Abstract
Background:
Little is known about the difference between black and non-black patients with EGFR-mutated NSCLC particularly regarding survival. We thus characterized the EGFR expression profile, clinical characteristics, and survival outcome in these patients.
Patient and Methods:
We reviewed the cancer registry and patient charts at a New York-Bronx network (n=2773) treating a large population of minority patients, for non-squamous NSCLC (n=1986) diagnosed between 2009 and 2015. Survival was adjusted for smoking, gender, age, weight, and stage.
Results:
The EGFR mutation rate was 15% (98/652) in tested patients (Black: 14%; non-black: 16%). There was no significant difference between two cohorts with respect to age at diagnosis, gender, presenting stages and socioeconomic status. On the other hand, weight was noted to be heavier in Black patients with EGFR-mutated NSCLC than their non-black counterparts (p=0.012). After adjusting for gender, age, smoking status, weight and stage, the multivariate analysis revealed no racial disparity in survival among patients with wild-type EGFR (p=0.774); However, among patients with EGFR-mutated NSCLC, black patients had shorter survival in comparison to non-black patients (p=0.001), with two-year survival rates being 33% vs. 61% respectively. Such shorter survival was also observed among EGFR-inhibitor treated patients with common EGFR-mutations (P=0.040).
Conclusions:
To our knowledge, this is the first report of inferior survival among Black NSCLC patients with EGFR mutations, relative to non-black patients. The survival disparities suggest the need of more tailored management for this patient population.
Keywords: EGFR, black patients, survival, lung cancer, uncommon EGFR mutations
MicroAbstract
Non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutation is a distinct molecular subgroup with effective targeted therapy. Inour study of 2773 patients, the survival was similar between races among EGFR wild-type NSCLC; however, black patients with EGFR-mutant NSCLC had inferior survival compared to non-black counterparts. More tailored management for this population is warranted.
Introduction
Lung cancer is the most common cause of cancer death, with only 18% of patients surviving 5 years or longer1. It has been reported that Black patients diagnosed with lung cancer may experience worse overall survival relative to their Caucasian counterparts2. Hypotheses have been put forth that this disparity may be attributed to socioeconomic status, access to care, or possible variations in tumor characteristics3.
Advances in lung cancer diagnosis and treatment have led to improved clinical outcome in certain molecular subgroups of lung cancer patients, including those with activating EGFR mutations4. The prevalence of these EGFR mutations in non-small cell lung cancer (NSCLC) is approximately 10%−15% in Caucasian patients and up to 50% in Asian patients5. Among them, deletions in exon 19 (Del 19) and L858R in exon 21 are the two most common EGFR mutations, representing ~85–90% of all EGFR mutations in NSCLC and convey sensitivity to EGFR tyrosine kinase inhibitors (TKI)6. About 10–15% of patients with EGFR mutated NSCLC have uncommon mutations, such as exon 20 insertions6. A recent pooled analysis of 260 Black patients with NSCLC found that only 5% harbored an EGFR mutation, which deviates from the mutational spectrum observed in Caucasians7.
Little is known, however, if the variations in mutational frequencies explain the ethnic disparity in overall survival experienced by Black patients. In particular, scarcity of data exists about the lung cancer survival among Black patients harboring EGFR mutations, relative to the non-black patients. Thus, in the current study, we determined the EGFR mutational profile in a large cohort of Black patients with NSCLC correlating mutational status with clinical, socioeconomic correlates and survival in these patients.
Patients and Methods
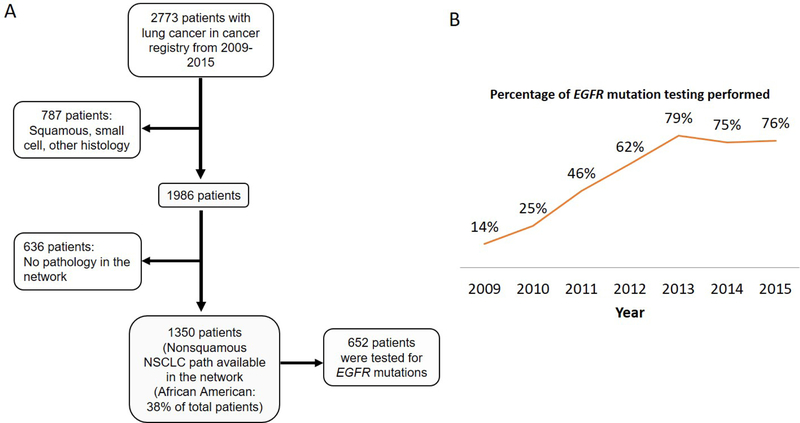
In order to further elucidate lung cancer survival associated with EGFR mutations among Black patients, we retrospectively reviewed a New York-Bronx (Montefiore) network cancer registry database and individual patient charts for non-squamous NSCLC patients diagnosed from 1/1/2009 to 12/31/2015. In total, 2,773 lung cancers were diagnosed at the network during this period, of whom 787 patients were diagnosed with squamous cell carcinoma, small cell carcinoma, or other. Out of the remaining 1,986 non-squamous NSCLC patients, 636 were excluded due to lack of pathology reports in the network. Of the remaining 1,350 eligible patients, 698 were excluded due to lack of EGFR mutation status. As such, our final analytic dataset consisted of only non-squamous NSCLC patients with available pathology and EGFR mutational data (n = 652) (Figure 1A). EGFR testing was performed by Integrated Oncology (Labcorp) via PCR-based technology. This assay analyzes exons 18–21 of the EGFR tyrosine kinase domain, and has been increasingly used as part of routine care in the network (14% of non-squamous NSCLC patients were tested in 2009, 25% in 2010, 46% in 2011, 62% in 2012, 79% in 2013, 75% in 2014, and 76% in 2015) (Figure 1B).
Figure 1.
A. Flowchart of study design and number of patients. B. Percentage of EGFR mutation testing by year among patients diagnosed with non-squamous non-small cell lung cancer
Statistical Analysis
Socioeconomic status was represented by annual household income based on home ZIP code. Differences in patient characteristic were compared by chi-squared and Mann-Whitney tests, when appropriate. Response was evaluated according to RECIST v1.1. Survival rates were compared by Kaplan-Meier curves and Cox proportional Hazard Models, adjusted for smoking status (never/ever), gender, age, weight and stage (using the 7th edition of AJCC criteria). P-values < 0.05 were considered significant.
Results
EGFR mutation profiles
The overall EGFR mutation rate was 15% (98/652) in all patients, with 14% (35/258) of Black patients and 16% (63/394) of non-black patients harboring mutations. The mutational spectrum with Del 19, L858R and uncommon mutations was found to be 46%, 23% and 31% in Black patients, compared to 43%, 35% and 22% in non-black patients. No significant difference was observed (p=0.39) (Table 1A).
Table 1: Patient demographics and clinical characteristics.
| Table 1A Patients with EGFR mutation | |||
|---|---|---|---|
| Non-Black (n=63) N (%) | Black (n=35) N (%) | P-Value | |
| Mean age at diagnosis | 68.2 | 63.2 | 0.06 |
| Gender | 0.84 | ||
| Male | 17(27%) | 8 (23%) | |
| Female | 46 (73%) | 27 (77%) | |
| Stage at diagnosisa | 0.85 | ||
| I+II | 19 (30%) | 11 (31%) | |
| III | 9 (14%) | 6 (17%) | |
| IV | 35 (54%) | 17 (49%) | |
| Smoking status | 1.00 | ||
| Never | 34 (54%) | 19 (54%) | |
| Ever | 29 (46%) | 16 (46%) | |
| Weight (kg) | 65.6 | 75.1 | 0.01 |
| Socioeconomic status (income, $) | 23853 | 21208 | 0.47 |
| Types of EGFR mutations | 0.39 | ||
| Del 19 | 27 (43%) | 16(46%) | |
| L858R | 22 (35%) | 8 (23%) | |
| Uncommon’ | 14 (22%)b | 11(31%)c | |
| Use of EGFR TKI | 32(51%) | 17(49%) | 0.42 |
| Table 1B Patients without EGFR mutation (EGFR wildtype) | |||
| Non-Black (n=331) N (%) | Black (n=223) N (%) | P-Value | |
| Mean age at Diagnosis | 67.4 | 64.7 | 0.83 |
| Gender | 0.62 | ||
| Male | 157(47%) | 122(55%) | |
| Female | 174 (53%) | 101 (45%) | |
| Stage at diagnosis | 0.40 | ||
| I+II | 94 (28%) | 54 (24%) | |
| III | 55 (17%) | 45 (20%) | |
| IV | 163 (49%) | 112(50%) | |
| Smoking status | 0.91 | ||
| Never | 42(13%) | 29(13%) | |
| Ever | 289 (87%) | 194 (87%) | |
| Weight (kg) | 69.3 | 70.6 | 0.40 |
| Table 1C: First line tyrosine kinase inhibitors used in EGFR mutated patients with metastatic disease. | |||
| non-Black N (%) | Black N (%) | P-value | |
| Total | 20 | 9 | |
| Erlotinib | 18(90.0) | 7 (77.8) | 0.57 |
| Afatinib | 2 (10.0) | 2 (22.2) | |
The stage at diagnosis for one EGFR-mutated black patient is not available.
Mutation types: Likely sensitive mutations to FDA-approved EGFR TKIs (SM): G719A in Exon 18 (n=3), E709K and G719A in Exon 18, Q701L and G719A in Exon 18, G719S in Exon 18 and S768I in Exon 20. Likely resistant mutations (RM): H773_V774insHPH mutation in Exon 20, H773dup mutation in Exon 20, N771_P772insH mutation in Exon 20, S768_D770dupSVD mutation in Exon 20 (n=3), N771, H773 dupNPH mutation in Exon 20, D770_N771insG mutation in Exon 20.
Mutation types: SM: G719A mutation in Exon 18 and L861Q mutation in Exon 21, L747_P753del7insS in Exon 19 and L833V mutation in Exon 21. S768I mutation in Exon 20 and L858R mutation in Exon 21, G719S mutation in Exon 18 and S768I mutation in Exon 20. RM: N771_H773dupNPH mutation in Exon 20, H773L and V774M mutations in Exon 20, P772_H773insGHP mutation in Exon 20, A763_Y764ins4 mutation in Exon 20. Undetermined or controversial sensitivity of mutations (UM): L833F mutation in Exon 21, S768I and V769L mutations in Exon 20 (n=2).
Abbreviations: SM, sensitive EGFR mutations to FDA approved EGFR TKIs. RM, resistant EGFR mutations. UM: undetermined or controversial sensitivity of EGFR mutations. TKI, tyrosine kinase inhibitor
Different from the common mutations (Del 19 or L858R), uncommon EGFR mutations are a heterogeneous group with diverse genetic composition and various responses to EGFR TKIs. In non-black patients with uncommon EGFR mutations, 6 (43%) had likely sensitive mutations to FDA-approved EGFR TKIs, including G719A in Exon 18 (n=3), E709K and G719A in Exon 18 (n=1), Q701L and G719A in Exon 18 (n=1), G719S in Exon 18 and S768I in Exon 20 (n=1); Eight (57%) had likely resistant mutations, including H773_V774insHPH mutation in Exon 20 (n=1), H773dup mutation in Exon 20 (n=1), N771_P772insH mutation in Exon 20 (n=1), S768_D770dupSVD mutation in Exon 20 (n=3), N771, H773 dupNPH mutation in Exon 20 (n=1), D770_N771insG mutation in Exon 20 (n=1).
In Black patients with uncommon EGFR mutations, 4 (36%) had likely sensitive mutations, including G719A in Exon 18 and L861Q in Exon 21(n=1), L858R in Exon 21 and S768I in Exon 20(n=1), G719A in Exon 18 and S768I in Exon 20(n=1), L747_P753del7insS in Exon 19 and L833V in Exon 21 (n=1) ; Three (27%) had likely resistant mutations, including N771_H773dupNPH mutation in Exon 20 (n=1), P772_H773insGHP mutation in Exon 20 (n=1), A763_Y764ins4 mutation in Exon 20 (n=1); Four (36%) had undetermined or controversial sensitivity of mutations, including L833F in Exon 21 (n=1), H773L and V774M in Exon 20 (n=1), S768I and V769L in Exon 20 (n=2).
Patient demographics, clinical characteristics and use of EGFR TKIs.
Black patients were similar to non-black patients with respect to age at diagnosis, gender, presenting stages and socioeconomic status (SES, represented by annual household income) (Table 1A and 1B). On the other hand, there was a significant weight difference between Black and non-black lung cancer patients harboring EGFR mutations (uncommon plus common), (75.1kg vs. 65.6 kg, p=0.012, Table 1A). On the other hand, such weight difference was not observed in EGFR wild-type patients (Table 1B). There was no significant difference in terms of exposure to EGFR TKIs (51% vs. 49%, p=0.42). Similarly, among patients harboring common EGFR mutations, there was a significant weight difference (black: 78.4kg vs. non-black: 65.1 kg, p=0.0051), whereas no significant difference in terms of the uses of EGFR TKI (p=0.40); Between Black and non-black patients with metastatic NSCLC harboring common EGFR mutations, neither was there a significant difference among the types of first-line TKI use (either erlotinib or afatinib) (p=0.57) (Table 1C).
Among those with metastatic or recurrent disease, 21 patients did not receive EGFR TKIs due to death before EGFR results coming back or patients’ wish to pursue hospice (non-black 38.5% vs. black 50.0%), loss to follow-up (23.1% vs. 50.0%), insensitive EGFR mutations (23.1% vs. 0.0%), and lack of oncology visit due to frequent hospitalizations (15.4% vs. 0.0%). No statistical significance was observed (p=0.230). Among 35 patients who progressed after first or second generation of TKI, the subsequent treatment was not significantly different (p=0.413) -osimertinib (non-black 28.6% vs. 14.3%), chemotherapy (33.3% vs. 14.3%), continuation of first/second generation of TKI (4.8% vs. 7.1%), other treatment (4.8% vs. 14.3%), and no treatment (28.6% vs. 50.0%). None of the patients received immunotherapy. Of 13 patients who did not receive treatment after TKI progression, all but one patient (patient refusal) were due to poor functional status or death following progression.
Survival analysis
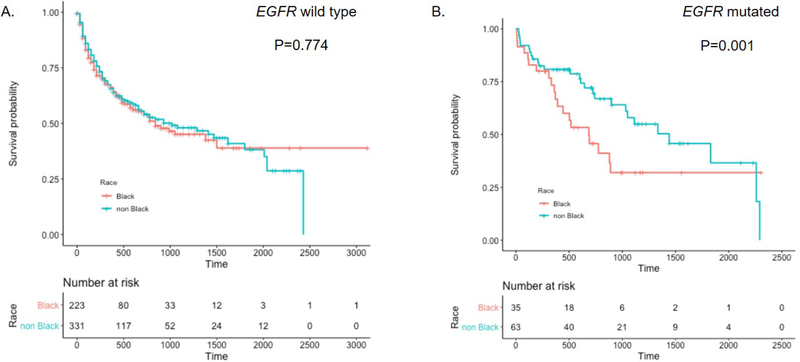
The objective response rate was 63.6% in black and 76.0% in non-black patients (p= 0.454). The two-year survival rate among EGFR wild-type patients was similar by race [Black = 38.2% (52/136); non-black = 39% (75/192), Figure 2A and supplementary for full Cox models]. In contrast, the two-year survival rates varied dramatically by race among patients harboring EGFR mutations [Black = 33.3% (9/27); non-black = 61.3% (27/44), Figure 2B]. Multivariate regression models, adjusting for gender, age, smoking status, weight and stage, showed similar racial differences in survival (EGFR wild-type Black vs. non-black, p=0.774; EGFR mutated Black vs. non-black, p=0.001.)
Figure 2.
Survival time associated with non-squamous NSCLC by ethnicity A. EGFR wild-type patients; B. EGFR mutated patients. *P-values from Cox proportional Hazard Models adjusting for smoking status, gender, age, weight and stage.
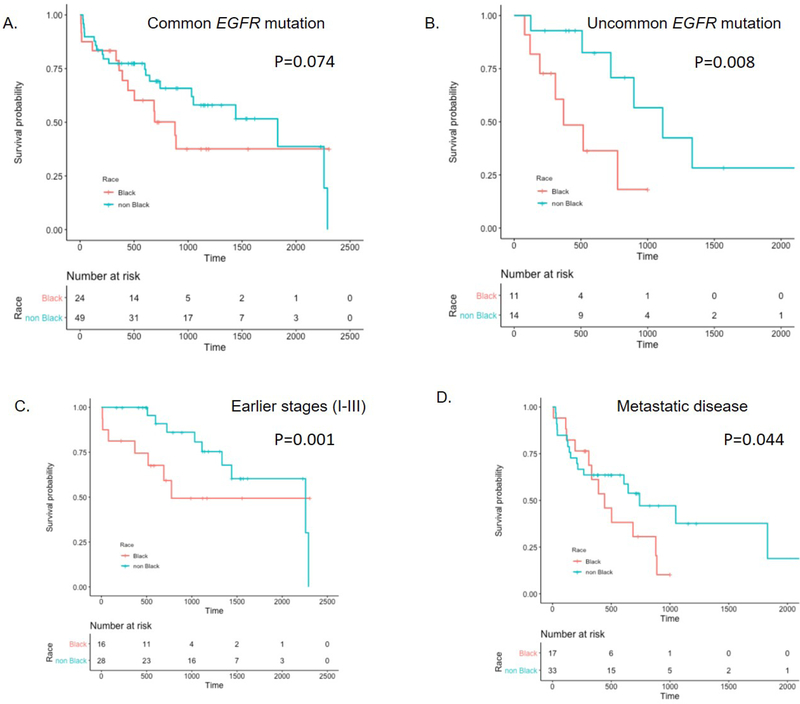
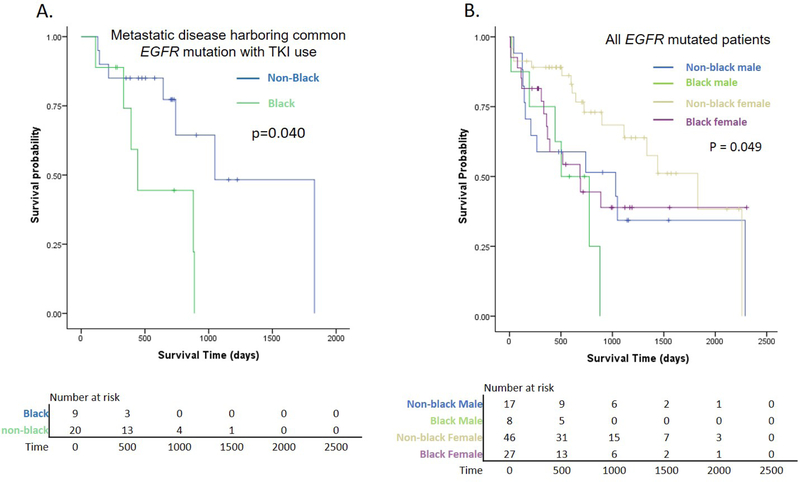
Interestingly, even with limited sample size, the survival difference was significant for patients with uncommon EGFR mutations (Black vs. non-black, p=0.008) (Figure 3B). There was also a trend for shorter survival in Black patients with common EGFR mutations (Black vs. non-black, p=0.074) (Figure 3A). Moreover, the survival disparity was particularly evident for earlier stages (I-III) (p=0.001) (Figure 3C), and was also observed amongst the group with metastatic disease (stage IV, p=0.044) (Figure 3D). The recurrence rate was similar by race (Black = 40%; non-black = 47%). For patients with metastatic disease harboring common EGFR mutation who were treated with EGFR TKIs as per standard practice at that time, Black patients had significantly inferior survival compared to non-black (p = 0.040, Figure 4A). Among those patients, the 2-year survival rate of Black patients was 44.4% compared to 77.3% of non-black patients.
Figure 3.
Survival of patients with EGFR mutation stratified by race. A. Patients with common EGFR mutations; B. Patients with uncommon EGFR mutations. C. Patients with early stages (I+II+III) disease. D. Patients with metastatic disease. *P-values from Cox proportional Hazard Models adjusting for smoking status, gender, age, weight and stage.
Figure 4.
Survival of patients with EGFR mutation A. EGFR-TKI treated patients with metastatic disease and common EGFR mutation stratified by race (p value from Cox proportional Hazard Model adjusting for smoking status, gender, age, weight and stage); B. Patients with EGFR mutation stratified by race and gender (P value from log-rank test). TKI: tyrosine kinase inhibitor
When patients with EGFR mutation were further stratified by gender, non-black female patients had significantly favorable survival (p=0.049), but black women did not appear to survive longer compared to black men (Figure 4B).
Discussion
Studies addressing racial disparities among lung cancer patients with EGFR mutation are limited. The survival comparison between different races is particularly lacking. We hereby determined the clinical attributes, socioeconomic characteristics and survival differences between Black vs. non-black lung cancer patients. Surprisingly, despite similar survival outcomes among wild-type patients in our cohort, Black patients with EGFR mutated NSCLC experienced significantly shorter survival than their non-Black counterparts. These results suggest that even in the era of molecularly targeted therapy, black patients face disparate survival outcomes and more tailored care is needed. To the best of our knowledge, this study is the first reporting racial differences in outcome for EGFR mutated non-squamous NSCLC.
The underlying cause of such disparity among patients harbouring EGFR mutation is not entirely clear. The fact that such disparity was not found among EGFR wild-type patients in the same study period suggests that differential access to cancer care may have not played a major role, although the access and compliance to oral medications may be different. Consistent with this observation, recent studies also showed that after controlling for stage, treatment, and comorbidities, racial disparity in overall lung cancer survival may disappear8–10. It should also be noted that we found no significant difference in terms of gender, socioeconomic status, smoking status, stage distribution, and use of EGFR TKI between black and non-black patients irrespective of EGFR mutation status. Although we cannot entirely exclude the possibility that small difference in TKI use may have impact on survival outcomes. In addition, among TKI-treated patients with metastatic disease and common EGFR mutations, a group that should derive clear and consistent clinical benefits, black patients still suffer poorer survival compared to non-black patients. However, we noted that although not statistically significant, there was a trend that black patients who progressed after front-line TKI were more likely to receive no treatment (50.0% vs. 28.6%). This might contribute to the inferior survival observed in blacks.
Racial difference of pharmacogenetic factors may contribute to such racial disparity. Erlotinib is known to be inactivated through CYP3A metabolism11, 12, the activity of which has been described higher in black patients13. Indeed, a recent phase II randomized study of African American patients with NSCLC (n = 55) found that erlotinib exposures were lower than those observed in previous studies, consistent with CYP3A pharmacogenetics implying higher metabolic activity and suboptimal dosing in African American patients14.
On the other hand, larger weight in black patients with EGFR-mutated lung cancer and set dosing for EGFR TKIs might lead to under-dosing issue of the cohort of black patients, but in our cohort, even after adjustment of weight, the survival difference still exists suggestive of other contributing factors. Another possible cause is medication adherence, which has been shown to be lower among black patients15. Unlike intravenous chemotherapy that can be easily tracked for adherence, the nature of being an oral medication makes assessing TKI adherence more difficult, which is now a subject to be further explored.
We also noted that black women do not gain similar survival benefits as non-black women (Figure 4B). Consistent with other studies reporting that women with lung cancer have favorable survival compared to men, our study showed that non-black women with EGFR mutation had the best survival compared to non-black men, black women, and black men. However, the survival between black men and women are quite similar in our population (Figure 4B), suggesting the need of further research on gender disparity.
Previous studies have reported the frequency of EGFR mutations greatly varying from 2 to 40% dependent on smoking history, histology and patient population (Summarized in Table 2), but none of the studies reported survival outcomes and comparisons to contemporaneous non-black patients. Although some studies reported lower frequency of EGFR mutation in black patients compared to white, this is not the case for a majority of studies.
Table 2:
Summarized studies reporting EGFR mutation in Black patients with non-small cell lung cancer
| Study | Year of Publication | Number of Blacks | % Female | % Ever Smoker | % Adenocarcinoma | % EGFR Mutation in Blacks | % Uncommon of EGFR Mutation in Blacks | % EGFR Mutation in Whites | Location |
|---|---|---|---|---|---|---|---|---|---|
| Leduc19 | 2017 | 169 | Not Reported | 37 | 100 | 40 | Not Reported | 19 | Martinique and Guadeloupe |
| Campbell20 | 2017 | 245 | 53 | 88 | 61 | 12 | Not Reported | 15 | Tennessee, USA |
| Steuer21 | 2016 | 66 | 58 | 23 | 100 | 22 | 25 | 14 US sites | |
| Legius22 | 2016 | 6 | 67 | 0 | 100 | 50 | 33 | Not Reported | Sub-Saharan Africa |
| Bollig-Fischer23 | 2015 | 137 | 58 | 90 | 67 | 15 | 67 | 7 | Wisconsin, USA |
| Araujo7 | 2015 | 260 | 37 | 90 | 53 | 5 | 8 | 6 | Tennessee, Michigan, and Ohio, USA |
| Phelps14 | 2014 | 45 | Not Reported | Not Reported | Not Reported | 4 | 2 | N/A | Ohio and North Carolina, USA |
| Yamaguchi24 | 2013 | 22 | Not Reported | 27 | Not Reported | 18 | Not Reported | N/A | Massachusetts, USA |
| Errihani25 | 2013 | 137 | 44 | 58 | 100 | 21 | 10 | N/A | Morocco |
| Bauml26 | 2013 | 67 | 63 | 84 | Not Reported | 4.8 | Not Reported | 14 | Pennsylvania, USA |
| Reinersman27 | 2011 | 121 | Not Reported | Not Reported | 100 | 19 | Not Reported | 13 | New York and Michigan, USA |
| Harada28 | 2011 | 16 | 56 | 50 | 75 | 31 | 40 | Not Reported | Maryland, USA |
| Cote29 | 2011 | 67 | 66 | 84 | 72 | 40 | 12 | 16 | Michigan, USA |
| Leidner30 | 2009 | 53 | 43 | 86 | 44 | 2 | Not Reported | Not Reported | Ohio, USA |
| Krishnaswamy31 | 2009 | 66 | 52 | 88 | 38 | 2 | 50 | 50 | Illinois, USA |
| Yang32 | 2005 | 41 | Not Reported | Not Reported | Not Reported | 2 | Not Reported | Not Reported | Maryland, USA |
Prior studies identified factors contributing to survival disparities. A study of a national cancer database showed that death from competing causes, including cardiovascular and other cancers can largely explain the notable racial disparities in early stage lung cancer patients10. Another study reported lower utilization of appropriate lung cancer screening among black patients16, but this is not the case in our community based on our prior report17. Older age, squamous histology, gender, larger tumor, and tendency to forgo treatment have also been reported18. However, a recent study from Vanderbilt showed that after controlling stage and treatment, racial disparities may disappear, although EGFR mutational status was not addressed in this particular study8. Stage and treatment were considered more important to predict survival.
Limitations:
Despite out efforts to control for common prognostic factors – gender, smoking status, stage, socioeconomic status, and weight, the nature of this retrospective study cannot eliminate all the potential biases. We were not able to assess the potential impact of pharmacokinetics, comorbidities and medication adherence that may affect survival disparity and these will be the focus of upcoming studies. The study is also limited in data retrieved from just one cancer registry with a relatively small sample size of EGFR mutated patients.
Conclusions
To our knowledge, this is the first report of inferior survival among black NSCLC patients with EGFR mutations, relative to non-black patients despite similar survival in wild-type patients. Black patients are heavier, but even after correcting weight, the survival disparity still exists. More studies on racial disparity specific to patients with targetable genetic alterations are needed, and subsequently more tailored management plans for this patient population are warrented.
Supplementary Material
Clinical Practice Points.
Racial disparity is known to exist among lung cancer patients, but its existence in patients harbouring EGFR mutation remains elusive.
This is the first study reporting that EGFR-mutated black patients had worse survival compared to non-black patients, after adjustment for smoking, gender, age, weight, and stage. Black women may not derive similar magnitude of survival benefit compared to non-black counterparts.
A more tailored care and further research is needed for black patients with EGFR mutations.
Acknowledgments
Funding: The work was supported by National Institutes of Health Paul Calabresi Career Development Award for Clinical Oncology [grant number: 5K12CA132783-04 (to R. Perez-Soler and H. Cheng)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no potential conflicts of interest.
References
- 1.Howlader NNA, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2014, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 2.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results From the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J Clin Oncol. 2018;36:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch FR, Bunn PA, Jr. EGFR testing in lung cancer is ready for prime time. The Lancet. Oncology 2009;10:432–433. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Alexander RE, Maclennan GT, et al. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol. 2012;25:347–369. [DOI] [PubMed] [Google Scholar]
- 7.Araujo LH, Lammers PE, Matthews-Smith V, et al. Somatic Mutation Spectrum of Non-Small-Cell Lung Cancer in African Americans: A Pooled Analysis. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:1430–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones CC, Mercaldo SF, Blume JD, et al. Racial Disparities in Lung Cancer Survival: The Contribution of Stage, Treatment, and Ancestry. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2018;13:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf A, Alpert N, Tran BV, Liu B, Flores R, Taioli E. Persistence of racial disparities in early-stage lung cancer treatment. The Journal of thoracic and cardiovascular surgery. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and Ethnic Disparities in Early-Stage Lung Cancer Survival. Chest. 2017;152:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rakhit A, Pantze MP, Fettner S, et al. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. European journal of clinical pharmacology. 2008;64:31–41. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Zhao M, He P, Hidalgo M, Baker SD. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:3731–3737. [DOI] [PubMed] [Google Scholar]
- 13.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics. 2001;27:383–391. [DOI] [PubMed] [Google Scholar]
- 14.Phelps MA, Stinchcombe TE, Blachly JS, et al. Erlotinib in African Americans with advanced non-small cell lung cancer: a prospective randomized study with genetic and pharmacokinetic analyses. Clin Pharmacol Ther. 2014;96:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farias AJ, Du XL. Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer. J Clin Oncol. 2017;35:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Japuntich SJ, Krieger NH, Salvas AL, Carey MP. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. Journal of the National Medical Association. 2018;110:424–427. [DOI] [PubMed] [Google Scholar]
- 17.Su CT, Bhargava A, Shah CD, et al. Screening Patterns and Mortality Differences in Patients With Lung Cancer at an Urban Underserved Community. Clinical lung cancer. 2018;19:e767–e773. [DOI] [PubMed] [Google Scholar]
- 18.Dalwadi SM, Lewis GD, Bernicker EH, Butler EB, Teh BS, Farach AM. Disparities in the Treatment and Outcome of Stage I Non-Small-Cell Lung Cancer in the 21st Century. Clinical lung cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 19.Leduc N, Atallah V, Agossou M, et al. Lung Adenocarcinoma Survival in EGFR-Mutated African-Caribbean Patients: A Multicenter Study in the French West Indies. Targeted oncology. 2017;12:689–693. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JD, Lathan C, Sholl L, et al. Comparison of Prevalence and Types of Mutations in Lung Cancers Among Black and White Populations. JAMA Oncol. 2017;3:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steuer CE, Behera M, Berry L, et al. Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: Results from the Lung Cancer Mutation Consortium. Cancer. 2016;122:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legius B, Van Den Broecke S, Muylle I, Ninane V. Driver oncogenes in Sub-Saharan African patients with non-small cell lung cancer. Lung Cancer (Auckland, N.Z.). 2016;7:149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollig-Fischer A, Chen W, Gadgeel SM, et al. Racial diversity of actionable mutations in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015;10:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi N, Vanderlaan PA, Folch E, et al. Smoking status and self-reported race affect the frequency of clinically relevant oncogenic alterations in non-small-cell lung cancers at a United States-based academic medical practice. Lung cancer (Amsterdam, Netherlands). 2013;82:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Errihani H, Inrhaoun H, Boukir A, et al. Frequency and type of epidermal growth factor receptor mutations in moroccan patients with lung adenocarcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2013;8:1212–1214. [DOI] [PubMed] [Google Scholar]
- 26.Bauml J, Mick R, Zhang Y, et al. Frequency of EGFR and KRAS mutations in patients with non small cell lung cancer by racial background: do disparities exist? Lung cancer (Amsterdam, Netherlands). 2013;81:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinersman JM, Johnson ML, Riely GJ, et al. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada T, Lopez-Chavez A, Xi L, Raffeld M, Wang Y, Giaccone G. Characterization of epidermal growth factor receptor mutations in non-small-cell lung cancer patients of African-American ancestry. Oncogene. 2011;30:1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cote ML, Haddad R, Edwards DJ, et al. Frequency and type of epidermal growth factor receptor mutations in African Americans with non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2011;6:627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leidner RS, Fu P, Clifford B, et al. Genetic abnormalities of the EGFR pathway in African American Patients with non-small-cell lung cancer. J Clin Oncol. 2009;27:5620–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic differences and functional analysis of MET mutations in lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5714–5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang SH, Mechanic LE, Yang P, et al. Mutations in the tyrosine kinase domain of the epidermal growth factor receptor in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:2106–2110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.