INTRODUCTION
Conventional teaching is that H. pylori (HP) infection is required for the development of non-cardia gastric adenocarcinoma (NCGC), through a sequence of atrophic gastritis, intestinal metaplasia, dysplasia, and finally, cancer.1–3 However, studies have not demonstrated a 100% rate of HP infection in gastric malignancy, hypothesized to be due to false negatives, gastric atrophy leading to apparent loss of infection, and inclusion of cardia, non-intestinal cancers.1–3
Two US studies have sought to identify the seroprevalence of HP in gastric cancer: one identified HP in 80.1% of 186 cancers (including cardia and non-adenocarcinomas) and one found 94% of intestinal and diffuse carcinomas with positive IgG in 109 Japanese-Americans.2, 3 These studies, however, are almost 30 years old and limited to California and Hawaii. Furthermore, there has been a changing incidence of gastric cancer in the US, yet large US studies have only used cancer databases without information on HP. The objective of this study was to use data from the Veterans Health Administration (VHA) to estimate the prevalence of HP in patients with NCGC.
METHODS
This retrospective cohort study was conducted within the VHA. (see Supplement). We first identified patients with testing that could indicate potential HP or gastric cancer, then identified HP infection based on administrative data, eradication prescription, or positive diagnostic test. We subsequently identified patients with NCGC via ICD codes and/or the Veterans Affairs Central Cancer Registry.4 We filtered to include intestinal type non-cardia gastric cancers, to avoid capturing non-adenocarcinomas and proximal tumors, which are less clearly associated with HP.1,5 Patients with NCGC were subcategorized on HP status.
Wilcoxon rank-sum and Pearson’s chi-squared tests were performed. The Institutional Review Boards of the Corporal Michael J. Crescenz VA Medical Center and the University of Pennsylvania approved this study.
RESULTS
Of 1,440,932 patients with potential HP or gastric cancer, 15,798 (1.1%) had NCGC (Figure 1). Among these, 4,749 (30.1%) had data to evaluate HP status, of which 1,071 were seronegative (22.6%). Despite seronegativity, 44 (<1%) had evidence documenting active infection. Additionally, 1,672 patients (35.2%) had seropositivity with or without additional evidence of HP, while 2,006 (42.2%) had no serum testing, but evidence of previous active HP.
Figure 1.
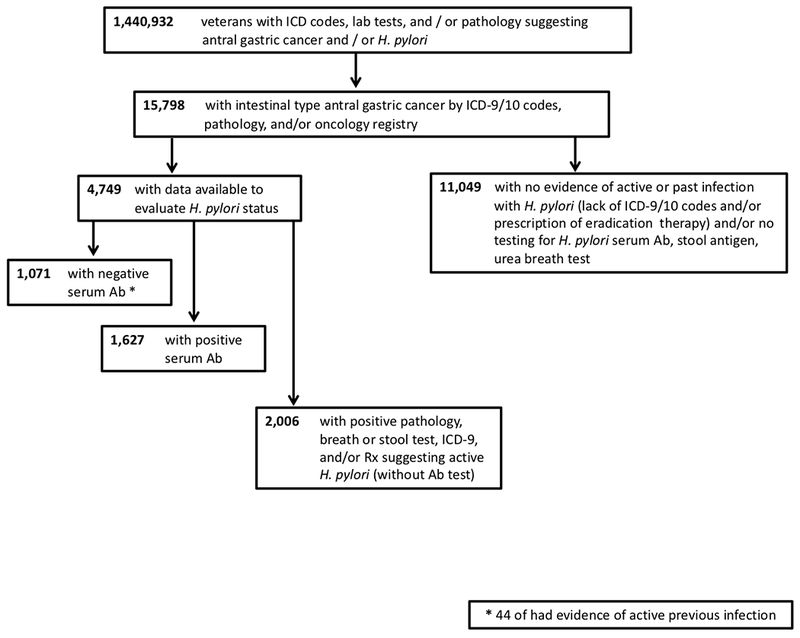
Among those with NCGC, there were significant differences seen in seronegative patients: 1) age at NCGC was two years younger; 2) higher proportion of white race; and 3) lower proportion of Hispanic ethnicity (Supplemental Table 1).
DISCUSSION
In a diverse US cohort of patients with NCGC, nearly one-quarter with HP testing data had no evidence of active or prior HP. Although carcinogenesis is complex, HP is presumed a necessary stimulus, and the World Health Organization classifies HP as a class 1 carcinogen.5–7 Previous studies have demonstrated that HP is important but not sufficient for carcinogenesis, and seronegative HP gastric cancer has been identified.1,3,5 Our findings demonstrate a similar prevalence of seronegative NCGC to previous US based studies, though we were able to exclude non-adenocarcinomas and proximal lesions.3
Seronegative patients were tested for antibodies within 0.1 years of cancer diagnosis (versus 3.8 years (p<0.001)). Seronegativity may indicate loss of antigenic drive as intestinal metaplasia replaces gastritis, acting as a less hospitable milieu for HP. The 44 with known history of active infection before seronegativity bolster this theory, and reiterate the imperfections of serum testing beyond false-negatives. Alternatively, these patients may have another cause of NCGC.
On the other hand, if HP continues to cause almost 80% of NCGC in the US, widespread testing may be indicated, even if overall gastric cancer incidence is declining. In the US, no test-and-treat policy exists, though testing is simple and non-invasive. This is in contrast to esophageal adenocarcinoma, which, despite lower incidence, carries invasive screening recommendations, with procedural risks and costs.
Limitations of this study include its retrospective nature and reliance on administrative codes, which may limit accurate diagnosis of histological subtypes or anatomic locations, but is likely not significantly different than other claims-based studies. A large number of patients had HP status that could not be assessed, possibly from care received outside of the VHA and/or imperfect data.
In a North American cohort with NCGC, 22.6% had no evidence of active or prior HP infection. A minority had evidence of previous active HP, reflecting probable burn out of their antigenic drive with worsening gastric atrophy. HP is still likely to account for the vast majority of distal gastric adenocarcinomas in the US. Novel risk factors in US patients should be explored, as should the value of a test-and-treat strategy to eradicate HP in patients at risk for gastric cancer.
Supplementary Material
Acknowledgments
Grant support:
Shria Kumar, MD is supported by an NIH training grant (5 T32 DK 7740-22)
Disclosures:
Shria Kumar, MD: Travel (Boston Scientific Corporation)
David C. Metz, MBBS: Consulting (Takeda, Lexicon, AAA. Novartis), Grant Support (Lexicon, Wren Laboratories, Ipsen, AAA)
David E. Kaplan, MD, MSc: Research grant support (Gilead, Bayer)
David S. Goldberg, MD, MSCE: Research grant support (Gilead, Merck, AbbVie, Zydus)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare they have no relevant conflicts of interests to this study.
REFERENCES
- 1.Helicobacter, Cancer Collaborative G. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–53. Epub 2001/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura A, Stemmermann GN, Chyou PH, et al. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325(16):1132–6. Epub 1991/10/17. [DOI] [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325(16):1127–31. Epub 1991/10/17. [DOI] [PubMed] [Google Scholar]
- 4.Zullig LL, Sims KJ, McNeil R, et al. Cancer Incidence Among Patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883–e91. Epub 2017/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JH, Cheung DY. Must-Have Knowledge about the Helicobacter pylori-Negative Gastric Cancer. Gut Liver. 2016;10(2):157–9. Epub 2016/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correa P Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S37–43. Epub 1995/01/01. [PubMed] [Google Scholar]
- 7.Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380(12):1158–65. Epub 2019/03/21. [DOI] [PubMed] [Google Scholar]
- 8.Moss SF. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol. 2017;3(2):183–91. Epub 2017/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


