Abstract
This review on the mechanisms of neuroinflammation following subarachnoid hemorrhage will focus mainly on toll‐like receptor 4 (TLR4), Heme Oxygenase‐1 (HO‐1), and the role of microglia and macrophages in this process. Vasospasm has long been the focus of research in SAH; however, clinical trials have shown that amelioration of vasospasm does not lead to an improved clinical outcome. This necessitates the need for novel avenues of research. Our work has demonstrated that microglial TLR4 and microglial HO‐1, not only affects cognitive dysfunction, but also circadian dysrhythmia in a mouse model of SAH. To attempt to translate these findings, we have also begun investigating macrophages in the cerebrospinal fluid of SAH patients. The goal of this review is to provide an update on the role of TLR4, HO‐1, and other signal transduction pathways in SAH‐induced neuroinflammation.
Keywords: adaptive immunity, CD163, Heme‐Oxygenase, innate immunity, microglia, subarachnoid hemorrhage, toll like receptor
Significance.
For the past 50 years, research in subarachnoid hemorrhage (SAH) has focused on angiographic vasospasm with the assumption that this was cause of delayed cerebral ischemia (DCI). After much research, this hypothesis has been debunked for the most part. Ameliorating angiographic vasospasm does not necessarily improve outcome after SAH, nor does all injury in the brain after SAH relate to ischemia. With this realization, came the understanding that alternate sources of injury after SAH must be sought, and thus neuroinflammation was targeted. Neuroinflammation after SAH has many monikers: Early Brain Injury, Cerebral Inflammatory Response, delayed neurological injury, et cetera. This review will focus on these alternative mechanisms of neuroinflammation after SAH and hopefully provide some insights to other researchers to allow us all to move the SAH research field forward.
1. INTRODUCTION
A total of 28,000 Americans will fall victim to an aneurysmal subarachnoid hemorrhage (SAH) this year, and one third of the survivors will have a poor cognitive outcome (Bederson et al., 2009; Etminan et al., 2019). While this is a small number compared to the total number that will have a stroke, SAH accounts for a disproportionately large health care cost because many of the patients are relatively young compared to other kinds of stroke (Bosetti et al., 2017; Reaven, Lovett, & Funk, 2009; Taylor et al., 1996). Furthermore, 65% of SAH patients will have severe constriction of their cerebral vasculature, otherwise known as vasospasm (Dankbaar, Rijsdijk, et al., 2009; Dankbaar, Rooij, et al., 2009; Rooij, Rinkel, Dankbaar, & Frijns, 2013; Vergouwen, 2011). In these studies, vasospasm has been found to be independently associated with mortality and poor neurological outcome. Remarkably, clinical trials that succeeded in reducing vasospasm showed no improvement in mortality or neurological outcome of SAH patients (Macdonald et al., 2011, 2012, 2008; Vergouwen, Ilodigwe, & Macdonald, 2011). Perhaps this is not surprising when one realizes that vasospasm could be an epiphenomenon of disease severity, and that treatments aimed solely at this sequalae of SAH do not address the underlying red blood cell (RBC)‐induced cerebral inflammation that persists.
The neuronal damage seen after SAH could be indirectly caused by an immune response initiated by danger proteins from lysed RBCs. Several pro‐inflammatory molecules such as heme, methemoglobin, and high mobility group box 1 bind to TLR4 and induce an inflammatory response (Figueiredo et al., 2007; Kwon et al., 2015; Takizawa et al., 2017). On the other hand, the presence of heme oxygenase‐1 (HO‐1) in microglia reduces inflammation by degrading heme and producing carbon monoxide (CO). CO has been found to play an important neuroprotective role by clearing subarachnoid blood (Schallner et al., 2015).
2. INNATE IMMUNE RESPONSES TO SAH
Macrophages are a member of the innate immune system, and one of the professional antigen presenting cells (APCs). Until recently, it was thought that all macrophages were derived from circulating monocytes, which in turn were generated by the bone marrow. Elegant experiments now reveal that tissue resident macrophages, such as Kupffer cells, Langerhans cells, and microglia are derived from the embryonic yolk sac, whereas circulating monocytes which after invasion of tissues become macrophages, are derived from the bone marrow (Gomez Perdiguero et al., 2015). Our work has shown that in a mouse model of SAH, microglia seem to have both protective and deleterious roles, depending on the time frame. Early in SAH, microglia seem to have a more deleterious role and eliminating them decreased neuronal apoptosis. Later on, neuronal apoptosis seemed to be independent of the existence of microglia (Hanafy, 2013). Furthermore, at least with respect to microglial heme oxygenase, a protective role was observed, which will be described in detail in the following section (Schallner et al., 2015). Using mouse chimeras, where only peripheral marrow was ablated and reconstituted with green fluorescent protein‐tagged leukocytes, our group found no significant GFP infiltrate 7 days after SAH induction (Schallner et al., 2015). This would suggest that microglia, at least at 7 days, are the only critical mediators of neuroinflammation in SAH.
On the other hand, others have shown an important role for neutrophils in SAH. Early in SAH, neutrophil depletion via Ly‐6G demonstrated normal cortical perfusion compared to neutrophil‐intact, SAH mice (Provencio, Altay, Smithason, Moore, & Ransohoff, 2011). This neutrophil‐induced cortical hypoperfusion is thought to be mediated by prostaglandin F2α (Neulen et al., 2019). Other studies have shown that the myeloperoxidase produced by neutrophils is found in human cerebral aneurysms and facilitates rupture of cerebral aneurysms in a mouse model. Other groups have found macrophages to be critical in aneurysm formation and rupture. None of these observations are mutually exclusive. Likely, many, if not all, components of the innate immune system have a part in neuroinflammation and possibly even neuroprotection, at different times, after SAH (Jassam, Izzy, Whalen, McGavern, & El Khoury, 2017; Kanazawa, Ninomiya, Hatakeyama, Takahashi, & Shimohata, 2017; Qin et al., 2017; Yao et al., 2017).
3. ADAPTIVE IMMUNE RESPONSES TO SAH
In contrast to innate immune responses, the adaptive immune system is based on antigen‐specific receptors such as T‐cell receptors and immunoglobulins. Therefore, several days are required to allow for memory of specific antigen and antigen‐driven clonal cell expansion (Abbas, 2010). In ischemic stroke, adaptive immune responses can be activated by multiple mediators generated by the innate immune system, leading to autoimmunity. With respect to hemorrhagic stroke, little has been done with respect to the adaptive immune system. Only one preclinical study has shown neuroprotective effects of statins via upregulation of regulatory T lymphocytes in rodent SAH models (Ayer et al., 2013). Furthermore, only two clinical studies have shown proliferation of CD4+ and CD8+ T cells in CSF and peripheral blood of SAH patients (Mathiesen, Andersson, Loftenius, & Holst, 1993; Moraes et al., 2015). Due to the paucity of research, the clinical implications of the adaptive immune system in SAH remain unexplored.
4. DELAYED NEUROLOGICAL DEFICITS
About 30% of surviving SAH patients will have delayed neurological deficits (DND) (Vergouwen, 2011). DND generally occurs 3–14 days after aneurysm rupture and carries a high morbidity and mortality (Bederson et al., 2009; Diringer et al., 2011). While DND was thought to be a direct result of large vessel vasospasm, evidence now exists that vasospasm can occur independently of DND (Macdonald et al., 2011, 2012, 2008; Pegoli, Mandrekar, Rabinstein, & Lanzino, 2015; Vergouwen et al., 2011). Likewise, DND can occur in the absence of vasospasm; this is where RBC‐induced cerebral inflammation, as well as cortical spreading depression and microcirculatory dysfunction could be culprits in DND, based on both clinical and preclinical studies (Hanafy, 2013; LeBlanc, Chen, Selim, & Hanafy, 2016; Macdonald et al., 2011, 2012, 2008; Schneider et al., 2015; Vergouwen, 2011; Vergouwen et al., 2011).
5. TOLL‐LIKE RECEPTOR 4 PATHWAY
Toll‐like receptors (TLRs) are membrane‐bound proteins that belong to the pattern recognition receptor (PRR) family, are ubiquitously expressed, and trigger an innate immune response when bound to their respective ligands (Vaure & Liu, 2014). Toll‐like Receptor 4 (TLR4), in an SAH mouse model, is predominantly expressed on APCs, such as microglia and macrophages, although it is also expressed to a lesser extent on astrocytes and neurons (Hanafy, 2013).
TLR4 recognizes a wide range of pathogenic components known as damage‐associated molecular patterns (DAMPs), with lipopolysaccharide (LPS) being the canonical agonist, as well as endogenous molecules such as heme and fibrinogen which are released during SAH (Vaure & Liu, 2014). The activation of TLR4 leads to the synthesis of pro‐inflammatory cytokines, chemokines, and the expression of co‐stimulatory molecules (Vaure & Liu, 2014). Thus, since neuroinflammation is a consequence of SAH, the study of TLR4‐mediated inflammation has drawn interest. Of note, heme has been shown to be a specific agonist of TLR4 expressed on APCs, stressing the need for further understanding of microglia in the heme‐induced cerebral inflammatory response (CIR) after SAH (Yao et al., 2017). Heme also has TLR4‐independent effects that could contribute to the CIR after SAH such as an oxidative burst, increased neutrophil recruitment, and increased HO‐1 expression (Yao et al., 2017). Our lab has shown that heme induces a significant amount of neuronal apoptosis in a mouse model of SAH, compared to LPS stimulation. These findings are not entirely surprising given that the toll receptor‐associated activator of interferon (TRIF) pathway, via interferon expression, does exert antiapoptotic effects (Blander, 2014; Hanafy, 2013; Shim et al., 2005).
Among all the TLRs, TLR4 is unique in the sense that it can signal through both the myeloid differentiation primary response protein 88 (MyD88) and the TRIF pathways to induce inflammatory responses (O'Neill & Bowie, 2007). Using an SAH mouse model, it was shown that in the early phase of SAH, neuronal apoptosis was mostly TLR4–MyD88‐dependent and microglial‐dependent, whereas, during late phase of SAH, neuronal apoptosis was largely TRIF dependent and microglia independent. This bimodal pattern of cerebral injury is important because it demonstrates that delayed neurological injury can occur in a mouse model of SAH as well (Hanafy, 2013; Vergouwen et al., 2011).
Both MyD88 and TRIF pathways trigger the expression of nuclear factor‐κB (NF‐κB), a key transcriptional regulator of inflammatory‐related genes (O'Neill & Bowie, 2007). However, unlike MyD88, TRIF also has the ability to induce interferon response elements, thereby producing anti‐apoptotic interferons. This antiapoptotic effect of TRIF ensures that inflammation from NF‐kB activation will be long lasting (O'Neill & Bowie, 2007). NF‐κB, in turn, triggers the transcription of pro‐inflammatory genes such as tumor necrosis factor (TNF‐α), interleukin‐1β (IL‐1β), and intercellular adhesion molecule‐1 (ICAM‐1). TNF‐α can induce (RAS‐related C3 Botulinum Toxin Substrate‐1) Rac‐1‐mediated oxidative stress and vasoconstriction (Vecchione et al., 2009). Moreover, increased levels of TNF‐α in brain interstitial fluid were found to correlate with worsened cerebral vasospasm (Hanafy et al., 2010). IL‐1β can also induce apoptosis and cyclooxygenase‐2‐facilitated inflammation. Finally, increased ICAM‐1 is but one of a multitude of endothelial proteins that can be upregulated in response to inflammation and is thought to have a critical role in microcirculatory dysfunction (Kong, Kim, Kim, Jang, & Lee, 2018).
6. MITOGEN‐ACTIVATED PROTEIN KINASES (MAPKS)
The MyD88‐dependent pathway also has effects on cell survival via the activation of mitogen‐activated protein kinases (MAPKs), such as the signal‐regulated kinase (ERK), p38, and c‐Jun N‐terminal kinase (JNK), which in turn leads to stimulation of the transcription factor activator protein‐1 (AP‐1) (Fang, Wang, Zhou, Wang, & Yang, 2013). MAPKs are directly involved in many cellular responses to a vast range of stimuli such as mitogens, heat shock, and inflammation (Pearson et al., 2001). Furthermore, the MAPK pathway seems to play a crucial role in the CIR. In a rat model of SAH, the MAPK pathway was critical to the regulation of cerebral blood flow (Maddahi, Ansar, Chen, & Edvinsson, 2011; Sun & Nan, 2016). Conversely, both the p38 and JNK MAPK pathways were also found to induce post‐SAH neuronal and endothelial cell apoptosis, inflammatory cytokine expression, and facilitate the CIR along with delayed neuronal injury (Huang et al., 2013; Sun & Nan, 2016; Zhang, Zhao, Shi, & Yin, 2011). To elucidate the role of MAPK pathways in post‐SAH injury, recombinant osteopontin (r‐OPN) was used in a rodent model. r‐OPN enhances the endogenous MAPK inhibitor, MKP‐1, which suppresses the phosphorylation of MAPKs, caldesmon, and heat shock protein 27 in spastic cerebral arteries of a rat model at 24‐hr post‐SAH (Suzuki, Hasegawa, Chen, Kanamaru, & Zhang, 2010). Interestingly, it was shown that administration of r‐OPN prior to SAH prevents vasospasm and neurological impairments at 24–72‐hr post‐SAH, in a rat model (Suzuki et al., 2010).
7. HIGH MOBILITY GROUP BOX 1
HMGB1 is a DNA‐binding protein that regulates gene expression. It is passively released during necrosis by cells in order to alert neighboring cells of cellular damage (Scaffidi, Misteli, & Bianchi, 2002). Some immune cells such as monocytes, macrophages, and dendritic cells secrete HMGB1 in an active manner, in response to various cellular stresses (Abraham, Arcaroli, Carmody, Wang, & Tracey, 2000; Lotze & Tracey, 2005; Wang et al., 1999). Important receptors such as the receptor for advanced glycation end products (RAGE), TLR2, TLR4, and TLR9 have been found to participate in HMGB1 signaling. RAGE is a receptor found at low levels in normal tissues, but upregulated at sites where its ligands concentrate (Chavakis, Bierhaus, & Nawroth, 2004). HMGB1 signaling through RAGE upregulates the production of chemotaxins and cytokines via NF‐kB (Palumbo et al., 2007; Park et al., 2003). The activation of TLR2 and TLR4 by HMGB1 leads to the upregulation of NF‐kB (Kokkola et al., 2005; Park et al., 2006, 2004); hence, HMGB1 likely leads to the release of pro‐inflammatory cytokines through these pathways. Furthermore, the interaction of IL‐1β, IFNγ, and TNFα with HMGB1 leads to an amplified inflammatory response compared with HMGB1 stimulation alone (Sha, Zmijewski, Xu, & Abraham, 2008). In addition, HMGB1 stimulates the release of reactive oxygen species by neutrophils via a TLR4‐dependent activation of (Nicotinamide adenine dinucleotide phosphate) NADPH oxidase (Fan et al., 2007) which results in further release of cytokines (Lotze & Tracey, 2005; Palumbo et al., 2007). HMGB1 also mediates the adhesion of inflammatory cells to the endothelial lumen by increasing the expression of ICAM‐1 and vascular cell adhesion molecule (VCAM‐1) (Fiuza et al., 2003; Treutiger et al., 2003).
It has been shown that HMGB1 is released from the nucleus of neuronal cells to the extracellular space during ischemic and traumatic brain injuries, and that the targeting of HMGB1 with monoclonal antibodies (mAb) reduces brain injury by preventing the breakdown of the blood–brain barrier (BBB) and reducing the inflammatory response (Liu et al., 2007; Okuma et al., 2012; Zhang, Takahashi, et al., 2011) In addition, data from several clinical studies indicate that HMGB1 could play a critical role in CIR and DND after SAH due to the high levels of HMGB1 found in plasma during the post‐SAH period (Murakami et al., 2011; Nakahara et al., 2009; Zhu et al., 2012).
Finally, in a rat SAH model, administration of anti‐HMGB1 antibodies decreased vasospasm by inhibiting HMGB1 translocation into arterial smooth muscle cells, thereby suppressing vasoconstriction and vascular inflammatory responses (Haruma et al., 2016).
8. HEME OXYGENASE (HO)
HO is an enzyme that catalyzes the degradation of heme. There are two isoforms of heme oxygenase (HO): HO‐1 and HO‐2. HO‐1, the inducible form, is found in neuronal cells, glial cells, and macrophages such as microglia, whereas HO‐2 is constitutively expressed in neuronal cells and vascular endothelial cells (Sutherland et al., 2009). Our lab demonstrated that microglial HO‐1 is necessary to alleviate neuronal cell death and cognitive dysfunction, as well as facilitate erythrophagocytosis (Schallner et al., 2015). Free heme released into the subarachnoid space during SAH is metabolized by HO‐1, releasing iron (Fe2+), biliverdin, and carbon monoxide (CO) (Kikuchi, Yoshida, & Noguchi, 2005).Free iron is thought to cause cell membrane damage via free radicals and the Fenton reaction (Loftspring, 2010). Previous studies have shown a causal relationship between free iron and brain injury following SAH (Gomes et al., 2014). Moreover, it has been shown that treatment with the iron‐chelating agent, deferoxamine (DFX), decreases brain edema, oxidative stress, and neuronal apoptosis (Lee et al., 2010; Yu, Jia, & Chen, 2014); further corroborating the damaging role of free iron (Loftspring, 2010; Selim, 2009). Our lab has shown that intrathecal administration of DFX may mediate some of its neuroprotective effects by increasing the expression of microglial HO‐1, as well as reducing neuronal apoptosis, reactive mitochondrial species, and improving cognitive function (LeBlanc et al., 2016).
To further elucidate the neuroprotective role of microglial HO‐1 after SAH, we investigated one of the by‐products of heme metabolism: CO. Despite the nefarious reputation of CO, we found it to be the neuroprotective by‐product of heme catabolism by microglial HO‐1 (Schallner et al., 2015). This was elucidated by exposing mice lacking microglial HO‐1 to gaseous CO, after SAH, which resulted in reduced injury, and improved cognitive function. This could be due to increasing erythrophagocytosis, although CO's effects are pleiotropic due to its gaseous nature. To this end, we found that administration of gaseous CO aids in normalizing circadian dysrhythmia after SAH (Schallner et al., 2017). We found that SAH induced at dawn compared to sunset resulted in worse cognitive function, more neuronal apoptosis, and an increased inflammatory milieu; all this correlated with reduced microglial HO‐1 expression at dawn and was rescued with exogenous CO administration (Schallner et al., 2017).
Additionally, CO seems to function similarly to nitric oxide (NO) as a vasodilator, neurotransmitter, and platelet aggregation inhibitor, as well as serving other anti‐inflammatory roles (Hanafy, Oh, & Otterbein, 2013). It is thought to act via soluble guanylyl cyclase (sGC), as well as cyclic GMP (cGMP), and BKca channels leading to vasodilation in the vascular smooth muscle cells (Hou, Xu, Heinemann, & Hoshi, 2008; Jaggar et al., 2005; Kaide et al., 2001; Wang, Wu, & Wang, 1997; Wu, Cao, Lu, & Wang, 2002), and thus the reduction of vasoconstriction. In addition, CO seems to inhibit TLR 2, 4, 5, and 9 signaling pathways in macrophages by interrupting their recruitment to membrane rafts (Nakahira et al., 2006). These rafts, are specialized lipid domains that contribute to immune signal transduction. CO was shown to inhibit TLR trafficking to lipid rafts by suppressing NADPH oxidase‐dependent ROS generation (Nakahira et al., 2006).
9. CD163
Haptoglobin is a protein found in the plasma that binds free hemoglobin (Hb) released from RBCs forming the hemoglobin–haptoglobin complex (Kristiansen et al., 2001). Cluster of differentiation 163 (CD163) was found to be a specific receptor of the hemoglobin–haptoglobin complex and is exclusively expressed on monocytes and macrophages. CD163 is involved in the clearance and endocytosis of hemoglobin–haptoglobin complexes, and thus it may protect tissues from hemoglobin‐mediated oxidative damage, serving as an alternative to the heme‐TLR4/HO‐1 pathway. To determine the potential role of CD163 in SAH patients, our lab performed flow cytometry on the cerebrospinal fluid (CSF) from SAH patients and found increased expression of CD163 on macrophages from SAH patients compared to unruptured aneurysm controls. To verify these findings, we then performed immunohistochemistry on the CSF macrophages from SAH patients with increasing modified Fisher scales, where the Fisher scale refers to the RBC burden of an SAH patient noted on CT scan. As expected, we found increased CD163 expression on macrophages which had phagocytosed more blood. Surprisingly, we found an inverse correlation between CSF macrophage CD163 expression measured on day 1 after SAH, and 90 day outcome of these patients as measured by the modified Rankin Scale (mRS). That is, increasing CD163 expression seemed to correlate with improved neurological outcome, or a lower mRS. With further study, CSF macrophage CD163 expression may prove to be an important biomarker for SAH prognostication (Thomas, Ogilvy, Griessenauer, & Hanafy, 2018). Understanding why this is so might lead to novel immunotherapies.
10. ANTI‐INFLAMMATORY TREATMENTS IN SAH PATIENTS
There is great interest in identifying an inflammatory biomarker that is associated with DND. Despite the fact that no biomarker has been validated to this end, a number of small scale clinical trials have attempted to use various anti‐inflammatory agents in SAH, but to no avail. Acetylsalicylic acid (Dorhout Mees, Bergh, Algra, & Rinkel, 2007), steroids (Chyatte, Fode, Nichols, & Sundt, 1987; Gomis et al., 2010; Mohney et al., 2018), various nonsteroid anti‐inflammatory agents (Nassiri et al., 2016), immunosuppressants (Manno, Gress, Ogilvy, Stone, & Zervas, 1997; Ryba, Pastuszko, Iwanska, Bidzinski, & Dziewiecki, 1991), and IL‐1 receptor antagonists (Singh et al., 2014) have all been failures. There are many potential explanations for these failures, but perhaps a more directed immune‐based approach might be necessary, mirroring novel therapies in the oncology world like chimeric antigen receptor T cells, but for the innate immune system.
11. CONCLUSION
The mechanisms behind the adverse sequalae of SAH are still poorly understood; although a summary of the cerebral inflammatory signal transduction pathways highlighted in this review are presented in Figure 1. While neuroinflammation itself is well known to cause cognitive dysfunction in diseases such as multiple sclerosis, poststroke recrudescence, and even systemic bacteremia; an exact mechanism behind the cognitive dysfunction in SAH has yet to be elucidated. Moreover, the high mortality rate of SAH patients makes it imperative to find new and better therapeutic treatments. SAH neuroinflammation seems to be caused primarily by the breakdown of hemoglobin in the subarachnoid space, which leads to the release of heme. Heme works as a potent TLR4 activator, and also activates the MyD88 and TRIF cascades. Microglia, macrophages, and neutrophils likely all have roles in potentiating heme‐mediated inflammation. While the involvement of the adaptive immune system in hemorrhagic stroke is not well understood, it could be important as well. RAGE, MAPK, and HMGB1 are involved in the initiation and propagation of inflammation, while CD163 and CO quell the inflammatory response. Despite the recent discovery of the neuroprotective effects presented by DFX and CO, there is still a clear need to further understand the neuroinflammation in SAH.
Figure 1.
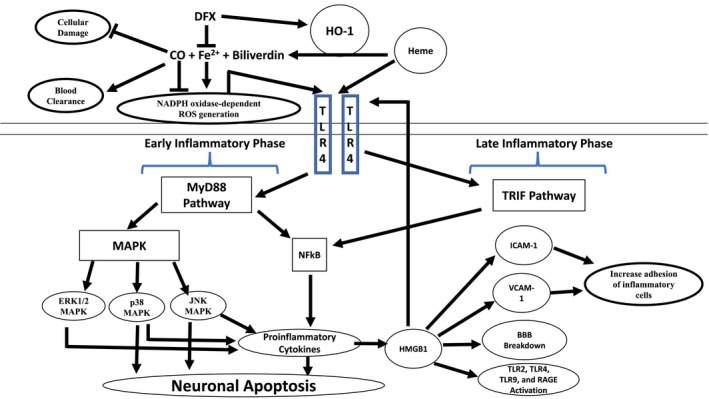
Heme metabolism and the microglial toll‐like receptor 4 (TLR4) signaling pathway following SAH: In the subarachnoid space, free heme is metabolized by heme oxygenase (HO)‐1, releasing iron (Fe2+), biliverdin, and carbon monoxide (CO). Deferoxamine (DFX), an iron‐chelating agent, decreases the oxidative toxicity of free iron and increase the HO‐1‐mediated neuroprotective effect. Low‐dose CO also has neuroprotective effect by increasing erythrophagocytosis. Heme initiates microglial TLR4 signaling and activates the myeloid differentiation primary response protein 88 dependent (MyD88) in early phase of SAH and the toll receptor associated activator of interferon‐dependent (TRIF) cascade in late phase of SAH. MyD88 triggers the expression of nuclear factor‐κB (NF‐κB) and mitogen‐activate protein kinase (MAPK), resulting in apoptosis and pro‐inflammatory gene expression. BBB, brain–blood barrier; ICAM‐1, intercellular adhesion molecule 1; and VCAM‐1, vascular cell adhesion molecule 1 [Color figure can be viewed at https://www.wileyonlinelibrary.com]
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Writing—Original Draft, V.P.; Writing—Review & Editing, Y.A. and K.A.H.
Supporting information
Transparent Peer Review Report.
Akamatsu Y, Pagan VA, Hanafy KA. The role of TLR4 and HO‐1 in neuroinflammation after subarachnoid hemorrhage. J Neuro Res. 2020;98:549–556. 10.1002/jnr.24515
Edited by Jerome Badaut. Reviewed by Paul Kasher and Marc J. Simard.
All peer‐reviewed communications can be found with the online version of the article.
Funding information
Dr. Hanafy is funded by the NINDS (R21 NS099606‐02 and 1R01NS109174‐01) and the American Heart Association Grant‐in‐Aid Award #17GRNT33670058
REFERENCES
- Abbas, A. K. (2010). Basic immunology updated edition: Functions and disorders of the immune system Ch. 5. Philadelphia, PA: Saunders. [Google Scholar]
- Abraham, E. , Arcaroli, J. , Carmody, A. , Wang, H. , & Tracey, K. J. (2000). HMG‐1 as a mediator of acute lung inflammation. The Journal of Immunology, 165, 2950–2954. [DOI] [PubMed] [Google Scholar]
- Ayer, R. E. , Ostrowski, R. P. , Sugawara, T. , Ma, Q. , Jafarian, N. , Tang, J. , & Zhang, J. H. (2013). Statin‐induced T‐lymphocyte modulation and neuroprotection following experimental subarachnoid hemorrhage. Acta Neurochirurgica. Supplementum, 115, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson, J. B. , Connolly, E. S. , Batjer, H. H. , Dacey, R. G. , Dion, J. E. , Diringer, M. N. , … Rosenwasser, R. H. (2009). Guidelines for the management of aneurysmal subarachnoid hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke, 40, 994–1025. [DOI] [PubMed] [Google Scholar]
- Blander, J. M. (2014). A long‐awaited merger of the pathways mediating host defence and programmed cell death. Nature Reviews Immunology, 14, 601–618. [DOI] [PubMed] [Google Scholar]
- Bosetti, F. , Koenig, J. I. , Ayata, C. , Back, S. A. , Becker, K. , Broderick, J. P. , … Corriveau, R. A. (2017). Translational stroke research: Vision and opportunities. Stroke, 48, 2632–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis, T. , Bierhaus, A. , & Nawroth, P. P. (2004). RAGE (receptor for advanced glycation end products): A central player in the inflammatory response. Microbes and Infection, 6, 1219–1225. 10.1016/j.micinf.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Chyatte, D. , Fode, N. C. , Nichols, D. A. , & Sundt, T. M. (1987). Preliminary report: Effects of high dose methylprednisolone on delayed cerebral ischemia in patients at high risk for vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery, 21, 157–160. [DOI] [PubMed] [Google Scholar]
- Dankbaar, J. W. , de Rooij, N. K. , Velthuis, B. K. , Frijns, C. J. M. , Rinkel, G. J. E. , & van der Schaaf, I. C. (2009). Diagnosing delayed cerebral ischemia with different CT modalities in patients with subarachnoid hemorrhage with clinical deterioration. Stroke, 40, 3493–3498. [DOI] [PubMed] [Google Scholar]
- Dankbaar, J. W. , Rijsdijk, M. , van der Schaaf, I. C. , Velthuis, B. K. , Wermer, M. J. H. , & Rinkel, G. J. E. (2009). Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology, 51, 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij, N. K. , Rinkel, G. J. E. , Dankbaar, J. W. , & Frijns, C. J. M. (2013). Delayed cerebral ischemia after subarachnoid hemorrhage: A systematic review of clinical, laboratory, and radiological predictors. Stroke, 44, 43–54. [DOI] [PubMed] [Google Scholar]
- Diringer, M. N. , Bleck, T. P. , Claude Hemphill, J. , Menon, D. , Shutter, L. , Vespa, P. , … Zipfel, G. (2011). Critical care management of patients following aneurysmal subarachnoid hemorrhage: Recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocritical Care, 15, 211–240. 10.1007/s12028-011-9605-9 [DOI] [PubMed] [Google Scholar]
- Dorhout Mees, S. M. , van den Bergh, W. M. , Algra, A. , & Rinkel, G. J. (2007). Antiplatelet therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database Systematic Review, 4, CD006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan, N. , Chang, H. S. , Hackenberg, K. , de Rooij, N. K. , Vergouwen, M. D. I. , Rinkel, G. J. E. , & Algra, A. (2019). Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: A systematic review and meta‐analysis. JAMA Neurology, 76, 588–597. 10.1001/jamaneurol.2019.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , Li, Y. , Levy, R. M. , Fan, J. J. , Hackam, D. J. , Vodovotz, Y. , … Wilson, M. A. (2007). Hemorrhagic shock induces NAD(P)H oxidase activation in neutrophils: Role of HMGB1‐TLR4 signaling. The Journal of Immunology, 178, 6573–6580. [DOI] [PubMed] [Google Scholar]
- Fang, H. , Wang, P.‐F. , Zhou, Y. , Wang, Y.‐C. , & Yang, Q.‐W. (2013). Toll‐like receptor 4 signaling in intracerebral hemorrhage‐induced inflammation and injury. Journal of Neuroinflammation, 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo, R. T. , Fernandez, P. L. , Mourao‐Sa, D. S. , Porto, B. N. , Dutra, F. F. , Alves, L. S. , … Bozza, M. T. (2007). Characterization of heme as activator of Toll‐like receptor 4. Journal of Biological Chemistry, 282, 20221–20229. [DOI] [PubMed] [Google Scholar]
- Fiuza, C. , Bustin, M. , Talwar, S. , Tropea, M. , Gerstenberger, E. , Shelhamer, J. H. , & Suffredini, A. F. (2003). Inflammation‐promoting activity of HMGB1 on human microvascular endothelial cells. Blood, 101, 2652–2660. [DOI] [PubMed] [Google Scholar]
- Gomes, J. A. , Selim, M. , Cotleur, A. , Hussain, M. S. , Toth, G. , Koffman, L. , … Provencio, J. J. (2014). Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH‐pilot (CSF iron in SAH). Neurocritical Care, 21, 285–293. 10.1007/s12028-014-9977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Perdiguero, E. , Klapproth, K. , Schulz, C. , Busch, K. , Azzoni, E. , Crozet, L. , … Rodewald, H. R. (2015). Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature, 518, 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis, P. , Graftieaux, J. P. , Sercombe, R. , Hettler, D. , Scherpereel, B. , & Rousseaux, P. (2010). Randomized, double‐blind, placebo‐controlled, pilot trial of high‐dose methylprednisolone in aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery, 112, 681–688. [DOI] [PubMed] [Google Scholar]
- Hanafy, K. A. (2013). The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. Journal of Neuroinflammation, 10, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy, K. A. , Oh, J. , & Otterbein, L. E. (2013). Carbon Monoxide and the brain: Time to rethink the dogma. Current Pharmaceutical Design, 19, 2771–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafy, K. A. , Stuart, R. M. , Khandji, A. G. , Connolly, E. S. , Badjatia, N. , Mayer, S. A. , & Schindler, C. (2010). Relationship between brain interstitial fluid tumor necrosis factor‐α and cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Journal of Clinical Neuroscience, 17, 853–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruma, J. , Teshigawara, K. , Hishikawa, T. , Wang, D. , Liu, K. , Wake, H. , … Nishibori, M. (2016). Anti‐high mobility group box‐1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Scientific Reports, 6, 37755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, S. , Xu, R. , Heinemann, S. H. , & Hoshi, T. (2008). The RCK1 high‐affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proceedings of the National Academy of Sciences of the United States of America, 105, 4039–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, L. , Wan, J. , Chen, Y. , Wang, Z. , Hui, L. , Li, Y. , … Zhou, W. (2013). Inhibitory effects of p38 inhibitor against mitochondrial dysfunction in the early brain injury after subarachnoid hemorrhage in mice. Brain Research, 1517, 133–140. 10.1016/j.brainres.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Jaggar, J. H. , Li, A. , Parfenova, H. , Liu, J. , Umstot, E. S. , Dopico, A. M. , & Leffler, C. W. (2005). Heme is a carbon monoxide receptor for large‐conductance Ca2+‐activated K+ channels. Circulation Research, 97, 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassam, Y. N. , Izzy, S. , Whalen, M. , McGavern, D. B. , & El Khoury, J. (2017). Neuroimmunology of traumatic brain injury: Time for a paradigm shift. Neuron, 95, 1246–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaide, J. I. , Zhang, F. , Wei, Y. , Jiang, H. , Yu, C. , Wang, W. H. , … Nasjletti, A. (2001). Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. Journal of Clinical Investigation, 107, 1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa, M. , Ninomiya, I. , Hatakeyama, M. , Takahashi, T. , & Shimohata, T. (2017). Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. International Journal of Molecular Sciences, 18, 2135 10.3390/ijms18102135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, G. , Yoshida, T. , & Noguchi, M. (2005). Heme oxygenase and heme degradation. Biochemical and Biophysical Research Communications, 338, 558–567. 10.1016/j.bbrc.2005.08.020 [DOI] [PubMed] [Google Scholar]
- Kokkola, R. , Andersson, A. , Mullins, G. , Ostberg, T. , Treutiger, C.‐J. , Arnold, B. , … Harris, H. E. (2005). RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scandinavian Journal of Immunology, 61, 1–9. 10.1111/j.0300-9475.2005.01534.x [DOI] [PubMed] [Google Scholar]
- Kong, D.‐H. , Kim, Y. K. , Kim, M. R. , Jang, J. H. , & Lee, S. (2018). Emerging roles of vascular cell adhesion molecule‐1 (VCAM‐1) in immunological disorders and cancer. International Journal of Molecular Sciences, 19, 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen, M. , Graversen, J. H. , Jacobsen, C. , Sonne, O. , Hoffman, H. J. , Law, S. K. , & Moestrup, S. K. (2001). Identification of the haemoglobin scavenger receptor. Nature, 409, 198–201. [DOI] [PubMed] [Google Scholar]
- Kwon, M. S. , Woo, S. K. , Kurland, D. B. , Yoon, S. H. , Palmer, A. F. , Banerjee, U. , … Simard, J. (2015). Methemoglobin is an endogenous toll‐like receptor 4 ligand‐relevance to subarachnoid hemorrhage. International Journal of Molecular Sciences, 16, 5028–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, R. H. , Chen, R. , Selim, M. H. , & Hanafy, K. A. (2016). Heme oxygenase‐1‐mediated neuroprotection in subarachnoid hemorrhage via intracerebroventricular deferoxamine. Journal of Neuroinflammation, 13, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.‐Y. , Keep, R. F. , He, Y. , Sagher, O. , Hua, Y. , & Xi, G. (2010). Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. Journal of Cerebral Blood Flow and Metabolism, 30, 1793–1803. 10.1038/jcbfm.2010.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K. , Mori, S. , Takahashi, H. K. , Tomono, Y. , Wake, H. , Kanke, T. , … Nishibori, M. (2007). Anti‐high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. The FASEB Journal, 21, 3904–3916. [DOI] [PubMed] [Google Scholar]
- Loftspring, M. C. (2010). Iron and early brain injury after subarachnoid hemorrhage. Journal of Cerebral Blood Flow and Metabolism, 30, 1791–1792. 10.1038/jcbfm.2010.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze, M. T. , & Tracey, K. J. (2005). High‐mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nature Reviews Immunology, 5, 331–342. 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- Macdonald, R. L. , Higashida, R. T. , Keller, E. , Mayer, S. A. , Molyneux, A. , Raabe, A. , … Marr, A. (2011). Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double‐blind, placebo‐controlled phase 3 trial (CONSCIOUS‐2). The Lancet Neurology, 10, 618–625. [DOI] [PubMed] [Google Scholar]
- Macdonald, R. L. , Higashida, R. T. , Keller, E. , Mayer, S. A. , Molyneux, A. , Raabe, A. , … Nowbakht, P. (2012). Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke, 43, 1463–1469. [DOI] [PubMed] [Google Scholar]
- Macdonald, R. L. , Kassell, N. F. , Mayer, S. , Ruefenacht, D. , Schmiedek, P. , Weidauer, S. , … Pasqualin, A. (2008). Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS‐1): Randomized, double‐blind, placebo‐controlled phase 2 dose‐finding trial. Stroke, 39, 3015–3021. [DOI] [PubMed] [Google Scholar]
- Maddahi, A. , Ansar, S. , Chen, Q. , & Edvinsson, L. (2011). Blockade of the MEK/ERK pathway with a raf inhibitor prevents activation of pro‐inflammatory mediators in cerebral arteries and reduction in cerebral blood flow after subarachnoid hemorrhage in a rat model. Journal of Cerebral Blood Flow and Metabolism, 31, 144–154. 10.1038/jcbfm.2010.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno, E. M. , Gress, D. R. , Ogilvy, C. S. , Stone, C. M. , & Zervas, N. T. (1997). The safety and efficacy of cyclosporine A in the prevention of vasospasm in patients with Fisher grade 3 subarachnoid hemorrhages: A pilot study. Neurosurgery, 40, 289–293. [DOI] [PubMed] [Google Scholar]
- Mathiesen, T. , Andersson, B. , Loftenius, A. , & von Holst, H. (1993). Increased interleukin‐6 levels in cerebrospinal fluid following subarachnoid hemorrhage. Journal of Neurosurgery, 78, 562–567. [DOI] [PubMed] [Google Scholar]
- Mohney, N. , Williamson, C. A. , Rothman, E. , Ball, R. , Sheehan, K. M. , Pandey, A. S. , … Rajajee, V. (2018). A propensity score analysis of the impact of dexamethasone use on delayed cerebral ischemia and poor functional outcomes after subarachnoid hemorrhage. World Neurosurgery, 109, e655–e661. [DOI] [PubMed] [Google Scholar]
- Moraes, L. , Grille, S. , Morelli, P. , Mila, R. , Trias, N. , Brugnini, A. , … Lens, D. (2015). Immune cells subpopulations in cerebrospinal fluid and peripheral blood of patients with Aneurysmal Subarachnoid Hemorrhage. Springerplus, 23, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, K. , Koide, M. , Dumont, T. M. , Russell, S. R. , Tranmer, B. I. , & Wellman, G. C. (2011). Subarachnoid hemorrhage induces gliosis and increased expression of the pro‐inflammatory cytokine high mobility group box 1 protein. Translational Stroke Research, 2, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara, T. , Tsuruta, R. , Kaneko, T. , Yamashita, S. , Fujita, M. , Kasaoka, S. , … Maekawa, T. (2009). High‐mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocritical Care, 11, 362. [DOI] [PubMed] [Google Scholar]
- Nakahira, K. , Kim, H. P. , Geng, X. H. , Nakao, A. , Wang, X. , Murase, N. , … Takahashi, T. (2006). Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS‐induced trafficking of TLRs to lipid rafts. Journal of Experimental Medicine, 203, 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri, F. , Ibrahim, G. M. , Badhiwala, J. H. , Witiw, C. D. , Mansouri, A. , Alotaibi, N. M. , & Macdonald, R. L. (2016). Propensity score‐matched study of the use of non‐steroidal anti‐inflammatory agents following aneurysmal subarachnoid hemorrhage. Neurocritical Care, 25, 351–358. [DOI] [PubMed] [Google Scholar]
- Neulen, A. , Pantel, T. , Kosterhon, M. , Kramer, A. , Kunath, S. , Petermeyer, M. , … Thal, S. C. (2019). Neutrophils mediate early cerebral cortical hypoperfusion in a murine model of subarachnoid haemorrhage. Scientific Reports, 11, 8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, L. A. J. , & Bowie, A. G. (2007). The family of five: TIR‐domain‐containing adaptors in Toll‐like receptor signalling. Nature Reviews Immunology, 7, 353–364. [DOI] [PubMed] [Google Scholar]
- Okuma, Y. , Liu, K. , Wake, H. , Zhang, J. , Maruo, T. , Date, I. , … Shima, K. (2012). Anti‐high mobility group box‐1 antibody therapy for traumatic brain injury. Annals of Neurology, 72, 373–384. [DOI] [PubMed] [Google Scholar]
- Palumbo, R. , Galvez, B. G. , Pusterla, T. , De Marchis, F. , Cossu, G. , Marcu, K. B. , & Bianchi, M. E. (2007). Cells migrating to sites of tissue damage in response to the danger signal HMGB1 require NF‐κB activation. Journal of Cell Biology, 179, 33–40. 10.1083/jcb.200704015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. S. , Arcaroli, J. , Yum, H.‐K. , Yang, H. , Wang, H. , Yang, K.‐Y. , … Abraham, E. (2003). Activation of gene expression in human neutrophils by high mobility group box 1 protein. American Journal of Physiology‐Cell Physiology, 284, C870–879. 10.1152/ajpcell.00322.2002 [DOI] [PubMed] [Google Scholar]
- Park, J. S. , Gamboni‐Robertson, F. , He, Q. , Svetkauskaite, D. , Kim, J.‐Y. , Strassheim, D. , … Abraham, E. (2006). High mobility group box 1 protein interacts with multiple Toll‐like receptors. American Journal of Physiology‐Cell Physiology, 290, C917–924. 10.1152/ajpcell.00401.2005 [DOI] [PubMed] [Google Scholar]
- Park, J. S. , Svetkauskaite, D. , He, Q. , Kim, J. Y. , Strassheim, D. , Ishizaka, A. , & Abraham, E. (2004). Involvement of toll‐like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry, 279, 7370–7377. 10.1074/jbc.M306793200 [DOI] [PubMed] [Google Scholar]
- Pearson, G. , Robinson, F. , Beers Gibson, T. , Xu, B. E. , Karandikar, M. , Berman, K. , & Cobb, M. H. (2001). Mitogen‐activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Reviews, 22, 153–183. [DOI] [PubMed] [Google Scholar]
- Pegoli, M. , Mandrekar, J. , Rabinstein, A. A. , & Lanzino, G. (2015). Predictors of excellent functional outcome in aneurysmal subarachnoid hemorrhage. Journal of Neurosurgery, 122, 414–418. 10.3171/2014.10.JNS14290 [DOI] [PubMed] [Google Scholar]
- Provencio, J. J. , Altay, T. , Smithason, S. , Moore, S. K. , & Ransohoff, R. M. (2011). Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. Journal of Neuroimmunology, 232, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, C. , Fan, W.‐H. , Liu, Q. , Shang, K. , Murugan, M. , Wu, L.‐J. , … Tian, D. S. (2017). Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke, 48, 3336–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaven, N. L. , Lovett, J. E. , & Funk, S. E. (2009). Brain injury and fever: Hospital length of stay and cost outcomes. Journal of Intensive Care Medicine, 24, 131–139. [DOI] [PubMed] [Google Scholar]
- Ryba, M. , Pastuszko, M. , Iwanska, K. , Bidzinski, J. , & Dziewiecki, C. (1991). Cyclosporine A prevents neurological deterioration of patients with SAH—A preliminary report. Acta Neurochirurgica, 112, 25–27. [DOI] [PubMed] [Google Scholar]
- Scaffidi, P. , Misteli, T. , & Bianchi, M. E. (2002). Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature, 418, 191–195. 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- Schallner, N. , Lieberum, J. L. , Gallo, D. , LeBlanc, R. H. 3rd , Fuller, P. M. , Hanafy, K. A. , & Otterbein, L. E. (2017). Carbon monoxide preserves circadian rhythm to reduce the severity of subarachnoid hemorrhage in mice. Stroke, 48, 2565–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallner, N. , Pandit, R. , LeBlanc, R. , Thomas, A. J. , Ogilvy, C. S. , Zuckerbraun, B. S. , … Hanafy, K. A. (2015). Microglia regulate blood clearance in subarachnoid hemorrhage by heme oxygenase‐1. Journal of Clinical Investigation, 125, 2609–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, U. C. , Davids, A.‐M. , Brandenburg, S. , Müller, A. , Elke, A. , Magrini, S. , … Vajkoczy, P. (2015). Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathologica, 130, 215–231. 10.1007/s00401-015-1440-1 [DOI] [PubMed] [Google Scholar]
- Selim, M. (2009). Deferoxamine mesylate: A new hope for intracerebral hemorrhage: From bench to clinical trials. Stroke, 40, S90–91. [DOI] [PubMed] [Google Scholar]
- Sha, Y. , Zmijewski, J. , Xu, Z. , & Abraham, E. (2008). HMGB1 develops enhanced proinflammatory activity by binding to cytokines. The Journal of Immunology, 180, 2531–2537. 10.4049/jimmunol.180.4.2531 [DOI] [PubMed] [Google Scholar]
- Shim, J.‐H. , Xiao, C. , Paschal, A. E. , Bailey, S. T. , Rao, P. , Hayden, M. S. , … Yamada, G. (2005). TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes & Development, 19, 2668–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, N. , Hopkins, S. J. , Hulme, S. , Galea, J. P. , Hoadley, M. , Vail, A. , … Tyrrell, P. J. (2014). The effect intravenous interleukin‐1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: A phase II randomised controlled trial. Journal of Neuroinflammation, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , & Nan, G. (2016). The mitogen‐activated protein kinase (MAPK) signaling pathway as a discovery target in stroke. Journal of Molecular Neuroscience, 59, 90–98. 10.1007/s12031-016-0717-8 [DOI] [PubMed] [Google Scholar]
- Sutherland, B. A. , Rahman, R. M. , Clarkson, A. N. , Shaw, O. M. , Nair, S. M. , & Appleton, I. (2009). Cerebral heme oxygenase 1 and 2 spatial distribution is modulated following injury from hypoxia‐ischemia and middle cerebral artery occlusion in rats. Neuroscience Research, 65, 326–334. 10.1016/j.neures.2009.08.007 [DOI] [PubMed] [Google Scholar]
- Suzuki, H. , Hasegawa, Y. , Chen, W. , Kanamaru, K. , & Zhang, J. H. (2010). Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Annals of Neurology, 68, 650–660. 10.1002/ana.22102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa, T. , Shibata, M. , Kayama, Y. , Shimizu, T. , Toriumi, H. , Ebine, T. , … Suzuki, N. (2017). High‐mobility group box 1 is an important mediator of microglial activation induced by cortical spreading depression. Journal of Cerebral Blood Flow and Metabolism, 37, 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, T. N. , Davis, P. H. , Torner, J. C. , Holmes, J. , Meyer, J. W. , & Jacobson, M. F. (1996). Lifetime cost of stroke in the United States. Stroke, 27, 1459–1466. [DOI] [PubMed] [Google Scholar]
- Thomas, A. , Ogilvy, C. S. , Griessenauer, C. J. , & Hanafy, K. A. (2018). Macrophage CD163 expression in cerebrospinal fluid: Association with subarachnoid hemorrhage outcome. Journal of Neurosurgery, 1, 1–7. [DOI] [PubMed] [Google Scholar]
- Treutiger, C. J. , Mullins, G. E. , Johansson, A.‐S.‐M. , Rouhiainen, A. , Rauvala, H. M. E. , Erlandsson‐Harris, H. , … Palmblad, J. E. W. (2003). High mobility group 1 B‐box mediates activation of human endothelium. Journal of Internal Medicine, 254, 375–385. [DOI] [PubMed] [Google Scholar]
- Vaure, C. , & Liu, Y. (2014). A comparative review of toll‐like receptor 4 expression and functionality in different animal species. Frontiers in Immunology, 5, 316 10.3389/fimmu.2014.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchione, C. , Frati, A. , Di Pardo, A. , Cifelli, G. , Carnevale, D. , Gentile, M. T. , … Antenucci, G. (2009). Tumor necrosis factor‐alpha mediates hemolysis‐induced vasoconstriction and the cerebral vasospasm evoked by subarachnoid hemorrhage. Hypertension, 54, 150–156. [DOI] [PubMed] [Google Scholar]
- Vergouwen, M. D. I. (2011). Participants in the International Multi‐Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocritical Care, 15, 308–311. [DOI] [PubMed] [Google Scholar]
- Vergouwen, M. D. I. , Ilodigwe, D. , & Macdonald, R. L. (2011). Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm‐dependent and ‐independent effects. Stroke, 42, 924–929. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Bloom, O. , Zhang, M. , Vishnubhakat, J. M. , Ombrellino, M. , Che, J. , … Manogue, K. R. (1999). HMG‐1 as a late mediator of endotoxin lethality in mice. Science, 285, 248–251. 10.1126/science.285.5425.248 [DOI] [PubMed] [Google Scholar]
- Wang, R. , Wu, L. , & Wang, Z. (1997). The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Archiv, 434, 285–291. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Cao, K. , Lu, Y. , & Wang, R. (2002). Different mechanisms underlying the stimulation of K(Ca) channels by nitric oxide and carbon monoxide. Journal of Clinical Investigation, 110, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, X. , Liu, S. , Ding, W. , Yue, P. , Jiang, Q. , Zhao, M. , … Zhang, H. (2017). TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice. Journal of Neuroimmunology, 310, 38–45. [DOI] [PubMed] [Google Scholar]
- Yu, Z.‐Q. , Jia, Y. , & Chen, G. (2014). Possible involvement of cathepsin B/D and caspase‐3 in deferoxamine‐related neuroprotection of early brain injury after subarachnoid haemorrhage in rats. Neuropathology and Applied Neurobiology, 40, 270–283. 10.1111/nan.12091 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Takahashi, H. K. , Liu, K. , Wake, H. , Liu, R. , Maruo, T. , … Nishibori, M. (2011). Anti‐high mobility group box‐1 monoclonal antibody protects the blood‐brain barrier from ischemia‐induced disruption in rats. Stroke, 42, 1420–1428. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Zhao, X. D. , Shi, J. X. , & Yin, H. X. (2011). Inhibition of the p38 mitogen‐activated protein kinase (MAPK) pathway attenuates cerebral vasospasm following experimental subarachnoid hemorrhage in rabbits. Annals of Clinical and Laboratory Science, 41, 244–250. [PubMed] [Google Scholar]
- Zhu, X.‐D. , Chen, J.‐S. , Zhou, F. , Liu, Q.‐C. , Chen, G. , & Zhang, J.‐M. (2012). Relationship between plasma high mobility group box‐1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. Journal of Neuroinflammation, 9, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparent Peer Review Report.


