Abstract
Purpose:
Prospectively compare parent/nurse controlled analgesia (PNCA) to continuous opioid infusion (COI) in the post-operative neonatal intensive care unit (NICU) population.
Design/Methods:
A randomized controlled trial compared neonates treated with morphine PNCA to those treated with morphine COI. The primary outcome was average opioid consumption up to 3 post-operative days. Secondary outcomes included 1) pain intensity, 2) adverse events that may be directly related to opioid consumption, and 3) parent and nurse satisfaction.
Results:
The sample consisted of 25 post-operative neonates and young infants randomized to either morphine PNCA (n = 16) or COI (n = 9). Groups differed significantly on daily opioid consumption, with the PNCA group receiving significantly less opioid (P = .02). Groups did not differ on average pain score or frequency of adverse events (P values > .05). Parents in both groups were satisfied with their infant’s pain management and parents in the PNCA group were slightly more satisfied with their level of involvement (P = .03). Groups did not differ in nursing satisfaction.
Conclusions:
PNCA may be an effective alternative to COI for pain management in the NICU population. This method may also substantially reduce opioid consumption, provide more individualized care, and improve parent satisfaction with their level of participation.
Clinical Implications:
Patients in the NICU represent one of our most vulnerable patient populations. As nurses strive to provide safe and effective pain management, results of this study suggest PNCA may allow nurses to maintain their patients’ comfort while providing less opioid and potentially improving parental perception of involvement.
Study Type:
Treatment study.
Level of Evidence:
I.
It has been only 30 years since misconceptions regarding infants not feeling pain were dispelled (Anand & Hickey, 1987). More recently, a systematic review has highlighted the short-term (e.g., poor early neurodevelopment, altered brain development) and long-term (e.g., delayed postnatal growth, poor quality cognitive and motor development) neurodevelopmental outcomes of poorly controlled pain in newborns (Valeri, Holsti, & Linhares, 2015). However, the benefits of analgesic agents are also being challenged by rising concerns regarding their effects on the developing nervous system (Taddio & Katz, 2004). Analgesics, particularly opioids, have been shown to decrease the stress response and improve clinical outcomes in post-surgical newborns (Anand & Hickey, 1987), yet the optimal dose for a given infant has not been determined (Taddio & Katz, 2004). Furthermore, the optimal opioid delivery system has yet to be identified. It stands to reason that the risk of adverse effects can be mitigated by using smaller bolus doses, shortened infusion times, or both (van Dijk et al., 2002), thus decreasing the total amount of opioids received by infants.
Currently, continuous opioid infusion (COI) continues to be a standard method of opioid delivery (Simons & Anand, 2006; van Dijk et al., 2002) in the neonatal intensive care unit (NICU). However, COI delivers a steady flow of medicine, thus potentially providing more or less opioid than the infant requires. Opioid COIs have not been shown to be more beneficial than intermittent bolus dosing (van Dijk et al., 2002). There is significant variability in opioid requirements (Howard et al., 2010; Simons & Anand, 2006) based on patient metabolism, physiology, previous opioid exposure, concomitant medical problems, and extent of the surgical procedure (Bell et al., 2014; Simons & Anand, 2006). All factors considered, the optimal delivery method for analgesia in NICU patients should be safe, prompt, adaptable, and able to address variability in patient status.
Parent/nurse-controlled analgesia (PNCA) allows for rapid opioid titration in response to pain or the anticipation of pain associated with care. Additionally, PNCA allows for increased involvement of parents in their infants’ pain management. For these reasons, PNCA has been a treatment option for children unable to operate conventional patient-controlled analgesia (PCA) in our organization for nearly 20 years. However, no studies have explicitly compared COI and PNCA in the neonatal population with regard to efficacy, side effects, or caregiver satisfaction.
PNCA has been shown to be safe and effective in several pediatric populations (Anghelescu et al., 2011; Czarnecki et al., 2008, 2011, 2014; Czarnecki, Hainsworth, Simpson, & Weisman, 2017; Malviya et al., 2001; Monitto et al., 2000; Voepel-Lewis, Marinkovic, Kostrzewa, Tait, & Malviya, 2008) and is endorsed by the American Society of Pain Management Nurses (Cooney et al., 2013). Howard et al. (2010) reviewed the use of Nurse Controlled Analgesia (NCA) for post-operative pain management in over 10,000 children, 510 of whom were neonates. The authors concluded that overall, NCA was safe and effective and resulted in high nurse satisfaction ratings. Key to maintaining safety and avoiding inappropriate administration or over-sedation with either PNCA or NCA are appropriate education and monitoring.
We have previously reported on our experience (Czarnecki et al., 2014) comparing morphine PNCA to fentanyl COI (our standard opioid infusion at the time). We found that neonates and young infants treated with PNCA received significantly less opioid than those treated with COI, suggesting a potential benefit for PNCA. However, that study was limited by the inability to control for the type of opioid, which may have influenced some of the results.
An important but often overlooked component of pediatric pain management is the role of parents. Franck, Oulton, and Bruce (2012) qualitatively evaluated parents’ perceptions of pain control in their post-surgical neonates. Parents expressed a strong desire to be more involved in their infants’ pain management and had concerns regarding the timeliness of information provided about their infants’ pain. The authors suggested that future studies should examine ways in which parents can “achieve their desired level of involvement” (p. 45). One of our recent studies examined differences in parent and nurse satisfaction associated with three opioid delivery systems: PNCA with basal, PNCA alone, and IV opioids administered by a nurse on an as-needed (PRN) basis (Czarnecki, Hainsworth, Jacobson, & Weisman, 2015). We demonstrated that parents were highly satisfied with all three methods of opioid delivery, but more parents in the PRN group reported a desire to be more involved. In addition, nurses were found to be least satisfied with PRN opioids owing to decreased parent involvement and increased time needed to administer opioids.
The purpose of this prospective, randomized controlled trial (RCT) was to compare morphine PNCA to morphine COI in postoperative infants (“infant” will be used to describe the neonates and young infants represented). Based on our previous studies, we hypothesized that there would be less opioid consumption (primary outcome) in PNCA patients, without differences in pain scores or adverse event profiles. We also expected to find a greater level of parent involvement and higher parent and nurse satisfaction in the PNCA group.
Methods
This single-center, prospective RCT was approved by the hospital’s institutional review board and was registered at www.clinicaltrials.gov (ID: ) by the principal investigator before study enrollment.
Setting
This study took place in the NICU at Children’s Hospital of Wisconsin (CHW), with data collected between April 2013 and April 2015. At the time of the study, the unit consisted of a 43-bed intensive care unit and a 16-bed step-down unit with approximately 600 admissions per year.
Procedure
Potential participants were identified from the surgery schedule and the surgical team’s work list that included any infant with a planned surgery. Initially, the study team also made contact with the mothers of potential participants in the hospital’s Fetal Concerns Clinic. However, this recruitment method was unsuccessful, and was therefore discontinued.
Inclusion criteria: infants 1) born no earlier than 34 weeks postmenstrual age and having a corrected age of no greater than 44 weeks at the time of enrollment, 2) having a birth or current weight of at least 2 kg, 3) undergoing an abdominal or thoracic surgical procedure conducted by the pediatric general and thoracic surgery team, and 4) expected to require opioids for at least 24 hours after the surgical procedure based on the opinion of the surgeon. Additionally, at least one parent was required to be able to read and speak English. Because recruitment proved more difficult than anticipated, the protocol was amended to remove the upper age limit, but the requirement of being cared for in the NICU remained.
Exclusion criteria: infants 1) requiring vasopressors, 2) with significant prior opioid exposure (defined as >2 days of continual exposure before surgery, or >3 doses over the 5 days before the surgery), and 3) who were expected to be chemically paralyzed after surgery.
Design
This study used an unblinded design. Parents were approached before surgery. After informed consent was obtained, infants were randomized to one of two arms: either the morphine PNCA with a basal infusion (PNCA) group or the morphine COI group. Randomization was stratified by gender and surgical location (thoracic, upper abdomen, or lower abdomen) in blocks of four. The randomization scheme was created by a statistician on the research team using Windows version 6.0 of “rand.exe” (http://block-stratified-randomization.software.informer.com/). The clinical research coordinator used the scheme to assign participants to groups.
Protocol Details
Morphine was administered using PNCA for group 1 and COI for group 2. Conditions were designed to replicate current pain management procedures in our NICU. For the PNCA group, orders were entered by a member of the Acute Pain Service (APS) or the patient’s anesthesiologist. Using a 250 mcg/mL solution, PNCAs were ordered as morphine 0.010-0.0125 mg/kg button dose, 8-minute lockout, 0.010-0.0125 mg/kg/hr basal rate, and 0.060 mg/kg/hour total maximum. Doses were rounded to the nearest microgram. Parents were instructed about signs of pain and told to push the PNCA button only when the child was awake and in pain, never to push the PNCA button when the child was asleep, and to notify their nurse if they felt their child’s pain was not well controlled. For the COI group, morphine was ordered at 0.030 mg/kg/hr, with a 0.030 mg/kg/hr nurse bolus available each hour to a maximum of 0.060 mg/kg/hr total. Parents were instructed on signs of pain and to notify their nurse if their child’s pain was not well controlled. The postsurgical opioid delivery system was initiated upon return to the NICU. Based on our current practice, PNCA patients were managed by the APS, and COI patients were managed by the neonatology team. COI patients were checked on daily by the APS, but opioid adjustments were made only by the neonatology team. Monitoring of all patients was consistent with current practice. Patients had continuous pulse oximetry and cardiopulmonary monitoring. Pain and sedation scores (Hoffman, Nowakowski, Troshynski, Berens, & Weisman, 2002) were recorded at least every 2 hours for the first 24 hours, with a minimum of every 4 hours thereafter. To calculate total opioid consumption, PNCA doses (PNCA group) and morphine boluses (COI group) were documented hourly.
Enrolled patients who at any point before PNCA or COI discontinuation subsequently required vasopressors, a high-frequency oscillating ventilator, benzodiazepines, acetaminophen, or antiinflammatory agents as adjuncts for pain were removed from the study. At the time of the study, acetaminophen was not used as routinely as it is now. In order to not confound pain assessment and opioid consumption by having some patients receive it and others not, the study team chose to stop data collection if acetaminophen (or benzodiazepine) was administered. Data collection terminated for all other patients when the PNCA or COI was discontinued. However, patient orders were monitored for 5 additional days to assess whether methadone was started.
Independent variables were collected to describe the sample and to determine whether groups were equivalent on all potentially confounding variables. Demographic and anthropometric data were extracted from the electronic health record, and included gender, race, ethnicity, gestational age, weight at time of surgery, total time PNCA or COI was used, and whether the current surgery was the infant’s first. We also recorded mechanical ventilation and any unplanned reintubation occurrences. PNCA injections and attempts, as well as COI boluses, were recorded for daytime (07:0018:59) and nighttime (19:00-06:59) shifts. Mothers were asked about perinatal use of antidepressant or illicit drugs.
Primary Outcome
Our primary outcome was opioid consumption, defined as the average amount of opioid consumed over the time of study, and reported in mg/kg/hr. For the PNCA group, it was calculated based on the number of hourly injections plus the basal rate. For the COI group, it was based on the infusion rate across the time on study and any boluses administered.
Secondary Outcomes
Average Pain Intensity Over the Time of the Study
Participant pain intensity was assessed using the Revised-Face, Legs, Activity, Cry, Consolability (Revised-FLACC) scale (Malviya, Voepel-Lewis, Burke, Merkel, & Tait, 2006), the scale currently used in our NICU for this patient population. The FLACC has been used previously for NICU infants (Ahn, Kang, & Shin, 2005), has been validated in numerous pediatric populations (McGrath et al., 2008), and was originally validated in children from 2 months to 7 years (Merkel, Voepel-Lewis, Shayevitz, & Malviya, 1997).
Adverse Events
Adverse events are defined a priori as naloxone rescue doses and any reintubation while on the opioid delivery system. Requiring methadone after the opioid delivery system was discontinued was used as a measure of opioid tolerance. A modified Ramsey scale was used (Hoffman et al., 2002). Nurses reported sedation scores from 1 to 6, with 1 to 3 indicating significant sedation, 4 indicating asleep and easily arousable or awake but drowsy, 5 indicating normal awake/alert, and 6 indicating anxiety or pain higher than baseline. Sedation scores below 4 were considered an indication of somnolence.
Parent and Nurse Satisfaction
No satisfaction tools in the extant literature would meet study requirements; therefore the investigators developed parent (Appendix A) and nurse (Appendix B) satisfaction surveys based on our previous studies (Czarnecki et al., 2017). Parents were asked about their satisfaction with 1) the pain management for their infant, and 2) their involvement in the pain management for their infant. Parents in the PNCA group were also asked how often they pushed the PNCA button. Nurses were asked about 1) their satisfaction with the method of pain management for their patient, 2) whether parents were present during their shift, 3) whether they provided education to parents, and if so, 4) how long it took to provide the education. Directions for both surveys were explained to parents at the time of consent, and to nurses during daily rounds. Additionally, the study team contacted nurses on “off shifts” by phone to remind them of the need to complete the surveys. Printed blank copies of the parent surveys were left in each infant’s NICU suite. Printed blank copies of the nurse survey were available in a two-sided envelope in each participant’s suite, with one side used to hold blank copies and the other side clearly labeled for completed surveys (to protect anonymity). Parents were asked to complete the survey once a day, and nurses once each shift. The research coordinator collected completed surveys daily.
Statistical Analysis and Data Management
Data were entered into a REDCap (http://www.project-redcap.org) database. Bimonthly reports were generated to look for missing data, outliers, and inconsistencies. Inconsistencies were checked against original data forms and corrections made as needed. CONSORT principles (Schulz, Altman, Moher, & Group, 2011) were used to assess recruitment success.
Statistical analyses were calculated using SPSS 24 (IBM SPSS Inc., Chicago, IL) and SAS 9.4 software (SAS Institute Inc.). An unadjusted two-sided p < .05 was used for statistical significance in all analyses. Unless otherwise noted, all analyses include data from postoperative days (POD) 0-3. All participants who received the allocated intervention and had at least one post-randomized outcome were included in the modified intent-to-treat analyses. In addition, descriptive and frequency analyses were used to provide summary information about patient characteristics. We report the median (Mdn) with interquartile range (IQR) throughout. A Wilcoxon signed rank test was used to compare paired data of daytime and nighttime injections and attempts for the PNCA group, and paired daytime and nighttime boluses for the COI group. A Mann Whitney test was used to compare PCNA and COI groups; comparisons based on categorical data were analyzed using Chi-square or Fischer’s exact tests. We used a mixed model with random post-operative day (POD) to examine the associations between opioid consumption and considered covariates, which included race, gender, surgery location, gestational age, study group, and POD. The covariance structure used was AR(1); the model was estimated by using maximum likelihood, while POD and intercept were modelled as random effects. To meet the normality assumption of the linear mixed model, we transformed the outcome opioid consumption by using logarithm at base 10. For model selection procedure, we first included one of the considered covariates in the model to examine any time-varying effects on the outcome (univariable model analysis), then repeated the same procedure for other covariates. Based on the univariable model analyses, any covariates and interaction terms with p < .20 were included in the final multivariable analysis. The final model includes only those covariates with p < .05. Adverse events are reported based on incidence collapsed across POD.
Sample Size/Power Analysis
Based on our retrospective study (Czarnecki et al., 2014), and using an estimate of a large effect size, we planned to include 30 patients in each group (60 patients total). This sample size would have allowed for an 80% power at 5% significance level using a t test to detect opioid consumption differences between groups of at least 0.74 SD. The study, however, was terminated early due to difficulty recruiting as well as time and cost constraints.
Results
Participants
There were 371 screened patients, with the final sample consisting of 25 infants, 16 in the PNCA group and nine in the COI group (CONSORT diagram shown in Fig. 1). Four participants from each group were removed from the study early, but all eight had at least one post-randomization outcome and were included in the modified intent-to-treat analyses. Early discontinuation occurred primarily owing to use of concomitant medications, such as acetaminophen or a benzodiazepine. Table 1 compares patient characteristics of both groups. The groups did not differ in gestational age, weight on day of surgery, or hours under study (p > .05). Groups did not differ in gender, age (days), gestational age (weeks), race, ethnicity, or surgical area (p > .05). Although there were slightly more patients in the PNCA group having their first surgery, the difference was not significant (p = .12). No mothers reported opioid or illicit drug use during pregnancy; one mother in the COI group reported use of a SSRI.
Figure 1.
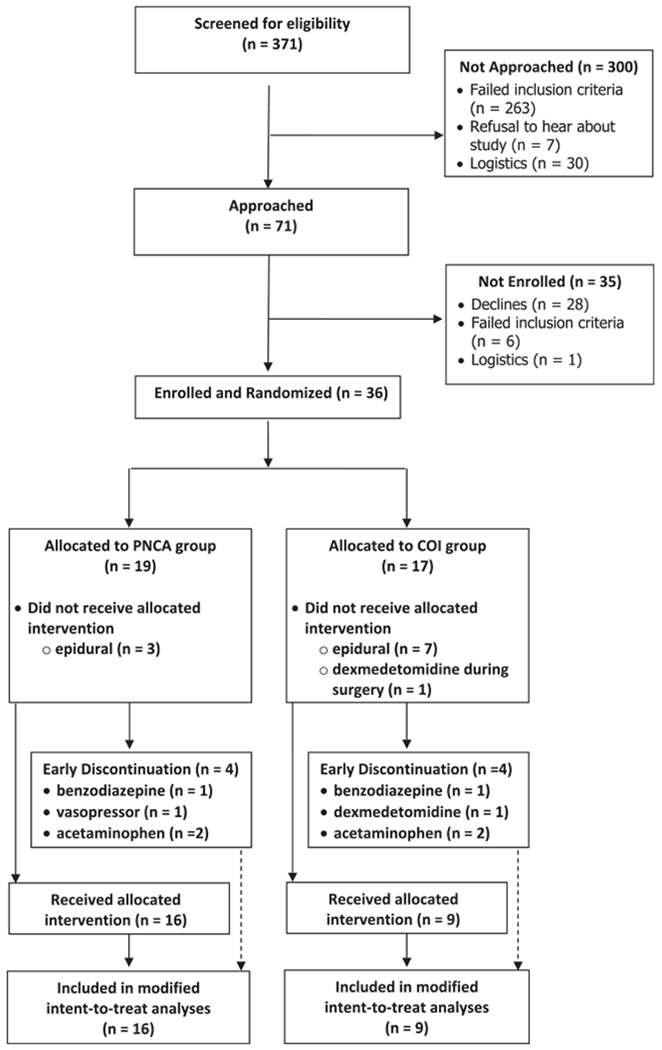
CONSORT diagram. COI = continuous opioid infusion; PNCA = parent/nurse-controlled analgesia.
Table 1.
Patient Demographics
| PNCA (n = 16) | COI (n = 9) | Total Sample (N = 25) | |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 9 (56.3) | 5 (55.6) | 14 (56.0) |
| Race, n (%) | |||
| White | 14 (87.5) | 7 (77.8) | 21 (84.0) |
| Black | 2 (12.5) | 2 (22.2) | 4 (16.0) |
| Ethnicity, n (%) | |||
| Hispanic | 2 (12.5) | 0 | 2 (8.0) |
| Not Hispanic | 14 (87.5) | 9 (100.0) | 23 (92.0) |
| Gestational Age (weeks) | |||
| Median | 37.4 | 39.2 | 37.4 |
| IQR | 37.1-39.3 | 35.9-45.8 | 36.9-39.5 |
| Age (days) | |||
| Median | 4.0 | 2.0 | 4.0 |
| IQR | 1.3-12.0 | 0.0-59.0 | 2.0-11.0 |
| Weight (kg) | |||
| Median | 2.8 | 3.0 | 2.9 |
| IQR | 2.3-3.3 | 2.8-3.7 | 2.4-3.4 |
| Surgical Area, n (%) | |||
| Thoracic | 3 (18.8) | 2 (22.2) | 5 (20.0) |
| Upper Abdomen | 10 (62.5) | 5 (55.6) | 15 (60.0) |
| Lower Abdomen | 3 (18.8) | 2 (22.2) | 5 (20.0) |
| Total Time PNCA or COI in place (hours) | |||
| Median | 64.0 | 66.0 | 65.0 |
| IQR | 39.3-70.3 | 12.5-89.5 | 30.5-71.5 |
| First Surgery, n (%) | |||
| Yes | 16 (100.0) | 7 (77.8) | 23 (92.0) |
| Intubated at start of PNCA/COI, n (%) | 16 (100.0) | 8 (88.9) | 24 (96.0) |
COI = continuous opioid infusion; IQR = interquartile range; PNCA = parent/nurse-controlled analgesia.
No mothers reported opioid use or illicit drug use during pregnancy.
Morphine Consumption
The PNCA group received significantly less opioid than the COI group (p = .02) over POD 0-3 (Fig. 2 and Table 2). Morphine consumption also differed across PODs, with significantly less morphine consumed with each subsequent POD (p < .0001). The interaction between study group and POD was significant, such that the reduction of morphine consumption over time was greater in the COI group than in the PNCA group (p < .001). In the PNCA group only, nighttime morphine consumption (0.012 mg/kg/hr; 0.0034-0.016) was significantly greater than daytime consumption (0.0098 mg/kg/ hr; 0.0029-0.016) (p = .03). For the COI group, nighttime (0.040 mg/ kg/hr; 0.017-0.039) did not differ from daytime consumption (0.034 mg/kg/hr; 0.020-0.043) across POD 0-3 (p > .05). Univariate analyses indicated that there was no significant difference in morphine consumption between groups based on gender, race, or surgery location (p > .05).
Figure 2.
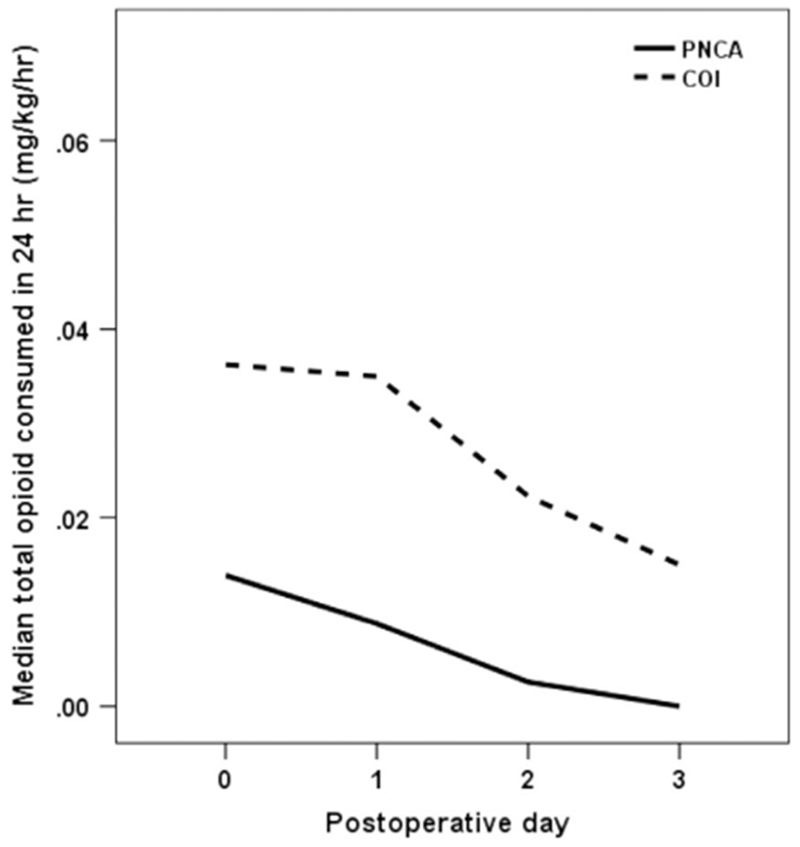
Average morphine consumption (mg/kg/hr). COI = continuous opioid infusion; PNCA = parent/nurse-controlled analgesia.
Table 2.
Average Morphine Consumption (mg/kg/hr) Reported as Median (IQR) for 24-hour, 12-hour Day, and 12-hour Night by Group and POD
| Opioid Consumption 24 Hours Median (IQR) |
Opioid Consumption Day Median (IQR) |
Opioid Consumption Night Median (IQR) |
|
|---|---|---|---|
| PNCA | |||
| POD 0 | 0.014 (0.013-0.017) | 0.015 (0.011-0.020) | 0.013 (0.012-0.017) |
| POD 1 | 0.0088 (0.0065-0.0015) | 0.011 (0.0070-0.015) | 0.010 (0.0033-0.015) |
| POD 2 | 0.0026 (0.0020-0.0046) | 0.0036 (0.0027-0.0061) | 0.0018 (0.0004-0.011) |
| POD 3 | 0.0000 (0.0000-0.0000) | 0.0000 (0.0000-0.010) | 0.0020 (0.016-—) |
| COI | |||
| POD 0 | 0.036 (0.036-0.040) | 0.045 (0.015-0.080) | 0.034 (0.030-0.040) |
| POD 1 | 0.035 (0.022-0.038) | 0.038 (0.024-0.041) | 0.030 (0.019-0.043) |
| POD 2 | 0.022 (0.019-0.039) | 0.026 (0.021-0.058) | 0.018 (0.014-0.058) |
| POD 3 | 0.015 (0.011-0.035) | 0.015 (0.013-0.052) | 0.010 (0.0099-—) |
COI = continuous opioid infusion; IQR = interquartile range; PNCA = parent/nurse-controlled analgesia; POD = postoperative day.
Data reported to 2 significant digits.
PNCA Group
The average number of injections and attempts was below one per hour for both daytime and nighttime shifts. There was no daytime vs. nighttime difference in injections (p = .42) or attempts (p = .86).
COI Group
Of the nine patients in the COI group, six received at least one morphine bolus. The average number of boluses per infant was below one per shift (range: 0-7 per shift), and administration did not differ between daytime and nighttime shifts (p = .09).
Pain Scores
Average daily pain scores were low (medians and IQR shown in Fig. 3 and Table 3) and did not differ between groups for the 24h periods (PNCA 1.6 [0.5-2.5] vs. COI 1.5 [0-2.6]), the 12-hour daytime shifts (PNCA 1.7 [0.0-2.6] vs CO11.7 [0.0-3.0]), or for the 12-hour nighttime shifts (PNCA 1.8 [0.8-2.8] vs COI 1.3 [0.0-2.6]; all p values > .05).
Figure 3.
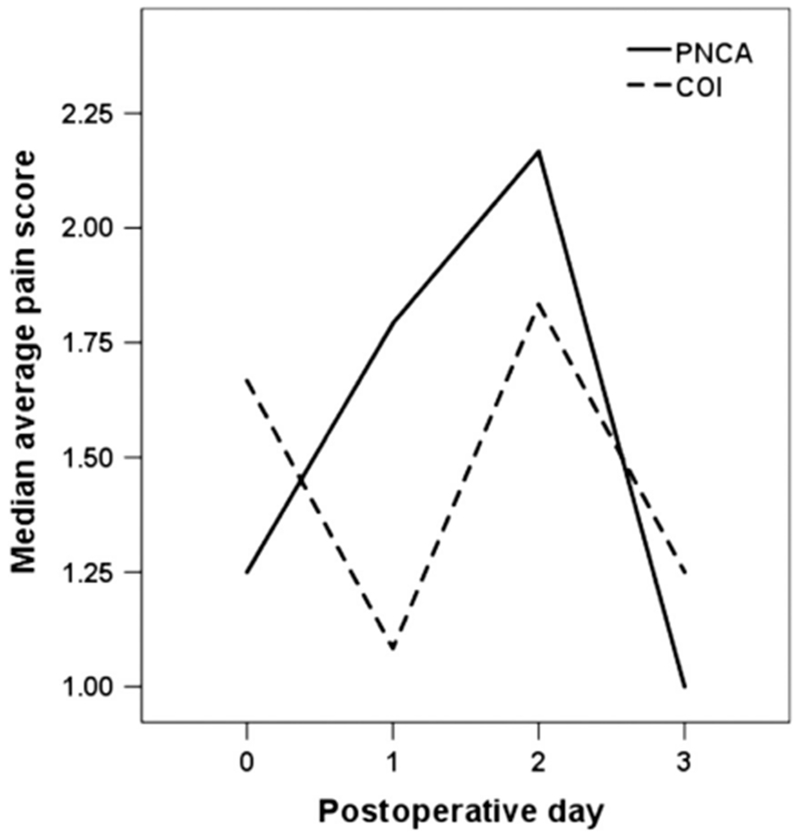
Average daily pain scores. COI = continuous opioid infusion; PNCA = parent/nurse-controlled analgesia.
Table 3.
Average Daily Pain Scores Reported as Median (IQR), by Group and POD
| Pain 24 hours Median (IQR) |
Pain Day Median (IQR) |
Pain Night Median (IQR) |
|
|---|---|---|---|
| PNCA | |||
| POD 0 | 1.25 (0.43-2.81) | 1.00 (0.00-3.33) | 1.33 (0.56-2.69) |
| POD 1 | 1.79 (1.37-2.55) | 1.50 (0.66-2.50) | 1.80 (1.55-3.00) |
| POD 2 | 2.17 (0.31-2.36) | 2.20 (0.33-2.29) | 2.00 (0.00-2.75) |
| POD 3 | 1.00 (0.00-2.44) | 1.00 (0.00-2.55) | 2.28 (1.80- —) |
| COI | |||
| POD 0 | 1.67 (0.00-4.42) | 2.00 (0.00-3.63) | 0.80 (0.00-5.00) |
| POD 1 | 1.083 (0.00-3.18) | 1.41 (0.00-3.67) | 0.67 (0.00-3.54) |
| POD 2 | 1.83 (0.58-3.42) | 1.86 (0.70-3.79) | 2.00 (0.00-3.071) |
| POD 3 | 1.25 (0.31-2.45) | 1.25 (0.00-2.81) | 1.67 (0.00—) |
COI = continuous opioid infusion; IQR = interquartile range; PNCA = parent/nurse-controlled analgesia; POD = postoperative day.
Data reported to 2 significant digits.
Adverse Events
Twelve infants (48%) had sedation scores of <4 during the study period, 7 (44%) in the PNCA group and 5 (56%) in the COI group (p = .57). No infants required naloxone, reintubation, or methadone after morphine was discontinued.
Parent Satisfaction
A total of 33 individual surveys were completed by parents, with at least one survey representing each of the 25 patients in the study. Twenty-one (range: 1-2 per patient) were completed by parents in the PNCA group and 12 (range: 1-2) were completed by parents in the COI group. No parent rated their satisfaction with the pain management of their child lower than 3 out of 4 (4 = very satisfied), and there were no differences between the groups (p > .05).
When asked about their satisfaction with their involvement in their infant’s pain management, 100% (13/13) of parents in the PNCA group reported that they were “very satisfied,” compared to 64% (7/11) of parents in the COI group (p = .03). No parent reported a satisfaction level lower than a 3 out of 4 (4 = very satisfied). Parents in the PNCA group were asked how often they pushed the PNCA button. Seven out of 11 parents (64%) indicated that they “never” pushed the button, and 4/11 (36%) indicated that they “sometimes” pushed the button.
Nursing Satisfaction
A total of 76 surveys were completed by nurses, with at least one survey representing each of the 25 patients in the study, with the exception of one patient in the PNCA group for whom no nurse satisfaction surveys were completed. Fifty-one (median 3.0 surveys per patient; IQR 1.0-5.0) were collected from nurses in the PNCA group and 25 (median 2.0 surveys per patient, IQR 1.0-3.5) from nurses in the COI group (p = .12).
Most nurses (n = 55; 72%) reported being “very satisfied” with the method of pain management used for their patient. No between-group differences were found for nurses’ satisfaction ratings (p = .87). Of the nurses in the PNCA group, 71% (36 of 51) reported that they were “very satisfied,” and of the nurses in the COI group, 76% (19 of 25) reported the same. Nurses also reported that the parents in the PNCA group (29%; 15 of 51 responses) were present “very little,” compared to 60% (15 of 25) of the nurse reports for the COI group (p = .03). The nurses reported whether they provided education to parents regarding pain cues, pain assessment, and the method of opioid delivery, and if so, how long (in minutes) it took. Based on the nurse report, parents of 21 infants (84%) received education regarding pain management, and four did not because they were not present. Nurses reported that education of parents took 5 to 10 minutes. No between-group differences were found in the number of nurses who provided education or in the amount of time it took to provide it (both p values > .05).
Discussion
Appropriate pain management in infants is extremely challenging. Side effects, development of tolerance, and the potential long-term effects of opioids on the developing brain are some of the unknown variables that need to be considered when we deliver opioids to infants (Allegaert & van den Anker, 2016; Drasner, 2010; Ward & Loepke, 2012). The purpose of this prospective, randomized controlled trial (RCT) was to compare morphine PNCA to morphine COI in post-operative infants, with opioid consumption as the primary outcome. The most important contributions of this study were the findings that, when compared with COI, significantly less opioid was consumed by infants in the PNCA group, and at no compromise to their level of comfort. On POD 0 and 1, infants in the COI group consumed 2.5 times the amount of morphine as infants in the PNCA group. These results suggest not only that PNCA is feasible in the NICU environment, but that it offers the opportunity to minimize opioid consumption by titrating the amount used to meet individual needs.
These findings are consistent with our previous retrospective comparison of PNCA to COI in the NICU (Czarnecki et al., 2014). In that study, we found that infants in the COI group consumed over six times the amount of opioid as those whose medication was delivered via PNCA, with no between-group differences in pain scores. At the time of that study, it was our hospital’s practice to use fentanyl for COI in the NICU. Although we used “morphine equivalents” in the analyses, we realized that the two groups received two different drugs, which have very different pharmacodynamic properties. The study was also potentially limited by physician bias and nursing partiality to COI. Surgeons and anesthesiologists may have felt that patients with more invasive procedures may have more pain, and may “deserve” COI.
Although the current study eliminated all of the limitations of our retrospective study, the current findings are limited by the small sample size. The current trial was originally designed to randomize 30 patients into each study arm based on an a priori power analysis. As stated previously, constraints in funding, and difficulty recruiting, required early termination of the trial. Although most parents were open to the idea when initially approached, they declined enrollment owing to the randomized nature of the study. In fact, 39% (28 of 71 approached) declined enrollment, with many stating they wanted their surgeon to make decisions regarding their infant’s pain management. Another reason for the small sample size was the number of enrolled patients who, despite their infants originally seeming likely to require a PNCA/COI for post-surgical pain, ultimately had an epidural placed during surgery. Of the 36 who were consented and randomized, 28% (n = 10) received an epidural. It is important to point out that difficulty recruiting for research involving neonates is recognized as a significant problem inherent in research on neonates and other vulnerable populations (Songstad et al., 2018).
In addition to the contributions involving opioid consumption and pain control, the current study has expanded our understanding of parent and nurse satisfaction when it comes to PNCA use in the NICU. Our most important finding with regard to satisfaction is that parents in the PNCA group were more satisfied with their involvement in their infants’ pain management than were parents in the COI group. We hypothesize that PNCA parents’ satisfaction may have been increased simply because they were given the choice to be involved. Importantly, this involvement was not trivial; rather, parents were given the opportunity to administer an opioid to their infant, and to play an active role in relieving their child’s pain. In support of this hypothesis, although parents in the PNCA group were present more often than parents in the COI group (by nurse report), by parents’ own reports, most never pushed the PNCA button. The pattern of our findings is consistent with the choice hypothesis if we consider the possibility that the increased satisfaction ratings by parents in the PNCA group may have been mediated by an increased sense of control and confidence in the overall care their infant was given. That is, simply giving parents the choice to be actively involved in their infants’ pain management may have resulted in an increased sense of involvement, even though the majority of parents in the PNCA group reported never actually using the PNCA button. Future studies would be necessary to empirically test these hypotheses, as well as to identify other factors that may influence parental satisfaction in this population.
Strengths and Limitations
Recruiting limitations notwithstanding, this study is the first prospective examination of the use of PNCA in the NICU environment. This study was carefully designed and executed, and all participants received morphine for post-surgical pain control. A further strength is that our institution is approximately 8 years into the use of PNCA in the NICU environment; therefore attitudes and perspectives toward this opioid delivery system in the NICU are likely stable. This study is also the first to examine parental satisfaction with their involvement in their infants’ pain care in the NICU environment, with parents offered the possibility of delivering opioid to their infants when they were in pain. This study was limited by its unblinded nature. Inherent biases on the part of nurses or parents could have affected results. However, parents and nurses were told that the study was being undertaken because we did not know if one system was better than the other. To model treatment consistent with our practice guidelines, the groups were followed by either the APS (PNCA) or by the neonatology team (COI). While potentially viewed as a limitation, we believe that this strengthened the design. Prior to study implementation, the team (which includes experts in pain, neonatology, and surgery) met to consider and design guidelines for the study and treatment of both groups. The research team considered it important to maintain our current system in order to avoid introducing a new paradigm in which one team managed both modalities. To have done so would have meant patients in one group or the other would have been managed by a team with less expertise, which could have influenced the results. Nonetheless, it is possible that the control of opioid management by two separate teams may have influenced the results. Another limitation is the use of non-validated questionnaires in an attempt to provide data regarding the satisfaction of bedside nurses, the satisfaction of parents, and the education the nurses were providing to parents regarding their infants’ pain control.
We also recognize that a limitation with our survey process is the potential for bias inherent in the study procedures. Parents in both groups underwent a consenting and survey process that clearly conveyed our interest in their infant’s pain experience, which in turn may have affected satisfaction ratings. As high parent satisfaction with pain management has been demonstrated in previous studies of various analgesic delivery systems (Choi et al., 2008; Czarnecki et al., 2015; Muthusamy et al., 2010; Twycross & Collis, 2013; Twycross & Finley, 2013), it is not known to what extent parents’ awareness of our interest may have contributed to the high satisfaction found in our current study. However, this would not explain the between-group differences in parents’ ratings of their satisfaction with their involvement in their infants’ care. Additionally, consent did not necessarily translate into a willingness to use the PNCA button when randomized to that group. While involvement in their infants’ pain management has been shown to be important to parents (Franck et al., 2012; Simons, Franck, & Roberson, 2001) and nurses (Czarnecki et al., 2015), and while parents in the PNCA group were more satisfied with their level of involvement than parents in the COI group, very few parents reported actually activating the PNCA button. Ultimately, we cannot know whether the low PNCA use by parents was related to the amount of time they were present or absent, the education they received, their preference for nurses to be entirely responsible for medication administration, or other causes. Coupled with results of our previous study (Czarnecki et al., 2015), these results call into question the extent to which parents’ access to PNCA affects their overall satisfaction with pain management.
Future Directions
Future studies should replicate these findings with a larger sample. Given the recruiting challenges, it may be possible to accomplish this with a multi-site trial. Additionally, it will be important to determine factors that contributed to parents’ satisfaction with their involvement in their infants’ pain care, as well as what factors may have inhibited parents’ use of the PNCA button. Future studies should also examine factors related to educating parents about PNCA in vulnerable populations, as well as the potential to develop interventions that may increase parents’ use of the PNCA button when appropriate, or interventions that help them achieve their desired level of involvement (Franck et al., 2012).
Conclusions and Clinical Implications
This RCT showed that in infants who underwent a variety of surgical procedures, the use of PNCA afforded less total opioid use than COI, with no compromise to pain control. The findings presented here are consistent with those of prior studies of infant PNCA (Czarnecki et al., 2014) and NCA (Howard et al., 2010). For both groups, opioids were only administered for ≤3 days on average, despite the infants having major surgical procedures such as thoracotomies and laparotomies. This would strongly suggest that it is possible to more effectively titrate opioid use to an individual patient’s requirements in the post-operative NICU population. These findings suggest that PNCA is a viable alternative to, and may offer advantages over, COI for post-operative pain management in the NICU environment.
Supplementary Material
Acknowledgments
The authors wish to thank Derek Jirovec, B.S., Ellen Edwards, B.S., Crystie Cowan, M.S., APNP, and the NICU nurses for their contributions to this study. Clinical Trial Registry and Number: www.clinicaltrials.gov # .
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR001436. The content is solely the responsibility of the author(s) and does not necessarily represent the official views of the NIH.
Footnotes
Supplementary Data
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.pmn.2019.08.002.
References
- Ahn Y, Kang H, & Shin E (2005). Pain assessment using CRIES, FLACC and PIPP in high-risk infants. Taehan Kanho Hakhoe Chi, 35(7), 1401–1409. [DOI] [PubMed] [Google Scholar]
- Allegaert K, & van den Anker JN (2016). Neonatal pain management: Still in search of the holy grail. International Journal of Clinical Pharmacology and Therapeutics, 54(7), 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ, & Hickey PR (1987). Pain and its effects in the human neonate and fetus. The New England Journal of Medicine, 317(21),1321–1329. [DOI] [PubMed] [Google Scholar]
- Anghelescu DL, Kaddoum RN, Oakes LL, Windsor KB, Faughnan LG, & Burgoyne LL (2011). An update: The safety of patient-controlled analgesia by proxy for pain management in pediatric oncology: 2004 to 2010. Anesthesia and Analgesia, 113(6), 1525–1526. [DOI] [PubMed] [Google Scholar]
- Bell GC, Crews KR, Wilkinson MR, Haidar CE, Hicks JK, Baker DK, Kornegay NM, Yang W, Cross SJ, Howard SC, Freimuth RR, Evans WE, Broeckel U, Relling MV, & Hoffman JM (2014). Development and use of active clinical decision support for preemptive pharmacogenomics. Journal of the American Medical Informatics Association: JAMIA, 21 (e1), e93–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee WK, Lee SJ, Bai SJ, Lee SH, Park BY, & Min KT (2008). Parent-controlled analgesia in children undergoing cleft palate repair. Journal of Korean Medical Science, 23(1), 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney MF, Czarnecki M, Dunwoody C, Eksterowicz N, Merkel S, Oakes L, & Wuhrman E (2013). American society for pain management nursing position statement with clinical practice guidelines: Authorized agent controlled analgesia. Pain Management Nursing: Official Journal of the American Society of Pain Management Nurses, 14(3), 176–181. [DOI] [PubMed] [Google Scholar]
- Czarnecki ML, Ferrise AS, Jastrowski Mano KE, Garwood MM, Sharp M, Davies H, & Weisman SJ (2008). Parent/nurse-controlled analgesia for children with developmental delay. The Clinical Journal of Pain, 24(9), 817–824. [DOI] [PubMed] [Google Scholar]
- Czarnecki ML, Hainsworth K, Simpson PM, Arca MJ, Uhing MR, Varadarajan J, & Weisman SJ (2014). Is there an alternative to continuous opioid infusion for neonatal pain control? A preliminary report of parent/nurse-controlled analgesia in the neonatal intensive care unit. Paediatric Anaesthesia, 24(4), 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki ML, Hainsworth KR, Jacobson AASPM, & Weisman SJ (2015). Opioid administration for postoperative pain in children with developmental delay. Parent and nurse satisfaction. Journal of Pediatric Surgical Nursing, 4(1), 15–27. [Google Scholar]
- Czarnecki ML, Hainsworth KR, Simpson PM, & Weisman SJ (2017). Parent/nurse-controlled analgesia compared with intravenous PRN opioids for post-surgical pain management in children with developmental delay: A randomized controlled trial. Pain Medicine (Malden, Mass.), 19, 742–752. [DOI] [PubMed] [Google Scholar]
- Czarnecki ML, Salamon KS, Jastrowski Mano KE, Ferrise AS, Sharp M, & Weisman SJ (2011). A preliminary report of parent/nurse-controlled analgesia (PNCA) in infants and preschoolers. The Clinical Journal of Pain, 27(2), 102–107. [DOI] [PubMed] [Google Scholar]
- Drasner K (2010). Anesthetic effects on the developing nervous system: If you aren’t concerned, you haven’t been paying attention. Anesthesiology, 113(1), 10–12. [DOI] [PubMed] [Google Scholar]
- Franck LS, Oulton K, & Bruce E (2012). Parental involvement in neonatal pain management: An empirical and conceptual update. Journal of Nursing Scholarship: An Official Publication of Sigma Theta Tau International Honor Society of Nursing, 44(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Hoffman GM, Nowakowski R, Troshynski TJ, Berens RJ, & Weisman SJ (2002). Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics, 109(2), 236–243. [DOI] [PubMed] [Google Scholar]
- Howard RF, Lloyd-Thomas A, Thomas M, Williams DG, Saul R, Bruce E, & Peters J (2010). Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children’s hospital. Paediatric Anaesthesia, 20(2), 126–134. [DOI] [PubMed] [Google Scholar]
- Malviya S, Voepel-Lewis T, Burke C, Merkel S, & Tait AR (2006). The revised FLACC observational pain tool: Improved reliability and validity for pain assessment in children with cognitive impairment. PaediatricAnaesthesia, 16(3), 258–265. [DOI] [PubMed] [Google Scholar]
- Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Lauer A, Munro H, & Farley F (2001). Pain management in children with and without cognitive impairment following spine fusion surgery. Paediatric Anaesthesia, 11 (4), 453–458. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L, Hertz SH, Ljungman G, Palermo T, Rappaport BA, Rhodes T, Schechter N, Scott J, Sethna N, Svensson OK, Stinson J, von Baeyer CL, Walker L, Weisman S, White RE, Zajicek A, Zeltzer L, & PedIMMPACT. (2008). Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The Journal of Pain: Official Journal of the American Pain Society, 9(9), 771–783. [DOI] [PubMed] [Google Scholar]
- Merkel SI, Voepel-Lewis T, Shayevitz JR, & Malviya S (1997). The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing, 23(3), 293–297. [PubMed] [Google Scholar]
- Monitto CL, Greenberg RS, Kost-Byerly S, Wetzel R, Billett C, Lebet RM, & Yaster M (2000). The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesthesia and Analgesia, 91(3), 573–579. [DOI] [PubMed] [Google Scholar]
- Muthusamy K, Recktenwall SM, Friesen RM, Zuk J, Gralla J, Miller NH, Galinkin JL, & Chang FM (2010). Effectiveness of an anesthetic continuous-infusion device in children with cerebral palsy undergoing orthopaedic surgery. Journal of Pediatric Orthopedics, 30(8), 840–845. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, & Group C (2011). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. International Journal of Surgery (London, England), 9(8), 672–677. [DOI] [PubMed] [Google Scholar]
- Simons J, Franck L, & Roberson E (2001). Parent involvement in children’s pain care: Views of parents and nurses. Journal of Advanced Nursing, 36(4), 591–599. [DOI] [PubMed] [Google Scholar]
- Simons SH, & Anand KJ (2006). Pain control: Opioid dosing, population kinetics and side-effects. Seminars in Fetal & Neonatal Medicine, 11(4), 260–267. [DOI] [PubMed] [Google Scholar]
- Songstad NT, Roberts CT, Manley BJ, Owen LS, Davis PG, & investigators HT (2018). Retrospective consent in a neonatal randomized controlled trial. Pediatrics, 141(1), 2017–2092. [DOI] [PubMed] [Google Scholar]
- Taddio A, & Katz J (2004). Pain, opioid tolerance and sensitisation to nociception in the neonate. Best Practice & Research: Clinical Anaesthesiology, 18, 291–302. [DOI] [PubMed] [Google Scholar]
- Twycross A, & Collis S (2013). How well is acute pain in children managed? A snapshot in one English hospital. Pain Management Nursing: Official Journal of the American Society of Pain Management Nurses, 14(4), e204–e215. [DOI] [PubMed] [Google Scholar]
- Twycross A, & Finley GA (2013). Children’s and parents’ perceptions of postoperative pain management: A mixed methods study. Journal of Clinical Nursing, 22(21-22), 3095–3108. [DOI] [PubMed] [Google Scholar]
- Valeri BO, Holsti L, &Linhares MB (2015). Neonatal pain and developmental outcomes in children born preterm: A systematic review. Clinical Journal of Pain, 31 (4), 355–362. [DOI] [PubMed] [Google Scholar]
- van Dijk M, Bouwmeester NJ, Duivenvoorden HJ, Koot HM, Tibboel D, Passchier J, & de Boer JB (2002). Efficacy of continuous versus intermittent morphine administration after major surgery in 0-3-year-old infants: A double-blind randomized controlled trial. Pain, 98(3), 305–313. [DOI] [PubMed] [Google Scholar]
- Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait AR, & Malviya S (2008). The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesthesia and Analgesia, 107(1), 70–75. [DOI] [PubMed] [Google Scholar]
- Ward CG, & Loepke AW (2012). Anesthetics and sedatives: Toxic or protective for the developing brain? Pharmacological Research, 65(3), 271–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


