Abstract
Hepatocellular carcinoma (HCC) is primarily diagnosed in the latter stages of disease progression and is the third leading cause of cancer deaths worldwide. Thus, there is a need to find biomarkers of early HCC as well as the development of more effective treatments for the disease. Sphingosine-1-phosphate (S1P) is a pleiotropic lipid signaling molecule produced by two isoforms of sphingosine kinase (SphK1 and SphK2) that is involved in regulation of many aspects of mammalian physiology and pathophysiology, including inflammation, epithelial and endothelial barrier function, cancer, and metastasis, among many others. Abundant evidence indicates that SphK1 and S1P promote cancer progression and metastasis in multiple types of cancers. However, the role of SphK/S1P in HCC is less well studied. Here, we review the current state of knowledge of SphKs and S1P in HCC, including evidence for the correlation of SphK1 expression and S1P levels with progression of HCC and negative outcomes, and discuss how this information could lead to the design of more effective diagnostic and treatment modalities for HCC.
Keywords: Hepatocellular carcinoma, S1P receptors, Sphingosine kinase, Sphingosine-1-phosphate (S1P)
1. Introduction
Hepatocellular carcinoma (HCC) is cancer of the liver arising from malignant hepatocytes and accounts for nearly 90% of all primary liver cancers. HCC has a high level of morbidity, being the third leading cause of cancer deaths (El-Serag 2011). Due in part to both delayed diagnosis of HCC as well as its general aggressiveness, the median 5-year survival rate is less than 7%. Major risk factors for HCC include hepatitis virus B and C infections, overconsumption of alcohol, and nonalcoholic fatty liver disease (NAFLD), particularly when it progresses to nonalcoholic steatohepatitis (NASH). NAFLD/NASH are often associated with obesity and type 2 diabetes, and the rapid increase in the occurrence of these two disorders may contribute to the rise in non-viral associated HCC observed in the industrialized countries (Satapathy and Sanyal 2015). The hallmarks of NAFLD progression including insulin resistance, oxidative stress, and inflammation are all factors that also promote cancer initiation and progression (Cohen et al. 2011).
Sphingolipids, including sphingomyelin and glycosphingolipids, are essential lipid components of mammalian membranes. Sphingolipids consist of various head groups attached to ceramide, which is structurally analogous to the glycerolipid backbone diacylglycerol and, like diacylglycerol, is a second messenger involved in signaling pathways, typically promoting apoptosis and suppressing cell growth (Newton et al. 2015; Coant et al. 2017; Ogretmen 2018). Deacylation of ceramide yields sphingosine that has also been implicated in cell signaling. Sphingosine can be reacylated back to ceramide by a salvage pathway or phosphorylated by one of two sphingosine kinases (SphK1 and SphK2) forming sphingosine-1-phosphate (S1P). There are two fates for S1P: irreversible degradation by S1P lyase or dephosphorylation back to sphingosine. The metabolism of S1P is critical because S1P is a potent pleiotropic signaling molecule that regulates many physiological and pathological processes that are important for cancer including cell growth, proliferation, and cell motility, immune cell recruitment, epithelial and endothelial barrier function, and angiogenesis and lymphangiogenesis, among many others (Ogretmen 2018; Pyne and Pyne 2010; Kunkel et al. 2013; Maceyka and Spiegel 2014). Multiple stimuli, including growth factors, cytokines, and hormones, stimulate phosphorylation and activation of cytosolic SphK1 leading to its translocation to the plasma membrane where its substrate sphingosine resides and/or is generated (Maceyka and Spiegel 2014). S1P can be transported out of the cell, either by a specific S1P transporter called spinster 2 (Spns2), a member of the major facilitator superfamily of transporters, or via a subset of ATP-binding cassette (ABC) transporters, including ABCC1 and ABCG2 (Takabe and Spiegel 2014). This S1P can activate a family of five, S1P-specific G protein-coupled receptors (S1PR1–5) that mediate many of its known actions in an autocrine or paracrine manner, termed “inside-out signaling by S1P” (Takabe et al. 2008). These receptors are differentially expressed and couple to a wide array of heterotrimeric G proteins, leading to a diverse, and at times opposing, range of cellular and physiological responses (Pyne and Pyne 2010; Maceyka and Spiegel 2014). S1P produced inside cells also has intracellular actions; however, only a handful of intracellular targets and pathways have been identified so far. For example, S1P produced by activation of SphK1 binds to and activates proteins such as TNF receptor-associated factor 2 (TRAF2) (Alvarez et al. 2010; Park et al. 2015, 2016; Liu et al. 2017), a key adaptor molecule in TNFR signaling complexes that promotes downstream signaling cascades leading to activation of the master transcription factor NF-κB (Alvarez et al. 2010; Park et al. 2015). S1P can also activate NF-κB through formation of a signaling complex, consisting of S1P, TRAF2, and RIP1 that further associates with heat shock proteins GRP94 and HSP90α and IRE1α (Park et al. 2016). S1P produced in the nucleus by SphK2 is an endogenous histone deacetylase inhibitor (Hait et al. 2009, 2014; Nguyen-Tran et al. 2014; Nagahashi et al. 2015; Gardner et al. 2016) and also binds to hTERT and increases telomerase activity and enhances cancer cell growth (Panneer Selvam et al. 2015).
S1P is present at high levels in the blood and lymph, and the functions of S1P and its receptors as well as Spns2 in the regulation of immune cell trafficking have recently been implicated in tumor immunology and metastasis (Fang et al. 2017; van der Weyden et al. 2017). Moreover, numerous studies have also shown that expression of SphK1 and S1P promotes progression and metastasis of many types of cancers and correlates with poor prognosis (Ogretmen 2018; Pyne and Pyne 2010; Maceyka and Spiegel 2014). However, while the SphK1/S1P/S1PR signaling axis has been associated with initiation, progression, and chemoresistance of a variety of cancers, much less is known about its role in HCC. SphK1 and S1P expression have been correlated with initiation and progression of NAFLD, which is a risk factor for HCC, and thus, it is not surprising that they would also play a role in HCC. Here we discuss current evidence indicating that SphK1 and S1P are upregulated in human HCC patients and that they may serve as valuable biomarkers for early stage disease detection. We also examine the molecular mechanisms in HCC leading to SphK1/S1P upregulation, evidence that their upregulation promotes epithelial-mesenchymal transition and HCC progression, and the participation of downstream effectors of SphK1/S1P in HCC progression.
2. SphK1 and S1P Are Upregulated in HCC
Several studies have suggested that the SphK1/S1P axis could play a role in the promotion of HCC based on the observations that SphK1 is overexpressed in HCC cell lines and in patient samples and that S1P is increased in both patient serum and HCC tumors (Shi et al. 2015; Cai et al. 2017). For example, using immunohistochemistry techniques, both SphK1 and S1P levels were observed to be upregulated in HCC (Reynolds et al. 2017). Moreover, expression of SphK1 in HCC tumors was higher than in normal adjacent tissues, and SphK1 expression positively correlated with tumor size, tumor stage, and histological differentiation and negatively correlated with overall survival (Cai et al. 2017). Similarly, it was shown that SphK1 levels correlated with increased recurrence and decreased survival of patients with portal vein tumor thrombosis after primary tumor resection (Shi et al. 2015). However, in one study, though HCC tissues had elevated levels of SphKs, they had reduced levels of S1P compared to normal adjacent tissues (Uranbileg et al. 2016), findings that were linked to the elevated expression of the S1P-degrading enzyme, S1P lyase. These results highlight the importance of localized coupling of S1P synthesis, release, and S1PR activation that may not correlate with bulk S1P levels but are still sufficient to promote S1P signaling and HCC progression. Importantly, however, several studies have shown by mass spectrometry that serum levels of S1P are elevated in HCC compared to patients with cirrhosis (Ressom et al. 2012) and significantly elevated compared to normal controls (Grammatikos et al. 2016; Zeng et al. 2016). These results suggest that serum S1P may be a useful diagnostic marker for patients with liver disease that has progressed to HCC, particularly for patients at risk for liver cancer given its typically late stage of diagnosis.
3. Inflammation and Fibrosis
Many studies have linked SphK1 and S1P to the promotion of inflammatory responses, and it is well known that inflammation can promote the initiation and progression of cancer (Kunkel et al. 2013; Pyne et al. 2016). Indeed, several studies have also linked SphK1/S1P signaling to liver inflammation, fibrosis, and NAFLD/NASH (Rohrbach et al. 2017). For example, SphK1 is elevated in NAFLD patients and in obese mice that have many of the hallmarks of the disease, including NF-κB activation, elevated cytokine production, and immune cell infiltration (Geng et al. 2015) (Fig. 1). SphK1 null mice are resistant to these obesity-driven effects, and inflammatory mediator production in hepatocytes is reduced when S1PR1 is knocked down. Similarly, liver fibrosis in humans and in CCl4-induced fibrosis in mice have been correlated with increased levels of S1P, which stimulate fibrotic angiogenesis through activation of S1PR1 and S1PR3 (Yang et al. 2013).
Fig. 1.
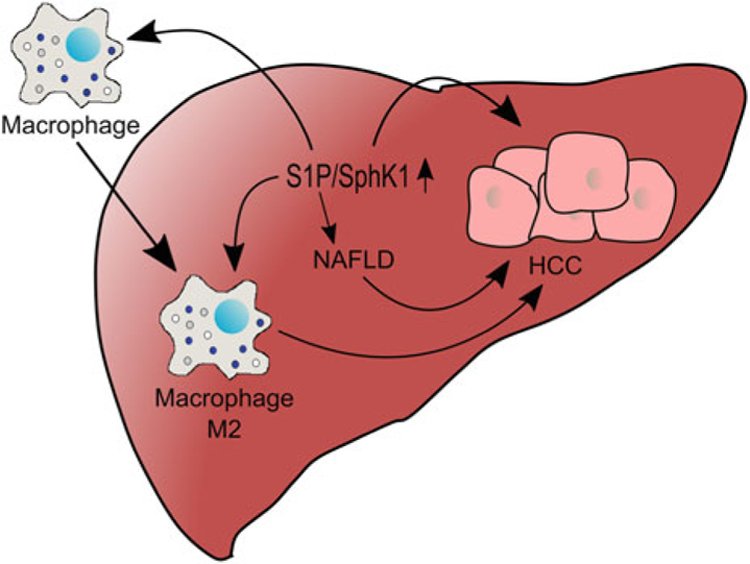
Role of S1P in promotion of liver cancer. SphK1 and S1P have been shown to promote NAFLD, which is a risk factor for HCC development. Moreover, increased SphK1 and S1P production by the liver can enhance recruitment of macrophages that migrate to the tumor site, remain there, and aid in angiogenesis, termed tumor-associated macrophages (TAMs), and are thought to express an M2 phenotype
Myeloid cells, particularly tumor-associated macrophages (TAMS), are frequently increased in the HCC microenvironment and are associated with worse prognosis (Wan et al. 2015). Although the role of the SphK/S1P axis in recruitment and function of TAMS has not been investigated in HCC, in many other cancers, S1P secreted by the tumor due to elevation of SphK1 plays a key role in the recruitment and phenotypic shift of the tumor macrophages from pro-inflammatory (M1) to anti-inflammatory (M2) that promotes tumorigenesis and angiogenesis (Rodriguez et al. 2016; Mrad et al. 2016) (Fig. 1).
4. Regulation of the S1P Signaling Axis in HCC
The observations that SphK1 and S1P are elevated in HCC tumor samples suggest that the tumors have mechanisms for increasing S1P signaling. One group has shown that the HCC cell line HepG2 secretes S1P and that inhibition of this secretion blocks cell proliferation that can be rescued by exogenous S1P, indicative of an autocrine S1P signaling loop (Jin et al. 2016). They linked the secretion of S1P to AKR1B10, a protein involved in lipid metabolism that is elevated in HCC, as siRNA against AKR1B10 reduced S1P levels and cell growth. They further showed that high expression of AKR1B10 correlated with both more negative outcomes and higher levels of S1P in tumor but not peripheral liver tissue. This study utilized an ELISA method to measure S1P that is not as sensitive as mass spectrometry methods and did not detect any changes in S1P synthesis or degradation that could affect S1P levels, although other studies have implicated pathways that control SphK1 expression in HCC. For example, similar to many other growth factors, hepatocyte growth factor (HGF) stimulates SphK activity, S1P production, and motility in HCC cells that can be blocked by downregulating SphK1. HGF induced translocation of SphK1, the S1P transporter Spns2, and the S1P receptor S1PR1 to the lamellipodia at the leading edge of moving lung vascular cells (Fu et al. 2016). This not only reaffirms the inside-out signaling hypothesis, but it also suggests that the pro-motility signaling occurs at distinct regions of the plasma membrane to promote directed motility up a chemoattractant gradient. Similarly, motility and invasion of several HCC cell lines were dependent on overexpression of SphK1 and signaling via S1PR1 (Bao et al. 2012), supporting a role for the SphK1/S1P/S1PR1 axis in liver metastasis and suggesting that this axis might be an attractive therapeutic target for the development of new anti-HCC drugs.
Expression of highly upregulated in liver cancer (HULC), which was originally identified as a very highly overexpressed long noncoding RNA in HCC, was suggested to correlate with both SphK1 mRNA and ELISA-measured S1P levels in patient samples (Lu et al. 2016). Functionally, HULC-induced angiogenesis in situ and in HCC xenografts depended on SphK1 expression. Mechanistically, HULC sequestered miR-107, increasing the transcription of E2F1, which activated the transcription of SphK1. Together, these results suggest that HULC promotes tumor angiogenesis in liver cancer in part by upregulation of SphK1 (Lu et al. 2016). Another miR, miR-506, was also claimed to downregulate SphK1 mRNA and protein, to reduce secreted S1P in vitro, and to negatively correlate with mRNA levels of SphK1 in clinical HCC samples (Lu et al. 2015). Thus, it was proposed that miR-506 depresses angiogenesis of liver cancer by targeting the 3′UTR of SphK1 mRNA. Interestingly, a second group has found a similar downregulation of miR-506 in pancreatic cancer that correlated with the upregulation of SphK1/AKT/NF-κB signaling (Li et al. 2016). Further studies are needed to support the significance of these miRs in HCC and conclusively demonstrate their specific targets.
5. Downstream Effectors/Pathways of SphK1/S1P that Promote HCC
5.1. S1PRs
The most obvious and druggable proximal targets of the S1P signaling axis are the cell surface S1P receptors, which have been linked to progression of many cancers (Ogretmen 2018; Pyne and Pyne 2010; Maceyka and Spiegel 2014; Patmanathan et al. 2017). Indeed, several studies have correlated expression of S1PR1 to HCC progression and poor prognoses. Consistent with previous studies suggesting that SphK1 produced S1P promotes tumor cell migration and invasion by activating S1PR1 (Bao et al. 2012), in diethylnitrosamine-induced HCC in mice, SphK1, SphK2, S1PR1, and S1PR3 were upregulated, while the S1P-degrading enzyme S1P lyase was downregulated (Sanchez et al. 2018). Other studies also suggested that S1P-S1PR1 signaling is involved in the progression of HCC in patients. miR-148a dysregulation discriminated overall survival and recurrence-free survival rates of HCC. In human HCC samples, both ubiquitin-specific protease 4 (USP4) and S1PR1 were identified as targets of miR-148a. USP4 and S1PR1 were upregulated in mesenchymal-type liver tumor cells with miR-148a dysregulation, facilitating migration and proliferation of tumor cells, though a significant negative correlation was not found between miR148a and S1PR1 in three heterotopic patient-derived HCC xenografts (Heo et al. 2014). Another putative tumor-suppressing microRNA, miR-363, decreased S1PR1 expression by binding its 3′UTR and also inhibited the proliferation of HCC cells (Zhou et al. 2014).
5.2. The S1P Signaling Axis Promotes EMT in HCC
The epithelial-mesenchymal transition (EMT), a process by which epithelial cells dedifferentiate to multipotent mesenchymal stem cells, is a hallmark of cancer initiation (Kalluri and Weinberg 2009). Cells that undergo EMT lose the expression of epithelial markers, polarity, and become more motile. Several studies suggest that the S1P signaling axis promotes EMT in HCC. For example, inhibition of SphK activity with N,N-dimethylsphingosine in HCC cells was originally shown to upregulate liver-specific markers of differentiation, including albumin and alpha-fetoprotein (AFP) (Osawa et al. 2001). Intriguingly, in a set of 77 HCC patient samples, not only did expression of SphK1 and SphK2 correlate with decreased differentiation so did expression of S1P lyase. However, in this sample set, there was no correlation between SphK1/2 expression and S1P levels in the tumor (Uranbileg et al. 2016). Once again, these results suggest that localized S1P production rather than absolute levels may be critical to its function in promoting HCC.
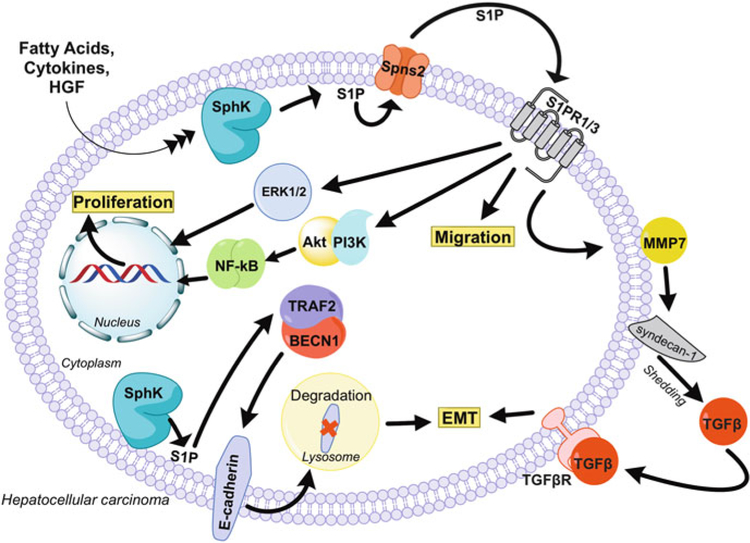
It was recently shown that SphK1 induces the EMT in HepG2 cells by accelerating lysosomal degradation of the cell-cell adhesion molecule E-cadherin (CDH1), thus facilitating their invasion and metastasis (Liu et al. 2017). Of note, in HepG2 cells, SphK1-produced S1P bound to TRAF2 and stimulated lysine 63-linked ubiquitination and activation of beclin1 that in turn stimulated autophagic degradation of E-cadherin (Liu et al. 2017). These intriguing findings define a novel mechanism for the regulation of the EMT via SphK1-TRAF2-beclin1-E-cadherin signaling cascade in HCC cells. Moreover, this study suggests that inhibition of SphK1 to attenuate autophagy may be a promising strategy for treatment of HCC. In agreement, a non-isozyme-specific SphK inhibitor promoted the Wnt5a-dependent degradation of the tumor-promoter β-catenin and inhibited HepG2 cell proliferation (Liu et al. 2016). Another group put forward a different mechanism to explain how S1P induces the EMT in HCC showing that signaling through S1PR1 stimulated the MMP7-dependent shedding of syndecan-1, leading to the activation of the TGF-β signaling pathway and the promotion of the EMT phenotype (Zeng et al. 2016) (Fig. 2). Although there is strong evidence for EMT in HCC patients, so far, data are missing that demonstrate that the process of EMT is reversible after intrahepatic or even distal metastatic colonization. Future studies are needed to clarify whether targeting the SphK/S1P axis and inhibition of EMT could lead to development of effective therapies against HCC.
Fig. 2.
S1P signaling in HCC progression. S1P can regulate signaling and physiological processes in several pathways to promote HCC. As indicated in the text, these include increasing cell motility and proliferation through S1PR1/3 activation, S1PR1/3-mediated activation of ERK and AKT, degradation of E-cadherin to promote EMT, and proteolysis of syndecan-1 to induce HGF signaling. The EMT can be regulated by a unique pathway involving the SphK1-TRAF2-beclin1-E-cadherin signaling cascade in HCC cells
6. Inhibition of the S1P Signaling Axis as Treatment for HCC
The studies discussed clearly demonstrate that SphK1 and S1P signaling axis are associated with and likely promote the initiation and progression of HCC. Therefore, it has been suggested that inhibiting SphK1 might be a useful approach for treatment of HCC. Preclinical studies have suggested that effects of drugs that reduce HCC tumor cell growth, such as melatonin (Sanchez et al. 2018) or the natural prenylflavonoid icaritin (Lu et al. 2017), might be due to inhibition of SphK1 and decreased activation of S1PRs. Several groups have investigated the efficacy of inhibiting S1P signaling more directly in vitro and for treatment of tumor-bearing mice. It has been shown that the dual SphK1/2 inhibitor SKI-II acts synergistically with 5-fluorouracil (5-FU), a common drug used for the treatment of advanced HCC, to both promote HCC cell death and inhibit migration of HCC cells through downstream inhibition of FAK, ERK, and NF-κB (Grbcic et al. 2017). Similar effects were obtained when SKI-II was used in combination with the MEK1/2 inhibitor U0126 (Zhang et al. 2013) (Table 1). It has also been shown that SKI-II, alone or in combination with the ROS-generating drug selenite, is effective at killing Huh7 HCC cells, but not non-tumorigenic hepatocyte cells (Chatzakos et al. 2012). Interestingly, ABC294640, which was developed as a SphK2 inhibitor and decreases serum S1P levels, was found to be effective in two HCC xenograft models when used in combination with sorafenib, a multikinase inhibitor and emerging drug for treatment HCC (Beljanski et al. 2011). It was further shown that ABC294640 decreased the level of S1P in the xenograft tumors themselves (Table 1). Furthermore, ABC294640 is currently in a phase II clinical trial for the treatment of patients with sorafenib-resistant HCC (https://clinicaltrials.gov/ct2/show/NCT02939807).
Table 1.
Compounds that target the S1P axis in liver fibrosis and in preclinical HCC animal models
| Compound | Target(s) | Mechanism | Effects | References |
|---|---|---|---|---|
| N,N-dimethylsphingosine | SphK1/SphK2 | SphK1 (Ki = 5 μM) and PKC inhibitor | Blocked bone marrow-derived mesenchymal stem cell differentiation to myofibroblasts during liver injury | Yang et al. (2012) |
| PF-543 | SphK1 | SphK1-specific inhibitor (Ki = 3.6 nM) | Reduced liver fibrosis | Gonzalez-Fernandez et al. (2017) |
| SEW2871 | S1PR1 | Agonist (EC50 = 14–140 nM) | Reduced hepatic parenchymal damage and fibrosis | Ding et al. (2016) |
| VPC23019 | S1PR1/3 | Antagonist (pKi = 7.9/5.9 for S1PR1/3) | Reduced liver fibrosis | Yang et al. (2013) |
| W146 | S1PR1 | Antagonist (Ki = 10–20 nM) | Reduced liver fibrosis | Yang et al. (2012) |
| JTE-013 | S1PR2 | Antagonist (Ki = 17 nM) | Reduced hepatic inflammation and fibrosis in mice with BDL ligation | Yang et al. (2015) |
| Reduced bile acid-induced cholangiocyte proliferation | Wang et al. (2017) | |||
| SKI-II | SphK1/SphK2 | SphK inhibitor (IC50 = 16 μM for SphK1; 8 μM for SphK2) | Reduced survival and migration of HCC cells | Lu et al. (2017) and Zhang et al. (2013) |
| Reduced liver injury | Yang et al. (2013) | |||
| FTY720/FTY720-P | S1PR1,3,4,5 | S1PR1 agonist/functional antagonist of S1PR1, 3, 4, 5 HDAC inhibitor | Reduced intrahepatic and lung metastases in orthotopic rat model | Li et al. (2012) |
| Extended life span of tumor-bearing rats | Ushitora et al. (2009) | |||
| ABC294640 | SphK2 | SphK2 inhibitor | Reduced HCC xenograft in mice | Beljanski et al. (2011) |
| ABC294640 | SphK2 | SphK2 inhibitor | Phase II clinical trial for sorafenib-resistant HCC patients | https://clinicaltrials.gov/ct2/show/NCT02939807 |
Several studies examined the potential of FTY720 as a therapeutic option for HCC. FTY720 is a prodrug that is phosphorylated by SphK2 to the S1P analog FTY720-phosphate, which acts a functional antagonist for S1PR1 (Brinkmann et al. 2010) and is also a class I HDAC inhibitor (Hait et al. 2015). FTY720 is orally available, generally well tolerated, and already widely used for treatment of multiple sclerosis. As such, it is an attractive drug for cancer chemotherapy, and it has been found to effectively inhibit proliferation of many types of cancer cells. In HCC xenograft models, FTY720 administration inhibited tumor growth, tumor microvessel density, and metastases (Ho et al. 2005), effects that were linked to inhibition of AKT activation (Lee et al. 2004). In an orthotopic rat model, FTY720 was shown to reduce both intrahepatic and lung metastases as well as reducing tumor angiogenesis, likely through its function as a S1PR1 modulator (Li et al. 2012) (Table 1). Consistent with these results, FTY720 also decreased circulating endothelial progenitor cells. In this model, FTY720 suppressed tumor proliferation index, as well as activation of AKT and FAK (Ng et al. 2007). FTY720 also effectively downregulated S1PR1, decreased cell migration and ERK activation, and markedly extended life span in rats whose cancerous liver was transplanted with a non-cancerous one (Ushitora et al. 2009). This result is important as liver transplantation after cancer resection can provide long-term survival if recurrence can be avoided. While the clinical translation of FTY720 for cancer treatment is currently limited due to its immune suppression effects, future studies aimed at more complete understanding of the mechanism of action of FTY720, developing approaches for its specific delivery, and reducing effective concentrations and its immunosuppressive effects by using it in synergistic combinations with other drugs could enhance its rapid acceptance as an important anti-HCC drug.
7. Future Perspectives
That multiple mechanisms that promote HCC converge on the activation of the S1P signaling axis suggests that targeting this axis may prove therapeutically useful. S1P is elevated in patient tumors, as is the enzyme that produces it, SphK1, indicating it may be a node for HCC progression and thus a promising drug target. Tumor profiling and published preclinical studies with the functional S1PR1 antagonist FTY720 suggest that S1PR1 may also be a target for HCC treatment. The results discussed here indicate the need for further studies of inhibition of the SphK/S1P/S1PR signaling axis as one arm of a combination therapy for the treatment of this deadly disease.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 GM043880 to S.S. T.R. was supported by T32 training grant in Digestive Disease and Liver Disease 5T32DK007150-39.
Abbreviations
- HCC
Hepatocellular carcinoma
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- S1P
Sphingosine-1-phosphate
- S1PR
S1P receptor
- SphK
Sphingosine kinase
- Spns2
Spinster 2
Footnotes
Conflict of Interest Statement The authors declare that they have no conflicts of interest with the contents of this article.
References
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S (2010) Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Chen Z, Xu Y, Zhao Y, Zha R, Huang S, Liu L, Chen T, Li J, Tu H, He X (2012) Sphingosine kinase 1 promotes tumour cell migration and invasion via the S1P/EDG1 axis in hepatocellular carcinoma. Liver Int 32:331–338 [DOI] [PubMed] [Google Scholar]
- Beljanski V, Lewis CS, Smith CD (2011) Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther 11:524–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov 9:883–897 [DOI] [PubMed] [Google Scholar]
- Cai H, Xie X, Ji L, Ruan X, Zheng Z (2017) Sphingosine kinase 1: a novel independent prognosis biomarker in hepatocellular carcinoma. Oncol Lett 13:2316–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzakos V, Rundlof AK, Ahmed D, de Verdier PJ, Flygare J (2012) Inhibition of sphingosine kinase 1 enhances cytotoxicity, ceramide levels and ROS formation in liver cancer cells treated with selenite. Biochem Pharmacol 84:712–721 [DOI] [PubMed] [Google Scholar]
- Coant N, Sakamoto W, Mao C, Hannun YA (2017) Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 63:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH (2011) Human fatty liver disease: old questions and new insights. Science 332:1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BS, Liu CH, Sun Y, Chen Y, Swendeman SL, Jung B, Chavez D, Cao Z, Christoffersen C, Nielsen LB, Schwab SR, Rafii S, Hla T (2016) HDL activation of endothelial sphingosine-1-phosphate receptor-1 (S1P1) promotes regeneration and suppresses fibrosis in the liver. JCI Insight 1:e87058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365:1118–1127 [DOI] [PubMed] [Google Scholar]
- Fang V, Chaluvadi VS, Ramos-Perez WD, Mendoza A, Baeyens A, Rivera R, Chun J, Cammer M, Schwab SR (2017) Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-gamma response. Nat Immunol 18:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu P, Ebenezer DL, Berdyshev EV, Bronova IA, Shaaya M, Harijith A, Natarajan V (2016) Role of sphingosine kinase 1 and S1P transporter Spns2 in HGF-mediated lamellipodia formation in lung endothelium. J Biol Chem 291:27187–27203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner NM, Riley RT, Showker JL, Voss KA, Sachs AJ, Maddox JR, Gelineau-van Waes JB (2016) Elevated nuclear and cytoplasmic FTY720-phosphate in mouse embryonic fibroblasts suggests the potential for multiple mechanisms in FTY720-induced neural tube defects. Toxicol Sci 150:161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng T, Sutter A, Harland MD, Law BA, Ross JS, Lewin D, Palanisamy A, Russo SB, Chavin KD, Cowart LA (2015) SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J Lipid Res 56:2359–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez B, Sanchez DI, Crespo I, San-Miguel B, Alvarez M, Tunon MJ, Gonzalez-Gallego J (2017) Inhibition of the SphK1/S1P signaling pathway by melatonin in mice with liver fibrosis and human hepatic stellate cells. Biofactors 43:272–282 [DOI] [PubMed] [Google Scholar]
- Grammatikos G, Dietz J, Ferreiros N, Koch A, Dultz G, Bon D, Karakasiliotis I, Lutz T, Knecht G, Gute P, Herrmann E, Zeuzem S, Mavromara P, Sarrazin C, Pfeilschifter J (2016) Persistence of HCV in acutely-infected patients depletes C24-ceramide and upregulates sphingosine and sphinganine serum levels. Int J Mol Sci 17:18095–18105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbcic P, Tomljanovic I, Klobucar M, Kraljevic Pavelic S, Lucin K, Sedic M (2017) Dual sphingosine kinase inhibitor SKI-II enhances sensitivity to 5-fluorouracil in hepatocellular carcinoma cells via suppression of osteopontin and FAK/IGF-1R signalling. Biochem Biophys Res Commun 487:782–788 [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S (2009) Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 325:1254–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Wise LE, Allegood JC, O’Brien M, Avni D, Reeves TM, Knapp PE, Lu J, Luo C, Miles MF, Milstien S, Lichtman AH, Spiegel S (2014) Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci 17:971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hait NC, Avni D, Yamada A, Nagahashi M, Aoyagi T, Aoki H, Dumur CI, Zelenko Z, Gallagher EJ, Leroith D, Milstien S, Takabe K, Spiegel S (2015) The phosphorylated prodrug FTY720 is a histone deacetylase inhibitor that reactivates ERalpha expression and enhances hormonal therapy for breast cancer. Oncogenesis 4:e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo MJ, Kim YM, Koo JH, Yang YM, An J, Lee SK, Lee SJ, Kim KM, Park JW, Kim SG (2014) microRNA-148a dysregulation discriminates poor prognosis of hepatocellular carcinoma in association with USP4 overexpression. Oncotarget 5:2792–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JW, Man K, Sun CK, Lee TK, Poon RT, Fan ST (2005) Effects of a novel immunomodulating agent, FTY720, on tumor growth and angiogenesis in hepatocellular carcinoma. Mol Cancer Ther 4:1430–1438 [DOI] [PubMed] [Google Scholar]
- Jin J, Liao W, Yao W, Zhu R, Li Y, He S (2016) Aldo-keto reductase family 1 member B 10 mediates liver cancer cell proliferation through sphingosine-1-phosphate. Sci Rep 6:22746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel GT, Maceyka M, Milstien S, Spiegel S (2013) Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12:688–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Man K, Ho JW, Sun CK, Ng KT, Wang XH, Wong YC, Ng IO, Xu R, Fan ST (2004) FTY720 induces apoptosis of human hepatoma cell lines through PI3-K-mediated Akt dephosphorylation. Carcinogenesis 25:2397–2405 [DOI] [PubMed] [Google Scholar]
- Li CX, Shao Y, Ng KT, Liu XB, Ling CC, Ma YY, Geng W, Fan ST, Lo CM, Man K (2012) FTY720 suppresses liver tumor metastasis by reducing the population of circulating endothelial progenitor cells. PLoS One 7:e32380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wu H, Li W, Yin L, Guo S, Xu X, Ouyang Y, Zhao Z, Liu S, Tian Y, Tian Z, Ju J, Ni B, Wang H (2016) Downregulated miR-506 expression facilitates pancreatic cancer progression and chemoresistance via SPHK1/Akt/NF-κB signaling. Oncogene 35:5501–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Zhang W, Gao S, Jiang Q, Xiao Z, Ye L, Zhang X (2015) MiR-506 suppresses liver cancer angiogenesis through targeting sphingosine kinase 1 (SPHK1) mRNA. Biochem Biophys Res Commun 468:8–13 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xiao Z, Liu F, Cui M, Li W, Yang Z, Li J, Ye L, Zhang X (2016) Long non-coding RNA HULC promotes tumor angiogenesis in liver cancer by up-regulating sphingosine kinase 1 (SPHK1). Oncotarget 7:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang CX, Ma Y, He HW, Wang JP, Shao RG (2016) SphK1 inhibitor SKI II inhibits the proliferation of human hepatoma HepG2 cells via the Wnt5A/beta-catenin signaling pathway. Life Sci 151:23–29 [DOI] [PubMed] [Google Scholar]
- Liu H, Ma Y, He HW, Zhao WL, Shao RG (2017) SPHK1 (sphingosine kinase 1) induces epithelial-mesenchymal transition by promoting the autophagy-linked lysosomal degradation of CDH1/E-cadherin in hepatoma cells. Autophagy 13:900–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PH, Chen MB, Liu YY, Wu MH, Li WT, Wei MX, Liu CY, Qin SK (2017) Identification of sphingosine kinase 1 (SphK1) as a primary target of icaritin in hepatocellular carcinoma cells. Oncotarget 8:22800–22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrad M, Imbert C, Garcia V, Rambow F, Therville N, Carpentier S, Segui B, Levade T, Azar R, Marine JC, Diab-Assaf M, Colacios C, Andrieu-Abadie N (2016) Downregulation of sphingosine kinase-1 induces protective tumor immunity by promoting M1 macrophage response in melanoma. Oncotarget 7:71873–71886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Takabe K, Liu R, Peng K, Wang X, Wang Y, Hait NC, Allegood JC, Yamada A, Aoyagi T, Liang J, Pandak WM, Spiegel S, Hylemon PB, Zhou H (2015) Conjugated bile acid activated S1P receptor 2 is a key regulator of sphingosine kinase 2 and hepatic gene expression. Hepatology 61:1216–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, Spiegel S (2015) Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy. Exp Cell Res 333:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KT, Man K, Ho JW, Sun CK, Lee TK, Zhao Y, Lo CM, Poon RT, Fan ST (2007) Marked suppression of tumor growth by FTY720 in a rat liver tumor model: the significance of downregulation of cell survival Akt pathway. Int J Oncol 30:375–380 [PubMed] [Google Scholar]
- Nguyen-Tran DH, Hait NC, Sperber H, Qi J, Fischer K, Ieronimakis N, Pantoja M, Hays A, Allegood J, Reyes M, Spiegel S, Ruohola-Baker H (2014) Molecular mechanism of sphingosine-1-phosphate action in Duchenne muscular dystrophy. Dis Model Mech 7:41–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogretmen B (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18:33–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa Y, Nagaki M, Banno Y, Nozawa Y, Moriwaki H, Nakashima S (2001) Sphingosine kinase regulates hepatoma cell differentiation: roles of hepatocyte nuclear factor and retinoid receptor. Biochem Biophys Res Commun 286(4):673–677 [DOI] [PubMed] [Google Scholar]
- Panneer Selvam S, De Palma RM, Oaks JJ, Oleinik N, Peterson YK, Stahelin RV, Skordalakes E, Ponnusamy S, Garrett-Mayer E, Smith CD, Ogretmen B (2015) Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci Signal 8:ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Choi S, Shin B, Yu J, Hwang JM, Yun H, Chung YH, Choi JS, Choi Y, Rho J (2015) Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J Biol Chem 290:9660–9673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, Lee YM, Yano T, Holleran WM, Elias P, Uchida Y (2016) ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A 113:E1334–E1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patmanathan SN, Wang W, Yap LF, Herr DR, Paterson IC (2017) Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell Signal 34:66–75 [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10:489–503 [DOI] [PubMed] [Google Scholar]
- Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S (2016) Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv Biol Regul 60:151–159 [DOI] [PubMed] [Google Scholar]
- Ressom HW, Xiao JF, Tuli L, Varghese RS, Zhou B, Tsai TH, Ranjbar MR, Zhao Y, Wang J, Di Poto C, Cheema AK, Tadesse MG, Goldman R, Shetty K (2012) Utilization of metabolomics to identify serum biomarkers for hepatocellular carcinoma in patients with liver cirrhosis. Anal Chim Acta 743:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GM, Visentin B, Sabbadini R (2017) Immunohistochemical detection of sphingosine-1-phosphate and sphingosine kinase-1 in human tissue samples and cell lines. Methods Mol Biol 1697:43–56 [DOI] [PubMed] [Google Scholar]
- Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, Alvarez SE (2016) Sphingosine-1 phosphate: a new modulator of immune plasticity in the tumor microenvironment. Front Oncol 6:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach T, Maceyka M, Spiegel S (2017) Sphingosine kinase and sphingosine-1-phosphate in liver pathobiology. Crit Rev Biochem Mol Biol 52:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez DI, Gonzalez-Fernandez B, San-Miguel B, de Urbina JO, Crespo I, Gonzalez-Gallego J, Tunon MJ (2018) Melatonin prevents deregulation of the sphingosine kinase/sphingosine 1-phosphate signaling pathway in a mouse model of diethylnitrosamine-induced hepatocellular carcinoma. J Pineal Res 62:12369. [DOI] [PubMed] [Google Scholar]
- Satapathy SK, Sanyal AJ (2015) Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis 35:221–235 [DOI] [PubMed] [Google Scholar]
- Shi J, He YY, Sun JX, Guo WX, Li N, Xue J, Cheng SQ (2015) The impact of sphingosine kinase 1 on the prognosis of hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol 14:198–206 [PubMed] [Google Scholar]
- Takabe K, Spiegel S (2014) Export of sphingosine-1-phosphate and cancer progression. J Lipid Res 9:1839–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe K, Paugh SW, Milstien S, Spiegel S (2008) “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev 60:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranbileg B, Ikeda H, Kurano M, Enooku K, Sato M, Saigusa D, Aoki J, Ishizawa T, Hasegawa K, Kokudo N, Yatomi Y (2016) Increased mRNA levels of sphingosine kinases and s1p lyase and reduced levels of s1p were observed in hepatocellular carcinoma in association with poorer differentiation and earlier recurrence. PLoS One 11:e0149462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushitora Y, Tashiro H, Ogawa T, Tanimoto Y, Kuroda S, Kobayashi T, Miyata Y, Itamoto T, Asahara T, Ohdan H (2009) Suppression of hepatocellular carcinoma recurrence after rat liver transplantation by FTY720, a sphingosine-1-phosphate analog. Transplantation 88:980–986 [DOI] [PubMed] [Google Scholar]
- van der Weyden L, Arends MJ, Campbell AD, Bald T, Wardle-Jones H, Griggs N, Velasco-Herrera MD, Tuting T, Sansom OJ, Karp NA, Clare S, Gleeson D, Ryder E, Galli A, Tuck E, Cambridge EL, Voet T, Macaulay IC, Wong K, Sanger Mouse Genetics P, Spiegel S, Speak AO, Adams DJ (2017) Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 541:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Kuo N, Kryczek I, Zou W, Welling TH (2015) Myeloid cells in hepatocellular carcinoma. Hepatology 62:1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, Qiang X, Sun L, Gurley EC, Lai G, Zhang L, Liang G, Nagahashi M, Takabe K, Pandak WM, Hylemon PB, Zhou H (2017) The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology 65:2005–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chang N, Liu X, Han Z, Zhu T, Li C, Yang L, Li L (2012) Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-beta1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol 181:85–97 [DOI] [PubMed] [Google Scholar]
- Yang L, Yue S, Yang L, Liu X, Han Z, Zhang Y, Li L (2013) Sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis is involved in liver fibrosis-associated angiogenesis. J Hepatol 59:114–123 [DOI] [PubMed] [Google Scholar]
- Yang L, Han Z, Tian L, Mai P, Zhang Y, Wang L, Li L (2015) Sphingosine 1-phosphate receptor 2 and 3 mediate bone marrow-derived monocyte/macrophage motility in cholestatic liver injury in mice. Sci Rep 5:13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Yao X, Chen L, Yan Z, Liu J, Zhang Y, Feng T, Wu J, Liu X (2016) Sphingosine-1-phosphate induced epithelial-mesenchymal transition of hepatocellular carcinoma via an MMP-7/syndecan-1/TGF-beta autocrine loop. Oncotarget 7:63324–63337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, He H, Zhang H, Yu D, Zhao W, Chen Y, Shao R (2013) The blockage of Ras/ERK pathway augments the sensitivity of SphK1 inhibitor SKI II in human hepatoma HepG2 cells. Biochem Biophys Res Commun 434:35–41 [DOI] [PubMed] [Google Scholar]
- Zhou P, Huang G, Zhao Y, Zhong D, Xu Z, Zeng Y, Zhang Y, Li S, He F (2014) MicroRNA-363-mediated downregulation of S1PR1 suppresses the proliferation of hepatocellular carcinoma cells. Cell Signal 26:1347–1354 [DOI] [PubMed] [Google Scholar]