Abstract
Background
The cation-chloride cotransporters Na+–K+–2Cl−–1 (NKCC1) and K+–2Cl−–2 (KCC2) critically regulate neuronal responses to gamma-aminobutyric acid (GABA). NKCC1 renders GABA excitatory in immature neurons while expression of KCC2 signals GABA maturation to its inhibitory role. Imbalances in NKCC1/KCC2 alter GABA neurotransmission, which may contribute to hyperexcitability and blunted inhibition in neurocircuitry after neonatal exposure to anesthesia. Thus, we hypothesized that anesthetics may dysregulate NKCC1 and/or KCC2 in developing brain.
Methods
We exposed postnatal day (PND) 7 mice to sevoflurane or carrier gases and assessed NKCC1 and KCC2 expression across three brain regions 6 hours and 24 hours after initial exposure. To test differences in behavior, we challenged pups receiving sevoflurane or carrier gases on PND7 with propofol on PND8 and recorded parameters of anesthesia induction and maintenance.
Results
Sevoflurane exposure increased cortical NKCC1 at 6 hours (p = .03) and decreased cortical and hippocampal KCC2 at 24 hours (p = .009 and p = .007, respectively). NKCC1/KCC2 ratio was significantly increased at both 6 hours (p = .02) and 24 hours (p = .03) in cortex and at 24 hours (p = .02) in hippocampus. After propofol challenge on PND8, pups previously exposed to sevoflurane on PND7 regained righting reflex significantly faster than their non-exposed cohort (p < .001).
Conclusions
Disturbing NKCC1/KCC2 balance may underlie circuit hyperexcitability and contribute to neurodevelopmental impairments we have observed in previous studies of neonatal anesthesia exposure. Human infants previously exposed to anesthesia may require higher concentrations of anesthetic drugs, potentially compounding their susceptibility for neurodevelopmental sequalae.
Introduction
Sedative and anesthetic drugs are administered routinely and frequently to infants and children for procedural sedation and surgical anesthesia. However, prolonged or repeated exposure to these agents cause widespread, pathological neuroapoptosis in neonatal rodents [1–3] and non-human primates [4–5]. There are now several clinical studies linking exposure to anesthetics in human infants with neuropathology and cognitive disturbances in later life [6–8]. Despite growing public concern, researchers have only begun to clarify mechanisms underlying the deleterious consequences of neonatal anesthesia exposure.
In embryonic and early postnatal development, gamma-aminobutyric acid (GABA) is the main excitatory neurotransmitter due to ubiquitous neuronal expression of the chloride importer Na+–K+–2Cl−–1 (NKCC1) [9]. NKCC1 maintains high intracellular chloride, establishing a concentration gradient across neuronal membranes that drives chloride efflux upon GABAA receptor activation. Net efflux of chloride depolarizes immature neurons, and perinatal GABA excitation is critical for several ontogenic processes. GABA excitation persists through the first week of life in rodents and the first few months in human infants before switching to its neuroinhibitory role [10]. This milestone is identified by upregulation of the chloride extruder K+–2Cl−–2 (KCC2). Thus, mature neurons maintain low intracellular chloride, and GABAA receptor activation causes chloride influx and neuroinhibition.
NKCC1 and KCC2 are sensitive to neuronal injury, and imbalances in expression are thought to contribute to several neuropathological disorders [10–11]. Furthermore, neurotoxins such as bisphenol A delay the developmental upregulation of KCC2, resulting in high neuronal intracellular chloride load [12]. Thus, an expected pathological outcome of reduced KCC2 expression is hyperexcitability in neurons that would otherwise be hyperpolarized by GABA. Indeed, we have reported that general anesthetics decrease synaptic inhibition in thalamocortical circuitry of developing rat brain [13], an effect that could be explained by increases in NKCC1 or decreases in KCC2 protein expression. Our objective in the current study was to assess whether neonatal exposure to a clinically-relevant concentration of sevoflurane – a common anesthetic in pediatric medicine – alters NKCC1 and KCC2 protein expression.
Methods
All experiments were approved by the Institutional Animal Care and Use Committee of the University of Colorado Anschutz Medical Campus. Breeding pairs of mice were maintained on a 12-hour light/dark cycle with ad libitum access to food and water. Efforts were made to minimize number of animals used and experimental procedures were in accordance with the Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Animals and Anesthesia Protocol
Litters of postnatal day (PND) 7 CD1 mice of both sexes were randomly assigned to receive sevoflurane for 6 hours (3% for 2 hours; 2.4% for 4 hours) delivered in carrier gases of 30% oxygen and compressed air, while control littermates received carrier gases only. Pups were placed in sealed anesthetic chambers (Scivena Scientific, USA) and kept normothermic and normoxic. We continuously monitored gas composition inside of chambers with an infrared analyzer (Datex Ohmeda, USA) for concentrations of sevoflurane, oxygen, carbon dioxide, and minimum alveolar concentration. During each experiment, we recorded SaO2, heart rate, and breathing rate per minute (MouseOx Plus, STARR Life Sciences Corp., USA) for at least two anesthetized animals at 2 hours as the anesthesia chambers were equilibrated to a lower concentration of sevoflurane and immediately at the end of the 6 hour exposure (Table 1).
Table 1.
Physiological Parameters of Sevoflurane Exposed Pups
| MAC | CO2 | SaO2 | HR | BRPM | |
|---|---|---|---|---|---|
| 2 Hours | 1.37 (0.02) | 0.05 (0.01) | 92.76 (3.79) | 293 (18.43) | 104.3 (16.33) |
| 6 Hours | 1.12 (0.01) | 0.05 (0.02) | 95.63 (1.92) | 293.2 (15.10) | 68.7 (10.92) |
Data are presented as Mean (SEM). MAC minimum alveolar concentration, CO2 carbon dioxide, SaO2 hemoglobin oxygen saturation, HR heart rate, BRPM breathing rate per minute monitored at 2 hours and 6 hours after initiation of sevoflurane
Tissue Isolation
We collected cortex, hippocampus, and thalamus at two time points: 1. Immediately at end of exposure (6 hours) to determine acute sevoflurane-induced dysregulation of NKCC1 and KCC2; and 2. Twenty-four hours later to assess longer-term alterations in these proteins.
At the experimental end points, pups were deeply anesthetized with isoflurane and transcardially perfused with ice cold phosphate buffered saline via the left ventricle. Brains were extracted, and cortex, hippocampus, and thalamus were rapidly dissected on ice and snap frozen in liquid nitrogen. Samples were stored at −80° C and processed for crude plasma membrane fraction according to standard protocol [14].
Crude Membrane Faction Western Immunoblot
Briefly, samples were homogenized in ice-cold sucrose buffer containing 10mM Tris pH 7.4, 320 mM sucrose, and protease and phosphatase inhibitors (Thermo Scientific, USA). Homogenates were centrifuged at 1,000 g for 10 minutes and supernatant S1 was transferred to a fresh tube for further centrifugation for 15 minutes at 10,000 g. The resultant P2 pellet was suspended in N-PER Neuronal Protein Extraction Reagent (Thermo Scientific), homogenized, and centrifuged for an additional 10 minutes at 10,000 RPM at 4° C. Plasma membrane samples were transferred to clean, RNA-free tubes for protein concentration determination via bicinchoninic acid assay (Thermo Scientific, USA). Samples were excluded from analysis if protein concentration was 2.5 SDs above or below the mean concentration for a given litter, indicative of aberrant protein extraction.
Five micrograms of samples were loaded with Laemmli sample buffer (Sigma-Aldrich, USA), and proteins were resolved in 4–20% Tris-Glycine eXtended Gels (Bio-Rad, USA) in sodium dodecyl sulfate running buffer. Proteins were electrophoretically separated at 120V for 2 hours using Bio-Rad PowerPac (Bio-Rad, USA). Proteins were then electrophoretically transferred overnight at 4° C to polyvinylidene difluoride membranes (Immobilon, Sigma-Aldrich, USA) in transfer buffer. The following day, membranes were dried at room temperature for at least 2 hours, briefly activated with 100% methanol, and washed with MilliQ water before confirming protein transfer with ponceau S (Sigma-Aldrich, USA). Membranes were then washed with 0.3% Tween-20 in tris-buffered saline (TBST) for 15 minutes followed by blocking for 1 hour in 3% bovine serum albumin (BSA) in 0.1% TBST at room temperature. For primary antibodies, we used rabbit anti-NKCC1 (1:1000; Cell Signaling, USA), anti-KCC2 (1:1000; Cell Signaling, USA), or anti-β-actin (1:10,000; Sigma-Aldrich, USA). Membranes were incubated in their respective primary antibodies in 1.5% bovine serum albumin (BSA) overnight at 4° C. The next day, membranes were washed in fresh 0.3% TBST every 5 minutes for 25 minutes, then incubated in 1.5% BSA plus mouse anti-rabbit secondary (1:15,000; Santa Cruz Biotechnology, USA) for 1 hour at room temperature. After further washes in 0.3% TBST (2 times, 15 minutes each), membranes were developed with SuperSignal Maximum Sensitivity Substrate (Thermo Scientific, USA). Immunoblot membrane images were captured in high-resolution using GBOX (Chemi XR5; Syngene, USA) and analyzed densitometrically via computerized image analysis program GeneSys (Syngene, USA) by a blinded observer. NKCC1 and KCC2 densitometry data for sevoflurane pups were analyzed as percent change of controls after normalization to β-actin. Adult mouse spinal cord, which is enriched in both NKCC1 and KCC2, served as a positive control for all immunoblots. Representative immunoblots and lanes of individual pups are presented in Fig. 1 to 3.
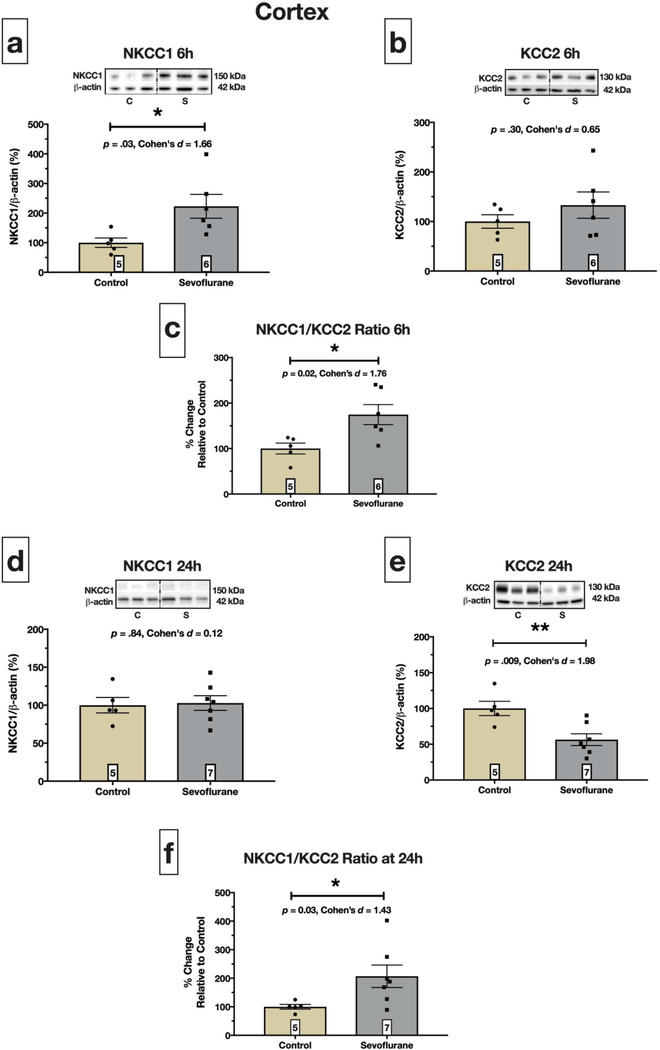
Fig. 1. Sevoflurane exposure dysregulates neonatal cortical NKCC1 and KCC2.
Immediately after 6 hours exposure to sevoflurane, cortical NKCC1 increased over two-fold compared to controls, p = .03 (a), but KCC2 was not affected, p = .30 (b). Upregulation of NKCC1 resulted in increased NKCC1/KCC2 ratio, p =.02 (c). Twenty-four hours after initial exposure, we observed no differences in NKCC1 protein expression between control and sevoflurane pups, p =.84 (d). However, at this time point, KCC2 was significantly reduced, p = .009 (e), leading to increased NKCC1/KCC2 ratio, p = .03 (f). Boxed numbers in bars represent n per group
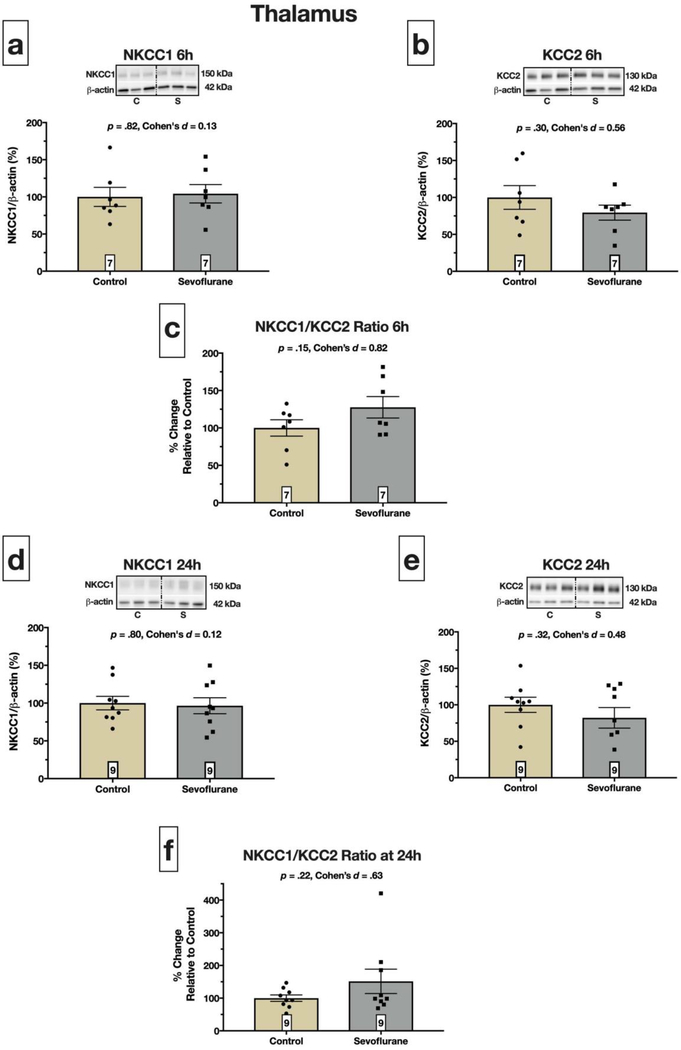
Fig. 3. Thalamic NKCC1 and KCC2 protein expression is not altered by sevoflurane.
NKCC1 protein expression was not statistically different between pups exposed to sevoflurane for 6 hours versus controls exposed to carrier gases only, p = .82 (a). Furthermore, sevoflurane had no effect on KCC2 protein levels, p = .30 (b), resulting in no change in the NKCC1/KCC2 ratio at 6 hours, p = .15; (c). (d) and (e) show that exposure to sevoflurane on PND7 did not alter NKCC1 (p = .80) or KCC2 (p = .32) protein expression of PND8 pups. The NKCC1/KCC2 ratio was not altered at this time point, p = .22 (f). Boxed numbers in bars represent n per group
To compare across brain regions and time points, we ran separate membranes with two control animals near the average protein expression values for each region and time point. Data were normalized to β-actin as before and analyzed as percent change of cortical protein expression at 6 hours after initial exposure (PND7).
Behavioral Assessment of Previous Anesthesia Exposure
Litters of PND7 CD1 mouse pups were subjected to our standard sevoflurane protocol (3% for 2 hours plus 2.4% for 4 hours delivered in carrier gases of 30% oxygen and compressed air) or control protocol of only carrier gases as described above. On PND8, all pups were challenged with an intraperitoneal dose of 100 mg/kg propofol (Baxter, USA). We recorded time to loss of righting reflex (LORR) as a parameter of anesthesia induction and duration of LORR as a parameter of anesthesia maintenance.
Statistical Analysis
Comparisons between groups in Western Blot and behavioral experiments were performed via Welsh corrected unpaired t-tests assuming unequal variances. A priori power analysis (G*Power, Duesseldorf, Germany) based on previous studies [12, 15] suggested n = 5 per group was sufficient to detect statistically significant differences in protein expression between groups. Likewise, we determined that n = 10 per group would be sufficient to observe differences in LORR behavior. To compare protein expression across brain regions, we used two-way ANOVA followed by simple effects analysis. The significance level was set at α = 0.05; thus, p-value less than 0.05 was considered statistically significant. Data are expressed as Mean ± SEM.
Results
We collected cortex, hippocampus, and thalamus at two time points: 1. Immediately at end of exposure (6 hours) to determine acute sevoflurane-induced dysregulation of NKCC1 and KCC2; and 2. Twenty-four hours later to assess longer-term alterations in these proteins. Our rationale for the 6 hour time point was that neurons are in the initial phase of apoptosis. Therefore, changes in NKCC1 or KCC2 protein expression are captured as cells are dying but before they are deleted from the brain. Our rationale for the 24 hour time point was based on our previous studies reporting that neurons surviving the acute neuroapoptotic insult exhibit ultrastructural, morphological, and neurophysiological abnormalities [16].
Cortex
Six hours of sevoflurane exposure significantly increased cortical NKCC1 protein expression over two-fold compared to controls (p = .03; Fig. 1a), while KCC2 was increased 33% but did not reach statistical significance (p = .30; Fig. 1b). We also observed a statistically significant increase in NKCC1/KCC2 ratio (p = .02; Fig. 1c) driven primarily by sevoflurane-induced upregulation of NKCC1 protein expression.
Twenty-four hours later, on PND8, cortical NKCC1 was not affected by sevoflurane exposure (p = .84; Fig. 1d). However, sevoflurane exposed pups had significantly reduced (~44%) KCC2 protein (p = .009; Fig. 1e) compared to controls, resulting in a significant increase in the NKCC1/KCC2 ratio (p = 0.03; Fig. 1f).
Hippocampus
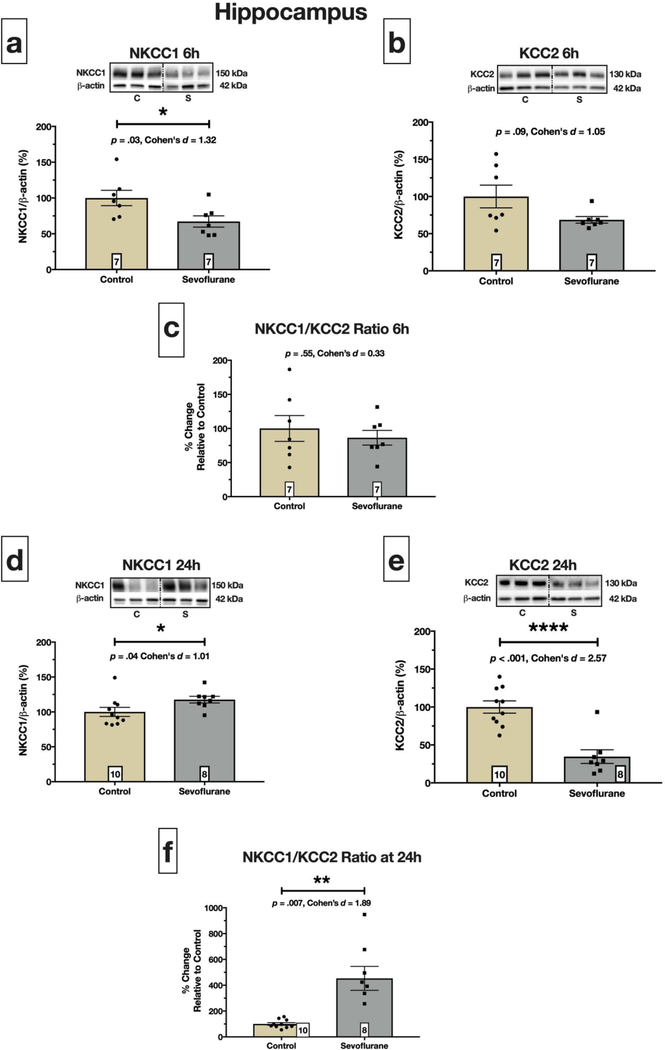
In hippocampus, there was a statistically significant 32% decrease of NKCC1 protein expression at 6 hours compared to controls (p = .03; Fig. 2a). Likewise, KCC2 protein levels exhibited a strong, but not statistically significant, trend of 31% reduction at 6 hours compared to controls (p = .09; Fig. 2b). When assessing the NKCC1/KCC2 ratio at 6 hours, we found that the ratio was unchanged between control and sevoflurane pups (p = .55; Fig. 2c).
Fig. 2. Perturbed hippocampal NKCC1 and KCC2 expression after sevoflurane exposure.
(a) displays a statistically significant decrease in NKCC1 protein at 6 hours after initiation of sevoflurane, p = .03. We also observed a 31% decrease in KCC2 in sevoflurane treated pups at this time point (b), but it did not reach statistically significance, p = .09. This led to no alteration of the NKCC1/KCC2 protein expression ratio at 6 hours, p = .55 (c). In contrast, sevoflurane caused a statistically significant reduction (~64%) in hippocampal KCC2 protein at 24 hours, p < .0001 (e), while NKCC1 was modestly, but significantly, increased, p = .04 (d). The result of upregulated NKCC1 and downregulated KCC2 was a 4.5-fold increase in hippocampal NKCC1/KCC2 ratio on PND8, p = .007 (f). Boxed numbers in bars represent n per group
Sevoflurane exposure on PND7 significantly increased NKCC1 protein levels at 24 hours (p = .04; Fig. 2d). Moreover, we observed a striking, 66% decrease in KCC2 protein at this time point (p < .0001; Fig. 2e). The significant reduction of hippocampal KCC2 led to a 4.5-fold increase in the NKCC1/KCC2 ratio at 24 hours (p = 0.007; Fig. 2f).
Thalamus
In contrast to cortex and hippocampus, sevoflurane exposure did not alter NKCC1 (Fig. 3a) or KCC2 (Fig. 3b) protein expression at 6 hours (p = .82 and p = .30, respectively). Therefore, the NKCC1/KCC2 ratio was unchanged (p = .15; Fig. 3c). Furthermore, sevoflurane exposure did not affect NKCC1 (Fig. 3d) or KCC2 (Fig. 3e) protein expression at 24 hours (p = .80 and p = .32, respectively) resulting in a lack of statistically significant difference in NKCC1/KCC2 ratio between control and sevoflurane exposed animals (p = .22; Fig. 3f).
NKCC1 and KCC2 Brain Region Comparison Across Time
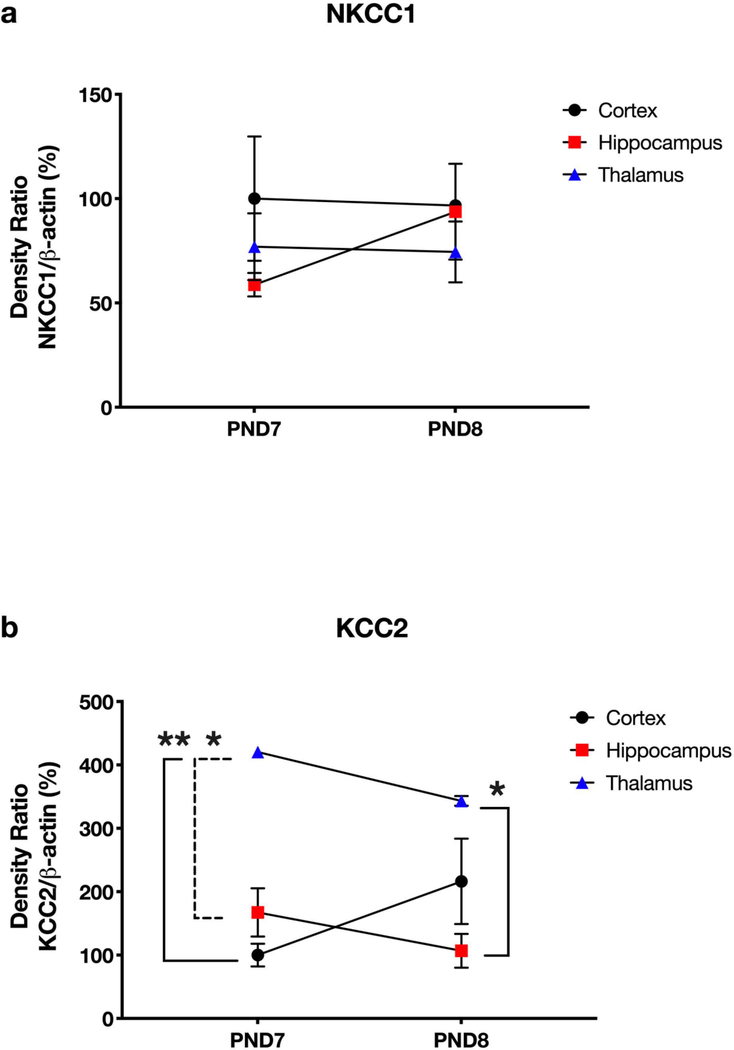
To compare NKCC1 and KCC2 expression in cortex, hippocampus, and thalamus, we ran separate immunoblot membranes with two control samples near the mean densitometry data for each region and time point (Fig. 4). In both panels, data are expressed as percent change versus cortical protein expression at 6 hours. We did not observe statistically significant changes in NKCC1 protein expression in any brain region from PND7 to PND8 (Fig. 4a), suggesting that neuronal NKCC1 levels do not fluctuate much during this developmental period. Also, we observed non-statistically significant decreases in KCC2 in hippocampus and thalamus and an increase in cortex (Fig. 4b). Interestingly, thalamic KCC2 levels were significantly higher than cortex and hippocampus on PND7 (p = 0.005 and p = 0.015, respectively) and hippocampus on PND8 (p = 0.02).
Fig. 4. Brain region comparison of NKCC1 and KCC2 across developmental timepoints.
NKCC1 protein expression was not different between regions or developmental timepoints (a). Our data highlight important developmental differences in membrane KCC2 protein expression between brain regions (b). Notably, KCC2 levels remained relatively steady between PND7 and PND8 within all regions. Interestingly, thalamic KCC2 levels were significantly higher than in cortex (solid left bracket, p = 0.005) and hippocampus on PND7 (dashed left bracket, p = 0.015) and hippocampus on PND8 (solid right bracket, p = 0.02). Thus, we suspect that thalamic nuclei may have greater magnitude of response to GABA hyperpolarization versus hippocampal or cortical neurons at these developmental ages
Behavioral Response to Subsequent Anesthesia Challenge
Thalamocortical circuits play a critical role in arousal, sleep, and anesthesia states [13]. We reasoned that our observed decrease at 24 hours in cortical KCC2 may interfere with normal responses to subsequent anesthesia exposure. Specifically, we hypothesized that the loss of approximately 44% of cortical KCC2 would retard GABA-mediated neuroinhibition and manifest behaviorally as prolonged anesthesia induction (time to LORR) or shortened anesthesia maintenance (duration of LORR) on PND8. Therefore, we exposed PND7 pups to sevoflurane or carrier gases, then challenged all pups on PND8 with a 100 mg/kg dose of propofol.
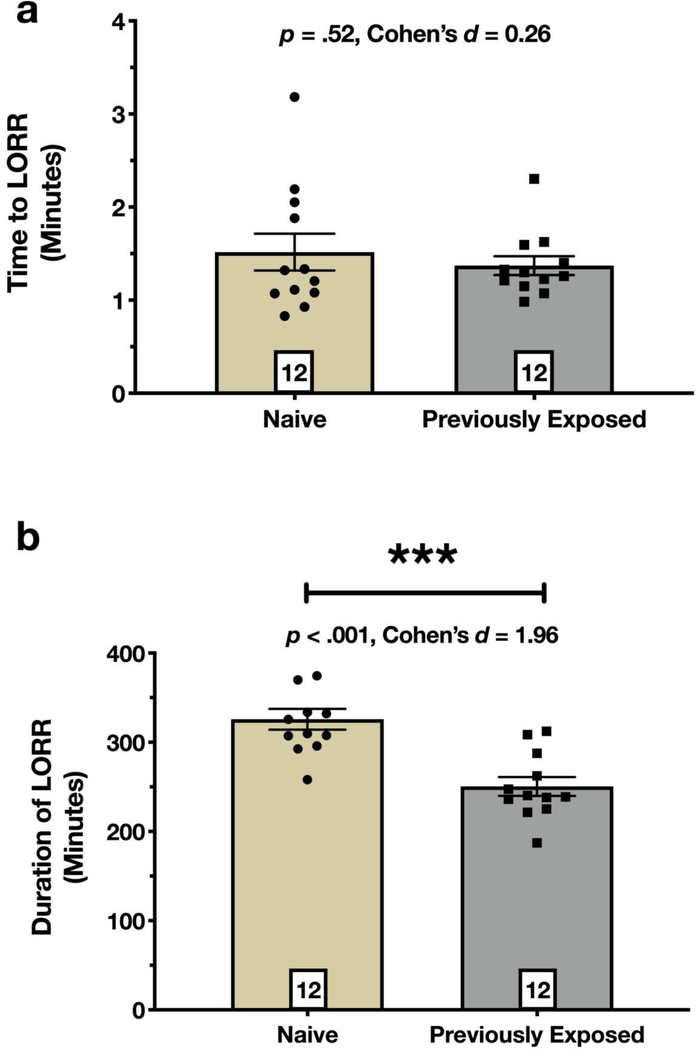
PND8 pups previously exposed to sevoflurane on PND7 did not differ in time to LORR versus their unexposed counterparts (p = .52; Fig. 5a), but duration of LORR was significantly (25%, approximately 75 minutes) shorter than their unexposed counterparts (p < .001; Fig. 5b).
Fig. 5. Previous exposure to sevoflurane shortens duration of propofol anesthesia.
On PND7, mouse pups were randomly assigned to our standard sevoflurane protocol or control group of carrier gases. We then challenged all pups with 100 mg/kg propofol and measured time to LORR and duration of LORR as parameters of anesthesia induction and maintenance, respectively. Anesthesia induction was not affected by previous exposure to sevoflurane, p = .52 (a). However, we observed a statistically significant effect on anesthesia maintenance (b). Specifically, Previously Exposed pups that received sevoflurane on PND7 had a shorter duration of LORR compared to their Naïve counterparts, p < .001. Boxed numbers in bars represent n per group
Discussion
Exposure of the immature brain to sevoflurane during early neurodevelopment causes tissue- and time-dependent dysregulation of cation-chloride cotransporters, NKCC1 and KCC2. In cortex, this dysregulation manifests as acutely increased NKCC1 protein expression and longer-term (24 hour) decreased KCC2 protein expression. In contrast, hippocampal NKCC1 was downregulated immediately after sevoflurane exposure (6 hours) on PND7, but modestly upregulated a day later on PND8. Furthermore, pups exposed to sevoflurane on PND7 gained their righting significantly faster than their naïve counterparts when challenged with 100 mg/kg propofol on PND8. Taken together, our findings suggest that altered cation-chloride cotransporter expression is yet another pathological consequence of neonatal exposure to drugs with sedative and anesthetic properties.
Immature neurons are enriched with NKCC1, but not KCC2, and are depolarized by GABA. Genetic interference of NKCC1 expression impairs GABA-mediated neurogenesis [11] and delays dendrite maturation [17]. Abnormal upregulation of NKCC1 either by experimental means or human genetic mutation facilitates seizure activity in developing hippocampus [18–19], an effect blocked by the NKCC1 inhibitor, bumetanide [19]. We report that, at 6 hours after initial exposure, the cortical NKCC1/KCC2 ratio was significantly increased due to upregulated expression of NKCC1 in sevoflurane exposed pups. In contrast, we observed a substantial decrease (~32%) in hippocampal NKCC1 at this time point. Because KCC2 was also decreased to a similar magnitude to that of NKCC1, the NKCC1/KCC2 ratio was unchanged, but it is unclear whether this affects neuronal responses to GABA in developing hippocampus. Our normalization to β-actin at this time point argues against neuronal loss as the culprit for changes in NKCC1. Furthermore, previous studies document that, at 6 hours, cortical neurons may be positive for cleaved caspase 3 – a marker of apoptosis – but are morphologically intact as the drug-induced apoptotic reaction is in its early phase [20–21]. Neurons at this early stage of commitment to apoptosis are likely to retain their full complement of membrane proteins until they are phagocytosed hours later. Yet, upregulation of NKCC1 while the anesthetic injury is ongoing is expected to cause neuronal hyperexcitability while downregulation may contribute to inappropriate neuroinhibition and cell silencing, which can be tested in future neurophysiological studies.
Interestingly, we observed longer-term changes in NKCC1 and KCC2 protein expression. Specifically, pups exposed to sevoflurane on PND7 and studied on PND8 had modestly upregulated NKCC1, but substantially downregulated KCC2. This imbalance of expression led to an NKCC1/KCC2 ratio that was 4.5-fold higher than the control cohort at 24 hours post-exposure. By 24 hours after initiation of anesthetic insult, the apoptotic reaction has waned, and phagocytosis has cleared the brain of many dead neurons. Again, because of normalization, we doubt that the observed changes in NKCC1 and KCC2 were due to neuronal loss. A plausible explanation is that, in surviving neurons, sevoflurane may cause KCC2 to be removed from the plasma membrane via dephosphorylation and endocytosis [10]. Additionally, sevoflurane may have genomic and epigenomic effects on NKCC1 and KCC2 gene expression [22] that can be tested in future studies. Indeed, we have previously reported that a clinically-relevant cocktail of midazolam, isoflurane, and nitrous oxide caused a significant decrease in brain-derived neurotrophic factor (BDNF) protein and mRNA in vivo [15]. The mechanism of BDNF dysregulation was traced to deleterious epigenetic modifications that repress gene transcription: Global histone 3 (H3) hypoacetylation and locus-specific H3 hypoacetylation of the BDNF gene. Given that BDNF regulates KCC2 gene expression during development [11] and that the NKCC1 and KCC2 genes are sensitive to epigenetic remodeling [23], sevoflurane may downregulate KCC2 indirectly by reducing neuronal BDNF protein levels or directly by changing acetylation or methylation status of the KCC2 gene Slc12A5.
Regardless of mechanism, sevoflurane-induced downregulated KCC2 or upregulated NKCC1 may have similar functional to those observed in other neuropathological disorders: Deviations from the normal developmental trajectory of NKCC1/KCC2 protein expression may produce depolarization of neurons that would otherwise be hyperpolarized by GABA [10–11]. For example, experimentally-induced cerebral ischemia in adult rats caused an abrupt re-expression of NKCC1 and downregulation of KCC2 [23]. A similar pattern of NKCC1 re-expression and KCC2 downregulation has been observed in rodent models of status epilepticus [24]. Studies using tissue from humans with temporal lobe epilepsy reveal a potentially complex pattern of KCC2 dysregulation, with increased expression in hippocampus [25] but decreased expression in subiculum [26]. Thus, neonatal sevoflurane exposure may cause neurons to adopt a more immature state in which GABA is depolarizing and supports neuronal hyperexcitability.
When comparing normal development in control animals, we noticed almost no change in NKCC1 expression from PND7 to PND8 in any brain region. These data suggest that perhaps NKCC1 has plateaued and may remain at consistent levels as maturation continues. Also, we observed no changes in cortical, hippocampal, or thalamic KCC2 across the PND7 to PND8 ages. Although KCC2 in adult rodent brain is well-characterized [27], relatively little is known about the precise spatiotemporal evolution of KCC2 expression during postnatal development. Generally, KCC2 expression and, therefore neuronal maturation, is thought to proceed in a caudal to rostral manner [28]. However, specific brain regions or sub-regions may significantly upregulate KCC2 at different developmental ages or, in some cases, not at all [28]. Because we used whole brain region homogenates, we cannot determine whether the stability of KCC2 we observed from PND7 to PND8 is sub-region specific (e.g., somatosensory cortex neurons may express KCC2 earlier or later versus motor cortex neurons). We did observe a four-fold higher expression of KCC2 in thalamus than in cortex or hippocampus at both PND7 and PND8. These data suggest that thalamic neurons are readily hyperpolarized by GABA at a relatively early postnatal age. Future studies are needed to determine the functional significance of this observation.
Finally, our behavioral data highlight potential consequences of anesthesia and KCC2 downregulation important to neonatal and pediatric medicine. Mouse pups previously exposed to sevoflurane on PND7 had a blunted response to propofol when challenged on PND8. When compared to humans, the difference between PND7 and PND8 amounts to approximately a month in the life of a human infant. Thus, our PND8 propofol challenge models a frequent scenario in which many sick babies require follow-up medical treatments for weeks or months that may necessitate anesthesia. Given that prolonged and/or multiple exposures to sedatives and anesthetic agents may be detrimental to human infant brain [29], while a single, very brief exposure may not [30], our translational interpretation of behavioral data is that infants who receive an initial anesthetic exposure may require higher concentrations, or longer durations, of anesthetic drugs during subsequent exposures.
Further studies are warranted to determine the mechanism(s) by which sevoflurane disturbs cation-chloride cotransporter expression and function. Dysregulation of cortical NKCC1 and KCC2 may interfere with arousal, sleep, and anesthetic states controlled by thalamocortical network activity. Likewise, decreased hippocampal KCC2 may lead to reduced GABA neuroinhibition and disturbances in learning and memory later in life. Future behavioral and neurophysiological experiments will investigate the functional consequences of cation-chloride cotransporter dysregulation caused by neonatal sevoflurane exposure.
Acknowledgements
Supported in part by funds from the Department of Anesthesiology at the University of Colorado Anschutz Medical campus, R0144517, R0144517-S, R01 GM118197, R01 GM118197, R21 HD080281 and March of Dimes National Award, USA (to VJT), CU Medicine Endowments (to VJT) and R01 GM118197–11S1 (OHC).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Cattano D, Young C, Straiko MMW, Olney JW (2008) Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg 106:1712–1714. 10.1213/ane.0b013e318172ba0a [DOI] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882. 10.1097/00008506-200307000-00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson SA, Young C, Olney JW (2008) Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol 20:21–28. 10.1097/ANA.0b013e3181271850 [DOI] [PubMed] [Google Scholar]
- 4.Creeley C, Dikranian K, Dissen G, et al. (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110:29–38. 10.1093/bja/aet173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenning KJ, Noguchi KK, Martin LD, et al. (2017) Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 10.1016/j.ntt.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ing C, DiMaggio C, Whitehouse A, et al. (2012) Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 10.1542/peds.2011-3822 [DOI] [PubMed] [Google Scholar]
- 7.Sun LS, Li G, Miller TLK, et al. (2016) Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA - J Am Med Assoc. 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, … Warner DO (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiol. 10.1097/SA.0b013e3181be865c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Ari Y (2002) Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci 3:728–739. 10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- 10.Kaila K, Ruusuvuori E, Seja P, et al. (2014) GABA actions and ionic plasticity in epilepsy. Curr Opin Neurobiol 26:34–41. 10.1016/j.conb.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 11.Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E (2012) The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neurosci 18:467–486. 10.1177/1073858412438697 [DOI] [PubMed] [Google Scholar]
- 12.Yeo M, Berglund K, Hanna M, et al. (2013) Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci U S A 110:4315–20. 10.1073/pnas.1300959110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiGruccio MR, Joksimovic S, Joksovic PM, et al. (2015) Hyperexcitability of rat thalamocortical networks after exposure to general anesthesia during brain development. J Neurosci 35:1481–1492. 10.1523/JNEUROSCI.4883-13.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng G, Orfila JE, Dietz RM, et al. (2017) Autonomous CaMKII activity as a drug target for histological and functional neuroprotection after resuscitation from cardiac arrest. Cell Rep 18:1109–1117. 10.1016/j.celrep.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalla Massara L, Osuru HP, Oklopcic A, et al. (2016) General anesthesia causes epigenetic histone modulation of c-Fos and brain-derived neurotrophic factor, target genes important for neuronal development in the immature rat hippocampus. Anesthesiology 124:1311–1327. 10.1097/ALN.0000000000001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez V, Feinstein SD, Lunardi N, et al. (2011) General anesthesia causes long-term impairment of mitochondrial morphogenesis and synaptic transmission in developing rat brain. Anesthesiology. 10.1097/ALN.0b013e3182303a63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young SZ, Taylor MM, Wu S, et al. (2012) NKCC1 knockdown decreases neuron production through GABAA-regulated neural progenitor proliferation and delays dendrite development. J Neurosci. 10.1523/JNEUROSCI.2864-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dzhala VI, Talos DM, Sdrulla DA, et al. (2005) NKCC1 transporter facilitates seizures in the developing brain. Nat Med 11:1205–1213. 10.1038/nm1301 [DOI] [PubMed] [Google Scholar]
- 19.Dzhala VI, Kuchibhotla KV., Glykys JC, et al. (2010) Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J Neurosci. 10.1523/JNEUROSCI.1769-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrera OH, O’Connor SD, Swiney BS, et al. (2017) Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity. J Matern Neonatal Med 30:2734–2741. 10.1080/14767058.2016.1261400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olney JW, Tenkova T, Dikranian K, et al. (2002) Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 9:205–19. 10.1006/nbdi.2001.0475 [DOI] [PubMed] [Google Scholar]
- 22.Ju LS, Yang JJ, Morey TE, et al. (2018) Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth 121:406–416. 10.1016/j.bja.2018.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HA, Hong SH, Kim JW, Jang IS (2010) Possible involvement of DNA methylation in NKCC1 gene expression during postnatal development and in response to ischemia. J Neurochem 114:520–529. 10.1111/j.1471-4159.2010.06772.x [DOI] [PubMed] [Google Scholar]
- 24.Li X, Zhou J, Chen Z, et al. (2008) Long-term expressional changes of Na+-K+-Cl- co-transporter 1 (NKCC1) and K+-Cl- co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE). Brain Res 1221:141–146. 10.1016/j.brainres.2008.04.047 [DOI] [PubMed] [Google Scholar]
- 25.Karlócai MR, Wittner L, Tóth K, et al. (2016) Enhanced expression of potassium-chloride cotransporter KCC2 in human temporal lobe epilepsy. Brain Struct Funct 221:3601–3615. 10.1007/s00429-015-1122-8 [DOI] [PubMed] [Google Scholar]
- 26.Karlócai MR, Wittner L, Tóth K, et al. (2016) Enhanced expression of potassium-chloride cotransporter KCC2 in human temporal lobe epilepsy. Brain Struct Funct 221:3601–3615. 10.1007/s00429-015-1122-8 [DOI] [PubMed] [Google Scholar]
- 27.Ben-Ari Y, Gaiarsa J-L, Tyzio R, Khazipov R (2007) GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev 87:1215–1284. 10.1152/physrev.00017.2006 [DOI] [PubMed] [Google Scholar]
- 28.Hübner CA, Lorke DE, Hermans-Borgmeyer I (2001) Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech Dev 102:267–269. 10.1016/S0925-4773(01)00309-4 [DOI] [PubMed] [Google Scholar]
- 29.Flick RP, Katusic SK, Colligan RC, et al. (2011) Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 128:e1053–e1061. 10.1542/peds.2011-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson AJ, Disma N, De Graaff JC, et al. (2016) Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 387:239–250. 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]