Abstract
Background
There is limited data regarding the use of emergency departments (EDs) for infectious disease screening and vaccination in resource-limited regions. In these settings, EDs are often the only contact that patients have with the healthcare system, turning an ED visit into an opportune time to deliver preventative health services.
Methods
In this pilot study, patients that met inclusion criteria were prospectively tested for hepatitis B surface antigen test (HBsAg). Previously unvaccinated patients who tested negative for HBsAg were offered HBV vaccination. The study setting was a public infectious disease hospital in Cordoba, Argentina. The primary outcomes were new HBV diagnoses, as well as vaccination completion between screening modalities (Point-of-Care-Testing-POCT vs. laboratory testing) and same vs. different day vaccination.
Results
We screened 100 patients for HBV (75 POCT & 25 laboratory). The median age of participants was 35 years (IQR 24–52) and 55% were male. No patients tested positive for HBsAg. All patients who completed first dose vaccination were initially screened with the POCT. No patients screened with laboratory testing returned for vaccination. Patients who were scheduled for vaccination the same day were more likely to complete vaccination compared to those scheduled for another day (75% vs. 14%, p<.001).
Conclusion
Our study supports the use of HBV POCTs in the ED in conjunction with vaccination of HBV-negative individuals. In regions with low HBV endemicity, direct vaccination without HBsAg testing may be more cost effective. We believe that this acute-care screening model is applicable to other resource-limited settings.
Keywords: hepatitis B virus, rapid diagnostic test, point-of-care test, hepatitis B vaccination
1. Introduction
1.1. Background
Screening for infectious diseases during emergency department (ED) visits has been studied in resource-rich countries (1–4). However, data about this approach in resource-limited settings is scarce, with very few studies exploring the use of ED visits as a setting to link patients to preventative services (5–7). In Argentina, EDs are often the first, and only contact that patients have with the healthcare system, as many patients do not seek primary care services and instead seek acute-care services only when they become ill. This is of particular importance in young populations who are at high-risk for sexually transmitted infections (STIs), such as hepatitis B virus (HBV), that would benefit from vaccination. Testing recommendations for HBV are similar between the Argentine Ministry of Health and the United States Centers for Disease Control and Prevention (CDC) (8, 9). We provide a comparative summary of these recommendations in Table 1.
Table 1.
Summary and comparison of hepatitis B virus testing recommendations from the Argentine Ministry of Health and the United States Centers for Disease Control and Prevention†
|
Overlapping Recommendations | |
| • Individuals with elevated AST/AST of unknown etiology* | |
| • Infants born to HBsAg positive mothers | |
| • Household or have sex contacts of persons known to be HBsAg positive** | |
| • HIV positive persons | |
| • Individuals with a history of intravenous drug use*** | |
| • Individuals with a history of hemodialysis | |
| • Pregnant women | |
| Additional Recommendations | |
| Argentina | United States |
| • Individuals with occupational exposure to HBV (i.e. needle stick injury) • Individuals with a history of another parenterally-transmittable hepatitis virus • Individuals with a history of another sexually-transmitted infection or with history of sexual intercourse without barrier contraception • Individuals who received a blood product transfusion prior to 1993 • Individuals with a history of recent invasive medical or surgical procedures within the last 6 months |
• Individuals who are the source of blood or body fluid exposure (i.e. sexual assault, needlesticks) • Individuals born in intermediate (HBsAg prevalence ≥2%) and high-endemicity (≥8%) countries • US-born individuals whose parents were born in high-endemicity countries • Men who have sex with men (MSM) • Individuals requiring immunosuppressive therapy • Donors of blood products, organs, tissues or semen |
Adapted from “Viral Hepatitis: Guide for Medical Teams,” published by the National Program for Viral Hepatitis of the Argentine Ministry of Health, 2015 and “Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection,” published by the US Centers for Disease Control and Prevention, 2008 (8, 9).
Argentine guidelines require LFTs to be >2.5x the level of normal and individuals must present with one of the following signs/symptoms: general malaise, myalgias, arthralgias, weakness, anorexia, nausea/vomiting or fever
CDC guidelines include needle-sharing contacts
Argentine guidelines include individuals with a history of intranasal drug use
1.2. Importance
The sequelae of untreated chronic HBV include cirrhosis and the development of hepatocellular carcinoma, both of which present insidiously and often do not manifest symptoms until they are irreversible or incurable (10). Similar to many countries, HBV was not added to the national Argentine vaccination schedule until the year 2000, leaving a large at-risk adult population susceptible to infection upon exposure (11). There have been subsequent efforts by the Argentine federal government to vaccinate unvaccinated adults, however, acute HBV incidence remained unchanged between 2007 and 2016, underlying the difficulty of vaccinating a young adult population (11, 12). While vaccination against the influenza virus in the ED has gained popularity in recent years, to our knowledge, there are no published data regarding vaccination for other infectious diseases, such as HBV, in an ED setting (13). As there is a significant time delay between when blood is drawn for HBV laboratory testing and a result being ready, we thought it was important to evaluate if the use of a HBV POCT test would provide superior linkage-to-care rates when compared to laboratory testing. Previous studies employing point-of-care testing for HBV in resource-limited settings have targeted linkage-to-care for treatment of HBV-positive persons, but have not addressed vaccination of HBV-negative persons (14). We believe that HBV testing in acute-care settings, with linkage to treatment if positive and vaccination if negative, can be an effective model of healthcare delivery in clinical settings with insufficient primary care services.
1.3. Goals of This Investigation
We aimed to explore the concept of HBV screening and vaccination during emergency department visits. The primary outcomes were new HBV diagnoses, as well as vaccination completion rates between screening modalities (POCT vs. laboratory) and timing of vaccination (same vs. different day).
2. Methods
2.1. Study Design and Setting
In this prospective pilot study, we recruited patients who presented to the ED of Hospital Rawson between April and May of 2018. This is a public infectious disease hospital located in Cordoba, Argentina that services a population of approximately 3.3 million people (15). This ED evaluates approximately 120 individuals a day with an admission rate of 6%. The median age of patients seen in the ED at the study institution is 45 years, and 51% of patients seen are women. This study was approved by the medical education committee of Hospital Rawson.
2.2. Selection of Participants
To meet inclusion for the HBV screening portion of our study, individuals had to be ≥18 years and deemed clinically stable by ED staff. As many patients had no documentation of previous vaccination, self-reported previous vaccination was not considered an exclusion criteria, and patients were still offered HBV testing. To meet inclusion for the vaccination portion of our study, individuals had to meet inclusion criteria for the HBV screening portion of the study, have either a negative HBV test result (laboratory or POCT) or no previous history of vaccination (including self-reported), and have no contraindications to vaccination. Following work-up and clearance by the ED provider, the research assistant approached individuals to offer participation in the study. All patients interested in participation underwent informed consent. Patients were recruited on weekdays between the hours of 8am and 5pm.
2.3. Interventions
In order to not disrupt ED workflow and efficiency, patients were screened for HBV using either HBV laboratory or a point-of-care test (POCT) using a practical sampling method. In patients who had a clinical indication for a blood-draw (as determined by the ED provider), the research assistant added HBV serology to their lab orders. In patients who did not have an indication for a blood-draw, the research assistant performed the POCT. This sampling method was chosen in favor of randomization because it preserved ED workflow by preventing the extension of ED patient wait times for patients who did not require blood draws. Point-of-care testing was performed using the PRECHEK Bio. Inc. HBsAg serum strip (Cat. NO. HBV 213, South Korea).
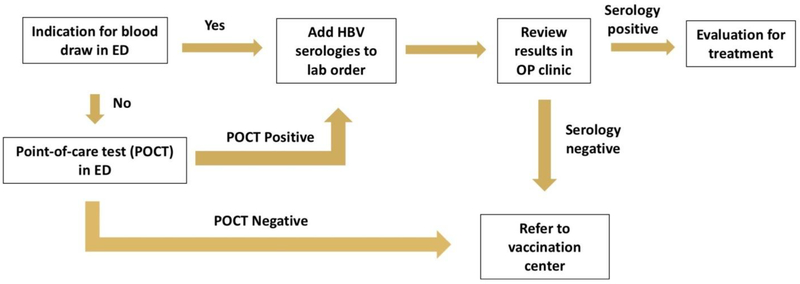
Patients who tested negative for HBsAg using the POCT and who were seen during the vaccination center’s operating hours (8am-2pm) were offered same-day vaccination. The vaccination center was located within the same facility as the ED. Patients who tested negative for HBsAg who were seen outside the vaccination center’s operating hours and patients who tested negative for HBsAg with laboratory testing were offered different-day vaccination. Since the result of HBV laboratory testing typically took one week to return, patients who tested negative for HBV were called one week after testing with instructions to return for vaccination. Patients who did not respond after daily phone calls on three separate days were considered lost to follow-up. A conceptual map of HBV screening and vaccination in the ED is provided in Figure 1.
Figure 1.
Model for hepatitis B virus screening in the emergency department. ED, Emergency Department; HBV, hepatitis B virus; POCT, point-of-care test; OP, outpatient.
2.4. Measurements
Demographic and clinical data were collected using standardized questionnaires at the time of patient enrollment. As the POCTs used in our study gave a result in 10 minutes, this data was recorded immediately by the research assistant. The results of the traditional laboratory testing were recorded by the research assistant when they became available (~typically one week later). The vaccination completion data were acquired by the research assistant after consulting the vaccination center’s digital vaccination registry.
2.5. Outcomes
The primary outcomes were new HBV diagnoses, as well as vaccination completion rates between screening modalities (POCT vs. laboratory) and timing of vaccination (same vs. different day).
2.6. Analysis
Statistical analyses were performed using STATA v15.1 (Statacorp, College Station, TX.).
3. Results
3.1. Characteristics of Study Subjects
We screened 100 patients for HBV. The median age of participants was 35 years (IQR 24–52) and 55% were male. Forty-one percent of individuals reported having no health insurance and 77% reported having no primary care provider. Fifty-six percent of patients reported having a doctor’s visit within the last six months.
3.2. Hepatitis Risk Factors
Twenty-two percent of individuals reported previous HBV testing (all negative), and 13 patients reported previous vaccination. Thirteen individuals presented to the ED for STI testing due to the presence of concerning symptoms or a recent high-risk exposure. Nine individuals reported having surgery or blood transfusions before 1992 [the year Argentina began testing for hepatitis C]. Two individuals reported using intravenous drugs. Thirteen patients had a previous diagnosis of an STI and no subjects had a known history of HBV.
3.3. Screening and Vaccination
Seventy-five individuals were screened for HBV (HBsAg) using the POCT, and 25 were screened with standard HBV testing. None tested positive for HBsAg. Thirteen subjects reported previous vaccination, seven were lost to follow-up and five had a contraindication to vaccination (i.e. active fever). Of the 75 individuals who were eligible for vaccination and/or still engaged in care, 59 (79%) agreed to HBV vaccination. Of those who agreed to vaccination, 16 were scheduled for same-day vaccination and 43 were scheduled for a future date. Those who were scheduled for vaccination the same day were significantly more likely to complete vaccination compared to those scheduled for another day (75% vs. 14%, p<.001, Table 2). All of the subjects that completed vaccination had been screened using the POCT. No patient screened with traditional laboratory testing went on to complete vaccination.
Table 2.
Hepatitis B virus screening and vaccination outcomes
| Outcome | POC Testing (n=75) | Standard Testing (n=25) | All (n=100) |
|---|---|---|---|
| Positive Test Result | 0/75 (0%) | 0/25 (0%) | 0/100 (0%) |
| Vaccination Status | |||
| Previous vacc. | 7/75 (13%) | 6/25 (24%) | 13/100 (13%) |
| Contraindication to vacc. | 4/75 (5%) | 1/25 (4%) | 5/100 (5%) |
| Refused vacc. | 13/75 (17%) | 3/25 (12%) | 16/100 (16%) |
| Lost to follow-up | 0/75 (0%) | 7/25 (28%) | 7/100 (7%) |
| Agreed to vacc. | 51/75 (68%) | 8/25 (32%) | 59/100 (59%) |
| Completed 1st vacc. | 18/51 (35%) | 0/8 (0%) | 18/59 (31%) |
| Date of Vaccine Appointment | |||
| Same day as test | 16/51 (31%) | 0/8 (0%) | 16/59 (21%) |
| Completed 1st vacc. | 12/16 (75%) | - | 12/16 (75%) |
| Different day as test | 35/51 (69%) | 8/8 (0%) | 43/59 (73%) |
| Completed 1st vacc. | 6/35 (17%) | 0/8 (0%) | 6/43 (14%) |
POC, point-of-care; Vacc., vaccination
4. Discussion
In our study, we did not diagnose any new cases of HBV. However, as Argentina is considered a country with low HBV endemicity (~0.7%), this is not surprising (16, 17). The median age of our study population was slightly younger than the median age of the whole ED population (35 vs. 45 years). One explanation is that older patients were more likely to present with unstable conditions, and thus were less likely to fit inclusion criteria for our study. The study population sex ratio was similar to the ED population. Interestingly, while most patients did not have established primary care providers, over half of the study population had seen a physician (non-PCP) within the last 6 months, indicating that most patients seek treatment in acute-care settings. This suggests that ED visits may be an ideal setting in which to provide this population with preventative health services (18).
It is notable that of the 25 patients who were screened using traditional laboratory testing, none completed the first dose of the HBV vaccine. Since the result of laboratory testing took approximately one week, these patients had to be called with the result of their test, and were required to return to the hospital for vaccination. This led to a high rate of loss to follow-up, as scheduling providers were often unable to contact the patient following discharge from the ED. At our institution, the vaccine center was only open from 8am to 2pm, which proved to be a major obstacle, as this was during normal work hours and patients were often unable to take time off from work. Patients who were screened with POCT after the vaccine center closed needed to return at a later date to receive the first dose of their HBV vaccine, leading to a decreased rate of completion. Unsurprisingly, individuals with same day appointments were significantly more likely to complete vaccination. This observation is consistent with non-ED, community-based programs, which have demonstrated that POC infectious disease testing can significantly improve linkage to care (19, 20). Offering subsequent HBV vaccinations (2nd and/or 3rd doses) through the ED could be envisioned if patient volumes and resources allowed, but the feasibility of such a practice would likely be site-specific. Our results, albeit from a small cohort, are important in designing public health programs that target young patient populations, as difficulties with follow-up will require innovative strategies to effectively deliver preventive services.
We believe that the use of POCTs for diseases like HBV facilitates improved linkage-to-care because of the immediacy and actionability of the result. We found that non-use of POCT and limited vaccine center hours were barriers to initiation of vaccination. We believe that even higher rates of linkage-to-care can be achieved if an ED were to stock its own HBV vaccines, rather than directing patients to a separate vaccination area. While this study was performed in a country with a low HBsAg prevalence, we hypothesize that the potential preventative impact of ED-based vaccination programs would be substantially greater in high prevalence areas, where the risk of HBV infection of non-infected individuals is much larger.
As there was often a single research assistant available to enroll patients in the study, and the assistant only worked daytime hours (8am-5pm) on weekdays, ED patient volumes and staff scheduling inhibited our capacity to include all eligible and interested patients beyond these times. Our study did not assess for HBV surface antibody (HBsAb) to reflect HBV immunity, due to poor reliability of commercial rapid tests available in the country. This assessment would have led to a better understanding of those in need of vaccination. It is important to note that HBsAg screening may be negative in patients with resolved infections and those with previous vaccination. While our methodology may result in the vaccination of patients who are already immunized, we believe these patients represent a minority of those screened, and this issue is outweighed by the benefit of vaccinating at-risk, unvaccinated persons. Nonetheless, future studies should include HBsAb if reliable testing is available. Patients with new HBV infections in the “window” period, those with HBsAg mutants and some with chronic HBV infections may have negative HBsAg tests. However, the frequency of individuals in this category is likely to be low.
Our study supports the use of HBV POCTs in the ED in conjunction with vaccination of HBV-negative persons. However, in low-endemicity regions, direct vaccination without HBsAg screening may be more cost effective in high-risk patients who do not present with signs, or symptoms of viral hepatitis or liver disease. Future steps include performing a regional cost-analysis study to determine whether POC testing with subsequent vaccination or direct vaccination without testing, is most practical and affordable. While we believe that the simplicity of our approach makes it transferable and applicable to other resource-limited settings, due to income and resource differences worldwide, our results should be interpreted with caution when implemented in different geographical areas. Larger studies will be needed to further identify barriers for vaccine program development and effectiveness. Lastly, this acute-care screening and linkage-to-care model could be useful in screening for non-communicable diseases such as diabetes (POC HgA1c) and anemia (POC hemoglobin).
Acknowledgments
Grant Support: This work was supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellowship Program at the University of Minnesota to JL, the Robert Wood Johnson Foundation, AFMDP, University of Minnesota Center for Global Health and Social Responsibility Global Health Seed Award and NIH-NCI R21 CA215883-01A1 to JDD.
Footnotes
Conflict of Interest Statement: The authors of this manuscript do not have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].White DA, Anderson ES, Pfeil SK, Trivedi TK, Alter HJ. Results of a Rapid Hepatitis C Virus Screening and Diagnostic Testing Program in an Urban Emergency Department. Annals of emergency medicine. 2016;67:119–28. [DOI] [PubMed] [Google Scholar]
- [2].Evans H, Balasegaram S, Douthwaite S, et al. An innovative approach to increase viral hepatitis diagnoses and linkage to care using opt-out testing and an integrated care pathway in a London Emergency Department. PloS one. 2018;13:e0198520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Orkin C, Leach E, Flanagan S, et al. High prevalence of hepatitis C (HCV) in the emergency department (ED) of a London hospital: should we be screening for HCV in ED attendees? Epidemiology and infection. 2015;143:2837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kelen GD, Green GB, Purcell RH, et al. Hepatitis B and hepatitis C in emergency department patients. The New England journal of medicine. 1992;326:1399–404. [DOI] [PubMed] [Google Scholar]
- [5].Hansoti B, Kelen GD, Quinn TC, et al. A systematic review of emergency department based HIV testing and linkage to care initiatives in low resource settings. PloS one. 2017;12:e0187443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hansoti B, Stead D, Parrish A, et al. HIV testing in a South African Emergency Department: A missed opportunity. PloS one. 2018;13:e0193858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bassett IV, Giddy J, Wang B, et al. Routine, voluntary HIV testing in Durban, South Africa: correlates of HIV infection. HIV medicine. 2008;9:863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. Centers for Disease Control and Prevention; 2008. [Google Scholar]
- [9].Viral Hepatitis” Guide for Medical Teams [Hepatitis Virales: Guia para los equipos de salud]. National Program for Viral Hepatitis, Argentine Ministry of Health 2016. [Google Scholar]
- [10].Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. Journal of hepatology. 2008;48:335–52. [DOI] [PubMed] [Google Scholar]
- [11].Stecher DK; Nathalia, Vizzotti Carla. Hepatitis B en Argentina. Situación actual y estrategia de vacunación universal para su control y eliminación / Hepatitis B in argentina. Current situation and strategy of universal vaccination for control and elimination. Actual SIDA infectol. 2014;22:18–21. [Google Scholar]
- [12].Vladimirsky SNSS, Otegui LO, Altabert NR, Minassian ML, Brajterman LS, González JE. Hepatitis B in Argentina: Cases characterization in the context of universal vaccination. World Hepatitis Summit. Sao Paulo, Brazil 2017. [Google Scholar]
- [13].Abraham MK, Perkins J, Vilke GM, Coyne CJ. Influenza in the Emergency Department: Vaccination, Diagnosis, and Treatment: Clinical Practice Paper Approved by American Academy of Emergency Medicine Clinical Guidelines Committee. The Journal of emergency medicine. 2016;50:536–42. [DOI] [PubMed] [Google Scholar]
- [14].Lemoine M, Shimakawa Y, Njie R, et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: the Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. The Lancet Global health. 2016;4:e559–67. [DOI] [PubMed] [Google Scholar]
- [15].Statistics) INdEyCNIoC. Proyecciones provinciales de poblacion por sexo y grupos de edad. (Provincial population projections by sex and age). In: MdEyPMoEa Industry, editor. Buenos Aires, Argentina2012. [Google Scholar]
- [16].Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet (London, England). 2015;386:1546–55. [DOI] [PubMed] [Google Scholar]
- [17].Patricia Angeleri EC, Joaquín Solari, Gabriela Vidiella,, Silvina Vulcano MB, Carlos Giovacchini,, Maria Pía Buyayisqui JA, Teresa Varela,, Herrmann J. Hepatitis Virales: Guia para los equipos de salud / Viral Hepatitis: A Guide for Medical Teams. In: Health Mo, editor.2016. [Google Scholar]
- [18].Thomas MC, Ademolu AO. Considerations for vaccine administration in the emergency department. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2014;71:231–6. [DOI] [PubMed] [Google Scholar]
- [19].Morano JP, Zelenev A, Lombard A, Marcus R, Gibson BA, Altice FL. Strategies for hepatitis C testing and linkage to care for vulnerable populations: point-of-care and standard HCV testing in a mobile medical clinic. Journal of community health. 2014;39:922–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. Journal of the International AIDS Society. 2014;17:18809. [DOI] [PMC free article] [PubMed] [Google Scholar]