Abstract
Background
Rotavirus vaccine introduction in the United States has reduced rotavirus disease burden, but outbreaks still occur. Complete-series rotavirus vaccination coverage is < 75% in the United States; it may be lower among vulnerable populations. We describe clinical characteristics and vaccination status of children during a rotavirus outbreak in a pediatric subacute care facility in 2017.
Methods
Clinical history, signs and symptoms, and vaccination history were abstracted for the 26 patients residing in the facility during the time of the outbreak. A case was defined as a patient experiencing 3 or more loose stools in a period of 24 hours, with onset April 17 – May 17, 2017. Stool samples from 14 resident patients were tested for rotavirus by reverse transcription polymerase chain reaction (RT-PCR).
Results
The median patient age at the facility was 2.9 years. Twenty-two of the 26 resident patients (85%) met the case definition. One child died. Stool samples from 11 cases were RT-PCR-positive for rotavirus. Fifteen cases were unvaccinated against rotavirus; 3 were partially vaccinated and 2 fully vaccinated. Vaccination status could not be completely determined for two cases.
Conclusions
An outbreak of rotavirus affected nearly all resident patients of a subacute care facility and caused one death. Due to recommendations against giving rotavirus vaccines in the intensive care setting, infants requiring prolonged intensive care stays may age out of rotavirus vaccine eligibility (1st dose must be given before 15 weeks, as per ACIP recommendations). This creates a vulnerable population of unvaccinated infants who may later congregate in another care setting.
Keywords: rotavirus, pediatric gastroenteritis, immunization, vaccines
SUMMARY:
In 2017, a rotavirus outbreak affected 22 of 26 patients in a pediatric subacute care facility, causing one death. The majority of children were unvaccinated; many of these had spent prolonged periods in ICUs, where live vaccine use is discouraged.
Introduction
Rotavirus is a common cause of severe pediatric gastroenteritis, and in vulnerable children, rotavirus diarrhea can quickly lead to serious dehydration and even death1. In the United States, two live, oral rotavirus vaccines–RotaTeq® (RV5, a pentavalent vaccine; Merck) and Rotarix® (RV1, a monovalent vaccine; GlaxoSmithKline)–are currently licensed and recommended for routine vaccination of U.S. infants by the Advisory Committee on Immunization Practices (ACIP)2. RV5 is given as a three-dose series (at 2, 4, and 6 months), and RV1 is given as a two-dose series (at 2 and 4 months)2. ACIP recommends age restrictions on the timing of rotavirus vaccination: the first dose is to be given by the age of 14 weeks and 6 days, and the last dose is to be completed by the age of 8 months and 0 days, with a minimum interval of 4 weeks between doses. These age restrictions were recommended because of the ages at which vaccine was administered in clinical trials and because of concerns about an association with intussusception3, a rare form of bowel obstruction whose natural incidence peaks between 4 and 9 months of age4. ACIP further cautions against the administration of the vaccine to infants in the neonatal intensive care unit (NICU) or nursery, due to the potential risk of horizontal transmission of the live vaccine-strain virus. The intersection of these recommendations can lead to a vulnerable population of children: those who were discharged from the NICU or pediatric intensive care unit (PICU) past 15 weeks of age—who may be at higher risk for severe rotavirus gastroenteritis (as compared to term, normal-weight, or otherwise healthy babies5–8), but were too old to begin the rotavirus vaccine series at discharge.
In the United States, substantial decreases in rotavirus disease burden have been noted since the introduction of rotavirus vaccine in 20069. However, disease activity persists in a biennial pattern with winter-spring seasonality9, and outbreaks continue to occur, affecting both vaccinated and unvaccinated individuals10. In April 2017, the Santa Clara County Public Health Department (SCCPHD) was notified of an outbreak of acute gastroenteritis (AGE) in a pediatric subacute care facility (Facility A). The present report describes this outbreak in detail and aims to characterize rotavirus vaccination status among the children resident in the facility at the time of the outbreak.
Patients and Methods
Setting
Facility A is a subacute care facility for children < 21 years of age with complex medical needs, e.g., children who are ventilator dependent or tracheostomy dependent. The facility provides 24-hour skilled nursing services, in addition to physical therapy, occupational therapy, and speech therapy. At any given time, the facility houses up to 27 patients. Most children are admitted to the facility during their first year of life, and reside there between a few months to a few years. During the time of the outbreak, Facility A employed 115 staff, including nurses, respiratory therapists, and other specialists.
Case definition
A suspected case was defined as a child experiencing 3 or more episodes of loose stools within a 24-hour period, with onset from April 17 through May 17, 2017. A confirmed case was defined as a suspected case with a stool sample that was positive by RT-PCR for rotavirus. Suspected and confirmed cases will be referred to as “case patients.” Three staff were also reported ill, but will not be discussed in the present manuscript, given that they would not have been eligible for rotavirus vaccination; further, limited clinical information was available about these adults.
Investigations and Interventions
This outbreak was initially reported to the SCCPHD by phone on April 27, 2017; at the time, the facility identified 5 children with vomiting and / or diarrhea of recent onset. SCCPHD conducted a site visit on May 3. Recommendations implemented included increased cleaning and disinfection with bleach solution, implementation of cohorting and isolation procedures, cancellation of group activities, and suspension of new admissions. Stool samples were collected and forwarded for testing at a local hospital. Because of the noted rotavirus positivity among the samples, rotavirus vaccination status was also ascertained at this time.
In February 2018, permission was obtained from the facility to perform additional chart reviews for the patients resident at the time of the outbreak. During a site visit March 5 – 9, 2018, a single data collector (RMB) abstracted data from medical charts using a standardized instrument. Fields included the patient’s age at admission to the facility, primary diagnoses, significant clinical history (including birth history, NICU and PICU stays and dates), vaccination history (rotavirus and other vaccines), and clinical signs and symptoms for the period 17 April through 17 May, 2017.
Laboratory Testing
At the time of the outbreak, stool samples were collected from 14 resident patients, not limited to suspected cases. These samples were initially sent to a local hospital, where they were tested using a gastroenteritis multipathogen polymerase chain reaction (PCR) panel (BioFire® GI Panel). Of these samples, 7 were then forwarded to the California Department of Public Health Viral and Rickettsial Disease Laboratory (VRDL) for additional RT-PCR testing and subsequently forwarded to the CDC Rotavirus Surveillance and Molecular Epidemiology Team Laboratory at CDC for confirmatory testing of rotavirus by enzyme immunoassay (EIA; Premiere® Rotaclone®, Meridian Bioscience, Inc.), and genotyping of rotavirus strains by qRT-PCR and Next Generation Sequencing (NGS) methods11.
Ethics
This outbreak investigation was considered public health practice, and so was exempt from IRB review.
Results
At the time of the outbreak, 26 patients were resident at the facility, with a median age of 2.9 years (Interquartile range [IQR]: 1.8 – 7.1 years) (Table 1). The primary admitting diagnoses varied, but all patients were tracheostomy dependent, 13 (50%) had chronic respiratory failure, and 25 (96%) had a gastrostomy tube. One half had a history of preterm birth (defined as birth before 37 completed weeks of gestation). No patients were noted to have an immunocompromising condition at the time of the outbreak.
Table 1:
Characteristics of the patients resident at the time of the outbreak
| Characteristic | Frequency (%) or Median (IQR) | |
|---|---|---|
| Residents (N = 26) | Case Patients (N = 22) | |
| Age in years | 2.9 (1.8, 7.1) | 2.4 (1.5, 5.4) |
| Male | 17 (65%) | 15 (68%) |
| Race | ||
| Hispanic / Latino | 12 (46%) | 12 (55%) |
| Asian / Pacific Islander | 11 (42%) | 7 (32%) |
| Black / African American | 2 (8%) | 2 (9%) |
| White non-Hispanic | 1 (4%) | 1 (5%) |
| Insurance Status | ||
| Public only | 22 (85%) | 18 (82%) |
| Public and Private | 3 (12%) | 3 (14%) |
| Unknown | 1 (4%) | 1 (5%) |
| Age in months at first admission to Facility A | 9.5 (5.9, 15.3) | 9.0 (5.9, 15.3) |
| History of NICU admission | 21 (81%) | 18 (82%) |
| History of PICU admission | 19 (73%) | 15 (68%) |
| Primary / Admitting Diagnosis | ||
| Bronchopulmonary Dysplasia | 4 (15%) | 4 (18%) |
| Chronic Respiratory Failure | 4 (15%) | 4 (18%) |
| Congenital Malformations | 3 (11%) | 3 (14%) |
| Congenital Myopathies | 2 (8%) | 0 (0%) |
| Other | 13 (50%) | 11 (50%) |
| Chronic Respiratory Failure | 13 (50%) | 9 (41%) |
| Tracheostomy Dependent | 26 (100%) | 22 (100%) |
| Gastrostomy Tube | 25 (96%) | 21 (95%) |
| Preterm birth (<37 weeks gestational age) | 13 (50%) | 10 (45%) |
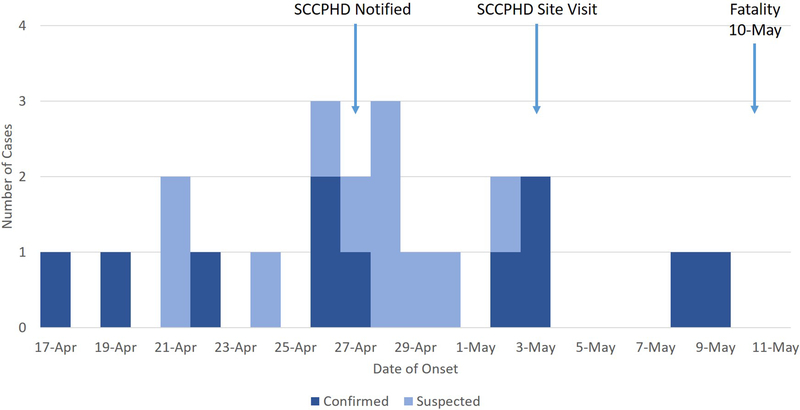
Out of 26 resident patients, 22 (85%) met the suspected case definition during the period of the outbreak; 11 were confirmed by RT-PCR (Table 2). The epidemic curve is presented in Figure 1. Of these children, 17 (77%) also had vomiting, and 19 (86%) had a fever (Table 2). The median number of days with diarrhea was 4, and the median number of days with vomiting was 2. Fifteen (68%) of the ill children received oral rehydration (typically as commercial electrolyte solution added to regular feeds). All children were managed at the facility during their illness. One toddler, aged 22 months and with pre-existing chronic respiratory failure, died from complications attributed to rotavirus-induced dehydration; though she was receiving rehydration therapy at Facility A, she declined rapidly and expired before a transfer to acute care at an outside hospital could be completed. All other children recovered.
Table 2:
Clinical information for case patients* (N = 22)
| Frequency (%) | Median (IQR) | |
|---|---|---|
| Diarrhea | 22 (100%) | |
| Days | 4.0 (2.3, 5.0) | |
| Max episodes / 24hr. | 5.5 (3.3, 8.8) | |
| Vomiting | 17 (77%) | |
| Days | 2.0 (2.0, 4.0) | |
| Max episodes / 24hr. | 5.0 (2.0, 6.0) | |
| Fever | 19 (86%) | |
| Max temperature | 101.0 (100.2, 102.1) | |
| Rehydration Therapy | 15 (68%) | |
| Outcome | ||
| Recovered | 21 (95%) | |
| Deceased | 1 (5%) |
Defined as residents experiencing ≥3 loose stools in a period of 24 hours, with onset April 17 – May 17, 2017.
Figure 1: Epidemic Curve.
Epidemic curve of suspected and confirmed rotavirus cases in an outbreak at a pediatric subacute care facility. Suspected cases were those children experiencing ≥3 loose stools within a 24-hour period, with onset from April 17 through May 17, 2017. A confirmed case was defined as a suspected case with a stool sample that was positive by RT-PCR for rotavirus.
Stool samples were collected from 14 resident patients based on suspected gastroenteritis. Upon chart review, 11 of these patients met the suspected case definition. All 11 of these samples tested positive for rotavirus by PCR at a local hospital. The remaining 3 samples tested negative, and were from children who did not meet our suspected case definition. No other pathogens were detected by multiplex PCR in any of these 14 samples. Seven samples were forwarded to California’s VRDL, where they all also tested positive for rotavirus by RT-PCR; adenovirus was also detected in 4 of these samples, but at higher Ct values. These 7 rotavirus-positive samples were subsequently forwarded to CDC, where all 7 were RT-PCR-positive, 6 were also EIA-positive, and 6 were genotyped as G12P[8]; the seventh was identified as G12, but its P type was not identified. Rotavirus vaccine strain was not detected in any samples.
Of the 26 children residing at the facility at the time of the outbreak, only 2 (8%) were documented to have completed the full series of rotavirus vaccination (Table 3). Another 2 children (8%) had each received 2 doses of rotavirus vaccine, but because the vaccine type was not documented, vaccination status could not be categorized definitively. Five children (19%) had received partial courses of rotavirus vaccination, and 17 (65%) were completely unvaccinated. Among these 22 undervaccinated children (5 partially and 17 unvaccinated against rotavirus), 12 (55%) were receiving care in the NICU or PICU during the age at which rotavirus vaccine would have been received (6 – 15 weeks for dose 1; 10 weeks – 8 months for dose 2), and aged out of eligibility by the time of discharge. Five (23%) undervaccinated children were still age-eligible to receive rotavirus vaccine upon discharge from the NICU or PICU, but did not. Two children began their routine immunizations on a delayed schedule, and aged out of rotavirus vaccine eligibility before beginning or completing the series. Finally, two children were born before rotavirus vaccine was available, and one child was born in a country where rotavirus vaccine was not widely available at the time. No contraindications for rotavirus vaccination were noted for any patient during medical chart review. All documented doses were received far in advance of the outbreak (> 1 year).
Table 3:
Rotavirus vaccination status of patients resident at time of outbreak (N = 26)
| Frequency (%) | ||
|---|---|---|
| Residents (N = 26) |
Case Patients (N = 22) |
|
| Complete (3 doses of RV5 or 2 doses of RV1) | 2 (8%) | 2 (9%) |
| Partial (1 – 2 doses of RV5 or 1 dose of RV1) | 5 (19%) | 3 (14%) |
| Child in NICU/PICU when doses would have been received | 3 | 1 |
| Missed opportunity (child could have received dose just after PICU discharge, but did not) | 1 | 1 |
| Delayed schedule; hospitalized during time of 2nd dose | 1 | 1 |
| Unvaccinated (0 doses of RV1 or RV5) | 17 (65%) | 15 (68%) |
| Child in NICU/PICU and aged out of eligibility | 9 | 9 |
| Missed opportunity (child could have received 1st dose just after NICU/PICU discharge, but did not) | 4 | 4 |
| Child not age-eligible (born before 2006) | 2 | 0 |
| Healthy child with delayed vaccinations (past 15 weeks of age) | 1 | 1 |
| Child born in a country where rotavirus vaccine not widely available | 1 | 1 |
| Unknown (at least partially vaccinated) | 2 (8%) | 2 (9%) |
| Child received 2 doses of rotavirus vaccine but vaccine product (RV1 vs. RV5) is unknown | 2 | 2 |
Among the 22 case patients, 2 (9%) were completely vaccinated, 3 (14%) were partially vaccinated, 15 (68%) were unvaccinated, and 2 (9%) had indeterminate vaccination status (Table 3). The one child who died was unvaccinated for rotavirus; she was admitted to the NICU soon after birth for complications related to prematurity, and was not discharged from intensive care until after 15 weeks of age.
Discussion
In this report, we describe a rotavirus outbreak among a vulnerable and largely unvaccinated population. Of 26 patients resident at this pediatric subacute care facility, 22 fell ill. The genotype associated with this outbreak, G12P[8], is the predominant genotype currently circulating in the US24, and estimated vaccine effectiveness against this genotype has been demonstrated to be high in US populations25. However, among case patients, only 2 had confirmed receipt of a full course of rotavirus vaccination. Fifteen case patients, including one toddler who died from rotavirus-induced dehydration, were unvaccinated against rotavirus. Out of 20 undervaccinated case patients, 15 (75%) were in the NICU or PICU during the time that they would have received rotavirus vaccine. Although 5 were still age-eligible to receive rotavirus vaccine upon discharge, as recommended, this opportunity was missed, and these children remained unvaccinated or undervaccinated.
Concerns around administration of rotavirus vaccination in the NICU/PICU are driven by the theoretical risk of the live-attenuated vaccine virus being transmitted to other infants in the same unit who are acutely ill and to preterm infants who are not age-eligible for vaccine2. While most NICU/PICUs in the United States refrain from administering rotavirus vaccination to admitted patients because of these concerns, some other countries provide the vaccine using standard infection control precautions. For instance, in Canada, rotavirus vaccination of age-eligible hospitalized preterm infants is permitted, with appropriate consultation with infection control and neonatologists12. Similarly, in Australia and England, rotavirus vaccination is encouraged for medically stable, age-eligible, hospitalized infants, provided that standard infection control procedures are maintained13, 14; vaccination is encouraged especially if delay could result in the child aging out of eligibility for rotavirus vaccination. Despite this guidance, rotavirus vaccination is not always administered to eligible infants: a 2013 survey of NICUs in the UK found that 20% did not administer rotavirus vaccine at all, while 29% administered the vaccine but with additional restrictions15.
Several studies have attempted to provide more data on the safety of rotavirus vaccine administration in the NICU. In the U.S., Monk et al. conducted a retrospective chart review in a NICU where RV5 administration was permitted for eligible infants receiving some enteral nutrition at the time of routine 2-month vaccinations, with standard precautions17. Records were reviewed for 96 NICU infants who received at least one dose of RV5 and their 801 NICU neighbors (those in the same “pod”) who remained unvaccinated against rotavirus. RV5 was well tolerated in vaccinated infants, and < 1% of neighboring unvaccinated infants experienced gastrointestinal symptoms; 2 unvaccinated infant stools were tested, and both were PCR-negative for rotavirus. Hofstetter et al. conducted prospective rotavirus surveillance among a cohort of PICU and NICU patients at Seattle Children’s Hospital, where hospital policy during the study period dictated that RV5 be administered with routine vaccinations to clinically stable, age-eligible infants regardless of hospital setting (ICU vs. non-ICU), and in accordance with standard precautions18. Over the study period, 385 eligible infants were enrolled and followed for the extent of their hospitalization or up to 245 days; stool samples were collected weekly and tested for rotavirus by RT-PCR, but clinical information was not recorded. Though shedding was detected in some vaccinated infants, no vaccine-type virus was detected in samples from unvaccinated infants, even among those in close geotemporal proximity to vaccinated infants. However, all patients were in double or single rooms, so opportunities for cross-exposure may have already been limited. Thrall et al. describe their experience administering RV5 in NICUs in two hospitals in Canada, according to national guidance19. RV5 was administered to age-eligible infants tolerating some enteral feeding, at the same time as routine immunizations and in conjunction with routine infection control procedures. Over the 20-month study period, 102 infants received at least 1 dose of RV5, and the vaccine appeared well tolerated, although the lack of a true control group is a limitation. No cases of nosocomial rotavirus gastroenteritis were identified through hospital surveillance in the post-vaccination period. Hiramatsu et al. employed a similar design as Monk et al., selecting vaccinated and neighboring unvaccinated infants from NICUs at two Japanese hospitals, but followed the two groups prospectively, adhered to contact precautions, and tested stool samples from all infants20. Over the 14-month study period, 19 vaccinated infants (9 with RV5, 10 with RV1) and 49 unvaccinated infants were enrolled; diarrhea was reported in 3 vaccinated and 2 unvaccinated infants, but no other gastrointestinal symptoms were reported. Though vaccine strain shedding was detected in >75% of vaccinated infants, no rotavirus viral genomes were detected in any stool samples collected from unvaccinated infants. Overall, these reports suggest that it may be possible to administer rotavirus vaccine in the NICU safely and without horizontal transmission17, 19, 20.
Although ACIP recommends that age-eligible children receive rotavirus vaccine upon discharge from the NICU, vaccination opportunities may be missed, as observed in our investigation. In addition, a study from a Texas hospital found that 33% of rotavirus vaccine age-eligible patients were not vaccinated upon discharge from the NICU21. These missed opportunities may occur at either the discharging facility or the admitting facility. For instance, immunization records were not immediately transferred to Facility A for two patients in our population, and by the time they received their next set of immunizations, they had already aged out of eligibility for rotavirus.
This report is subject to several limitations. First, although medical records were reviewed for all children resident at the facility, it was sometimes difficult to define cases, as these children were already extremely ill, and some had abnormal existing stooling patterns, or histories of intermittent fever or emesis. For this report, we applied a standardized case definition based on symptoms, but given the population, this case definition may not have been fully sensitive or specific. Further, not all children were tested for rotavirus. Second, the level of detail found in Facility A’s medical records regarding previous hospitalizations and clinical history varied for each child. It is possible that additional information would have uncovered additional reasons for which some children remained unvaccinated. Further, although careful clinical management is critical, especially amongst vulnerable populations such as this one, the authors were unable to retrospectively make determinations regarding the appropriateness of clinical management. Third, because rotavirus vaccine type was not recorded for 2 infants, their vaccination status could not be fully determined. However, each infant had 2 recorded doses, so both were at least partially vaccinated against rotavirus. Fourth, although it is possible that inadequate infection control practices contributed to this outbreak, this was not possible to assess given the retrospective nature of this report; while SCCPHD conducted a site visit and investigation, the outbreak was already peaking by the time SCCPHD was notified. It should also be noted that this report is descriptive in nature—the small size of the outbreak prohibited any analysis of vaccine effectiveness, and no conclusions regarding vaccine efficacy should be drawn from this report.
Conclusion
This outbreak of rotavirus in a pediatric subacute facility demonstrates how the convergence of two policies—not vaccinating against rotavirus in the NICU/PICU, and not beginning rotavirus vaccine series after 15 weeks of age2—can result in a select population of vulnerable children who are unvaccinated against rotavirus and who may later congregate in a single setting. Premature and low birth weight infants are at greater risk of severe outcomes of rotavirus gastroenteritis5, 6, 8, 22, 23, as underscored by the fatality reported in our study cohort. Despite the demonstrated reduction in rotavirus burden in the post-vaccine era9, rotavirus outbreaks can and do continue to occur in the US10, leaving unvaccinated and vulnerable children at higher risk of severe outcomes. Given these considerations, and emerging data on the safety of rotavirus vaccination in the NICU setting, the current guidelines for vaccination of these vulnerable infants might be re-examined to ensure optimal protection by rotavirus vaccination in US children.
ACKNOWLEDGMENTS
We thank Chao-Yang Pan, MPH and Thalia Huynh from the VRDL, and Brandon Bonin from the Santa Clara County Public Health Laboratory for their technical and analytical assistance. We also gratefully acknowledge the clinical and administrative staff of Facility A for their cooperation and assistance throughout the investigation.
Footnotes
DISCLOSURE
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
REFERENCES
- 1.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization-Coordinated Global Rotavirus Surveillance N. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis. 2016;62 Suppl 2:S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortese MM, Parashar UD, CDC. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2009;58(RR-2):1–25. [PubMed] [Google Scholar]
- 3.Kassim P, Eslick GD. Risk of intussusception following rotavirus vaccination: An evidence based meta-analysis of cohort and case-control studies. Vaccine. 2017;35(33):4276–4286. [DOI] [PubMed] [Google Scholar]
- 4.Tate JE, Simonsen L, Viboud C, Steiner C, Patel MM, Curns AT, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008;121(5):e1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman RD, Grupp-Phelan J, Shay DK, Davis RL. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics. 1999;103(1):E3. [DOI] [PubMed] [Google Scholar]
- 6.Dennehy PH, Cortese MM, Begue RE, Jaeger JL, Roberts NE, Zhang R, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J. 2006;25(12):1123–1131. [DOI] [PubMed] [Google Scholar]
- 7.Dennehy PH. Rotavirus Infection: A Disease of the Past? Infect Dis Clin North Am. 2015;29(4):617–635. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Hudak ML, Premachandra BR, Stevens G, Monteiro CB, Bradshaw JA, et al. Clinical manifestations of rotavirus infection in the neonatal intensive care unit. Pediatr Infect Dis J. 2002;21(12):1099–1105. [DOI] [PubMed] [Google Scholar]
- 9.Aliabadi N, Tate JE, Haynes AK, Parashar UD, CDC. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64(13):337–342. [PMC free article] [PubMed] [Google Scholar]
- 10.Burke RM, Tate JE, Barin N, Bock C, Bowen MD, Chang D, et al. Three Rotavirus Outbreaks in the Postvaccine Era - California, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(16):470–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gautam R, Mijatovic-Rustempasic S, Esona MD, Tam KI, Quaye O, Bowen MD. One-step multiplex real-time RT-PCR assay for detecting and genotyping wild-type group A rotavirus strains and vaccine strains (Rotarix(R) and RotaTeq(R)) in stool samples. PeerJ. 2016;4:e1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canadian Immunization Guide: Part 3 - Vaccination of Specific Populations. Government of Canada; 2015. [Google Scholar]
- 13.The Australian Immunisation Handbook: Rotavirus. 10th ed2017. [Google Scholar]
- 14.Green Book Chapter 27b: Rotavirus. Public Health England; 2013. [Google Scholar]
- 15.Jaques S, Bhojnagarwala B, Kennea N, Duffy D. Slow uptake of rotavirus vaccination in UK neonatal units. Arch Dis Child Fetal Neonatal Ed. 2014;99(3):F252. [DOI] [PubMed] [Google Scholar]
- 16.Rotavirus vaccines. WHO position paper - January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64. [PubMed] [Google Scholar]
- 17.Monk HM, Motsney AJ, Wade KC. Safety of rotavirus vaccine in the NICU. Pediatrics. 2014;133(6):e1555–1560. [DOI] [PubMed] [Google Scholar]
- 18.Hofstetter AM, Lacombe K, Klein EJ, Jones C, Strelitz B, Jacobson E, et al. Risk of Rotavirus Nosocomial Spread After Inpatient Pentavalent Rotavirus Vaccination. Pediatrics. 2018;141(1). [DOI] [PubMed] [Google Scholar]
- 19.Thrall S, Doll MK, Nhan C, Gonzales M, Perreault T, Lamer P, et al. Evaluation of pentavalent rotavirus vaccination in neonatal intensive care units. Vaccine. 2015;33(39):5095–5102. [DOI] [PubMed] [Google Scholar]
- 20.Hiramatsu H, Suzuki R, Nagatani A, Boda H, Miyata M, Hattori F, et al. Rotavirus Vaccination Can Be Performed Without Viral Dissemination in the Neonatal Intensive Care Unit. J Infect Dis. 2018;217(4):589–596. [DOI] [PubMed] [Google Scholar]
- 21.Stumpf KA, Thompson T, Sanchez PJ. Rotavirus vaccination of very low birth weight infants at discharge from the NICU. Pediatrics. 2013;132(3):e662–665. [DOI] [PubMed] [Google Scholar]
- 22.Bruijning-Verhagen P, Mangen MJ, Felderhof M, Hartwig NG, van Houten M, Winkel L, et al. Targeted rotavirus vaccination of high-risk infants; a low cost and highly cost-effective alternative to universal vaccination. BMC Med. 2013;11:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parashar UD, Kilgore PE, Holman RC, Clarke MJ, Bresee JS, Glass RI. Diarrheal mortality in US infants. Influence of birth weight on risk factors for death. Arch Pediatr Adolesc Med. 1998;152(1):47–51. [DOI] [PubMed] [Google Scholar]
- 24.Bowen MD, Mijatovic-Rustempasic S, Esona MD, Teel EN, Gautam R, Sturgeon M, et al. Rotavirus Strain Trends During the Postlicensure Vaccine Era: United States, 2008–2013. J Infect Dis. 2016;214(5):732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne DC, Selvarangan R, Azimi PH, Boom JA, Englund JA, Staat MA, et al. Long-term Consistency in Rotavirus Vaccine Protection: RV5 and RV1 Vaccine Effectiveness in US Children, 2012–2013. Clin Infect Dis. 2015;61(12):1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]