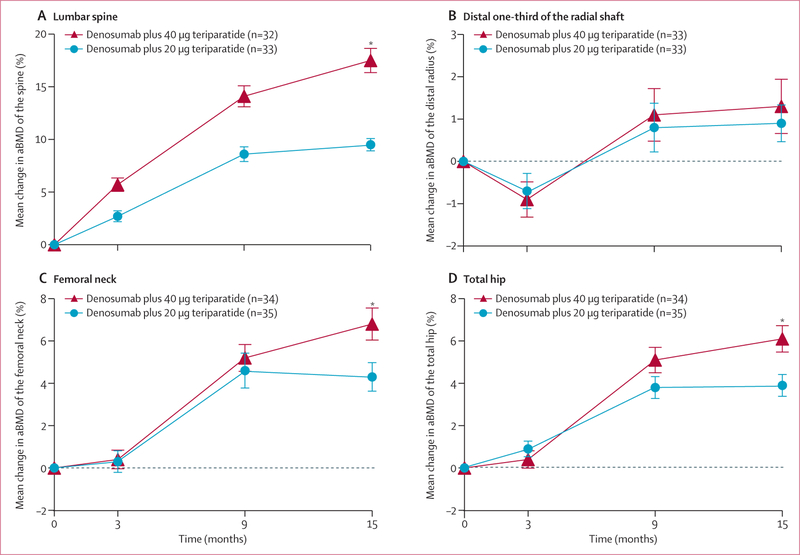
At 15 months, aBMD had increased from baseline in both treatment groups at all measured sites (figure 2). The mean percentage increase in lumbar spine aBMD was significantly higher in the 40 μg group (17·5% [SD 6·0]) than the 20 μg group (9·5% [3·2]; difference 8·1%, 95% CI 5·5 to 10·6, p<0·0001).
Figure2: Mean percentage change in aBMD of the lumbar spine (A), distal radius (B), femoral neck (C), and total hip (D).
Error bars show standard error. For the lumbar spine and distal one-third of the radial shaft, patient numbers differ from the number included in the intention-to-treat population because dual x-ray absorptiometry was not possible at these sites in some patients. aBMD=areal bone mineral density. *p<0·05 for between-group comparison.
The mean percentage increase in femoral neck aBMD was also significantly higher in the 40 μg group (6·8% [SD 4·1]) than the 20 μg group (4·3% [3·7]; difference 2·5%, 95% CI 0·5 to 4·5, p=0·04). Total hip aBMD also increased by 6·1% (3·4) in the 40 μg group compared with 3·9% (2·9) in the 20 μg group (difference 2·2%, 95% CI 0·6 to 3·8, p<0·0001). At 9 months, total hip and femoral neck aBMD had increased by more than 5% and spine aBMD increased by around 14% in the 40 μg group. At the distal one-third of the radial shaft, aBMD transiently decreased in both groups after 3 months of treatment with teriparatide alone, but had increased to a similar extent by 15 months (difference 0·5%, 95% CI −1·1 to 2·1, p=0·43).
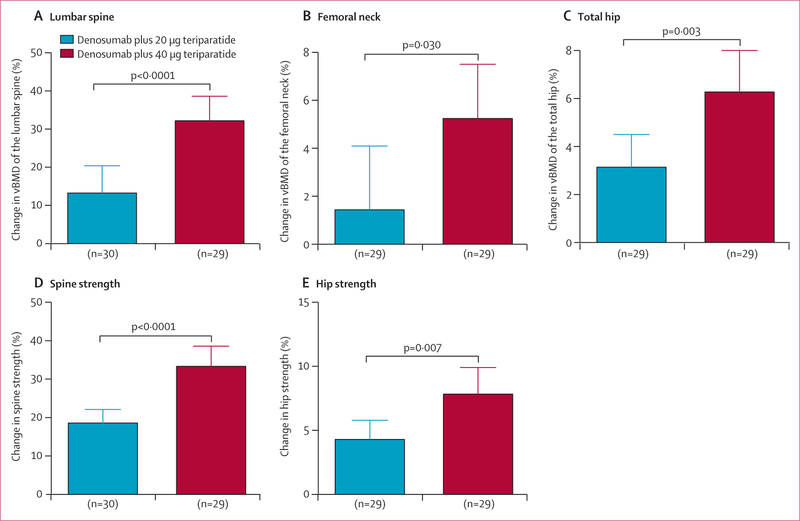
At 15 months, vBMD increased in both treatment groups with significantly larger increases observed in the 40 μg group than in the 20 μg group at all sites (figure 3). Mean spine vBMD increased by 32·2% (SD 17·0) in the 40 μg group and 13·3% (18·8) in the 20 μg group (difference 18·2%, 95% CI 10·4 to 26·0, p<0·0001), femoral neck vBMD increased by 5·2% (6·0) in the 40 μg group and 1·4% (6·7) in the 20 μg group (difference 3·8%, 0·4 to 7·2, p=0·030), and total hip vBMD increased by 6·3% (4·4) in the 40 μg group and 3·1% (3·5) in the 20 μg group (difference 3·1%, 1·0 to 5·3, p=0·003).
Figure3: Mean percentage change in volumetric bone mineral density of the lumbar spine (A), femoral neck (B), and total hip (C) and estimated strength of the spine (D) and hip (E).
Error bars show 95% CIs. Patient numbers differ from the number included in the modified intention-to-treat population because quantitative CT was only done at months 0 and 15, and quantitative CT was not possible in some patients.
At 15 months, the mean increase in spine strength was significantly higher in the 40 μg group (33·3% [SD 14·0]) than the 20 μg group (18·5% [9·5]; difference 14·8%, 95% CI 3·1 to 12·0, p<0·0001), and mean total hip strength had also increased to a greater extent in the 40 μg group (7·9% [5·3]) than the 20 μg group (4·3% [4·0]; difference 3·6%, 1·3 to 4·7, p=0·007)
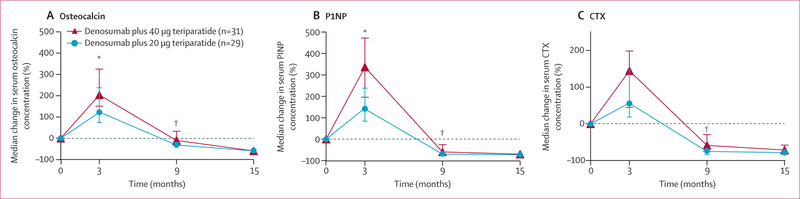
At 3 months, median bone formation marker concentrations had increased to a greater extent in the 40 μg than the 20 μg group (204% vs 123% for osteocalcin; 337% vs 142% for P1NP; p<0·0001 for both comparisons; figure 4). At 9 months, concentrations of these markers were suppressed to below the original pre-treatment baseline concentrations in both groups and the extent of suppression was greater in the 20 μg group than the 40 μg group. At 15 months, all markers were similarly suppressed in both groups.
Figure 4: Median percentage change in serum concentrations of biochemical markers.
Change in biochemical markers of bone formation, osteocalcin (A) and P1NP (B), and biochemical marker of bone resorption CTX (C). Error bars show IQR. P1NP=N-terminal propeptide of type 1 procollagen. CTX=C-Terminal Telopeptide of Type I Collagen. *p<0·001 for between-group comparison. †p<0·05 for between-group comparison.
30 (77%) of 39 participants in the 20 μg group and 29 (78%) of 37 participants in the 40 μg group had adverse events, and nine (23%) and two (5%) patients had serious adverse events (table 2). No participants developed hypercalcemia (measured 24 h after teriparatide administration). Seven patients in the 20 μg group had serious adverse events, which included fractures in three patients (two at the radius, one at the fibula), new diagnoses of malignancy in three patients (transitional cell carcinoma of the kidney diagnosed after the 3 month visit in one patient, squamous cell carcinoma of the tongue diagnosed after the 3 month visit in one patient, and ductal carcinoma in situ of the breast diagnosed on screening mammogram shortly before the 15 month visit in one patient), and shoulder dislocation, pancreatitis, and non-cardiac chest in a single patient. Two patients in the 40 μg group had serious adverse events, which included 1 week of musculoskeletal back pain that required an emergency room visit in one patient and a scheduled hip replacement for a congenital malformation in one patient. One participant in the 20 μg group discontinued within 1 month of enrolment due to headache. Three participants in the 40 μg group discontinued due to transient eczema rash, hypertension, and asthma. Specifically, the participant who had hypertension in the 40 μg group was diagnosed with hypertension at a routine primary care visit within 1 month of enrolment. The other participant in the 40 μg group chose to discontinue at 8 months due to a cough that led to a new diagnosis of asthma. All serious adverse events were considered unrelated to treatment, with the exception of headache and rash, as assessed by study investigators and an independent safety monitoring board. Additionally, one participant in the 20 μg group was withdrawn due to non-adherence; she presented to her 3 month visit late (after 5 months) and reported approximately 50% adherence due to travel commitments.
Table 2:
Adverse events
| Denosumab plus teriparatide 20 μg (n=39) |
Denosumab plus teriparatide 40 μg (n=37) |
|
|---|---|---|
| Any adverse event during treatment | 30 (77%) | 29 (78%) |
| Deaths | 0 (0) | 0 (0) |
| Any serious adverse event during treatment | 7 (18%) | 2 (5%) |
| Discontinuations due to adverse events | 4 (10%) | 2 (5%) |
| Adverse events occurring in >10% of patients | ||
| Fatigue | 12 (31%) | 14 (38%) |
| Nausea | 8 (21%) | 16 (43%) |
| Joint pain | 15 (38%) | 17 (46%) |
| Rhinorrhoea | 8 (21%) | 11 (30%) |
| Dizziness | 9 (23%) | 8 (22%) |
| Eczema | 7 (18%) | 11 (30%) |
| Paraesthesia | 9 (23%) | 3 (8%) |
| Muscle cramp | 15 (38%) | 13 (35%) |
| Abnormal heart rate | 6 (15%) | 2 (5%) |
| Data are n (%). | ||
Discussion
In this 15 month randomised controlled trial, we showed that combined treatment with teriparatide 40 μg and denosumab increases BMD at the hip and spine to a greater extent than a similar regimen with 20 μg teriparatide and denosumab. Although comparisons in BMD increases across different studies should be made with caution, notably, the increases in spine aBMD observed after 12 months of treatment with currently approved anabolic or antiresorptive therapies range from 4 to 14%,1–10 which is significantly lower than the increase in lumbar spine BMD observed with the 40 μg regimen in the current trial (17·5%). Similarly, at 12 months, increases in femoral neck aBMD with most currently approved anabolic or antiresorptive therapies range from 2 to 3% compared with an increase of 6·1% in total hip aBMD with the 40 μg regimen, and these increases are comparable to increases in total hip aBMD observed with the mixed anabolic and antiresorptive sclerostin-inhibitor, romosozumab.1–10 Furthermore, mean spine vBMD increased by more than 30% in the 40 μg group, which is several times higher than that reported with other approved drugs (including romosozumab) after 12 months of treatment (range 4—13%). Total hip vBMD increased by 6·3%, which is several times higher than that reported for single drugs or combinations (range 1·3%).11,25–28 The increases in vBMD observed in the 40 μg group translated to spine and hip strength increases of more than 30% and 7%, respectively, which are also greater than that reported with other drugs, including oral bisphosphonates, denosumab, teriparatide, and romosozumab, which after treatment for 12·18 months range from approximately 4·27% and 2·6% at the spine and hip, respectively.20,26–32
The precise cellular and molecular mechanisms underlying the efficacy of combined denosumab and teriparatide cannot be completely defined in the absence of histomorphometric and molecular analysis of bone biopsy tissue. However, the changes in biochemical markers of osteoclast and osteoblast activity continue to suggest that the efficacy is derived from optimising the balance between bone formation and bone resorption, particularly as it relates to modelling-based bone formation. In the original DATA study,15,16 the extent of bone resorption was identical in the denosumab monotherapy and combination groups, whereas bone formation markers were less suppressed in the combination group than the monotherapy group. An increase in modelling-based bone formation has been observed in patients who took either teriparatide and denosumab, although this increase was limited to the cancellous bone in patients who received denosumab.33
The findings of the current study suggest that high dose teriparatide stimulates even greater separation between bone resorption and formation than that of standard dose teriparatide and that denosumab maintains its capacity to inhibit bone resorption even in the presence of high dose teriparatide. Furthermore, the between-group difference in spine and hip BMD increases continued to increase between 9 and 15 months although both groups were receiving identical treatment during this period (denosumab monotherapy). This continued increase in BMD might be explained by mineralisation of a large amount of new undermineralised bone formed during high dose teriparatide therapy or the filling of an increased amount of remodelling space and reduction in cortical porosity during the denosumab-induced low bone turnover state.
The 40 μg daily dose of teriparatide used in this study is twice that of the dose approved by the US Food and Drug Administration. In a phase 3 trial4 in which women with osteoporosis received placebo, teriparatide 20 μg, or teriparatide 40 μg daily, both doses of teriparatide decreased vertebral and non-vertebral fracture risk compared with placebo, but fracture risk did not differ between the teriparatide groups, despite greater BMD increases with 40 μg teriparatide than 20 μg teriparatide. Although the study was not specifically powered to detect difference in fracture risk reduction between teriparatide 20 μg and 40 μg, it has been hypothesised that the absence of greater fracture risk reduction, despite larger BMD increases in the 40 μg group, was due to the higher teriparatide dose stimulating greater cortical porosity and larger decreases in BMD in anatomical sites consisting of predominately cortical bone, which has been demonstrated in numerous human and animal studies.17,34–37 In the present study, bone resorption in both treatment groups was potently suppressed by the concomitantly administered denosumab and thus the results of the phase 3 study are likely not applicable. Evidence suggests that the correlation between changes in BMD, particularly at the hip, correlate strongly across all osteoporosis treatments, regardless of underlying mechanism. Specifically, in a large pooled analysis38 of 21 osteoporosis drug trials including 83 395 participants, 69% and 74% of the non-vertebral anti-fracture efficacy was explained by the change in total hip or femoral neck BMD, respectively. The US patent for teriparatide will expire in August 2019, and thus potential generic preparations of teriparatide might make this combination regimen more affordable for patients.
In the previously reported phase 3 trial,4 more participants withdrew due to adverse events in the 40 μg dose group (11%) than the 20 μg (6%) or placebo (6%) groups. Although our study size was small, no differences in withdrawal rate due to side-effects were observed between the 40 μg group and 20 μg group and no difference in serum calcium concentrations were identified between either group at any timepoint, although concentrations were assessed 24 h after teriparatide injection (data not shown). The absence of observed hypercalcaemia might have been due to the inhibition of bone resorption (and hence calcium release from the bone matrix) by denosumab. Additionally, all fractures and cancer diagnoses were reported in the 20 μg group. Considering the sample size, conclusions regarding differences in safety between these two dose regimens cannot be made.
This study has some limitations. The study was done at a single site in a predominantly white population, which might limit the generalisability of the results. It should also be noted that the analysis of biochemical markers of bone turnover were only done in participants who completed all study visits. However, the baseline characteristics of this subset of women were similar to the complete study population (data not shown). Additionally, decreases in bone marrow fat might lead to an overestimate of increases in vBMD by single-energy quantitative CT and parathyroid hormone has been reported to reduce bone marrow fat in animal studies.39,40 The relatively small size of the study does not allow for direct assessment of fracture benefit nor does it allow for rigorous evaluation of the tolerability and safety of this treatment.
In summary, the combination of denosumab and high dose teriparatide results in large increases in aBMD and vBMD of the hip and spine resulting in increases in estimated bone strength, which has not been achieved with any previously evaluated monotherapy or combination therapy approaches. Considering that skeletal integrity cannot be fully restored in most patients with established osteoporosis at present, this 40 μg regimen is likely to provide benefits for women at the highest risk of fragility fracture. Direct assessment of the potential of this combination to reduce the incidence of fractures compared with the current standard of osteoporosis care is warranted.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from database inception until Jan 31, 2019, using the search terms “anabolic”, “antiresorptive”, “osteoporosis”, and “combination therapy”, without language restrictions. We reviewed all randomised, controlled trials and pertinent animal studies published in peer-reviewed journals. Our group previously demonstrated that in postmenopausal women with osteoporosis, the combination of teriparatide and denosumab increases bone density more than either drug alone.
Added value of this study
This study shows that combined treatment with high dose teriparatide and denosumab increased bone mineral density at the spine and hip to a greater extent than did combined treatment with standard dose teriparatide and denosumab in postmenopausal women with osteoporosis. Furthermore, these rapid increases in bone density are larger than that observed with any currently available drug or other combination therapy approaches.
Implications of all the available evidence
This novel combination of high dose anabolic and antiresorptive treatment could have important therapeutic implications for the treatment of women at high risk of fragility fractures.
Acknowledgments
We thank the staff at the Bone Density Center for measuring bone density for the study and the study volunteers for their participation.
We thank Fatma Gossiel for assistance with the bone turnover marker analysis. The project described was supported by the Dart Family Foundation, the National Institutes of Health (grant 1UL1TR001102), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants K23AR068447 and K24AR067847). Study drugs were supplied by Eli Lilly and Amgen.
Funding National Institutes of Health and the Dart Foundation.
RE reports grants and personal fees from Amgen, Alexion, and Nittobo; grants from AstraZeneca, Medical Research Council (MRC)-Arthritis Research UK Centre for Excellence in Musculoskeletal Ageing Research, the National Institute for Health Research, MRC/AstraZeneca Mechanisms of Diseases, the MRC, and the Department of Health; grants, personal fees, and non-financial support from Immunodiagnostic Systems and Roche; personal fees and advisory board fees from Eli Lilly; GlaxoSmithKline Nutrition, D-STAR, Mereo, Sandoz, AbbVie, Haoma Medica, Samsung, and Elsevier; reimbursement for travel expenses from Radius Health; has received grants from, and served on the Clinical and Scientific Committee for the Royal Osteoporosis Society; has served on the advisory board for the European Calcified Tissue Society; has served on the Committee of Scientific Advisors of the International Osteoporosis Foundation; served as the treasurer for the Institute of Biomedical Science; and served on the programme planning committee for the American Society for Bone and Mineral Research. BZL has served on an advisory board for Amgen.
Footnotes
Declaration of interests
All other authors declare no competing interests.
Data sharing
Requests for access to de-identified participant data should be directed to jntsai@mgh.harvard.edu.
Contributor Information
Joy N Tsai, Department of Medicine, Endocrine Unit, Massachusetts General Hospital, Havard Medical School, Boston, MA, USA.
Hang Lee, Biostatistics Center, Massachusetts General Hospital, Havard Medical School, Boston, MA, USA.
Natalie L David, Department of Medicine, Endocrine Unit, Massachusetts General Hospital, Havard Medical School, Boston, MA, USA.
Richard Eastell, Academic Unit of Bone Metabolism, University of Sheffield, Sheffield, UK.
Benjamin Z Leder, Department of Medicine, Endocrine Unit, Massachusetts General Hospital, Havard Medical School, Boston, MA, USA.
References
- 1.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535–41. [DOI] [PubMed] [Google Scholar]
- 2.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999; 282: 134–52. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282: 637–45. [DOI] [PubMed] [Google Scholar]
- 4.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–41. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–65. [DOI] [PubMed] [Google Scholar]
- 6.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–22. [DOI] [PubMed] [Google Scholar]
- 7.Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 2016; 316: 722–33. [DOI] [PubMed] [Google Scholar]
- 8.Eisman JA, Civitelli R, Adami S, et al. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 2008; 35: 488–97 [PubMed] [Google Scholar]
- 9.Chesnut CH 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 2004; 19: 1241–49. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532–43. [DOI] [PubMed] [Google Scholar]
- 11.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 2003; 349: 1207–15. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2010; 95: 1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res 2011; 26: 503–11. [DOI] [PubMed] [Google Scholar]
- 14.Finkelstein JS, Leder BZ, Burnett SM, et al. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 2006; 91: 2882–87 [DOI] [PubMed] [Google Scholar]
- 15.Tsai JN, Uihlein AV, Lee H, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 2013; 382: 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leder BZ, Tsai JN, Uihlein AV, et al. Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab 2014; 99: 1694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai JN, Uihlein AV, Burnett-Bowie SM, et al. Effects of two years of teriparatide, denosumab, or both on bone microarchitecture and strength (DATA-HRpQCT study). J Clin Endocrinol Metab 2016; 101: 2023–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai JN, Uihlein AV, Burnett-Bowie SA, et al. Comparative effects of teriparatide, denosumab, and combination therapy on peripheral compartmental bone density, microarchitecture, and estimated strength: the DATA-HRpQCT Study. J Bone Miner Res 2015; 30: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black DM, Steinbuch M, Palermo L, et al. An assessment tool for predicting fracture risk in postmenopausal women. Osteoporos Int 2001; 12: 519–28. [DOI] [PubMed] [Google Scholar]
- 20.Keaveny TM, McClung MR, Genant HK, et al. Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res 2014; 29: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res 2012; 27: 808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyak JH, Sigurdsson S, Karlsdottir G, et al. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone 2011; 48: 1239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orwoll ES, Marshall LM, Nielson CM, et al. Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res 2009; 24: 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin S, Kopperdhal DL, Melton LJ 3rd, et al. Association of hip strength estimates by finite-element analysis with fractures in women and men. J Bone Miner Res 2011; 26: 1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eastell R, Lang T, Boonen S, et al. Effect of once-yearly zoledronic acid on the spine and hip as measured by quantitative computed tomography: results of the HORIZON Pivotal Fracture Trial. Osteoporos Int 2010; 21: 1277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McClung MR, Zanchetta JR, Hoiseth A, et al. Denosumab densitometric changes assessed by quantitative computed tomography at the spine and hip in postmenopausal women with osteoporosis. J Clin Densitom 2013; 16: 250–56. [DOI] [PubMed] [Google Scholar]
- 27.Genant HK, Engelke K, Bolognese MA, et al. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J Bone Miner Res 2017; 32: 181–87. [DOI] [PubMed] [Google Scholar]
- 28.Lewiecki EM, Keaveny TM, Kopperdahl DL, et al. Once-monthly oral ibandronate improves biomechanical determinants of bone strength in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2009; 94: 171–80. [DOI] [PubMed] [Google Scholar]
- 29.Keaveny TM, Hoffmann PF, Singh M, et al. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res 2008; 23: 1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keaveny TM, Donley DW, Hoffmann PF, et al. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 2007; 22: 149–57. [DOI] [PubMed] [Google Scholar]
- 31.Keaveny TM, Crittenden DB, Bolognese MA, et al. Greater gains in spine and hip strength for romosozumab compared with teriparatide in postmenopausal women with low bone mass. J Bone Miner Res 2017; 32: 1956–62. [DOI] [PubMed] [Google Scholar]
- 32.Kleerekoper M, Greenspan SL, Lewiecki EM, et al. Assessing the effects of teriparatide treatment on bone mineral density, bone microarchitecture, and bone Strength. J Bone Joint Surg Am 2014; 96: e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dempster DW, Zhou H, Recker RR, et al. Remodeling- and modeling-based bone formation with teriparatide versus denosumab: a longitudinal analysis from baseline to 3 months in the AVA study. J Bone Miner Res 2018; 33: 298–306. [DOI] [PubMed] [Google Scholar]
- 34.Fox J, Miller MA, Newman MK, Recker RR, Turner CH, Smith SY Effects of daily treatment with parathyroid hormone 1–84 for 16 months on density, architecture and biomechanical properties of cortical bone in adult ovariectomized rhesus monkeys. Bone 2007; 41: 321–30. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int 2011; 22: 357–62. [DOI] [PubMed] [Google Scholar]
- 36.Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res 2013; 28: 736–45. [DOI] [PubMed] [Google Scholar]
- 37.Zebaze R, Takao-Kawabata R, Peng Y, et al. Increased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatide. Bone 2017; 99: 80–84. [DOI] [PubMed] [Google Scholar]
- 38.Black D, Vittinghoff E, Eastell R, et al. Change in BMD as a surrogate for fracture risk reduction in osteoporosis trials: results from pooled, individual-level patient data from the FNIH Bone Quality Project. American Society for Bone and Mineral Research Annual Meeting; Montreal; Sept 29—Oct 1, 2018. 1070. [Google Scholar]
- 39.Sfeir JG, Drake MT, Atkinson EJ, et al. Evaluation of cross-sectional and longitudinal changes in volumetric bone mineral density in postmenopausal women using single-versus dual-energy quantitative computed tomography. Bone 2018; 112: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Hanai JI, Le PT, et al. Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab 2017; 25: 661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.