Abstract
Ischemic brain damage is triggered by glutamate excitotoxicity resulting in neuronal cell death. Previous research has demonstrated that NMDA receptor activation triggers downstream calcium-dependent signaling pathways, specifically Ca2+/calmodulin-dependent protein kinase II (CaMKII). Inhibiting CaMKII is protective against hippocampal ischemic injury, but there is little known about its role in the cerebellum. To examine the neuroprotective potential of CAMKII inhibition in Purkinje cells we subjected C57BL/6 or CAMKIIα KO male mice (8–12 weeks old) to cardiac arrest followed by cardiopulmonary resuscitation (CA/CPR). We performed a dose-response study for tat-CN19o and cerebellar injury was analyzed at 7 days after CA/CPR. Acute signaling was assessed at 6 hours after CA/CPR using Western blot analysis. We observed increased phosphorylation of the T286 residue of CAMKII, suggesting increased autonomous activation. Analysis of Purkinje cell density revealed a decrease in cell density at 7 days after CA/CPR that was prevented with tat-CN19o at doses of 0.1 and 1 mg/kg. However, neuroprotection in the cerebellum required doses that were 10-fold higher than what was needed in the hippocampus. CAMKIIα KO mice subjected to sham surgery or CA/CPR had similar Purkinje cell densities, suggesting CAMKIIα is required for CA/CPR induced injury in the cerebellum. We also observed a CA/CPR-induced activation of death associated protein kinase (DAPK1) that tat-CN19o did not block. In summary, our findings indicate that inhibition of autonomous CAMKII activity is a promising therapeutic approach that is effective across multiple brain regions.
Keywords: Ischemia, cerebellum, calcium/calmodulin-dependent protein kinase, excitotoxicity, neuroprotection
Introduction
In the United States, there are approximately 560,000 cardiac arrests each year, resulting in high rates of morbidity and mortality (1). Advances in resuscitation research and increased accessibility to defibrillators has improved survival rates, however neurological outcomes in survivors remain poor. The neurological sequelae following cardiac arrest and cardiopulmonary resuscitation (CA/CPR) include cognitive, executive and motor deficits (2–7). Therapy to improve outcomes following CA is currently limited to hypothermia; however, the benefit on neurological outcomes remains unclear (8–14). The loss of blood flow during cardiac arrest results in global cerebral ischemia. There are neuronal populations that are particularly sensitive to global ischemic injury: CA1 hippocampal neurons, striatal medium spiny neurons and cerebellar Purkinje cells (15–19). Neurons in these brain areas undergo delayed cell death resulting from glutamate excitotoxicity, oxidative stress, DNA damage and inflammatory processes (18, 20–22). One approach aimed at improving neurological outcomes is to administer pharmacological agents to prevent cell death of sensitive neuronal populations. Despite data indicating high vulnerability of Purkinje cells in cardiac arrest victims, many preclinical studies using global ischemia models to test neuroprotective agents have focused on injury in the hippocampus and striatum. Purkinje cells are the sole output of the cerebellar cortex and are integral to the cerebellum’s function in motor coordination, motor learning, gait and postural control (23–26). Mechanisms of Purkinje cell death following CA/CPR remain unclear.
One of the early triggers for neuronal cell death is over-activation of N-methly-D-aspartate (NMDA) receptors by glutamate (22, 27–30). We previously tested the NMDA-receptor dependence of Purkinje cell and CA1 cell death following CA/CPR (31). While NMDA receptor activation contributes to cell death in both regions, inhibition with a GluN2B specific antagonist was protective only in the CA1. GluN2B activation is also implicated in striatal injury (32), making the cerebellum unique in the lack of contribution of this receptor subtype to ischemic damage. It is possible that cell death processes downstream of the NMDA receptor is also different in cerebellar Purkinje cells. Calcium/calmodulin-dependent protein kinase (CAMKII) is an intracellular signaling molecule that is activated by calcium that enters through NMDA receptors (33). CAMKII activation mediates several neuronal processes, including synaptic plasticity (33–37). Calcium-stimulated activity of CAMKII can be perpetuated by auto-phosphorylation of its T286 residue, resulting in calcium-independent autonomous activity of CAMKII (36–38). We recently reported that CA/CPR in mice resulted in autonomous activation of CAMKII that contributes to CA1 injury and that inhibition with the novel inhibitor, tat-CN19o, is neuroprotective in the hippocampus (8). Purkinje cells express high levels of CAMKII that is critical to synaptic plasticity processes in these neurons (39–41). Another calcium/calmodulin dependent kinase that interacts contributes to ischemia-induced cell death in the hippocampus is death-associated protein kinase (DAPK). In particular, phosphorylation of serine residue 305 inhibits DAPK activity and dephosphorylation of this residue is required for DAPK activation (42). In this study, we aimed to test the contribution of CAMKII and DAPK in Purkinje cell degeneration following CA/CPR.
Materials and Methods
For all experiments, 8–12 week male (20–25g) C57Bl/6 mice (Charles River Laboratories) or CAMKIIα homozygous knockouts (CAMKIIα KO; kindly provided by Dr. Ulli Bayer) were used. All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Colorado, School of Medicine and were performed according to the guidelines from the National Institutes of Health. Mice were group housed until the time of surgery, after which they were housed individually. Animals were kept on a 12 hour light/dark cycle and allowed free access to food and water.
CA/CPR surgery
CA/CPR was induced as previously described (16, 31, 43). Anesthesia was induced with isoflurane at 3% and then maintained at 1.5–2% in oxygen enriched air (20% O2/80% compressed medical air) using a nose cone. Eye lubrication was applied and temperature probes are inserted into the left ear and rectum to monitor head and body temperature, respectively. A PE-10 catheter was inserted into the right jugular vein for drug administration. Animals were endotracheally intubated, connected to a mouse ventilator (MiniVent Ventilator, Harvard Apparatus) ventilated at a rate of 150 breaths/min. Needle electrodes were placed subcutaneously on the chest for continuous EKG monitoring. Cardiac arrest was induced by injection of 50 μL KCl (0.5 M) via the jugular catheter and confirmed by asystole on the EKG monitor. The respirator was disconnected and anesthesia turned off throughout the cardiac arrest. Head temperature was maintained at 37.5 °C (±0.2 °C) using a heated water head coil, while the body temperature was allowed to fall spontaneously to a minimum of 35.5 °C. After 7.5 minutes of cardiac arrest the respirator was turned back on with 100% oxygen at a respiratory rate of 200/min and 25% greater tidal volume. Resuscitation efforts began after 8 total minutes of cardiac arrest by injection of 0.05–1.0 ml epinephrine solution (16 ug/ml in 0.9% saline), chest compressions, and ventilation with 100% oxygen. Chest compressions were stopped as soon as spontaneous circulation was restored and confirmed on the EKG. Resuscitation was abandoned if spontaneous circulation was not restored within 2.5 min. Once the mice had recovered a spontaneous breathing rate of 60 breaths/min they were extubated and disconnected from the ventilator. Drug administration (tatCN19o, tat-scramble) was performed intravenously 30 minutes after return of spontaneous circulation. The animals were placed in a single housed static recovery cage on a heated water blanket (35°C) for 24 h recovery and at room temp for long-term recovery. Mice received soft food and subcutaneous saline for three days after surgery and had free access to water and regular chow. Sham animals received the same procedure including IV access, intubation, EKG and temperature control except were not given KCl, epinephrine or chest compressions. Anesthesia times were kept to similar times between sham and cardiac arrest surgeries.
Drugs
All animals and treatment groups were randomized and blinded to the investigator. Tat-CN19o was tested at three different concentrations (0.01, 0.1 and 1.0 mg/kg) as well as a scrambled protein group (0.1 mg/kg) and were dissolved in saline vehicle and administered via the right jugular vein catheter 30 minutes post resuscitation.
Western blot analysis
At 6 hours after CA/CPR mice were deeply anesthetized with isoflurane (5%) and decapitated. Cerebellar vermis was isolated and snap-frozen in dry ice with methylbutane and stored at −80°C. Tissues were homogenized with tissue isolation pestles using neuronal protein extraction reagent (NPER) buffer containing protease and phosphatase inhibitors. Protein extracts were centrifuged at 1000×g for 10 minutes to remove nuclei and debris. Protein concentrations were quantified using BCA assay (Thermo Scientific) and diluted to 5 mg/ml. 50 ug of protein was loaded onto a 10% polyacrylamide gel and electrophoresis was performed for 1 hour (160V). Protein was transferred to a PDVF membrane (0.3A for 1 hour) and the membrane was blocked in 10% BSA overnight at 4°C for phospho-T286 CAMKII or 5% blocking grade milk for hour at room temperature for total CAMKII and β-actin. Primary antibody incubations were performed overnight at 4°C, followed by 3 washes in Tris buffered saline and secondary antibody incubations for 1 hour at room temperature. Bands were detected using chemiluminescent substrate and integrated volumes of appropriately sized bands were quantified using Image Lab software (Biorad).
Purkinje cell density analysis
At 7 days after CA/CPR mice were transcardially perfused with phosphate buffered saline (PBS) followed by paraformaldehyde (4% in phosphate buffer) under isoflurane anesthesia and decapitated. Brains were removed and post-fixed in paraformaldehyde for 24 hours after which time cerebellums were processed and paraffin embedded. Coronal sections (6 μm thickness at 100 μm intervals) were cut, mounted onto charged slides and deparaffinized. Antigen retrieval was performed using sodium citrate buffer, pH 6.0 at 95°C for 30 minutes. Sections were blocked and permeabilized (10% normal donkey serum/0.5% Triton-X in PBS) for 1 hour and primary antibody incubation with mouse monoclonal calbindin D28K antibody (Santa Cruz Biotechnology, 1:1000) was performed overnight at 4°C. The sections were incubated in donkey anti-mouse biotinylated secondary antibody (Jackson Immuno) for 1 hour and detected using ABC kit (Vector Laboratories) with DAB chromophore (Sigma-Aldrich). Images (10x objective) of cerebellar cortex vermis were acquired with QCapture Pro software (Leica) and linear density of calbindin-positive cells in the Purkinje cell layer was quantified using FIJI software (ImageJ, NIH).
Statistics
Each n represents an individual subject and data are represented as mean± standard error. Student’s t-test was used to compare two groups (sham vs. cardiac arrest) and one-way ANOVA with Dunnet’s posthoc analysis was used to compare tat-CN19o to tat-scrambled.
Results
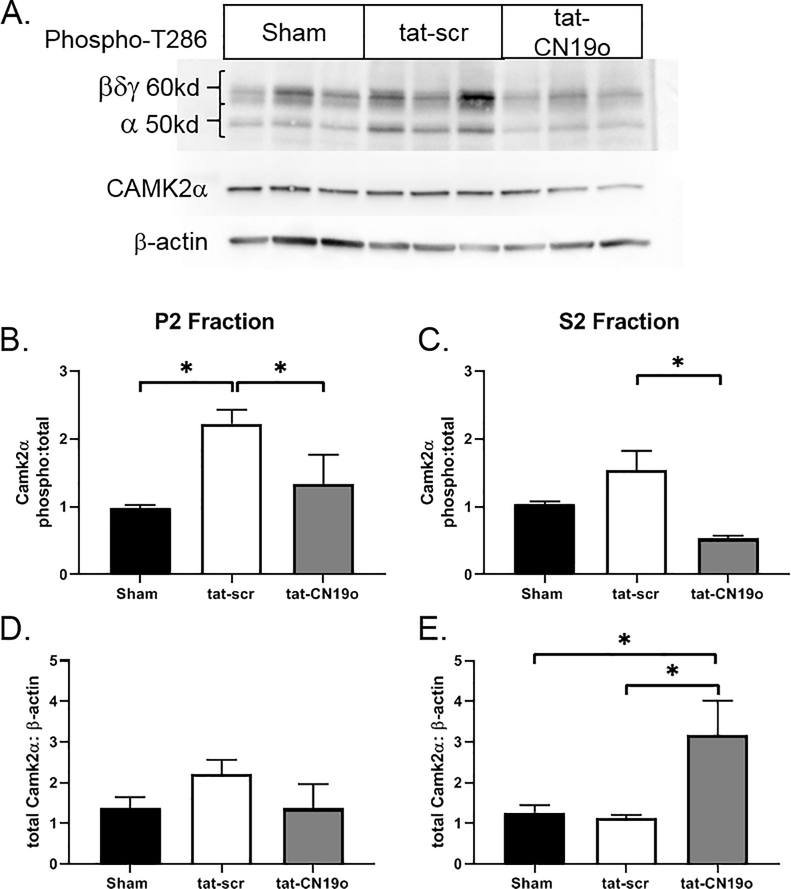
We previously demonstrated that loss of Purkinje cell begins at 24 hours after CA/CPR and peaked at 7 days after recovery (31). Within the cerebellar cortex, CAMKIIα is predominantly expressed by Purkinje cells, with very low or absent expression in granule cells and interneurons (40). We first sought to determine whether there was CAMKIIα activation that preceded the loss of Purkinje cells. We used T286 phosphorylation as an indicator of autonomous CAMKII activation (Figure 1A). We observed a 114% increase in T286 phosphorylation of the CAMKIIα (50 kD) isoform in the P2 membrane fraction of cerebellar homogenates at 6 hours after CA/CPR (Figure 1B). CAMKIIα phosphorylation in the S2 cytosolic fraction was not significantly increased by CA/CPR (Figure 1C). We did not observe significant CA/CPR-induced changes in T286 phosphorylation of the 60 kD isoforms of CAMKII (Figure 1A). Total levels of CAMKIIα were not different between shams and after CA/CPR (p=0.40 and p=0.96 in P2 and S2 fractions, respectively).
Figure 1.
CA/CPR induces CAMKIIα activation in the cerebellum. Membrane (P2) and cytosolic (S2) fractions of cerebellar protein homogenates were probed for phosphorylated T286, total CAMKIIα and β-actin. A) Representative blots (P2 fraction) from mice subjected to sham, CA/CPR +tat-scr, CA/CPR+tat-CN19o. B, C) Quantification of the integrated volumes of phospho/total CAMKIIα in P2 and S2 fractions. D, E) Quantification of the integrated volumes of total CAMKIIα/β-actin in P2 and S2 fractions. N=5–6 per group. * indicate p<0.05.
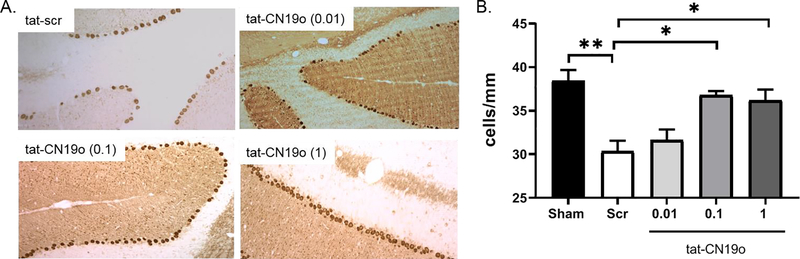
We next tested whether inhibition of stimulated and autonomous activation of CAMKII with the peptide inhibitor tat-CN19o (38) altered Purkinje cell loss after CA/CPR. We administered tat-CN19o (0.01, 0.1 and 1.0 mg/kg) at 30 minutes after resuscitation and analyzed Purkinje cell densities 7 days later, time of maximal Purkinje cell loss (31). Purkinje cell density in sham controls was 38.5±1.2 cells/mm and decreased to 30.4±1.2 cells/mm in mice that were subjected to CA/CPR and received tat-scrambled (p=0.003 compared to sham)(Figure 2A and 2C). Reduced staining of dendrites in the molecular layer were also observed in mice that received tat-scr (Figure 2A). Mice that received 0.01 mg/kg of tat-CN19o had a similar Purkinje cell density (31.7±1.2 cells/mm) to those that received tat-scrambled (p=0.91). We observed significant protection against Purkinje cell loss after CA/CPR in mice that received 0.1 mg/kg (36.8±0.5 cells/mm) and 1.0 mg/kg (36.2±1.2 cells/mm) compared to tat-scrambled (p=0.005 and 0.01, respectively) (Figure 2B and 2C). We used western blot analysis to determine if tat-CN19o altered T286 phosphorylation seen at 6 hours after CA/CPR. Tat-CN19o (0.1 mg/kg) significantly reduced T286 phosphorylation of CAMKIIα compared to tat-scr (p=0.04 and p=0.01 for P2 and S2 fractions, respectively) (Figure 1A–C). We also observed an increase in total CAMKIIα levels in the S2 fraction in mice that received tat-CN19o compared to tat-scr (p=0.03) (Figure 1E).
Figure 2.
CAMKII inhibition with tat-CN19o provides neuroprotection from CA/CPR induced Purkinje cell loss. A. Representative images of calbindin labeling used to quantify Purkinje cell density in the cerebellum at 7 days after CA/CPR. B. Quantification of Purkinje cell densities at 7 days after surgery. Mice that were subjected to CA/CPR and received tat-scr or 0.01 mg/kg tat-CN19o had lower cell densities. Tat-CN19o doses of 0.1 and 1.0 mg/kg had similar cell density to sham controls. N=4–8 per group. One-way ANOVA with Tukey multiple comparisons. * indicates p<0.05. ** indicates p<0.01.
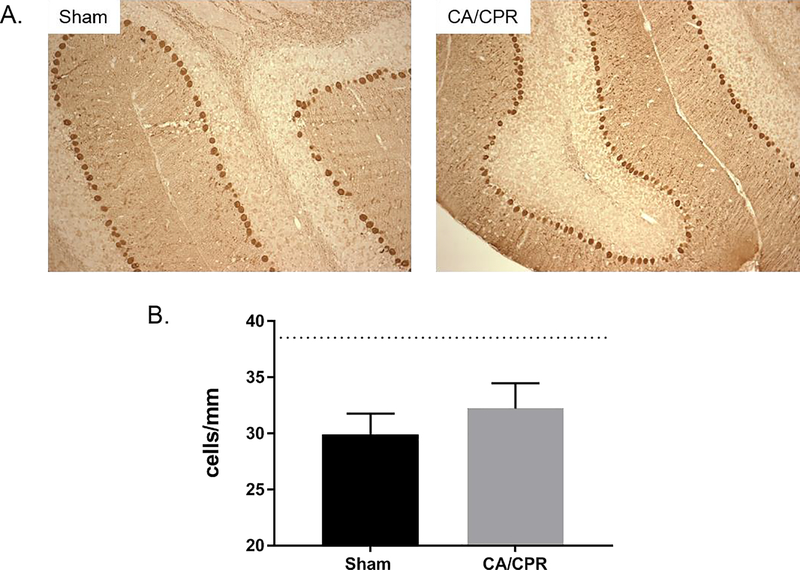
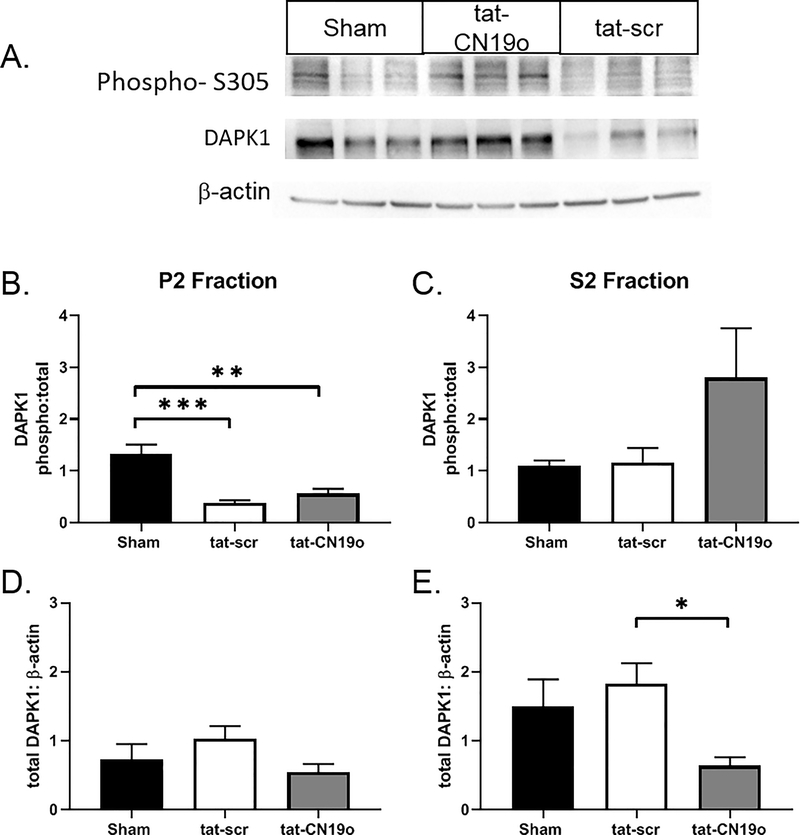
Our western blot analysis suggests activation of the CAMKIIα isoform following CA/CPR. To our knowledge, tat-CN19o does not have specificity for any single CAMKII isoform (38). To determine the role of CAMKIIα in CA/CPR induced injury we performed CA/CPR in mice lacking CAMKIIα (CaMKIIα KO). Purkinje cell density in CAMKIIα shams was 29.9±0.9 cells/mm (Figure 3A), which was significantly lower than that observed in wild-type shams (p=0.001). Purkinje cell density in knockouts subjected to CA/CPR was similar to that of shams (32.2±1.1 cells/mm, p=0.17), indicating a lack of ischemia-induced injury (Figure 3B). To assess the role of a second calcium/calmodulin dependent cell death pathway, we used western blot analysis to determine the impact of CA/CPR on DAPK activity in the cerebellum. We quantified S305 phosphorylation relative to total DAPK expression (Figure 4A). At 6 hours after CA/CPR we observed a 71% reduction (p=0.0002 compared to sham) in DAPK phosphorylation in the membrane fraction (P2) of cerebellar homogenates from mice that received tat-scr (0.1 mg/kg) (Figure 4B), indicating that CA/CPR activates DAPK signaling. No change in DAPK phosphorylation was observed in the S2 cytosolic fraction (p=0.99 compared to sham) (Figure 4C). We observed a 57% reduction in DAPK phosphorylation in the P2 membrane fraction in mice that received tat-CN19o (0.1 mg/kg) compared to sham (p=0.0016), suggesting that CAMKII inhibition does not prevent CA-CPR induced activation of membrane-associated DAPK1 (p=0.51 compared to tat-scr)(Figure 4B). Total levels of DAPK1 in the P2 or S2 fractions were not different between sham and cardiac arrest animals that received tat-scr (p=0.5 and p=0.72, respectively)(Figure 4D and 4E), however tat-CN19o reduced total DAPK levels in the S2 fraction by 57% (p=0.03 compared to tat-scr)(Figure 4E).
Figure 3.
CAMKIIα KO mice lack CA/CPR induced Purkinje cell loss. A. Representative images of calbindin labeling used to quantify Purkinje cell density in the cerebellum at 7 days after CA/CPR. B. Quantification of Purkinje cell densities at 7 days after surgery. CAMKIIα mice were subjected to sham surgery or CA/CPR. Dotted line represents Purkinje cell density in wildtype shams. N=4 per group.
Figure 4.
CA/CPR induces DAPK1 activation in the cerebellum. Membrane (P2) and cytosolic (S2) fractions of cerebellar protein homogenates were probed for phosphorylated S305, total DAPK1 and β-actin. A) Representative blots from mice subjected to sham, CA/CPR +tat-scr, CA/CPR+tat-CN19o. B, C) Quantification of the integrated volumes of phospho/total DAPK1 in P2 and S2 fractions. D, E) Quantification of the integrated volumes of total CAMKIIα/β-actin in P2 and S2 fractions. N=5–6 per group. ** indicate p<0.01. *** indicates p<0.001
Discussion
Our results demonstrate an increase in CAMKIIα activity following CA/CPR that contributes to loss of Purkinje cells following CA/CPR. Pharmacological inhibition of CAMKII activity with the peptide inhibitor, tat-CN19o, prevented the loss of Purkinje cells following CA/CPR. This effect is likely mediated by the α isoform of CaMKII as CA/CPR did not cause Purkinje cell loss in mice lacking CAMKIIα. We also observed an ischemia-induced activation of DAPK1 in the cerebellum that was not blocked by tat-CN19o administration, suggesting that DAPK1 activation alone is not sufficient to induced Purkinje cell death after CA/CPR.
CAMKII is highly expressed throughout the brain and plays a key role in induction of synaptic plasticity in many brain regions, including the cerebellum (33, 34, 37, 40, 41, 44, 45). Prolonged calcium increases during ischemia result in pathophysiological activation of many intracellular signaling cascades, including the CAMKII pathway (8, 46). Our results suggest that CAMKII activation following CA/CPR contributes to the loss of Purkinje cells. This is consistent with other studies that have identified a pro-apoptotic role for CAMKII activation following ischemia (8, 46–51). However, there are some studies that suggest that CAMKII can engage pro-survival signaling and that prolonged inhibition or knockout of CAMKII exacerbates ischemic damage (52). Our results with tat-CN19o suggest that acute inhibition of CAMKII activity is beneficial for Purkinje cell survival following CA/CPR. We saw no decrease in Purkinje cells density in CAMKIIα KO mice subjected to CA/CPR compared to KO shams, suggesting that CAMKIIα is required for ischemia-induced Purkinje cells loss. However, we did see a reduced Purkinje cell density in CAMKII KO shams compared to wild-types. This difference between genotypes was not seen previously, however Purkinje cell density was compared at P17-p28 (45), suggesting delayed alterations in Purkinje cells in CAMKIIα KO mice. Our calbindin staining in CAMKII KO mice did not reveal obvious gaps in the Purkinje cell layer like those seen after an ischemic insult. Therefore, morphological studies are necessary to understand differences in Purkinje cell density seen between adult wild-type and CAMKIIα KO mice.
Our results with T286 phosphorylation in the cerebellum following CA/CPR are consistent with other brain areas following ischemic or hypoxic insults. The cerebellum has high expression of multiple CAMKII isoforms, with CAMKIIα only expressed in Purkinje cells. Interestingly, we only observed an increase in T286 phosphorylation of CAMKIIα. These results are consistent with a lack of Purkinje cell loss following CA/CPR in CAMKIIα KO mice. Mice that received tat-CN19o after CA/CPR had reduced phosphorylation of T286 in the cerebellum compared to mice that received tat-scr. This observation demonstrates that post-CA/CPR treatment with tat-CN19o reduces autonomous CaMKII activity and is likely the mechanism of neuroprotection observed. This is in contrast to our previous findings in the hippocampus, where despite observing neuroprotection, we did not observe an effect of tat-CN19o inhibition on T286 phosphorylation (8). We administered the CAMKII inhibitor at 30 minutes after CA/CPR in both studies. Tat-CN19o is expected to block the stimulated and autonomous activity of CAMKII, but not reverse the phosphorylation of T286 (38). It is possible that the kinetics of T286 phosphorylation in the hippocampus are more rapid than in the cerebellum and there is phosphorylation that precedes drug delivery. We did not analyze other phosphorylation sites of CAMKII, but others have suggested ischemia-induced phosphorylation of other residues that may contribute to autonomous activation of CAMKII (49, 51).
We observed dephosphorylation of residue S305 of DAPK after CA/CPR suggesting increased kinase activity. This is consistent with others that have reported DAPK activation following in vitro and in vivo ischemia (53–56). Activation of CAMKII and DAPK1 was only seen in the membrane fractions of cerebellar homogenates, suggesting membrane-associated signaling activates them. Like CAMKII, DAPK1 is a calcium/calmodulin-dependent protein kinase that can interact with the GluN2B subunit of the NMDA receptor (42, 54). Our previous findings that Purkinje cells lack GluN2B mediated currents and that pharmacological inhibition of GluN2B does not prevent Purkinje cell loss after CA/CPR (31) suggest that CAMKII and DAPK activation may occur via other receptor pathways. Tat-CN190 administered at a neuroprotective dose did not alter the phosphorylation of DAPK1, suggesting there is not a direct interaction between CAMKII and DAPK1. This also suggests that DAPK1 alone is not sufficient to induce Purkinje cell degeneration. Others have reported that DAPK1 inhibition or knockout protects against ischemic injury (53–55, 57). It is possible that S305 is not the only residue regulating DAPK1 activity; therefore, future studies will investigate the role of DAPK inhibition or knockdown on Purkinje cell death after CA/CPR. We did observe a reduction in cytosolic expression of DAPK1, however the mechanism of this downregulation remains unclear. Increased activation of CAMKII and DAPK1 are likely to amplify excitotoxic signals through phosphorylation of substrates such as glutamate receptors, voltage-dependent calcium channels and acid sensing ion channels (46, 48, 58). Intracellular pathways may also be engaged by CAMKII and DAPK1 to activate cell death mechanisms (57, 59–61)
Our data show that inhibiting CAMKII activity provides robust neuroprotection against CA/CPR-induced Purkinje cell loss. It is important to highlight that doses of tat-CN190 that were required to provide neuroprotection in the cerebellum were 10-fold higher than those needed in the CA1 region of the hippocampus (8). It is unclear whether the difference between brain regions due to differences in CAMKII expression/activation by CA/CPR or to differential drug delivery between the forebrain and cerebellum. Regardless of the cause, this finding highlights the need for preclinical studies of neuroprotection in global ischemia models to examine all affected brain regions prior to clinical translation.
Funding Acknowledgements
This work was supported by NINDS K01 NS086969 (NQ); NINDS R01 NS080851 (PSH, UB).
Footnotes
Publisher's Disclaimer: Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Buanes EA, Gramstad A, Søvig KK, Hufthammer KO, Flaatten H, Husby T, et al. Cognitive function and health-related quality of life four years after cardiac arrest. Resuscitation. 2015;89:13–8. [DOI] [PubMed] [Google Scholar]
- 3.Khot S, and Tirschwell DL. Long-term neurological complications after hypoxic-ischemic encephalopathy. Seminars in neurology. 2006;26(4):422–31. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesan A, and Frucht S. Movement disorders after resuscitation from cardiac arrest. Neurologic clinics. 2006;24(1):123–32. [DOI] [PubMed] [Google Scholar]
- 5.Bunch TJ, White RD, Smith GE, Hodge DO, Gersh BJ, Hammill SC, et al. Long-term subjective memory function in ventricular fibrillation out-of-hospital cardiac arrest survivors resuscitated by early defibrillation. Resuscitation. 2004;60(2):189–95. [DOI] [PubMed] [Google Scholar]
- 6.Lim C, Verfaellie M, Schnyer D, Lafleche G, and Alexander MP. Recovery, long-term cognitive outcome and quality of life following out-of-hospital cardiac arrest. J Rehabil Med. 2014;46(7):691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madl C, and Holzer M. Brain function after resuscitation from cardiac arrest. Current opinion in critical care. 2004;10(3):213–7. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Orfila JE, Dietz RM, Moreno-Garcia M, Rodgers KM, Coultrap SJ, et al. Autonomous CaMKII Activity as a Drug Target for Histological and Functional Neuroprotection after Resuscitation from Cardiac Arrest. Cell reports. 2017;18(5):1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cronberg T, Lilja G, Horn J, Kjaergaard J, Wise MP, Pellis T, et al. Neurologic Function and Health-Related Quality of Life in Patients Following Targeted Temperature Management at 33 degrees C vs 36 degrees C After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA Neurol. 2015;72(6):634–41. [DOI] [PubMed] [Google Scholar]
- 10.Lilja G, Nielsen N, Friberg H, Horn J, Kjaergaard J, Nilsson F, et al. Cognitive function in survivors of out-of-hospital cardiac arrest after target temperature management at 33 degrees C versus 36 degrees C. Circulation. 2015;131(15):1340–9. [DOI] [PubMed] [Google Scholar]
- 11.Dell’anna AM, Scolletta S, Donadello K, and Taccone FS. Early neuroprotection after cardiac arrest. Curr Opin Crit Care. 2014;20(3):250–8. [DOI] [PubMed] [Google Scholar]
- 12.Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. The New England journal of medicine. 2002;346(8):549–56. [DOI] [PubMed] [Google Scholar]
- 13.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. The New England journal of medicine. 2002;346(8):557–63. [DOI] [PubMed] [Google Scholar]
- 14.Arrich J, Holzer M, Herkner H, and Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009(4):CD004128. [DOI] [PubMed] [Google Scholar]
- 15.Ng T, Graham DI, Adams JH, and Ford I. Changes in the hippocampus and the cerebellum resulting from hypoxic insults: frequency and distribution. Acta neuropathologica. 1989;78(4):438–43. [DOI] [PubMed] [Google Scholar]
- 16.Kofler J, Hattori K, Sawada M, DeVries A, Martin L, Hurn P, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. Journal of neuroscience methods. 2004;136(1):33–44. [DOI] [PubMed] [Google Scholar]
- 17.Horn M, and Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathologica. 1992;85:79–87. [DOI] [PubMed] [Google Scholar]
- 18.Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, and Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain research bulletin. 1998;46(4):281–309. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Hashimoto H, and Kosaka F. Histological changes of neuronal damage in vegetative dogs induced by 18 minutes of complete global brain ischemia: two-phase damage of Purkinje cells and hippocampal CA1 pyramidal cells. Acta Neuropathol. 1990;80(5):527–34. [DOI] [PubMed] [Google Scholar]
- 20.Martin LJ, Sieber FE, and Traystman RJ. Apoptosis and necrosis occur in separate neuronal populations in hippocampus and cerebellum after ischemia and are associated with differential alterations in metabotropic glutamate receptor signaling pathways. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20(1):153–67. [DOI] [PubMed] [Google Scholar]
- 21.Globus MY, Busto R, Martinez E, Valdes I, Dietrich WD, and Ginsberg MD. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. Journal of neurochemistry. 1991;57(2):470–8. [DOI] [PubMed] [Google Scholar]
- 22.Hertz L Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology. 2008;55(3):289–309. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert PF, and Thach WT. Purkinje cell activity during motor learning. Brain research. 1977;128(2):309–28. [DOI] [PubMed] [Google Scholar]
- 24.Llinás R, and Welsh JP. On the cerebellum and motor learning. Current opinion in neurobiology. 1993;3(6):958–65. [DOI] [PubMed] [Google Scholar]
- 25.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16(6):645–9. [DOI] [PubMed] [Google Scholar]
- 26.Thach WT. A role for the cerebellum in learning movement coordination. Neurobiology of learning and memory. 1998;70(1–2):177–88. [DOI] [PubMed] [Google Scholar]
- 27.Ikonomidou C, and Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet neurology. 2002;1(6):383–6. [DOI] [PubMed] [Google Scholar]
- 28.Lynch DR, and Guttmann RP. Excitotoxicity: perspectives based on N-methyl-D-aspartate receptor subtypes. The Journal of pharmacology and experimental therapeutics. 2002;300(3):717–23. [DOI] [PubMed] [Google Scholar]
- 29.Arundine M, and Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cellular and Molecular Life Sciences (CMLS). 2004;61(6):657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szydlowska K, and Tymianski M. Calcium, ischemia and excitotoxicity. Cell calcium. 2010;47(2):122–9. [DOI] [PubMed] [Google Scholar]
- 31.Quillinan N, Grewal H, Deng G, Shimizu K, Yonchek JC, Strnad F, et al. Region-specific role for GluN2B-containing NMDA receptors in injury to Purkinje cells and CA1 neurons following global cerebral ischemia. Neuroscience. 2015;284:555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L, et al. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(12):3992–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayer KU and Schulman H CaM kinase: Still intriguing at 40. Neuron. 2019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HK, Barbarosie M, Kameyama K, Bear MF, and Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405(6789):955–9. [DOI] [PubMed] [Google Scholar]
- 35.Barria A, and Malinow R. NMDA Receptor Subunit Composition Controls Synaptic Plasticity by Regulating Binding to CaMKII. Neuron. 2005;48(2):289–301. [DOI] [PubMed] [Google Scholar]
- 36.Buard I, Coultrap SJ, Freund RK, Lee YS, Dell’Acqua ML, Silva AJ, et al. CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(24):8214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucchesi W, Mizuno K, and Giese KP. Novel insights into CaMKII function and regulation during memory formation. Brain Res Bull. 2011;85(1–2):2–8. [DOI] [PubMed] [Google Scholar]
- 38.Coultrap SJ, and Bayer KU. Improving a natural CaMKII inhibitor by random and rational design. PloS one. 2011;6(10):e25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Woerden GM, Hoebeek FE, Gao Z, Nagaraja RY, Hoogenraad CC, Kushner SA, et al. betaCaMKII controls the direction of plasticity at parallel fiber-Purkinje cell synapses. Nature neuroscience. 2009;12(7):823–5. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang C, Szabo G, and Sun QQ. Distribution of CaMKIIalpha expression in the brain in vivo, studied by CaMKIIalpha-GFP mice. Brain research. 2013;1518:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagasaki N, Hirano T, and Kawaguchi SY. Opposite regulation of inhibitory synaptic plasticity by alpha and beta subunits of Ca(2+)/calmodulin-dependent protein kinase II. The Journal of physiology. 2014;592(22):4891–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair S, Hagberg H, Krishnamurthy R, Thornton C, and Mallard C. Death associated protein kinases: molecular structure and brain injury. International journal of molecular sciences. 2013;14(7):13858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutchens MP, Traystman RJ, Fujiyoshi T, Nakayama S, and Herson PS. Normothermic cardiac arrest and cardiopulmonary resuscitation: a mouse model of ischemia-reperfusion injury. Journal of visualized experiments : JoVE. 2011(54). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014;81(2):249–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hansel C, Jeu M, Belmeguenai A, Houtman SH, Buitendijk GS, Andreev D, et al. αCaMKII Is Essential for Cerebellar LTD and Motor Learning. Neuron. 2006;51(6):835–43. [DOI] [PubMed] [Google Scholar]
- 46.Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, and Bayer KU. CaMKII in cerebral ischemia. Acta pharmacologica Sinica. 2011;32(7):861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano H, Fukushi H, Morishima Y, and Shirasaki Y. Calmodulin and calmodulin-dependent kinase II mediate neuronal cell death induced by depolarization. Brain research. 2003;962(1–2):41–7. [DOI] [PubMed] [Google Scholar]
- 48.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, et al. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–46. [DOI] [PubMed] [Google Scholar]
- 49.Gurd JW, Rawof S, Zhen Huo J, Dykstra C, Bissoon N, Teves L, et al. Ischemia and status epilepitcus result in enhanced phosphorylation of calcium and calmodulin-stimulated protein kinase II on threonine 253. Brain research. 2008;1218:158–65. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed ME, Dong Y, Lu Y, Tucker D, Wang R, and Zhang Q. Beneficial Effects of a CaMKIIalpha Inhibitor TatCN21 Peptide in Global Cerebral Ischemia. Journal of molecular neuroscience : MN. 2017;61(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rostas JA, Hoffman A, Murtha LA, Pepperall D, McLeod DD, Dickson PW, et al. Ischaemia- and excitotoxicity-induced CaMKII-Mediated neuronal cell death: The relative roles of CaMKII autophosphorylation at T286 and T253. Neurochem Int. 2017;104:6–10. [DOI] [PubMed] [Google Scholar]
- 52.Ashpole NM, Song W, Brustovetsky T, Engleman EA, Brustovetsky N, Cummins TR, et al. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. The Journal of biological chemistry. 2012;287(11):8495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shamloo M, Soriano L, Wieloch T, Nikolich K, Urfer R, and Oksenberg D. Death-associated protein kinase is activated by dephosphorylation in response to cerebral ischemia. The Journal of biological chemistry. 2005;280(51):42290–9. [DOI] [PubMed] [Google Scholar]
- 54.Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarap MM, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140(2):222–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He C, Stroink AR, and Wang CX. The role of DAPK-BimEL pathway in neuronal death induced by oxygen-glucose deprivation. Neuroscience. 2014;258:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita Y, and Yamashita T. Role of DAPK in neuronal cell death. Apoptosis. 2014;19(2):339–45. [DOI] [PubMed] [Google Scholar]
- 57.Pei L, Shang Y, Jin H, Wang S, Wei N, Yan H, et al. DAPK1-p53 interaction converges necrotic and apoptotic pathways of ischemic neuronal death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(19):6546–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meng F, Guo J, Zhang Q, Song B, and Zhang G. Autophosphorylated calcium/calmodulin-dependent protein kinase II alpha (CaMKII alpha) reversibly targets to and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in cerebral ischemia and reperfusion in hippocampus of rats. Brain research. 2003;967(1–2):161–9. [DOI] [PubMed] [Google Scholar]
- 59.Jiang H, Fang J, Xing J, Wang L, Wang Q, Wang Y, et al. Tilianin mediates neuroprotection against ischemic injury by attenuating CaMKII-dependent mitochondrion-mediated apoptosis and MAPK/NF-kappaB signaling. Life Sci. 2019;216:233–45. [DOI] [PubMed] [Google Scholar]
- 60.Lu Q, Harris VA, Sun X, Hou Y, and Black SM. Ca(2)(+)/calmodulin-dependent protein kinase II contributes to hypoxic ischemic cell death in neonatal hippocampal slice cultures. PloS one. 2013;8(8):e70750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CH, Wang WJ, Kuo JC, Tsai HC, Lin JR, Chang ZF, et al. Bidirectional signals transduced by DAPK-ERK interaction promote the apoptotic effect of DAPK. EMBO J. 2005;24(2):294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]