Abstract
Recently, dimethyl fumarate (DMF) and Korean red ginseng (Ginseng), based on their purported antioxidative and anti-inflammatory properties, have exhibited protective potential in various neurological conditions. Their effects on cerebral ischemia and underlying mechanisms remain inconclusive; however increasing evidence indicates the involvement of the transcriptional factor Nrf2. This study evaluated the preventive effects of DMF and Ginseng on hippocampal neuronal damage following hypoxia-ischemia (HI) and assessed the contributions of reactive gliosis and the Nrf2 pathway. Adult wildtype (WT) and Nrf2−/− mice were pretreated with DMF or Ginseng for 7 days prior to HI. At 24 h after HI, DMF or Ginseng significantly reduced infarct volume (52.5±12.3% and 47.8±10.7%), brain edema (61.5±17.4% and 39.3±12.8%), and hippocampal CA1 neuronal degeneration, and induced expressions of Nrf2 target proteins in WT, but not Nrf2−/−, mice. Such hippocampal neuroprotective benefits were also observed at 6 h and 7 days after HI. The dynamic attenuation of reactive gliosis in microglia and astrocytes correlated well with this sustained neuroprotection in an Nrf2-dependent manner. In both early and late stages of HI, astrocytic dysfunctions in extracellular glutamate clearance and water transport, as indicated by glutamine synthetase and aquaporin 4, were also attenuated after HI in WT, but not Nrf2−/−, mice treated with DMF or Ginseng. Together, DMF and Ginseng confers robust and prolonged Nrf2-dependent neuroprotection against ischemic hippocampal damage. The salutary Nrf2-dependent attenuation of reactive gliosis may contribute to this neuroprotection, offering new insight into the cellular basis of an Nrf2-targeting strategy for stroke prevention or treatment.
Keywords: Astrocyte, Astrogliosis, Microglia, Oxidative stress, Stroke, Transcriptional factor
Introduction
Cerebral ischemia is one of the leading causes of death and disability worldwide. Despite its global ramifications, effective clinical intervention remains limited [1]. This incongruity catalyzes exploration of novel neuroprotection strategy. To this end, there is a great need to study natural brain repair processes and endogenous neuroprotective mechanisms following ischemic insult, which remains an enormous challenge for translational and clinical research [2–5]. Multiple lines of evidence from our laboratory and others indicate that the transcriptional factor Nrf2 plays an important role in cellular defense against oxidative stress and inflammation [6], two key pathophysiological mechanisms that are involved in cerebral ischemia initiation, progression, and repair; consequently, targeting Nrf2 has emerged as a promising strategy for stroke prevention or reversal [7–12]. However, the exact regulatory mechanism of Nrf2 activation in stroke attack and neuroprotection remains inconclusive. Ischemic injury evokes an extensive glial cell response referred to as reactive gliosis that impacts neuronal function [3, 13]. Gliosis occurring after central nervous system (CNS) insults, mainly involves activated microglia and astrocytes characterized by hypertrophic and proliferating astrocytes, and proliferating microglia [14]. Its contribution to the pathophysiological process of stroke, particularly associated with Nrf2-mediated endogenous protection, is of increasing interest in better understanding and ameliorating ischemic affliction [15, 16].
Our laboratory has been interested in exploring the Nrf2-targeted neuroprotective strategy against stroke. Recently, both dimethyl fumarate (DMF) and Korean red ginseng (Ginseng), based on their antioxidative and anti-inflammatory properties, have revealed promising protective potential in various neurological conditions [17–21]. DMF is the dimethyl ester derivative of fumaric acid and was approved by the Food and Drug Administration for multiple sclerosis in 2013 and psoriasis in 1994 [21, 22]. Ginseng has been used in traditional herbal medicine for thousands of years in East Asia and is the most widely used medicinal and nutritional supplement worldwide [23–25]. Ginseng, extracted from the root of Panax ginseng C.A. Meyer, exhibits a potential protective efficacy in animal and human studies [26, 27]. Their effects on cerebral ischemia and underlying cellular and molecular mechanisms are still inconclusive; although, increasing evidence suggest the involvement of Nrf2. Glial cells play a pivotal role in antioxidant and anti-inflammation defenses, much of which are regulated by Nrf2; nevertheless, mounting evidence implies that Nrf2 target genes are particularly enriched in glial cells [7, 9].
Cerebral ischemia initiates complex pathophysiological events that ultimately result in brain damage and functional impairment, alternations involving mobilization of resident cells like glial cells, accumulation of oxidants and production of inflammatory mediators, and interaction between endogenous protective mechanisms and the ischemic noxious cascade [2, 13, 15]. Endogenous protective mechanisms by which the brain prevents itself from noxious stimuli and repairs the ischemic damage are critical targets for stroke research [28–30]. The pharmacological pretreatment for ischemic attack is expected to, like ischemic preconditioning, elicit a cascade involving the boosting of an intrinsic endogenous protective process that rescues the brain from a severe ischemic insult and eventually promotes recovery [4, 31]. Such process-induced neuroprotection contains a rapid phase and a delayed phase [30, 32, 33]. The rapid phase occurs instantly after a stimulus and lasts hours through interfering RNA, phosphorylation targets, and transporter regulation, and the delayed phase occurs after a delay of 1 to 3 days by action on gene activation and de novo protein synthesis.
In this study, by using a cerebral hypoxia-ischemia (HI) mouse model and transgenic loss-of-function of Nrf2 mice, we hypothesized that pretreatment with DMF or Ginseng sustainably protects, in an Nrf2-dependent manner, against ischemic hippocampal neuronal damage through consecutive attenuation of reactive gliosis progression. By working under this assumption, we will answer the following questions in this study: (1) Does pretreatment with DMF or Ginseng protect against ischemic damage in initial, acute, and extended phases after HI; (2) what is the role of the Nrf2-dependent pathway in this process and (3) does reactive gliosis, including astrocytic function in regulation of glutamate metabolism and water homeostasis, involve Nrf2-dependent neuroprotective mechanisms? Additionally, in proof-of-principle experiments, (4) we explore the specific contribution of reactive gliosis progress and Nrf2-mediated endogenous protection during ischemic onset, progress, and repair in the context of an HI mouse model.
Materials and Methods
Animals
All procedures were approved by the University of Florida Institutional Animal Care and Use Committee. We conducted the experiments according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The Nrf2−/− mice were generated as described [12, 23]. Cohorts of adult Nrf2−/− and matched wildtype (WT) C57BL/6 male mice (10–18 weeks old) were used for this study.
Cerebral Hypoxia-Ischemia (HI) Model
Mice were subjected to cerebral hypoxia-ischemia as described before [34], and this transient unilateral HI model generated a reproducible ischemic lesion in the ipsilateral hemisphere. Each mouse was anesthetized with 2% to 4% isoflurane during surgery. The right side of the common carotid artery was separated and occluded permanently with a 6–0 nylon suture, and the incision was closed. Artificial tear ointment was used for protection and lubrication. The mouse was allowed to recover for 2 h with free access to water and food. The mouse was exposed to hypoxia (8% O2/balance N2) in a Plexiglas chamber for 1 h and was then taken back to its home cage. Sham surgery (sham) animals underwent anesthesia followed by exposure of the artery without ligation or hypoxia. The surgeon was blinded to the treatment and genotype.
Pretreatment with Dimethyl Fumarate (DMF) or Korean Red Ginseng (Ginseng)
Vehicle (double-distilled water), DMF (Sigma, St. Louis, MO; 100 mg/kg/day, suspended in 0.08% methylcellulose), or Ginseng (the commercially available standardized Korean red ginseng extract was kindly provided by Dr. Hocheol Kim; 100 mg/kg/day, dissolved in double-distilled water) was orally administered for 7 days to C57BL/6 WT and Nrf2−/− mice prior to HI [18, 19]. The Ginseng is a water-soluble extract that was prepared and standardized [23, 27].
Evaluation of Infarct Volume and Brain Edema
Brain infarct volume and brain edema were measured by 2,3,5-triphenyltetrazolium chloride (TTC) [35, 36]. Mouse brains were sliced into 1-mm-thick coronal sections with a brain matrix and were then incubated in a 2% TTC solution (Sigma-Aldrich) at 37°C for 20 min. The infarct size per section was delineated and analyzed using image analysis software (ImageJ, National Institutes of Health, Bethesda, MD). To minimize the distortion of the infarct area by brain edema, an indirect method was selected for calculating infarct volume. Corrected infarct volume (%) = [volume of contralateral hemisphere – (volume of ipsilateral hemisphere – volume of infarct)] / volume of contralateral hemisphere × 100. The experimenter for the analysis of infarct volume was blinded to treatment and genotype. Edema volume (%) = [(volume of ipsilateral hemisphere – volume of contralateral hemisphere) / volume of contralateral hemisphere] × 100.
Experimental Design
The overall experimental design for this study is as follows. For each cohort in this study, the mice were randomly distributed into groups of both genotypes and treatments. Mice were blinded to experimenters. Experiment I: Twenty-four h after HI, infarct volume and brain edema were evaluated by TTC staining (n=4–6 per group), mRNA levels of hippocampal CA1 region were measured by quantitative real-time PCR (n=3–4 per group), and neuronal loss and other immunostaining markers in the hippocampal CA1 region were examined by Cresyl violet (CV) staining and immunostaining with corresponding antibodies (n=4–5 per group). Experiment II: Six h after HI, neuronal injury and other immunostaining markers in the hippocampal CA1 region were examined by CV staining and immunostaining with corresponding antibodies (n=3–5 per group). Experiment III: Seven days after HI, the same markers as in Experiment II were measured (n=5–7 per group).
Histology and Immunohistochemistry
Staining was performed on frozen coronal sections as described previously [37]. Mice were anesthetized and transcardially perfused with PBS (pH 7.4) and 4% paraformaldehyde (pH 7.4). Brains were collected, post-fixed, cryoprotected in 30% sucrose solution (w/v), and sliced into 30-μm-thick coronal sections with a rotary microtome cryostat (Leica Biosystems, Buffalo Grove, IL). Neuronal death and degeneration in the hippocampal CA1 region were examined by CV staining according to the manufacturer’s protocol (IHC World, Woodstock, MD).
CV stains the Nissl substance (i.e., granular endoplasmic reticulum and ribosomes that occur in the soma and dendrites) of neurons and displays dark blue, making the cytoplasm appear mottled. These features were weakened or disappeared in degenerated or dead neurons. CV-positive pyramidal cells (large in size, strongly stained) were quantified in a 250-×250-μm square. Three consecutive sections of each brain were analyzed and given a single value for each mouse. Immunostaining studies were performed using standard protocols. The primary antibodies were rabbit polyclonal ionized calcium-binding adapter protein 1 (Iba1; 1:5000, Wako Bioproducts, Richmond, VA), rabbit polyclonal glial fibrillary acidic protein (GFAP; 1:3000; DAKO, Carpinteria, CA), mouse monoclonal glutamine synthetase (GS; 1:3000; EMD Millipore, Billerica, MA), and rabbit polyclonal aquaporin 4 (AQP4; 1:3000; EMD Millipore). Coronal sections were incubated with the appropriate secondary antibodies. The immunoreaction was visualized by 3,3-diaminobenzidine chromogen solution (DAB substrate kit; Vector Laboratories, Burlingame, CA). The immunoreactive intensity of Iba1, GFAP, GS, or AQP4 in CA1 hippocampal sub-region was quantified. Three consecutive sections at Bregma −1.70 ~ −2.06 were quantified and provided a single value for each mouse. The image was captured using ScanScope CS and analyzed using ImageJ software (National Institute of Health) or ImageScope Software (Aperio Technologies, Vista, CA).
Statistical Analysis
The statistical analyses were performed using PRISM software (GraphPad, La Jolla, CA). Multiple comparisons were carried out using a two-way ANOVA followed by a Bonferroni post hoc test or using a one-way ANOVA followed by a Tukey-Kramer post hoc test. All data are expressed as group mean±SEM and P ≤ 0.05 is considered statistically significant. Results were analyzed by investigators blinded to treatments and genotypes.
Results
Pretreatment with DMF or Ginseng Reduces Ischemic Infarct Volume, Brain Edema, and Hippocampal CA1 Neuron Loss 24 h after HI but not under Nrf2−/− Deficiency.
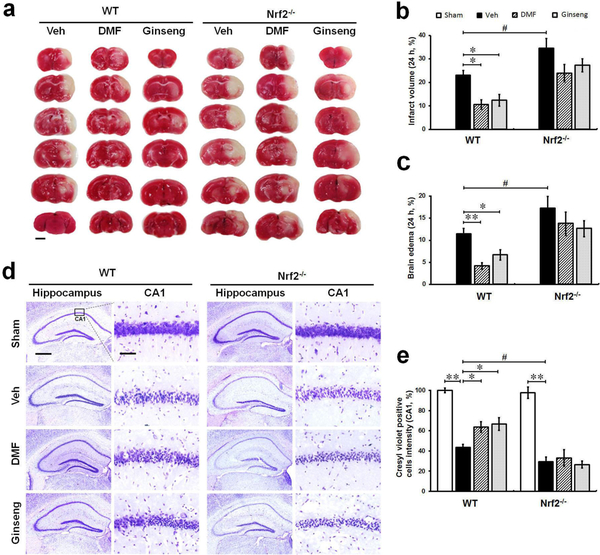
We first examined whether pretreatment with DMF or Ginseng could reduce ischemic infarct volume, brain edema, and hippocampal CA1 neuron loss 24 h after HI and whether these effects could be lost under deficiency (Fig. 1). WT and Nrf2−/− mice were pretreated for 7 days with DMF or Ginseng and were subsequently subjected to HI. Gross infarct volume and brain edema, estimated by TTC staining, was significantly reduced in ischemic WT mice (infarct volume: 10.37±1.95%, 12.11±2.43% vs 23.34±3.17%, P<0.05; brain edema: 4.05±0.74%, 6.82±1.14% vs 12.27±2.03%, P<0.01 and P<0.05; Fig. 1 a–c) but not Nrf2−/− mice (infarct volume: 22.37±6.65%, 28.59±2.48% vs 33.78±5.26; brain edema: 14.35±3.93%, 12.729±2.88% vs 16.72±3.14%; Fig. 1a–c) pretreated with DMF or Ginseng, whereas Nrf2 deficiency exacerbated such ischemia-induced damage after HI compared to corresponding WT controls. In normal conditions, grossly abnormal anatomy in the hippocampal CA1 neurons of WT and Nrf2−/− mice was absent. Ischemia led to a profound decrease in surviving cells in the pyramidal layer, which is more evident in Nrf2−/− mice as indicated by CV-positive cells intensity. Consistent with the results above, pretreatment with DMF or Ginseng significantly decreased ischemia-induced hippocampal CA1 neuronal loss and degeneration in WT mice (CV-positive cells: 63.76±5.34%, 66.66±6.33% vs 43.57±3.10%, P<0.05; Fig. 1d–e) but, more importantly, not in Nrf2−/− mice (CV-positive cells: 33.15±8.27%, 26.55±3.56% vs 29.39±4.63%; Fig. 1d–e), whereas Nrf2 deficiency itself exacerbated the deterioration of neuron loss compared to the corresponding WT control group. These data reveal the robust protective efficacy of pretreatment with DMF or Ginseng on acute ischemic brain damage and hippocampal CA1 neuron injury. Notably, such neuroprotective efficacy was not detected in Nrf2−/− mice, suggesting the possible involvement of the Nrf2 pathway in such a neuroprotective process. In addition, these findings also confirm the protective role of Nrf2 on ischemic damage under HI.
Fig. 1. Pretreatment with DMF or Ginseng reduces ischemia-induced infarct volume, brain edema, and hippocampal CA1 neuron loss 24 h after HI in WT mice, but not in Nrf2−/− mice.
(a) Representative images of TTC-stained brain sections (n=4–6 per group). (b and c) Quantitative analyses of infarct volume and brain edema. (d) Representative images of CV-stained hippocampus (n=3 per sham group and n=4–5 per ischemic group). (e) Quantitative analyses of hippocampal CA1 neurons indicated by CV staining. 24 h after HI, ischemia-induced infarct volume, brain edema, and hippocampal CA1 neuronal loss were significantly reduced in WT, but more importantly, not in Nrf2−/− mice pretreated with DMF or Ginseng, whereas Nrf2 deficiency significantly exacerbated the deterioration process. *P<0.05, **P<0.01, #P<0.05. TTC: 2,3,5-triphenyltetrazolium chloride; CV: Cresyl violet. Scale bar, 2 mm (a), 500 μm (d, hippocampus) and 50 μm (d, CA1 sub-region).
Protein Expression Profile Induced by Pretreatment with DMF or Ginseng Suggests Activation of Nrf2 and Antioxidant Activity.
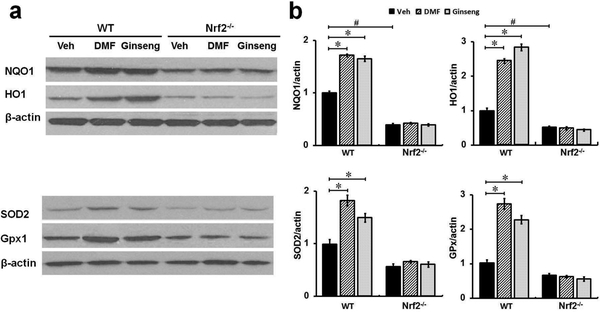
Nrf2 is a major regulator of redox homeostasis through a battery of cytoprotective genes in neurological conditions [11]. To examine whether Nrf2 activation contributes to the neuroprotection by pretreatment with DMF or Ginseng, we measured and contrasted the Nrf2 target proteins expression pattern between WT and Nrf2−/− mice. As shown in Fig. 2a, b, ipsilateral hippocampal region extracts were subjected to Western blot analysis. The results revealed that, compared to the corresponding controls 24 h after HI, Nrf2 target antioxidant proteins NQO1, HO1, GPx1, and SOD2 were significantly upregulated in WT mice but, more importantly, not in Nrf2−/− mice pretreated with DMF or Ginseng, supporting the vital role of the Nrf2-dependent pathway underlying the neuroprotection. In particular, these Nrf2-regulated alterations by pretreatment with DMF or Ginseng were not observed under Nrf2 deficiency indicated by similar expression levels in all ischemic Nrf2−/− mice groups, further emphasizing the Nrf2-dependent manner of their beneficial effects. The protein profile assessment strongly supported the Nrf2-dependent neuroprotective mechanism in this process.
Fig. 2. In WT but not Nrf2−/− mice pretreated with DMF or Ginseng, Nrf2 target antioxidant protein are significantly increased 24 h after HI.
(a, b) At 24 h after HI, the tissue from the ipsilateral hippocampal CA1 region was used for analysis of Nrf2 target proteins levels. Pretreatment with DMF or Ginseng significantly increased the expression levels of Nrf2 target antioxidant proteins NQO1, HO1, GPx1, and SOD2. More importantly, such benefits were not detected under Nrf2 deficiency. n=3–4 per group. *P<0.05, #P<0.05.
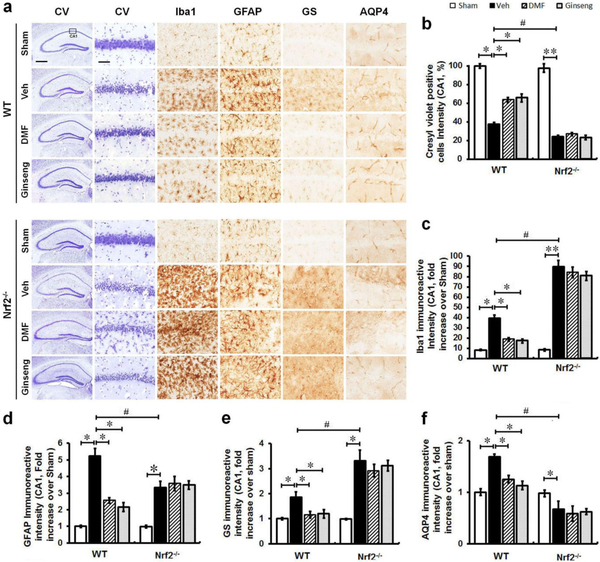
Pretreatment with DMF or Ginseng Attenuated Ischemia-induced Reactive Gliosis in the Hippocampal CA1 Region, but not under Nrf2−/− Deficiency.
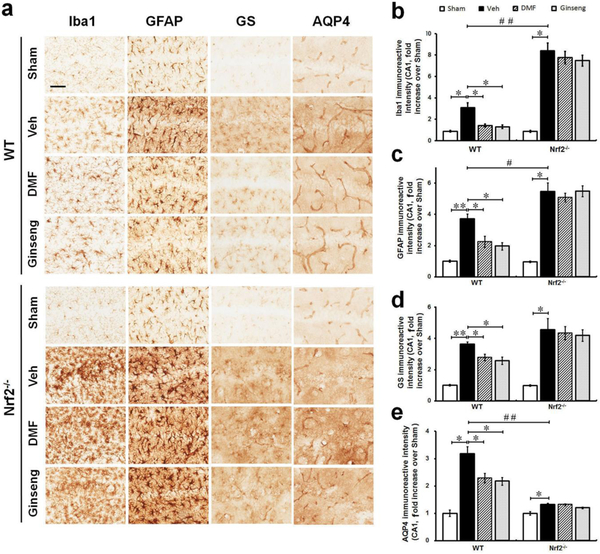
Reactive gliosis, which mainly refers to the biochemical, morphological, and functional changes in microglia and astrocytes, is often a prominent feature of cerebral ischemia, and exhibits critical functions in ischemic onset, progression, repair, and neuroprotection [3]. To examine the roles of glial cells, associated with Nrf2-mediated protection, in the neuroprotection of pretreatment with DMF or Ginseng against acute ischemic damage, the phenotypes of microglia and astrocytes were examined by immunohistochemical staining in the hippocampal CA1 region 24 h after HI (Fig. 3a–e). These include astrocytic function on regulating glutamate metabolism and water homeostasis. Microglia, which respond quickly to a brain insult, are the main cell type in ischemia-induced neuroinflammation. In normal conditions, microglia display a small soma with high branches and distribution in a non-overlapping fashion in both WT and Nrf2−/− mice. It is shown by Iba1 immunostaining that, when compared with sham controls, ischemic injury triggered significant activation of microglia characterized by hypertrophic soma with thickened and retracted processes, which was more obvious in Nrf2−/− mice. Pretreatment with DMF or Ginseng significantly attenuated ischemia-induced microglia activation in WT mice, but not in Nrf2−/− mice. In the sham mice, as indicated by GFAP antibody, astrocytes tiled the whole CA1 region in a pattern of regular distribution in both genotypes, most exhibiting a nonreactive status with small soma and fine processes. In both WT and Nrf2−/− mice, ischemia evoked astrocytic activation and proliferation 24 h after HI as indicated by the significant increase in the immunoreactivity of astrocytes, with a larger proportion of reactive astrocytes with hypertrophic soma and highly stained processes. Comparably, a more severe condition of reactive astrocytes was detected in Nrf2−/− mice than in WT mice, suggesting more severe deterioration triggered by Nrf2 deficiency. In comparison, pretreatment with DMF or Ginseng significantly reduced the drastic growth in the immunoactivity of GFAP-positive cells in WT mice, but not in Nrf2−/− mice. Astrocytes govern extracellular levels of glutamate via GS and maintain brain water homeostasis through AQP4 that greatly influences ischemic damage and repair. The expression level of GS was significantly reduced in WT mice but not in Nrf2−/− mice pretreated with DMF or Ginseng. Pretreatment with DMF or Ginseng significantly protected against the sharp decline of AQP4 expression level in WT mice but not in Nrf2−/− mice. In contrast, the Nrf2−/− mice exhibited a significantly lower AQP4 expression level compared to WT mice, signifying the importance of Nrf2 in water transport during HI hippocampal injury. These findings indicate that pretreatment with DMF or Ginseng attenuated hippocampal CA1 reactive gliosis in a seemingly Nrf2-dependent manner at 24 h following HI, which may contribute to the neuroprotection. In addition, Nrf2 deficiency exacerbated ischemia-induced reactive gliosis in our setting, implying that the reactive gliosis is possibly involved in the Nrf2-mediated neuroprotective process.
Fig. 3. Pretreatment with DMF or Ginseng attenuates ischemia-induced reactive gliosis in microglia and astrocytes, including astrocytic dysfunction of glutamate metabolism and water homeostasis 24 h after HI in WT but not Nrf2−/− mice.
(a) Representative images of Iba1, GFAP, GS, and AQP4 stained hippocampal CA1 regions (n=4–5 per group). (b–e) Quantitative analyses of (a). In normal conditions, microglia and astrocytes tiled the whole CA1 region in a regular distribution pattern in both WT and Nrf2−/− mice, mostly exhibiting a nonreactive status with small soma and fine processes. Ischemia triggered apparent proliferation 24 h after HI as indicated by increased immunoreactivity of glial cells, with hypertrophic soma and highly stained processes. Pretreatment with DMF or Ginseng significantly attenuated such process in WT mice, but failed to do so in Nrf2−/− mice. In addition, pretreatment with DMF or Ginseng significantly protected against the sharp increase of GS and overt decline of AQP4 expression levels in WT but not Nrf2−/− mice. n=4–5 per group. *P<0.05, **P<0.01, #P<0.05, ##P<0.01. Iba1, ionized calcium-binding adapter protein 1; GFAP, glial fibrillary acidic protein; GS, glutamine synthetase; AQP4, aquaporin 4. Scale bar, 50 μm.
The Early Phase of Ischemia-induced Reactive Gliosis and Hippocampal CA1 Neuronal Injury is Profoundly Mitigated by Pretreatment with DMF or Ginseng, whereas Loss of Nrf2 Reduces such Protection.
To further understand such neuroprotective properties on ischemia initiation, we sought to determine whether pretreatment with DMF or Ginseng could confer prompt neuroprotection against the very early phase of ischemic injury on hippocampal CA1 pyramidal neurons, which are extremely vulnerable to ischemic insults. For this, we examined neuronal death and degeneration 6 h after HI onset by CV staining of nearby sections (Fig 4a and b). After the onset of ischemia, both genotypes of mice displayed apparent neuronal death, which was more evident in Nrf2−/− mice, indicating the protective role of Nrf2 in ischemia after HI. Neuronal death and degeneration was significantly abated in the WT mice but not in Nrf2−/− mice pretreated with DMF or Ginseng, indicating that the rapid neuroprotection properties conferred by pretreatment with DMF or Ginseng were also Nrf2-dependent. To further examine the natural properties of reactive gliosis in this Nrf2-dependent neuroprotection 6 h after HI, the structural and functional changes of microglia and astrocytes in the initial phase of ischemia were analyzed (Fig 4a, c–f). Both immunoactivities of Iba-1- and GFAP-positive cells were significantly reduced in the WT mice but not in the Nrf2−/− mice pretreated with DMF or Ginseng, whereas Nrf2 deficiency itself dramatically increased these progressions. Morphologically, pretreatment with DMF or Ginseng apparently reduced the activation of astrocytes following ischemia, revealed by the small soma and fine processes in WT mice, and failed to do so in Nrf2−/− mice. In the very early phase of ischemia, both GS and AQP4 expression levels were notably increased, which was more evident in Nrf2−/− mice. In comparison, pretreatment with DMF or Ginseng significantly suppressed both GS and AQP4 immunostaining signals in WT mice but not in Nrf2−/− mice, while Nrf2 deficiency itself led to significant increases. This pattern of GS and AQP4 staining, along with the morphological features of microglia and astrocytes, correlated well with the early phase of ischemic damage and neuroprotection. These results provide further evidence for the contribution of reactive gliosis progression in the hippocampal CA1 region, associated with Nrf2-dependent mechanisms, in the neuroprotection process by pretreatment with DMF or Ginseng in the very early phase of ischemia under HI.
Fig. 4. The early phase of reactive gliosis and ischemia-induced neuronal injury are profoundly mitigated by pretreatment with DMF or Ginseng, whereas Nrf2 deficiency reduces such effects.
(a) Representative images of CV-, Iba1, GFAP, GS, and AQP4 stained hippocampal CA1 regions at 6 h after HI (n=4–5 per group). (b–f) Quantitative analyses for (a). The early phase of ischemia-induced hippocampal CA1 neuronal injury was significantly attenuated in WT but not Nrf2−/− mice pretreated with DMF or Ginseng, whereas Nrf2 deficiency exacerbated such an injury. Consistently, reactive gliosis in microglia and astrocytes including astrocytic dysfunction of glutamate metabolism and water homeostasis was significantly reduced by pretreatment with DMF or Ginseng, but not under Nrf2 deficiency, as revealed by the intensity of Iba1, GFAP, GS, and AQP4 staining. *P<0.05, #P<0.05. CV: Cresyl violet; Iba1, ionized calcium-binding adapter protein 1; GFAP, glial fibrillary acidic protein; GS, glutamine synthetase; AQP4, aquaporin 4. Scale bar, 500 μm (a, hippocampus) and 50 μm (a, CA1 sub-region).
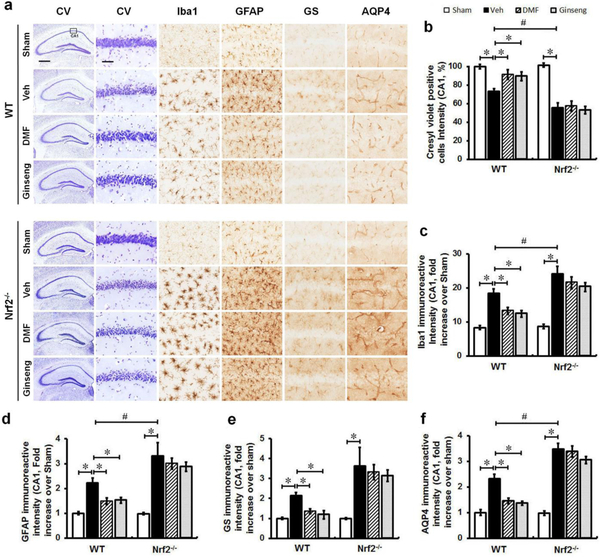
The Attenuated Reactive Gliosis Contributed to the Late-Stage of Nrf2-dependent Neuroprotection by Pretreatment with DMF or Ginseng, but not under Nrf2 Deficiency.
Finally, while the above studies revealed that the pretreatment is remarkably neuroprotective against acute ischemic neuronal deterioration, it could be more therapeutically relevant if such neuroprotection could be extended to the late phase of ischemia because long-term evaluation is a key component for testing the efficacy of potential therapeutics in translational stroke research. We therefore sought to examine whether such Nrf2-dependent neuroprotective efficacy on hippocampal neuron damage could be sustained to 7 days after HI (Fig. 5a–f). Indeed, compared to 24 h after HI, there was sustained hippocampal neuron damage in ischemic mice 7 days after HI, revealed by the density of hippocampal CA1 neurons. In particular, pretreatment with DMF or Ginseng dramatically protected against the damage in WT mice, but failed in Nrf2−/− mice, while ischemic Nrf2−/− still displayed worse conditions compared to corresponding WT controls. These findings suggest that pretreatment with DMF or Ginseng has long-lasting neuroprotective efficacy against ischemic hippocampal CA1 neuron damage after HI in an Nrf2-dependent manner and confirm the critical role of Nrf2 activation and its important effect on reactive gliosis in ischemia progression and repair in our setting. Meanwhile, ischemic glial cells appeared to experience prolonged activation and proliferation; comparably, Nrf2−/− mice exhibited much worse reactive gliosis conditions when compared with ischemic WT mice. In addition, we found that both immunoactivities of Iba-1- and GFAP-positive cells were significantly lower in WT mice but not in Nrf2−/− mice pretreated with DMF or Ginseng. Comparably, more degenerated astrocytes with broken down cell bodies and relatively lower overall expression were detected in Nrf2−/− mice than in WT mice, suggesting more severe deterioration triggered by Nrf2 deficiency. Interestingly, in WT but not Nrf2−/− mice pretreated with DMF or Ginseng, AQP4 and GS expression levels were significantly lower, correlating well with the attenuated reactive gliosis. The above findings further support our hypothesis that the attenuated reactive gliosis pattern correlated well with the long-lasting neuroprotection by pretreatment with DMF or Ginseng in an Nrf2-dependent manner.
Fig. 5. The attenuated reactive gliosis contributed to the late-stage of Nrf2-dependent neuroprotection by pretreatment with DMF or Ginseng, but not under Nrf2 deficiency.
(a) Representative images of CV-positive neurons in hippocampal CA1 neurons. (b–f) Quantitative analyses of (a). It appears that in the late phase of ischemia, hippocampal CA1 injury had a slight recovery 7 days after HI. After pretreatment with DMF or Ginseng, WT but not Nrf2−/− mice display a significantly better recovery process, as revealed by the average density of hippocampal CA1 neurons. However, Nrf2 deficiency significantly worsened the injury compared to ischemic WT controls. n=5–7 per group. *P<0.05, #P<0.05. CV: Cresyl violet; Iba1, ionized calcium-binding adapter protein 1; GFAP, glial fibrillary acidic protein; GS, glutamine synthetase; AQP4, aquaporin 4. Scale bar, 500 μm (a, hippocampus) and 50 μm (a, CA1 sub-region).
Discussion
The present study shows that pretreatment with DMF or Ginseng elicited robust and prolonged neuroprotection against hippocampal neuronal loss over a 7-day observation period of ischemia after HI in WT mice, but not in Nrf2−/−, demonstrating the involvement of an Nrf2-dependent mechanism in this neuroprotection. The WT mice but not Nrf2−/− pretreated with DMF or Ginseng indeed showed increased expression levels of Nrf2 target antioxidant proteins in the hippocampal CA1 region after HI, further supporting their beneficial effects in an Nrf2-dependent manner. The temporal pattern of attenuated reactive gliosis progression including abated dysfunction of glutamate metabolism and perturbed water transport in WT mice but not Nrf2−/− pretreated with DMF or Ginseng correlated well with such an Nrf2-dependent neuroprotection process. In addition, at different phases of ischemia, Nrf2−/− mice displayed exacerbated ischemic damage and deteriorated reactive gliosis progression in the ischemic hippocampus after HI, further highlighting the important role of Nrf2-dependent endogenous neuroprotection that is closely associated with glial cells by reactive gliosis in the context of ischemia under HI.
Pretreatment with DMF or Ginseng Reveals Robust and Prolonged Neuroprotective Properties against Ischemic Damage in an Nrf2-dependent Manner.
Recently, both DMF and Ginseng have gained considerable attention for their pharmacological properties that cannot only scavenge excessive free radicals but also boost the endogenous protective mechanism such as cellular antioxidant capacity by inducing de novo synthesis of antioxidant enzymes, potentially preventing the brain from subsequent severe damage [17]. We opted for pretreatment with DMF or Ginseng orally for several reasons, principally to prevent the surge in free radicals by boosting the endogenous antioxidant capability. The penumbral brain tissues are viable and salvageable when compared with the irreversible ischemic core insult, therefore emerging as one primary target for preventive treatment [38, 39]. We proposed that both DMF and Ginseng not only could enhance the brain’s resistance to acute ischemic insult, but also extend their beneficial effects to later phases after HI. The ischemic cascade usually continues for hours but can persist for days, even after the restoration of blood circulation [6]. Indeed, acute ischemic insult quickly led to a progressive deterioration of hippocampal CA1 neurons and peaked at 24 h, followed by gradual progression within 7 days after HI. In agreement with our assumption, pretreatment with DMF or Ginseng exhibited robust and sustained neuroprotective efficacy at different times, extents, and types. It essentially prevents the brain from acute ischemic insult possibly through limiting stroke initiation and rapid deteriorative processes, indicated by a significant reduction of the prompt deteriorative progression of hippocampal neuronal loss from 6 to 24 h after HI, and such favorable effects extended to the 7-day observation time. Nevertheless, demonstration of long-term protection is more meaningful for potential future use in humans. These data are consistent with recent reports that revealed the neuroprotective efficacy of DMF or Korean red ginseng in different stroke models, but all were conducted through post-stroke administration [21, 40–43]. Conversely, the Nrf2−/− mice are significantly more prone to ischemic insults than WT mice, as the loss of Nrf2 renders animals more vulnerable to various stressors through the failure to induce downstream phase II enzymes. Importantly, such beneficial effect was shown in an Nrf2-dependent manner by contrasting the outcomes between WT and Nrf2−/− groups. Here, we also need to point out that there is another possibility of an Nrf2-independent mechanism underlying this neuroprotection that was concealed by excessive severe ischemic damage induced by the lack of Nrf2.
The Nrf2-dependent Pathway Constitutes the Neuroprotection Mechanism.
Accumulated evidence shows that Nrf2 is a master regulator of redox homeostasis and inflammation during normal or pathological conditions by inducing the expression of a large variety of cytoprotective and detoxification genes encoding for phase II defense enzymes and antioxidant stress proteins [6, 8, 10, 11, 44]. The noted resistance of Nrf2 from oxidative stress-induced neurotoxicity is especially important for brain tissue that is not well equipped with antioxidant defenses. After activation, Nrf2 specifically regulates genes bearing an antioxidant response element within their promoter region like NQO1, HO1, GPx1, and SOD2, which control redox homeostasis. Our in vivo data reveals the Nrf2-dependent neuroprotective effects by DMF or Ginseng on hippocampal neuron damage in the context of ischemia under HI. Further studies will be helpful to demonstrate the clarify the cellular mechanism underlying the neuroprotection.
The Temporal Pattern of Attenuated Reactive Gliosis Progression Correlates well with Nrf2-dependent Neuroprotection.
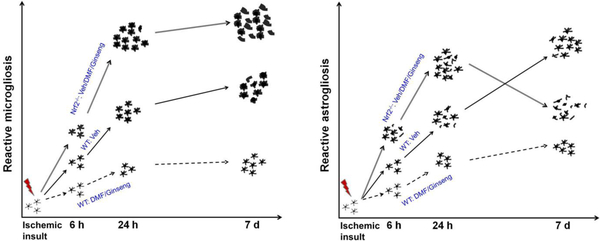
Glial cells are crucial for maintaining brain homeostasis, including energy metabolism, synaptic transmission, extracellular glutamate level, potassium buffering, and water homeostasis in various CNS conditions. In response to CNS insults, reactive microglia and astrocytes display hypertrophy, proliferation, and migration. It has been reported that many Nrf2 target antioxidant and detoxification genes are preferentially expressed in glial cells [45]; therefore, activation of Nrf2 may be closely associated with the reactive gliosis progression in the pathophysiology of cerebral ischemia, strongly suggesting the potential involvement of glial cells in Nrf2-dependent neuroprotection. Although studies have reported the vital role of glial cells in the pathophysiological progression of CNS injury, little is known about their contribution, associated with Nrf2 activation, in the context of ischemia under HI, and if this process contributes to the neuroprotection of pretreatment with DMF or Ginseng. Cerebral ischemia causes acute-phase cellular-to-brain injury within hours and late-phase brain damage that continues in the days after ischemia [39]. As we summarized in Fig. 6, during the acute deterioration of neuronal damage, pretreatment with DMF or Ginseng apparently attenuated the early and delayed phase progression of reactive gliosis in the hippocampal CA1 region within our 7-day observation period, but not under Nrf2 deficiency. The temporal pattern of sustained attenuated reactive gliosis progression correlated well with the Nrf2-dependent reduction of ischemic hippocampal CA1 neuronal loss. Recent reports indicated that in the initial early phase of ischemia insult around 6 h, microglia activation displayed by the expansion of microglia mainly consisted of the local existing resident microglia, not the microglial progenitors from the blood [46–49].
Fig. 6. Schematic representation of dynamic reactive gliosis in response to hypoxia-ischemia insults.
Following hypoxia-ischemia, acute ischemic insults promptly trigger activation of microglia and astrocytes, characterized by hypertrophic soma with thickened and retracted processes. With the rapid ischemic injury progression, the activated glial cells proliferate especially at the lesion sites to limit the injury and promote the repair. Nrf2 deficiency markedly exacerbates the progression above, along with the more severe neuronal degeneration in ischemic hippocampal CA1 region. In contrast, Nrf2 activation by DMF or Ginseng dramatically attenuates reactive gliosis, correlated well with the neuronal protection.
We also investigated selected astrocytic functions that involve extracellular glutamate clearance and water transport. AQP4, predominantly distributed in the perivascular astrocyte end feet and perisynaptic astrocytic terminals, is the most abundant water channel in the brain [50–52]. It is considered to play a key role in ischemia-induced edema, which remarkably affects the extent of brain lesion and repair. Astrocytes control extracellular glutamate levels through the ATP-dependent enzyme GS, which is exclusively expressed in astrocytes, and they are most sensitive to the oxidative insult during cerebral ischemia [53]. Our study revealed that pretreatment with DMF or Ginseng inhibited the rapid increases in the early phase and preserved levels from the sharp decline in later phases in GS and AQP4 expression caused by acute ischemia after HI, eventually attenuating the development and progression of ischemic injury. Our results are consistent with previous recent studies on DMF and Korean red ginseng [41, 54, 55]. The osmotic overload induced by the increase of glutamate and potassium ions may result in the initial swelling of astrocytes, followed by activation of a water influx through AQP4 that exacerbated the swelling, eventually leading to neuronal death [51, 56–58]. The coordinated AQP4 regulation by neuroprotection could compensate for the decrease of the intracellular hyperosmotic pressure triggered by astrocytic dysfunction of glutamate clearance. Importantly, these effects were absent under Nrf2 deficiency. Together, pretreatment with DMF or Ginseng attenuated the deteriorative disruption of microglia activation and astrocytic structural integrity and dysfunction, including water transport and glutamate clearance, correlating well with the Nrf2-dependent neuroprotection.
The Impact of Nrf2-dependent Neuroprotection on Temporal Reactive Gliosis Progression in the Hippocampus is Well Revealed in the Context of Ischemia under HI.
Although reactive gliosis, Nrf2 activation, and the role of Nrf2 action on glial cells have been considered to be implicated in the pathogenesis of various CNS diseases, there are no findings available for these claims in ischemia initiation, progress, and repair in an HI mouse model. In the proof-of-principle experiments with Nrf2−/− mice, we provided in vivo evidence for the different phases of ischemia-induced reactive gliosis, along with an assessment of the role of an Nrf2-dependent mechanism in this process after HI. As we proposed, Nrf2-deficient mice displayed greater susceptibility to ischemic insult in both the early and later phases after HI, as indicated by exacerbated ischemic neuronal loss and reactive gliosis. In addition, Nrf2 displays an important role in glutamate metabolism and water transport, indicated by delayed progression of GS and AQP4 expression following ischemic insults. These findings confirm the important role of Nrf2-dependent neuroprotection and its impact on ischemia-induced reactive gliosis progression in an HI mouse model, further supporting our assumption.
Ischemic stroke induces significant short- and long-term deficits in sensory, motor, perceptual, and cognitive functions, associated with related affected brain regions. Indeed, our previous study indicated that DMF or Ginseng elicited sustained neuroprotection against the deterioration in both ischemic striatum and cortex through Nrf2 pathway, eventually ameliorating sensorimotor deficits after HI [59]. The dynamic pattern of attenuated reactive gliosis was also involved in this beneficial effect. The present study showed the beneficial effect of DMF or Ginseng on ischemic hippocampal injury, potentially ameliorating associated cognitive impairment. The contribution of reactive gliosis and Nrf2 mechanism in this neuroprotection also correlated well with this previous study. This further supports the unique protective role of Nrf2 and the promising perspective of Nrf2-targeting therapeutic in stroke field.
A limitation of our work that should be mentioned is that the time-dependent nuclear Nrf2 protein translocation was not scrutinized. This could help to clarify whether the Nrf2 signal is activated in response to DMF/Ginseng treatment during ischemic injury. Due to the issue of specificity and selectivity of commercially available mouse Nrf2 antibodies [60, 61], for the next steps we plan to examine Nrf2 specific target protein levels in various brain cell types. Here, we mainly focused on the in vivo evidence of temporal reactive gliosis and Nrf2-dependent neuroprotection by DMF and Ginseng. We characterized the morphological progression of microglia and astrocytes in response to an ischemic insult in the early and late stages of ischemia following HI. To further illuminate the accurate roles of reactive gliosis in microglia and astrocytes in the Nrf2-dependent neuroprotection, we plan to next investigate the Nrf2 pathway and ischemic cascade markers that may uniquely mediate reactive gliosis, as well as the functional alterations of glial cells.
In conclusion, by using hypoxic-ischemic and transgenic loss-of-function of Nrf2 mouse models, we demonstrate that DMF or Ginseng elicited robust and prolonged neuroprotection against the early and late phases of ischemic hippocampal CA1 neuronal degeneration, while temporal attenuation of reactive gliosis in microglia and astrocytes played a critical role in this Nrf2-dependent neuroprotection. Moreover, Nrf2 could effectively regulate dynamic GS and AQP4 expressions that affect the extent of ischemic damage and repair. This study provides new insights into the role of reactive gliosis in Nrf2-dependent endogenous neuroprotection, enriching our understanding of the Nrf2-targeting therapeutic strategy in the context of ischemia.
Funding Statement
This study was supported in part by the National Institutes of Health (R01AT007429 and R01NS046400, S.D.) and the American Heart Association (16POST31220032, L.L.).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Author Disclosure Statement
The authors declare no conflicts of interests.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. (2016) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133:e38–360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 2.Bosetti F, Koenig JI, Ayata C, et al. (2017) Translational stroke research: vision and opportunities. Stroke 48:2632–2637. doi: 10.1161/STROKEAHA.117.017112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248. doi: 10.1016/j.neuron.2013.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dirnagl U, Becker K, Meisel A (2009) Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8:398–412. doi: 10.1016/S1474-4422(09)70054-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palace J (2008) Neuroprotection and repair. J Neurol Sci 265:21–25. doi: 10.1016/j.jns.2007.08.039 [DOI] [PubMed] [Google Scholar]
- 6.Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 7:97. doi: 10.1186/1479-5876-7-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calkins MJ, Johnson DA, Townsend JA, et al. (2009) The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal 11:497–508. doi: 10.1089/ars.2008.2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar H, Kim I-S, More SV, et al. (2014) Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat Prod Rep 31:109–139. doi: 10.1039/c3np70065h [DOI] [PubMed] [Google Scholar]
- 9.Leonardo CC, Doré S (2011) Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr Neurosci 14:226–236. doi: 10.1179/1476830511Y.0000000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by nrf2. Antioxid Redox Signal 29:1727–1745. doi: 10.1089/ars.2017.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Cao W, Biswal S, Doré S (2011) Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke 42:2605–2610. doi: 10.1161/STROKEAHA.110.607101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263. doi: 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pekny M, Pekna M (2016) Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta 1862:483–491. doi: 10.1016/j.bbadis.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 15.Gao Z, Zhu Q, Zhang Y, et al. (2013) Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol Neurobiol 48:690–701. doi: 10.1007/s12035-013-8460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sims NR, Yew WP (2017) Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function. Neurochem Int 107:88–103. doi: 10.1016/j.neuint.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 17.Brennan MS, Matos MF, Li B, et al. (2015) Dimethyl fumarate and monoethyl fumarate exhibit differential effects on KEAP1, NRF2 activation, and glutathione depletion in vitro. PLoS One 10:e0120254. doi: 10.1371/journal.pone.0120254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan MS, Patel H, Allaire N, et al. (2016) Pharmacodynamics of Dimethyl Fumarate Are Tissue Specific and Involve NRF2-Dependent and -Independent Mechanisms. Antioxid Redox Signal 24:1058–1071. doi: 10.1089/ars.2015.6622 [DOI] [PubMed] [Google Scholar]
- 19.Lastres-Becker I, García-Yagüe AJ, Scannevin RH, et al. (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in parkinson’s disease. Antioxid Redox Signal 25:61–77. doi: 10.1089/ars.2015.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastogi V, Santiago-Moreno J, Doré S (2014) Ginseng: a promising neuroprotective strategy in stroke. Front Cell Neurosci 8:457. doi: 10.3389/fncel.2014.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Sun G, Zhang J, et al. (2015) Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving nrf2. Stroke 46:1923–1928. doi: 10.1161/STROKEAHA.115.009398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheremata W, Brown AD, Rammohan KW (2015) Dimethyl fumarate for treating relapsing multiple sclerosis. Expert Opin Drug Saf 14:161–170. doi: 10.1517/14740338.2015.977251 [DOI] [PubMed] [Google Scholar]
- 23.Liu L, Vollmer MK, Fernandez VM, et al. (2018) Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front Cell Neurosci 12:74. doi: 10.3389/fncel.2018.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai Y-L, Qiao M-D, Yu P, et al. (2017) Comparing eight types of ginsenosides in ginseng of different plant ages and regions using RRLC-Q-TOF MS/MS. J Ginseng Res. doi: 10.1016/j.jgr.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li TSC (1995) Asian and American ginseng: a review. Horttechnology 5:27–34. [Google Scholar]
- 26.Lee SM, Bae B-S, Park H-W, et al. (2015) Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res 39:384–391. doi: 10.1016/j.jgr.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim YT, Yi Y-J, Kim M-Y, et al. (2008) Neuroprotection and enhancement of spatial memory by herbal mixture HT008-1 in rat global brain ischemia model. Am J Chin Med 36:287–299. doi: 10.1142/S0192415X08005771 [DOI] [PubMed] [Google Scholar]
- 28.Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26:248–254. doi: 10.1016/S0166-2236(03)00071-7 [DOI] [PubMed] [Google Scholar]
- 29.Fan Y-Y, Hu W-W, Nan F, Chen Z (2017) Postconditioning-induced neuroprotection, mechanisms and applications in cerebral ischemia. Neurochem Int 107:43–56. doi: 10.1016/j.neuint.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 30.Hausenloy DJ, Yellon DM (2016) Ischaemic conditioning and reperfusion injury. Nat Rev Cardiol 13:193–209. doi: 10.1038/nrcardio.2016.5 [DOI] [PubMed] [Google Scholar]
- 31.Li S, Hafeez A, Noorulla F, et al. (2017) Preconditioning in neuroprotection: From hypoxia to ischemia. Prog Neurobiol 157:79–91. doi: 10.1016/j.pneurobio.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7:437–448. doi: 10.1038/nrn1927 [DOI] [PubMed] [Google Scholar]
- 33.Kuzuya T, Hoshida S, Yamashita N, et al. (1993) Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res 72:1293–1299. [DOI] [PubMed] [Google Scholar]
- 34.Koh HS, Chang CY, Jeon S-B, et al. (2015) The HIF-1/glial TIM-3 axis controls inflammation-associated brain damage under hypoxia. Nat Commun 6:6340. doi: 10.1038/ncomms7340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang J, Li Y, Tang Y, et al. (2013) CXCR4 antagonist AMD3100 protects blood-brain barrier integrity and reduces inflammatory response after focal ischemia in mice. Stroke 44:190–197. doi: 10.1161/STROKEAHA.112.670299 [DOI] [PubMed] [Google Scholar]
- 36.Munakata M, Shirakawa H, Nagayasu K, et al. (2013) Transient receptor potential canonical 3 inhibitor Pyr3 improves outcomes and attenuates astrogliosis after intracerebral hemorrhage in mice. Stroke 44:1981–1987. doi: 10.1161/STROKEAHA.113.679332 [DOI] [PubMed] [Google Scholar]
- 37.Glushakov AV, Robbins SW, Bracy CL, et al. (2013) Prostaglandin F2α FP receptor antagonist improves outcomes after experimental traumatic brain injury. J Neuroinflammation 10:132. doi: 10.1186/1742-2094-10-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandera E, Botteri M, Minelli C, et al. (2006) Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke 37:1334–1339. doi: 10.1161/01.STR.0000217418.29609.22 [DOI] [PubMed] [Google Scholar]
- 39.Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67:181–198. doi: 10.1016/j.neuron.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ban JY, Kang SW, Lee JS, et al. (2012) Korean red ginseng protects against neuronal damage induced by transient focal ischemia in rats. Exp Ther Med 3:693–698. doi: 10.3892/etm.2012.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunze R, Urrutia A, Hoffmann A, et al. (2015) Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp Neurol 266:99–111. doi: 10.1016/j.expneurol.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 42.Lee JS, Choi HS, Kang SW, et al. (2011) Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med 39:83–94. doi: 10.1142/S0192415X1100866X [DOI] [PubMed] [Google Scholar]
- 43.Yao Y, Miao W, Liu Z, et al. (2016) Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl Stroke Res 7:535–547. doi: 10.1007/s12975-016-0496-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caso JR, Moro MA, Lorenzo P, et al. (2007) Involvement of IL-1beta in acute stress-induced worsening of cerebral ischaemia in rats. Eur Neuropsychopharmacol 17:600–607. doi: 10.1016/j.euroneuro.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 45.Vargas MR, Johnson JA (2009) The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med 11:e17. doi: 10.1017/S1462399409001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ajami B, Bennett JL, Krieger C, et al. (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10:1538–1543. doi: 10.1038/nn2014 [DOI] [PubMed] [Google Scholar]
- 47.Hambardzumyan D, Gutmann DH, Kettenmann H (2016) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19:20–27. doi: 10.1038/nn.4185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290–292. [DOI] [PubMed] [Google Scholar]
- 49.Priller J, Flügel A, Wehner T, et al. (2001) Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med 7:1356–1361. doi: 10.1038/nm1201-1356 [DOI] [PubMed] [Google Scholar]
- 50.Shin JA, Choi JH, Choi Y-H, Park E-M (2011) Conserved aquaporin 4 levels associated with reduction of brain edema are mediated by estrogen in the ischemic brain after experimental stroke. Biochim Biophys Acta 1812:1154–1163. doi: 10.1016/j.bbadis.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 51.Vella J, Zammit C, Di Giovanni G, et al. (2015) The central role of aquaporins in the pathophysiology of ischemic stroke. Front Cell Neurosci 9:108. doi: 10.3389/fncel.2015.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zador Z, Stiver S, Wang V, Manley GT (2009) Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol 159–170. doi: 10.1007/978-3-540-79885-9_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeitner TM, Battaile K, Cooper AJL (2015) Critical evaluation of the changes in glutamine synthetase activity in models of cerebral stroke. Neurochem Res 40:2544–2556. doi: 10.1007/s11064-015-1667-1 [DOI] [PubMed] [Google Scholar]
- 54.Chen-Roetling J, Song W, Schipper HM, et al. (2015) Astrocyte overexpression of heme oxygenase-1 improves outcome after intracerebral hemorrhage. Stroke 46:1093–1098. doi: 10.1161/STROKEAHA.115.008686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko HM, Joo SH, Kim P, et al. (2013) Effects of Korean Red Ginseng extract on tissue plasminogen activator and plasminogen activator inhibitor-1 expression in cultured rat primary astrocytes. J Ginseng Res 37:401–412. doi: 10.5142/jgr.2013.37.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagelhus EA, Ottersen OP (2013) Physiological roles of aquaporin-4 in brain. Physiol Rev 93:1543–1562. doi: 10.1152/physrev.00011.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277. doi: 10.1038/nrn3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verkman AS, Anderson MO, Papadopoulos MC (2014) Aquaporins: important but elusive drug targets. Nat Rev Drug Discov 13:259–277. doi: 10.1038/nrd4226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu L, Vollmer MK, Ahmad AS, et al. (2019) Pretreatment with Korean red ginseng or dimethyl fumarate attenuates reactive gliosis and confers sustained neuroprotection against cerebral hypoxic-ischemic damage by an Nrf2-dependent mechanism. Free Radic Biol Med 131:98–114. doi: 10.1016/j.freeradbiomed.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kemmerer ZA, Ader NR, Mulroy SS, Eggler AL (2015) Comparison of human Nrf2 antibodies: A tale of two proteins. Toxicol Lett 238:83–89. doi: 10.1016/j.toxlet.2015.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau A, Tian W, Whitman SA, Zhang DD (2013) The predicted molecular weight of Nrf2: it is what it is not. Antioxid Redox Signal 18:91–93. doi: 10.1089/ars.2012.4754 [DOI] [PMC free article] [PubMed] [Google Scholar]