Abstract
Background.
Previous research suggests that racial disparities in the report of analgesic adverse effects are partially mediated by the type of opioid prescribed to African Americans despite the presence of certain comorbidities, such as renal disease.
Aims.
We aimed to identify independent predictors of the type of opioid prescribed to cancer outpatients and determine if race and chronic kidney disease (CKD) independently predict prescription type, adjusting for relevant sociodemographic and clinical confounders.
Design and Methods.
We conducted secondary analysis of a 3-month observational study. Cancer patients (N=241) were from outpatient oncology clinics within a large mid-Atlantic healthcare system. Patients were older than 18 years of age, self-identified as African Americans or Whites, and had an analgesic prescription for cancer pain.
Results.
Consistent with published literature, most patients (75.5%) were prescribed either morphine or oxycodone preparations as oral opioid therapy for cancer pain. When compared to Whites, African Americans were significantly more likely to be prescribed morphine (14% vs. 33%) and less likely to be prescribed oxycodone (64% vs. 38%, respectively, p<0.001). The estimated odds for African Americans to receive morphine were 2.573 times that for Whites (95% CI = 1.077 and 6.145) after controlling for insurance type, income, and pain levels. In addition, presence of private health insurance was negatively associated with the prescription of morphine and positively associated with prescription of oxycodone in separate multivariable models. Presence of CKD did not predict type of analgesic prescribed.
Conclusions.
Both race and insurance type independently predict type of opioid selection for cancer outpatients. Future larger clinical studies are needed to fully understand the sources and clinical consequences of racial differences in opioid selection for cancer pain.
Keywords: Cancer pain, opioid, race, ethnicity, socioeconomic, disparities
Introduction
The National Academy of Medicine report, “Relieving Pain in America” (Simon, 2012), and other systematic reviews (Karen O Anderson, Green, & Payne, 2009; Cintron & Morrison, 2006) have found that one of the most consistent findings on pain treatment disparities pertains to African Americans. A meta-analysis of over 20 years of published studies (Meghani, Byun, & Gallagher, 2012) on analgesic disparities in pain treatment found that when compared to other racial and ethnic groups, African American patients have one of the highest risks for pain undertreatment despite lacking the linguistic barriers experienced by other minority patients (Meghani et al., 2012).
Most of the existing pain management disparities research focuses on racial and ethnic disparities in analgesic treatment of pain and have compared outcomes such as receipt of any analgesia (Meghani et al., 2012; Todd, Samaroo, & Hoffman, 1993), receipt of opioid analgesia (Chen et al., 2005; A. Heins et al., 2006; J. K. Heins et al., 2006; Pletcher, Kertesz, Kohn, & Gonzales, 2008; Singhal, Tien, & Hsia, 2016; Tamayo-Sarver et al., 2003), availability of opioids in predominantly minority neighborhoods (Green, Ndao-Brumblay, West, & Washington, 2005; Morrison, Wallenstein, Natale, Senzel, & Huang, 2000), wait time in receiving analgesia in emergency department (Epps, Ware, & Packard, 2008; Ware, Epps, Clark, & Chatterjee, 2012), and pain management index, i.e., highest reported pain levels in relation to the strength of analgesics prescribed (Cintron & Morrison, 2006; Meghani, Thompson, Chittams, Bruner, & Riegel, 2015; Minick et al., 2012).
While we know that African Americans are less likely to receive opioids for pain across care settings (Meghani et al., 2012) and more likely to have a negative pain management index (Cintron & Morrison, 2006; Meghani et al., 2015; Minick et al., 2012), it is not clear if there are also disparities in the types of strong opioids or World Health Organization Step 3 opioids (World Health Organization, 1996) (e.g. morphine, oxycodone) prescribed to African Americans with cancer pain. This understanding is important for a few reasons: Emerging literature points to poor adherence to prescribed analgesia among African Americans even with a higher burden of cancer-related pain (Meghani & Knafl, 2016; Meghani et al., 2015; Rhee, Kim, & Kim, 2012; Stout, Sexton, & Meghani, 2017; Yeager et al., 2018). These studies suggest that experience of analgesic side-effects secondary to the type of analgesia prescribed to African Americans despite clinical risks may contribute to poor adherence and lack of pain control. A study employing discrete choice experiment found that African Americans with cancer pain were more likely than Whites to make analgesic use decisions based on analgesic side effects (Meghani, Chittams, Hanlon, & Curry, 2013). In a subsequent analysis, despite a diagnosis of chronic kidney disease (CKD), African Americans were significantly more likely to receive morphine, with known toxic metabolites, morphine-3-glucuronide (M3G), and morphine-6-glucuronide (M6G)], which accumulate in renal disease (Meghani et al., 2014). Furthermore, authors found that racial disparities in patients’ reported analgesic adverse effects was partially mediated by the type of opioid prescribed to African Americans versus Whites (Meghani et al., 2014). The authors concluded that reducing racial disparities in the type of opioid prescribed and understanding sources of disproportionate opioid-related side-effects among African-Americans may reduce the observed clinical disparities in cancer pain outcomes (Meghani et al., 2014).
Some emerging literature suggests that disparities in type of opioid prescribed may influence a number of other clinical outcomes. For instance, long-acting opioid use has been correlated with increased risk of serious infection, with patients given oxycodone demonstrating a lower rate of infection than those prescribed morphine (Wiese et al., 2018). Additionally, a large retrospective cohort study recently reported that the risk of out-of-hospital death was lower in those filling long-acting oxycodone than those filling long-acting morphine, potentially pointing to an association between type of opioid prescribed and subsequent mortality outcomes (Chung et al., 2019).
This paper extends previous published work on analgesic treatment disparities to identify independent predictors of the selection of strong opioids prescribed to cancer outpatients and determine if race and the presence of CKD independently predicts the type of opioid prescription, while adjusting for relevant sociodemographic and clinical confounders.
Methods
Study Design
This is a secondary analysis of a study to understand racial disparities in adherence to prescribed oral analgesics for cancer pain among cancer outpatients using a 3-month observational, repeated measures design (baseline and 3 months) (Meghani et al., 2014; Meghani et al., 2015). The study was approved by the Institutional Review Board of the University of Pennsylvania. Patients were recruited from two outpatient medical oncology clinics within a large mid-Atlantic healthcare system. Inclusion criteria for this study included self-identified African-Americans and Whites; at least 18 years of age; diagnosed with solid tumors or multiple myeloma with cancer-related pain requiring opioid therapy; and a prescription of an oral analgesic for cancer pain.
Measures
Race.
Study participants self-identified race based on the National Institutes of Health racial categories (Health & Health, 2015)
Prescribed Opioids.
Analgesic prescription information was gathered from patient’s medical records and validated with patients during study-related home visits. We coded prescribed analgesics according to the World Health Organization analgesic steps (World Health Organization, 1996). The majority of the patients in our study were prescribed either morphine or oxycodone preparations in immediate or extended release forms. Thus the prescribed analgesics were coded as “morphine,” “oxycodone,” or “others.” The “others” category was small and comprised of a heterogeneous mix of opioid and non-opioid analgesics.
Chronic Kidney Disease.
We used the 4-variable abbreviated Modification of Diet in Renal Disease (aMDRD) study formula to estimate glomerular filtration rate (eGFR), which takes into account gender, age, ethnicity, and serum creatinine levels (National Kidney Foundation). We extracted up to seven serum creatinine values from patients’ electronic medical records. All eGFR values for a single patient were then averaged to determine CKD status (No CKD = eGFR > 90 mL/min/1.73 m2; CKD= eGFR <89 mL/min/1.73 m2). We chose MDRD over the Cockcroft-Gault formula since the precision of the MDRD is better in patients with cancer (Kleber et al., 2007) as well as in certain populations, including older adults and obese patients (Pöge et al., 2005). The 4-variable aMDRD has also shown comparable performance as a more precise MDRD7 equation, which takes into account additional parameters (Pöge et al., 2005).
Pain Intensity.
We measured pain intensity and impact using the Brief Pain Inventory (BPI) (Cleeland & Ryan, 1994). The psychometric properties of the BPI is well-established with cancer patients, including racial and ethnic minority patients with cancer (K. O. Anderson et al., 2000; Meghani & Keane, 2007; Meghani et al., 2015; Rhee et al., 2012; Yeager et al., 2018).
Health Insurance Type.
We collected health insurance information via self-report by study participants and validated it with information in patients’ electronic medical records. The insurance variable was coded as “private,” “Medicare,” “Medicaid,” and “other.” Managed Medicare or Medicaid plans (e.g. Bravo, HealthPartners, and Keystone Mercy) were classified as Medicare or Medicaid, respectively. Plans that were not private, Medicare, or Medicaid (such as VA, COBRA) were classified as other.
Data Analysis
A step-wise logistic model was generated by adding one variable at a time to identify the most significant to least significant variables. We ran two separate binary logistic regressions-one for the outcome of the prescription of morphine and other for prescription of oxycodone. The variables age, gender, health insurance, household income, pain levels in the past week (worst, least and average pain), duration of cancer pain, and past history of substance abuse were tested individually towards developing the final multivariable model including race and CKD status. All data were analyzed using SAS version 9.3.(SAS Institute Inc, 2012)
Results
The average age of the sample was 55 years (SD=10). There were no differences between African Americans and Whites regarding age, gender, and education (Table 1). However, African Americans were less likely to report having private health insurance when compared to Whites (p<0.001) and more likely to belong to a lower income bracket (p<0.001). African Americans reported poor cancer pain control in all BPI pain measures, including worst pain (p<0.001), average pain (p<0.001), and least pain (p<0.001) scores, indicating greater pain severity and lower pain relief in the previous week as well as greater pain-related functional interference (p=0.048) (Table 2). There were also significant differences by race in the type of opioid prescribed (Figure 1). When compared to Whites, African Americans were significantly more likely to be prescribed morphine (14% vs. 33%, respectively) and less likely to be prescribed oxycodone (64% vs. 38%, respectively, p<0.001).
Table 1.
Characteristics of Study Participants
| Variable | Total (N=182) | African Americans (N=73) | Whites (N=109) | P-values1 |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age | 55.1 (10.1) | 54.0 (9.3) | 55.8 (10.6) | 0.253 |
| Frequency (%) | ||||
| Gender | 0.074 | |||
| Male | 92 (51) | 31 (42) | 61 (56) | |
| Female | 90 (49) | 42 (58) | 48 (44) | |
| Marital Status | <0.001 | |||
| Married | 109 (60) | 24 (33) | 85 (78) | |
| Separated/Divorced/Widowed | 47 (26) | 33 (45) | 14 (13) | |
| Never Married | 26 (14) | 16 (22) | 10 (9) | |
| Education | 0.079 | |||
| Elementary | 3 (2) | 2 (3) | 1 (1) | |
| High School | 60 (33) | 27 (37) | 33 (30) | |
| College/Trade School | 89 (49) | 38 (52) | 51 (47) | |
| More Than College | 30 (16) | 6 (8) | 24 (22) | |
| Income | <0.001 | |||
| <$30, 000 | 57 (31) | 38 (52) | 19 (17) | |
| $30–50,000 | 36 (20) | 21 (29) | 15 (14) | |
| $50–70,000 | 32 (18) | 10 (13) | 22 (20) | |
| $70–90,000 | 20 (11) | 2 (3) | 18 (17) | |
| >$90,000 | 37 (20) | 2 (3) | 35 (32) | |
| Health Insurance | 0.683 | |||
| Yes | 178 (98) | 71 (97) | 107 (98) | |
| No | 4 (2) | 2 (3) | 2 (2) | |
| Type of Health Insurance | <0.001 | |||
| Private | 97 (54) | 23 (31) | 74 (69) | |
| Medicaid | 18 (10) | 16 (22) | 2 (2) | |
| Medicare | 42 (23) | 21 (29) | 21 (19) | |
| Other | 24 (13) | 13 (18) | 11 (10) | |
P-values are based on t-tests for continuous variables and chi-squared for categorical variables.
Table 2.
Clinical Variables
| Variable | Total (N=182) | African Americans (N=73) | Whites (N=109) | P-values1 |
|---|---|---|---|---|
| Mean (SD) | ||||
| Worst pain (0–10) | 6.8 (2.3) | 7.6 (2.0) | 6.2 (2.5) | <0.001 |
| Least pain (0–10) | 3.3 (2.0) | 4.1 (2.0) | 2.7 (1.8) | <0.001 |
| Average pain (0–10) | 4.7 (2.0) | 5.4 (1.9) | 4.2 (2.0) | <0.001 |
| Pain interference (0–10) | 35.4 (16.0) | 38.3 (16.5) | 33.5 (15.4) | 0.048 |
| Frequency (%) | ||||
| Type of Opioid Prescribed | <0.001 | |||
| Oxycodone | 128 (70) | 39 (53) | 89 (82) | |
| Morphine | 54 (30) | 34 (47) | 20 (18) | |
| Presence of metastatic disease | .030 | |||
| Yes | 142 (78) | 51 (70) | 91 (83) | |
| No | 40 (22) | 22 (30) | 18 (17) | |
| Past History of Substance Abuse | .040 | |||
| Yes | 32 (18) | 18 (25) | 14 (13) | |
| No | 150 (82) | 55 (75) | 95 (87) | |
| Estimated Glomerular Filtration Rate (eGFR) | 0.054 | |||
| ≥90 | 100 (55) | 46 (63) | 54 (49) | |
| 60–89 | 53 (29) | 14 (19) | 39 (36) | |
| <60 | 29 (16) | 13 (18) | 16 (15) | |
P-values are based on t-tests for continuous variables and chi-squared for categorical variables. CKD=Chronic Kidney Disease (estimated using eGFR; No CKD = eGFR >90 mL/min/1.73 m2; CKD= eGFR <89 mL/min/1.73 m2)
Figure 1. Type of Pain Medication by Race (%) (N=241)*.
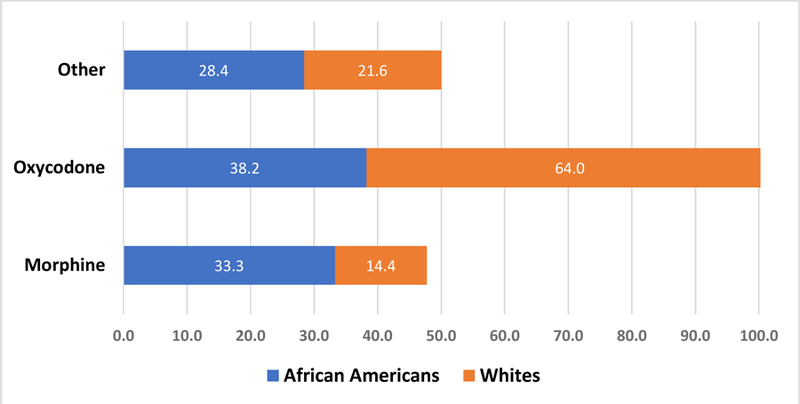
*The other group (n=59) was excluded from subsequent analysis.
Independent Correlates of Morphine Prescription for Cancer Pain
Based on step-wise analyses, the final model consisted of race, income, insurance type, average pain levels, and CKD status. Of these, average pain intensity was the strongest correlate of morphine prescription (p=0.0005) (Table 3). The point estimate of the odds ratio for average pain in the last week was 0.716. This suggests that for a one-unit increase in average pain score, we see about a 28% decrease in the odds of receiving morphine as an index drug (95% CI, 0.594 and 0.864). Also, when compared to patients on Medicaid, patients on private insurance were 78% less likely to receive morphine (OR=0.225, 95% CI, 0.077–0.662). In addition, race was an independent predictor of morphine prescription even after controlling for insurance type, income, and pain levels. The estimated odds for African Americans to receive morphine were 2.573 times that of the estimated odds for Whites (95% CI = 1.077 and 6.145) (Table 3).
Table 3.
Independent Correlates of Morphine Prescription for Cancer Pain
| Variable (reference) | Levels | OR | 95% CI | Wald Chi-Square | Pr > ChiSq | |
|---|---|---|---|---|---|---|
| Average pain | (0–10) | 0.716 | 0.594 | 0.864 | 12.2101 | 0.0005 |
| Race (ref: Whites) | African Americans | 2.570 | 1.077 | 6.134 | 4.5261 | 0.0334 |
| Insurance Type (ref: Medicaid) | Private | 0.225 | 0.077 | 0.662 | 4.0304 | 0.0447 |
| Other | 0.199 | 0.021 | 1.909 | 1.0047 | 0.3162 | |
| Multiple | 0.731 | 0.216 | 2.480 | 0.8559 | 0.3549 | |
| Medicare | 0.805 | 0.288 | 2.249 | 1.9787 | 0.1595 | |
| Income (ref: <50K) | >=$50, 000 | 0.565 | 0.241 | 1.327 | 1.7184 | 0.1899 |
| Chronic Kidney Disease (ref: No) | Yes | 1.855 | 0.910 | 3.781 | 2.8883 | 0.0892 |
Independent Correlates of Oxycodone Prescription for Cancer Pain
The presence of private health insurance was the only significant predictor in the multivariable model for oxycodone prescription for cancer pain. The point estimate of the odds ratio for insurance type (private vs. Medicaid) was 8.437. This suggests that the estimated odds of people with private insurance type receiving oxycodone is 8.437 times that of the estimated odds for people with Medicaid insurance type (95% CI, 2.814–25.297) (Table 4).
Table 4.
Independent Correlates of Oxycodone Prescription for Cancer Pain
| Variable (reference) | OR | 95% CI | Wald Chi-Square | Pr > ChiSq | ||
|---|---|---|---|---|---|---|
| Race (ref: Whites) | African Americans | 0.700 | 0.366 | 1.337 | 1.1682 | 0.2798 |
| Income (ref: <$50,000) | >=$50,000 | 1.510 | 0.805 | 2.834 | 1.6483 | 0.1992 |
| Insurance Type (ref: Medicaid) | Private | 8.437 | 2.814 | 25.297 | 9.9166 | 0.0016 |
| Other | 3.252 | 0.574 | 18.438 | 0.0093 | 0.9231 | |
| Multiple | 3.738 | 1.066 | 13.102 | 0.0455 | 0.8311 | |
| Medicare | 4.765 | 1.540 | 14.739 | 1.1211 | 0.2897 | |
| Chronic Kidney Disease (ref: No) | Yes | 0.683 | 0.383 | 1.216 | 1.6778 | 0.1952 |
Of note, in separate univariate logistic regression analyses, a past history of substance abuse was negatively associated with the prescription of oxycodone (likelihood estimate, - 0.4177, SE 0.172; p=0.0154) and positively associated with the prescription of morphine (likelihood estimate, 0.435, SE 0.180; p=0.015). However, the effect disappeared in multivariable analysis in each model. Also, the presence of CKD did not predict type of analgesic prescribed, while controlling for race and insurance status.
Discussion
Despite recent initiatives to spare opioids in clinical practice (Bohnert, Guy, & Losby, 2018; Dowell, Haegerich, & Chou, 2016), opioids remain important frontline treatment for the management of moderate to severe cancer pain (American Society of Clinical Oncology, 2016). Previous studies have shown that the types of opioids prescribed to patients with cancer pain relate to both their experience of analgesic adverse effects (Meghani et al., 2014) and their level of analgesic adherence for cancer pain (K. O. Anderson et al., 2000; Meghani et al., 2015; Rhee et al., 2012). Our findings indicate that, controlling for income, health insurance, and a number of relevant clinical correlates, race is independently associated with the type of opioid prescribed to cancer outpatients.
This findings are important from several perspectives: About two in three patients with cancer have a creatinine clearance of <90 ml/min and, of these, 20% of patients have a creatinine clearance of <60 ml/min (Janus et al., 2010; Launay-Vacher, 2010; Launay-Vacher et al., 2007). Morphine has known toxic metabolites that can accumulate in the presence of renal insufficiency, which is highly prevalent in cancer patients (Janus et al., 2010; Launay-Vacher, 2010; Launay-Vacher et al., 2007). A relatively higher prevalence of renal impairment among African Americans in the general population (Lipworth et al., 2012) may imply that many African American patients with cancer may be subjected to hidden opioid toxicities due to the choice of opioid selection (Meghani et al., 2014). Thus, the racial difference in the choice of opioid selection and associated outcomes warrants further studies, especially in the context of clinical comorbidities, such as renal disease.
A Cochrane systematic review compared controlled release oxycodone with morphine (Schmidt-Hansen, Bennett, Arnold, Bromham, & Hilgart, 2015) and found similar levels of pain relief and adverse events for both opioids. However, most studies that informed this conclusion excluded patients with various levels of renal impairment (Bruera et al., 1998; Mercadante et al., 2010; Riley et al., 2015). Similarly, a recent study that compared the tolerability profile of morphine and oxycodone found no statistically significant difference in the tolerability of morphine or oxycodone across renal function status (Zecca et al., 2016). The authors acknowledged that the study was underpowered and the subgroup of patients with renal impairment was small (n=29) (Zecca et al., 2016). The study also excluded patients with significant renal impairment. Further investigation in larger, more resourced studies are needed, which includes serum M3G and M6G levels while testing race as a moderator of CKD and type of opioid prescribed to cancer patients.
Research is also warranted into why such systematic clinical differences exist and the extent to which they are consequential in patients’ adherence to prescribed analgesia, pain control, function, and side effects. The differential opioid prescription to cancer patients (despite health insurance and income levels as demonstrated in this study) may relate to prescriber’s implicit bias in the clinical treatment of cancer pain. For instance, prescribers may be more reluctant to prescribe opioids to a historically stereotyped group (Meghani et al., 2012) or prescribe an opioid with lesser street value, such as morphine (Street Rx, 2017). Dual process theory in cognitive psychology suggests that implicit biases are automatic and unconscious (Burgess, van Ryn, Crowley-Matoka, & Malat, 2006), e.g. a social construction of a phenomenon may unconsciously seep into clinical decision-making and may affect certain groups disproportionately (Ezenwa & Fleming, 2012; Meghani et al., 2012). Published studies provide support for this clinical phenomenon articulated by African Americans being treated for cancer pain: “…a lot of black people feel, especially when we’re in pain, that we aren’t believed, and that is the main problem with us. And we accept that, that we’re not gonna be believed… And then when anything is offered to us, the first thing that’s being thrown up in our face is that, well, it’s got a street value. You don’t need to hear that.” (Meghani & Houldin, 2007). Our findings are particularly relevant in the current context of social stigma and policy flux related to the opioid crisis (Bohnert et al., 2018; Dowell et al., 2016; Guy et al., 2017) and there may be a risk for widening pain care disparities impacting already vulnerable groups (Meghani & Vapiwala, 2018).
In addition to race, type of health insurance was an independent factor in the choice of opioid prescribed to cancer patients across our analyses. Health insurance is part of the broad socioeconomic status (SES) category, which also includes variables such as income, education, wealth, and neighborhood characteristics (Meghani & Chittams, 2015). An ongoing debate in racial disparities research is whether observed racial disparities are in effect SES disparities (Meghani & Chittams, 2015). In the studies where the effect of race on poor outcomes disappears in a statistical model controlling for SES variables (Meghani & Chittams, 2015), researchers may inadvertently conclude that SES is a confounder of race. We have previously illustrated why such an interpretation may be misguided given the intricate relationship of race and SES in the United States (Meghani & Chittams, 2015; Williams, Mohammed, Leavell, & Collins, 2010). In effect, SES disparities affect racial and ethnic subgroups disproportionately (Meghani, 2011; Meghani & Chittams, 2015). For instance, in the present study, when compared to patients on Medicaid, patients with private insurance were significantly more likely to receive an oxycodone prescription for pain. However, 89% of those who were Medicaid-insured in this study were African Americans.
Finally, our study found a negative association between prescription of morphine and reported pain levels. The significance of this finding remains unclear but may suggest that patients may be receiving strong opioids from other routes, not captured in this study.
Study Limitations
The findings of this study must be interpreted in the context of the following limitations and perspectives: 1) while we are able to demonstrate that the significance of race remained after accounting for income and insurance type in the model (Table 3), it is possible that other unmeasured SES factors may relate to the effect of race on type of opioid prescription found in this study. To this end, 2) the current analyses does not allow us to assign a moral value to the sources of the observed racial differences. Our interpretation of findings is based on clinical experience and previous research on racial disparities in pain care for African Americans. Thus the observed differences may be mere differences or value-laden differences (Meghani & Gallagher, 2008) deserving further investigation. 3) Our findings may suffer from type II error due to a small sample size. For instance, certain variables, such as past history of substance abuse may become statistically significant in a larger sample. 4) We combined our analysis of immediate release and extended release preparations for the opioids due to a small clinical sample. This may explain the findings of the role of private insurance in predicting oxycodone prescription. At the time the study (Meghani et al., 2014), only extended release morphine was available in the generic forms and it was relatively inexpensive. Extended release oxycodone was available as brand name only and was significantly more expensive (ConsumerReports(R) Health, 2012). However, the fact that we were able to identify racial differences in type of opioid prescription controlling for type of insurance and despite a small sample size indicates a clinical phenomenon of sizeable effect. 5) Consistent with published literature, most patients were prescribed morphine or oxycodone in various forms as first line oral opioid therapy (Schmidt-Hansen et al., 2015). Thus we limited our analysis to these opioids. This excluded patients who were only prescribed opioids through non-oral routes, e.g. intravenous, subcutaneous, rectal, transdermal, and transmucosal (we do not expect this to exclude a significant number of patients as the oral route is most common in outpatient clinical practices as underscored in the National Comprehensive Cancer Network Adult Cancer Pain guidelines (National Comprehensive Cancer Network, 2015)
Conclusions
This is one of the few studies to identify differences in the selection of opioids prescribed to African Americans and Whites with cancer pain after adjusting for insurance type and relevant clinical correlates. Due to lack of consistent clinical data on morphine and its glucuronide metabolites (Smith, 2009) and research studies excluding patients with various levels of renal insufficiency status, future larger clinical studies are needed to fully understand the sources and clinical consequences of racial differences in opioid selection for cancer pain.
Additionally, there is lack of empirical evidence guiding multiple aspects of opioid prescribing practices for cancer patients (Paice et al., 2016) and a number of inconsistencies among existing national pain management guidelines (Meghani & Vapiwala, 2018) underscoring a need to further expand knowledge on opioid management for cancer pain. Careful pharmacological management of cancer pain continues to be important for optimal pain control and function, especially until there is consistent access to non-opioid, and non-pharmacological treatments for chronic pain—which still continues to remain limited in the United States (Becker et al., 2017; Meghani & Vapiwala, 2018).
Acknowledgments
Funding: This study was supported by a National Institutes of Health (NIH) Grant to Dr. Salimah H. Meghani (NIH/NINR RC1-NR011591).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Society of Clinical Oncology. (2016). ASCO Policy Statement: Opioid Therapy: Protecting Access to Treatment for Cancer-Related Pain https://www.asco.org/advocacy-policy/policies-positions-guidance/policy-statements
- Anderson KO, Green CR, & Payne R (2009). Racial and ethnic disparities in pain: causes and consequences of unequal care. The Journal of Pain, 10(12), 1187–1204. [DOI] [PubMed] [Google Scholar]
- Anderson KO, Mendoza TR, Valero V, Richman SP, Russell C, Hurley J, … Cleeland CS (2000). Minority cancer patients and their providers: pain management attitudes and practice. Cancer, 88(8), 1929–1938. [PubMed] [Google Scholar]
- Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, & Fraenkel L (2017). Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Family Practice, 18(1), 41. doi: 10.1186/s12875-017-0608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert ASB, Guy GP Jr., & Losby JL (2018). Opioid Prescribing in the United States Before and After the Centers for Disease Control and Prevention’s 2016 Opioid Guideline. Annals of Internal Medicine, 169(6), 367–375. doi: 10.7326/M18-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruera E, Belzile M, Pituskin E, Fainsinger R, Darke A, Harsanyi Z, … Ford I (1998). Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. Journal of Clinical Oncology, 16(10), 3222–3229. doi: 10.1200/JCO.1998.16.10.3222 [DOI] [PubMed] [Google Scholar]
- Burgess DJ, van Ryn M, Crowley-Matoka M, & Malat J (2006). Understanding the provider contribution to race/ethnicity disparities in pain treatment: insights from dual process models of stereotyping. Pain Medicine, 7(2), 119–134. doi: 10.1111/j.1526-4637.2006.00105.x [DOI] [PubMed] [Google Scholar]
- Chen I, Kurz J, Pasanen M, Faselis C, Panda M, Staton LJ, … Cykert S (2005). Racial differences in opioid use for chronic nonmalignant pain. Journal of General Internal Medicine, 20(7), 593–598. doi: 10.1111/j.1525-1497.2005.0106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CP, Dupont WD, Murray KT, Hall K, Stein CM, & Ray WA (2019). Comparative out-of-hospital mortality of long-acting opioids prescribed for non-cancer pain: A retrospective cohort study. Pharmacoepidemiology and drug safety, 28(1), 48–53. doi: doi: 10.1002/pds.4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron A, & Morrison RS (2006). Pain and ethnicity in the United States: A systematic review. Journal of Palliative Medicine, 9(6), 1454–1473. doi: 10.1089/jpm.2006.9.1454 [DOI] [PubMed] [Google Scholar]
- Cleeland C, & Ryan K (1994). Pain assessment: global use of the Brief Pain Inventory. Annals, Academy of Medicine, Singapore, 23(2), 10. [PubMed] [Google Scholar]
- ConsumerReports(R) Health. (2012). Using Opioids to Treat Chronic Pain: Comparing Effectiveness, Safety and Price http://consumerhealthchoices.org/wp-content/uploads/2012/08/BBD-Opioids-Full.pdf
- Dowell D, Haegerich TM, & Chou R (2016). CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA, 315(15), 1624–1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps CD, Ware LJ, & Packard A (2008). Ethnic wait time differences in analgesic administration in the emergency department. Pain Management Nursing, 9(1), 26–32. doi: 10.1016/j.pmn.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Ezenwa MO, & Fleming MF (2012). Racial Disparities in Pain Management in Primary Care. Journal of Health Disparities Research and Practice, 5(3), 12–26. [PMC free article] [PubMed] [Google Scholar]
- Green CR, Ndao-Brumblay SK, West B, & Washington T (2005). Differences in prescription opioid analgesic availability: comparing minority and white pharmacies across Michigan. Journal of Pain, 6(10), 689–699. doi: 10.1016/j.jpain.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Guy GP Jr., Zhang K, Bohm MK, Losby J, Lewis B, Young R, … Dowell D (2017). Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep, 66(26), 697–704. doi: 10.15585/mmwr.mm6626a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health N. I. o., & Health N. I. o. (2015). Racial and Ethnic Categories and Definitions for NIH Diversity Programs and for Other Reporting Purposes (NOT-OD-15–089) [Google Scholar]
- Heins A, Grammas M, Heins JK, Costello MW, Huang K, & Mishra S (2006). Determinants of variation in analgesic and opioid prescribing practice in an emergency department. Journal of Opioid Management, 2(6), 335–340. [DOI] [PubMed] [Google Scholar]
- Heins JK, Heins A, Grammas M, Costello M, Huang K, & Mishra S (2006). Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. Journal of Emergency Nursing, 32(3), 219–224. doi: 10.1016/j.jen.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Janus N, Launay-Vacher V, Byloos E, Machiels JP, Duck L, Kerger J, … Nortier J (2010). Cancer and renal insufficiency results of the BIRMA study. British Journal of Cancer, 103(12), 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleber M, Cybulla M, Bauchmüller K, Ihorst G, Koch B, & Engelhardt M (2007). Monitoring of renal function in cancer patients: an ongoing challenge for clinical practice. Annals of Oncology, 18(5), 950–958. [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V (2010). Epidemiology of chronic kidney disease in cancer patients: lessons from the IRMA study group Paper presented at the Seminars in nephrology. [DOI] [PubMed] [Google Scholar]
- Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, … Group, C. M. S. (2007). Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer, 110(6), 1376–1384. [DOI] [PubMed] [Google Scholar]
- Lipworth L, Mumma MT, Cavanaugh KL, Edwards TL, Ikizler TA, Tarone RE, … Blot WJ (2012). Incidence and predictors of end stage renal disease among low-income blacks and whites. PLoS One, 7(10), e48407. doi: 10.1371/journal.pone.0048407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani SH (2011). Corporatization of pain medicine: implications for widening pain care disparities. Pain Medicine, 12(4), 634–644. doi: 10.1111/j.1526-4637.2011.01074.x [DOI] [PubMed] [Google Scholar]
- Meghani SH, Byun E, & Gallagher RM (2012). Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Medicine, 13(2), 150–174. doi: 10.1111/j.1526-4637.2011.01310.x [DOI] [PubMed] [Google Scholar]
- Meghani SH, & Chittams J (2015). Controlling for Socioeconomic Status in Pain Disparities Research: All-Else-Equal Analysis When “All Else” Is Not Equal. Pain Medicine, 16(12), 2222–2225. doi: 10.1111/pme.12829 [DOI] [PubMed] [Google Scholar]
- Meghani SH, Chittams J, Hanlon AL, & Curry J (2013). Measuring preferences for analgesic treatment for cancer pain: how do African-Americans and Whites perform on choice-based conjoint (CBC) analysis experiments? BMC Medical Informatics and Decision Making, 13, 118. doi: 10.1186/1472-6947-13-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani SH, & Gallagher RM (2008). Disparity vs inequity: toward reconceptualization of pain treatment disparities. Pain Medicine, 9(5), 613–623. [DOI] [PubMed] [Google Scholar]
- Meghani SH, & Houldin AD (2007). The meanings of and attitudes about cancer pain among African Americans. Oncology Nursing Forum, 34(6), 1179–1186. doi: 10.1188/07.ONF.1179-1186 [DOI] [PubMed] [Google Scholar]
- Meghani SH, Kang Y, Chittams J, McMenamin E, Mao JJ, & Fudin J (2014). African Americans with cancer pain are more likely to receive an analgesic with toxic metabolite despite clinical risks: a mediation analysis study. Journal of Clincical Oncology, 32(25), 2773–2779. doi: 10.1200/JCO.2013.54.7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani SH, & Keane A (2007). Preference for analgesic treatment for cancer pain among African Americans. Journal of Pain and Symptom Management, 34(2), 136–147. doi: 10.1016/j.jpainsymman.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Meghani SH, & Knafl GJ (2016). Patterns of analgesic adherence predict health care utilization among outpatients with cancer pain. Patient Preference and Adherence, 10, 81–98. doi: 10.2147/PPA.S93726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani SH, Thompson AM, Chittams J, Bruner DW, & Riegel B (2015). Adherence to Analgesics for Cancer Pain: A Comparative Study of African Americans and Whites Using an Electronic Monitoring Device. Journal of Pain, 16(9), 825–835. doi: 10.1016/j.jpain.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani SH, & Vapiwala N (2018). Bridging the Critical Divide in Pain Management Guidelines From the CDC, NCCN, and ASCO for Cancer Survivors. JAMA Oncology, 4(10), 1323–1324. doi: 10.1001/jamaoncol.2018.1574 [DOI] [PubMed] [Google Scholar]
- Mercadante S, Tirelli W, David F, Arcara C, Fulfaro F, Casuccio A, & Gebbia V (2010). Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clinical Journal of Pain, 26(9), 794–797. [DOI] [PubMed] [Google Scholar]
- Minick P, Clark PC, Dalton JA, Horne E, Greene D, & Brown M (2012). Long-bone fracture pain management in the emergency department. Journal of Emergency Nursing, 38(3), 211–217. doi: 10.1016/j.jen.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Morrison RS, Wallenstein S, Natale DK, Senzel RS, & Huang LL (2000). “We don’t carry that”--failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. New England Journal of Medicine, 342(14), 1023–1026. doi: 10.1056/NEJM200004063421406 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2015). Adult Cancer Pain (Version 2.2015). NCCN Clinical Practice Guidelines in Oncology www.nccn.org [Google Scholar]
- National Kidney Foundation. Calculators for Health Care Professionals: GFR Calculators Retrieved November 19, 2013, from http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm
- Paice JA, Portenoy R, Lacchetti C, Campbell T, Cheville A, Citron M, … Bruera E (2016). Management of Chronic Pain in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology doi: 10.1200/JCO.2016.68.5206 [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Kertesz SG, Kohn MA, & Gonzales R (2008). Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA, 299(1), 70–78. doi: 10.1001/jama.2007.64 [DOI] [PubMed] [Google Scholar]
- Pöge U, Gerhardt T, Palmedo H, Klehr H-U, Sauerbruch T, & Woitas RP (2005). MDRD Equations for Estimation of GFR in Renal Transplant Recipients. American Journal of Transplantation, 5(6), 1306–1311. doi: doi: 10.1111/j.1600-6143.2005.00861.x [DOI] [PubMed] [Google Scholar]
- Rhee YO, Kim E, & Kim B (2012). Assessment of pain and analgesic use in African American cancer patients: factors related to adherence to analgesics. Journal of Immigrant and Minority Health, 14(6), 1045–1051. doi: 10.1007/s10903-012-9582-x [DOI] [PubMed] [Google Scholar]
- Riley J, Branford R, Droney J, Gretton S, Sato H, Kennett A, … Ross J (2015). Morphine or oxycodone for cancer-related pain? A randomized, open-label, controlled trial. Journal of Pain and Symptom Management, 49(2), 161–172. doi: 10.1016/j.jpainsymman.2014.05.021 [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2012). SASÆ Component Language 9.3. Cary, NC: SAS Institute. [Google Scholar]
- Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, & Hilgart JS (2015). Oxycodone for cancer-related pain. Cochrane Database Syst Rev(2), CD003870. doi: 10.1002/14651858.CD003870.pub5 [DOI] [PubMed] [Google Scholar]
- Simon LS (2012). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Journal of pain & palliative care pharmacotherapy, 26(2), 197–198. [Google Scholar]
- Singhal A, Tien YY, & Hsia RY (2016). Racial-Ethnic Disparities in Opioid Prescriptions at Emergency Department Visits for Conditions Commonly Associated with Prescription Drug Abuse. PLoS One, 11(8), e0159224. doi: 10.1371/journal.pone.0159224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HS (2009). Opioid metabolism. Mayo Clinic Proceedings, 84(7), 613–624. doi: 10.1016/S0025-6196(11)60750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout E, Sexton P, & Meghani SH (2017). Racial Differences in Adherence to Prescribed Analgesia in Cancer Patients: An Integrated Review of Quantitative Research. JCOM, 24(1), 39–48. [Google Scholar]
- Street Rx. (2017). Latest Street Prices for Prescription Drugs http://streetrx.com/
- Tamayo-Sarver JH, Dawson NV, Hinze SW, Cydulka RK, Wigton RS, Albert JM, … Baker DW (2003). The effect of race/ethnicity and desirable social characteristics on physicians’ decisions to prescribe opioid analgesics. Academy of Emergency Medicine, 10(11), 1239–1248. [DOI] [PubMed] [Google Scholar]
- Todd KH, Samaroo N, & Hoffman JR (1993). Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA, 269(12), 1537–1539. [PubMed] [Google Scholar]
- Ware LJ, Epps CD, Clark J, & Chatterjee A (2012). Do ethnic differences still exist in pain assessment and treatment in the emergency department? Pain Management Nursing, 13(4), 194–201. doi: 10.1016/j.pmn.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Wiese AD, Griffin MR, Schaffner W, Stein CM, Greevy RA, Mitchel EF, & Grijalva CG (2018). Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clinical Infectious Diseases [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, & Collins C (2010). Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences, 1186, 69–101. doi: 10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (1996). Cancer Pain Relief - with a guide to opioid availability Geneva: World Health Organization, . [Google Scholar]
- Yeager KA, Williams B, Bai J, Cooper HLF, Quest T, Meghani SH, & Bruner DW (2018). Factors related to Adherence to Opioids in Black Patients with Cancer Pain. Journal of Pain and Symptom Management doi: 10.1016/j.jpainsymman.2018.10.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca E, Brunelli C, Bracchi P, Biancofiore G, De Sangro C, Bortolussi R, … Caraceni A (2016). Comparison of the Tolerability Profile of Controlled-Release Oral Morphine and Oxycodone for Cancer Pain Treatment. An Open-Label Randomized Controlled Trial. Journal of Pain and Symptom Management, 52(6), 783–794 e786. doi: 10.1016/j.jpainsymman.2016.05.030 [DOI] [PubMed] [Google Scholar]


