Abstract
DNA double-strand breaks (DSBs) must be rejoined properly to prevent the occurrence of serious genomic rearrangements associated with many human diseases. Non homologous end joining (NHEJ) is a DSB repair mechanism known to protect genomic integrity that is also implicated in creating genomic translocations, inversions, deletions, and insertions. We recently investigated the impact of the pre-damage spatial proximity of DSB-bearing loci on the frequency of trans repair by NHEJ and surprisingly found no correlation between them. In this review we consider various models that might account for these unexpected results. While DSB movement is necessary to explain our findings, many questions remain about the nature and timing of that motion.
Keywords: DNA repair, non-homologous end joining, homologous recombination, nucleus, movement
DNA double-strand breaks (DSBs) are highly genotoxic lesions that lead to genomic rearrangements if repaired improperly. These genomic aberrations act as drivers in many human diseases including cancer. It is important to understand the underlying molecular and genomic mechanisms of chromosomal rearrangements so that preventive strategies may be designed.
The paradox: translocation potential independent of DSB locations
Two highly conserved mechanisms are used in eukaryotic cells to repair DSBs: homologous recombination (HR) and non-homologous end joining (NHEJ) (Paques & Haber 1999; Jackson 2001). In a recent paper (Sunder and Wilson 2019), we used yeast as a model system to explore the impact that the spatial proximity between two loci suffering DSBs has on the likelihood that those DSBs will be mispaired, i.e. that NHEJ repair will occur in trans (Fig. 1A). Using modifications of our “suicide deletion” assay (Fig. 1B), which traps precise NHEJ repair events at endonuclease-mediated DSBs to enable their detection by reporter genes, we found no correlation between trans repair and the pre-damage contact frequency of DSB loci as predicted by chromatin crosslinking. We did see a preference for joining of DSB ends arising from the same locus, i.e. cis repair (Fig. 1A), consistent with prior studies (Lee et al. 2008; Gao et al. 2016).
Fig. 1. Logic of the system to study trans NHEJ.
Schematics show (A) definitions of cis and trans repair with respect to the pairing of ends between two DSBs, and (B) configuration of the reporter assay used to detect rearrangements in Sunder and Wilson 2019.
Our results were distinct from earlier studies in yeast by Agmon et al. (2013), Lee et al. (2016), and Wang et al. (2017), who found a strong influence of DSB and donor locus proximity on ectopic gene conversion by HR. With those studies in mind, we entered our experiments with a hypothesis that similar proximity phenomena would prevail during NHEJ. However, the lack of correlation between locus proximity and trans repair frequency was a consistent finding in our system after many repetitions, locus variations and control comparisons (Sunder and Wilson 2019). Here, we provide an extended, often speculative, discussion of models that might explain the paradoxical finding that distant DSB pairs are as likely to form translocations/inversions as closely spaced pairs.
Limitations of our experimental system
We first recognize that our suicide deletion system in fact suffered two closely spaced DSBs at the “query” locus, which served to delete the endonuclease gene responsible for making the DSBs, while the trans repair “target” locus had one DSB (Fig. 1B). Even though we confirmed our core observation using a query allele with only one DSB, future direct DSB monitoring with single-DSB alleles will be important to confirm the universality of our findings. Also, we have not yet repeated chromatin crosslinking experiments in our strains. Comparisons of intra- and inter-chromosomal DSB pairs, which undoubtedly have different pre-damage proximities, alleviated this concern, but direct monitoring of allelic contacts before and during repair will be important to further understand the processes that lead to repair outcomes.
The necessity of DSB movement
The limitations of our study notwithstanding, the observed equal propensity toward trans repair regardless of DSB locus placement was compelling and can only be explained by a model in which DSBs, or DSB ends, move after breakage and before repair. It is not possible to rationalize our results without DSB motion, as trans repair would otherwise require co-localization prior to DSB formation and we examined allele pairs with different probabilities of pre-damage co-localization. Fortunately, there is abundant precedent for DSB movement. The search for a homology donor during HR is facilitated by chromosomal mobility, which is significantly enhanced in damaged chromosomes (reviewed in Mine-Hattab & Rothstein 2013; Dion & Gasser 2013). Overall, studies suggest that DSBs exhibit confined Brownian motion while engaged in a homology search. DSBs have also been observed to cluster over time by fluorescence microscopy in yeast (Lisby et al. 2003) and mammals (Aymard et al. 2017). Notably, DNA damage also enhances global chromatin mobility (Mine-Hattab & Rothstein 2012; Seeber et al. 2013, Zimmer & Fabre 2019). However, there are many unknown properties of the requisite DSB motion in the context of trans NHEJ – for instance whether it is random or directed, immediate or delayed – which have substantial implications for repair by both NHEJ and HR.
Simple models of DSB movement
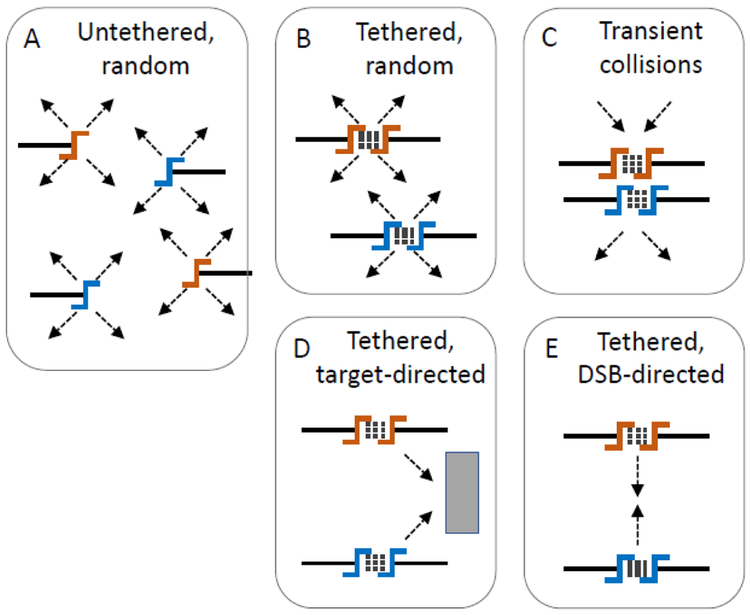
Perhaps the simplest model would be that soon after breakage, DSB ends separate and diffuse freely throughout the nucleus seeking repair partners by random collision (Fig 2A). However, this model predicts an equal likelihood of cis and trans repair, contrary to ours and prior observations (Lee et al. 2008). The unsurprising predominance of cis repair suggests either that DSB movement initiates relatively late, after a time window of limited motion when easily repaired DSBs undergo almost exclusively cis repair (see more below), or that DSBs move as tethered end pairs, maintaining a possibility for cis preference despite the movement.
Fig. 2. Simple models of possible DSB movement leading to trans NHEJ.
Colored symbols indicate cut ends from two loci, black lines indicate duplex DNA, stacked dashed lines indicate DSB end tethering, and arrows indicate movement, which might be random (A to C) or directional (D, E). Inter-DSB contact is required for trans NHEJ, which might be transient (C) or stabilized by DSB affinity for a nuclear sub-structure (D) or other DSBs (E).
Indeed, several studies including Lee et al. (2008) have demonstrated that DSB ends are tethered, as facilitated by Tel1 and other proteins, and that this configuration favors cis over trans repair. A distinct model therefore predicts that DSBs move as unit, as has been observed in human cells (Roukos et al. 2013). In such a model, tethered DSBs, while still moving randomly (Fig. 2B), could encounter each other based on their interim motion. DSB collisions could be random and transient (Fig. 2C), providing a finite probability of trans repair at each collision, even if cis repair remained the predominant outcome due to persistent tethering. However, within limited time frames Brownian motion would result in DSB positions and collision probabilities that maintained a dependence on pre-damage locus positions, which is inconsistent with our observations. With enough elapsed time prior to repair by NHEJ, interim motion could truly randomize post-damage positions. However, DSBs are not as fully and freely diffusible as extrachromosomal chromatin rings since they are of course still attached to their chromosomes (Neumann et al. 2012).
The fundamental alternative to random motion is that DSB movement is somehow directed, which could provide a ready resolution to the paradoxical independence of trans repair and DSB locus proximity. While the significance of random motion during homology search is well documented (Mine-Hattab & Rothstein 2013; Dion & Gasser 2013) recent reports have also suggested the existence of directed motion during DNA repair (Cho et al. 2014; Caridi et al. 2018), which imposes substantial additional requirements on the underlying mechanism. Most importantly, directed motion must, by definition, be mechanistically driven by some force toward some location with definable properties. That location could be external to the DSBs such as an ultrastructural element of the nucleus (Fig 2D). Indeed, DSBs can be driven by motor proteins to the nuclear periphery along induced nuclear actin filaments (Caridi et al. 2018).
Alternatively, DSBs could be directed to a dynamic structure created to accommodate repair. The most parsimonious model to explain our results would be that DSBs have an inherent affinity for each other, supported by a mechanism that drives tethered DSBs toward each other (Fig 2E). The mechanism of such movement could be active, for example through associations of DSB repair proteins with the actin or microtubule cytoskeleton (Lottersberger et al. 2015; Caridi et al. 2018; Schrank et al. 2018). The mechanism of DSB association could also be more passive and less purposeful, perhaps driven by phase separation of DSB ultrastructural assemblies similar to a favored model of the forces that drive heterochromatin self-assembly and nuclear organization (Larson & Narlikar 2018; Falk et al. 2019). These models highlight a critical distinction between our experiments and those addressing ectopic gene conversion in that the movement of two DSBs in relationship to each other could be fundamentally different than between a DSB and a donor locus. In further support of a distinct cellular handling of multiple DSBs, yeast cells with two or more, but not one, DSBs have been observed to show enhanced chromosome mobility throughout the genome (Dion et al. 2012; Mine-Hattab & Rothstein 2012, 2013).
Extended models with staged DSB processing
The above models each invoke one pervasive mechanism of movement. However, DSBs might behave differently with regard to movement potential either as a function of time or based on the status of the cell or DSB and the likelihood of repair by HR (Fig. 3). First, DSB motion in NHEJ might be delayed, only occurring at DSBs that fail rapid cis repair. This model implies that cis repair usually occurs close to the pre-damage location of a DSB (Fig. 3, left side). General support for this idea has been provided by Jeggo and colleagues who distinguish fast and slow NHEJ repair phases based on DSB end complexity and the ease of subsequent NHEJ (Lobrich & Jeggo 2017). In the context of our experiments, only the later repair phase might include increased DSB motion that drives tethered DSBs together into clusters as discussed above (Fig. 3, top track). Unfortunately, our suicide deletion system did not allow us to determine the relative timing of cis vs. trans NHEJ joint formation.
Fig. 3. Multi-stage models of possible DSB disposition in NHEJ and HR.
Flow charts (similar to Fig. 2) depict potential steps leading to NHEJ and HR repair of DSBs; see text for detailed discussion.
In a distinct model, increased motion might only apply to DSBs that were never tethered properly to begin with or that fail to repair before tethering diminishes to make way for DSB end processing and HR. In this case (Fig. 3, bottom track), we return to our first model of random untethered motion (Fig. 2A) but only apply it to a small subset of DSBs that failed cis repair yet retained the potential for NHEJ. Notably, this model directly evokes the impact of a tel1 mutation, which has been seen to result in both reduced tethering and increased trans repair (Lee et al. 2008).
Inter-relationships between NHEJ and HR
Importantly, NHEJ and HR share a common initial DSB substrate. It is now understood that 5’ resection is the key regulated step that commits a DSB to attempted HR while abrogating further possibilities for NHEJ (Mine-Hattab & Rothstein 2013; Dion & Gasser 2013, Bordelet & Dubrana 2019). We must therefore consider our results and the above models in the context of DSB resection and HR.
We first note that our NHEJ results are similar to those obtained by Haber and Leung (1996) for single strand annealing (SSA) of multiple DSBs, where rates of trans repair were independent of the genomic position of the two loci, a finding we also confirmed for SSA (Sunder and Wilson 2019). Like our NHEJ results, these SSA experiments used two DSB loci. However, unlike NHEJ, SSA requires 5’ resection to support annealing of strands across the two DSB ends. The overall implication is that the DSB movement necessary to explain our NHEJ results likely applies similarly to resected DSBs shuttled into SSA. It is unlike HR where the repair efficiency of a single DSB is dependent on the physical proximity of its unbroken homology donor.
Interestingly, HR provides important precedents for many motion models discussed above, albeit at different points in the lifetime of a DSB. Rad51 wraps around ssDNA to form nucleoprotein filaments that facilitate homology searching by promoting Brownian DSB motion (Dion et al. 2012; Mine-Hattab and Rothstein 2012). Moreover, Rad51 has been reported to promote directional movement and co-association of telomeres in the alternative lengthening of telomeres (ALT) pathway (Cho et al. 2014). However, while conceptually relevant, Rad51 acts downstream of 5’ resection and so necessarily promotes distinct movements from those we infer for trans NHEJ. Consistently, rad51 mutation had no effect on trans NHEJ frequencies (Sunder and Wilson 2019). Similarly, Lisby et al. (2003) reported that yeast suffering multiple DSBs show Rad52 foci that co-localize to form only one or two “repair centers”, reminiscent of portions of Figs 2 and 3. Because Rad52 functions during HR, such DSB clusters must again be distinct from any DSB association that supports trans NHEJ, and may only arise after the homology-driven capture of recombination donor alleles. Finally, DSBs in Drosophila heterochromatin are relocalized outside of heterochromatin before Rad51 is recruited, reminiscent of the types of phase transitions mentioned above (Chiolo et al. 2011).
Perhaps the most challenging point is the relative disposition of DSBs between NHEJ and HR. If there is an obligatory sequence of events such that HR only proceeds via resection of DSBs after NHEJ has failed, then any movement that happened at DSBs during attempted NHEJ would influence any subsequent HR. Specifically, if DSBs moved to a distant location for attempted NHEJ, then HR potential would be determined by the new, not the original, locations of the DSBs (Fig. 3, right side). As noted above, studies of donor proximity in gene conversion have only monitored a single DSB locus interacting with an intact donor (Agmon et al. 2013; Lee et al. 2016; and Wang et al. 2017). It is unknown if HR proximity results would change in cells with multiple DSBs, where DSB clustering prior to 5’ resection might influence the measured gene conversion frequencies.
A fundamental alternative is that DSB repair does not behave sequentially and that some fraction of cells or DSBs engage HR quickly and independently of the motion pathway(s) that drive trans NHEJ (Fig. 3, left side). In such a model, only rare cells would be exposed to the potential for trans NHEJ. There is substantial precedent that DSB repair pathway choice is strongly context dependent, including important factors such as cell cycle stage (Aylon et al. 2004; Mjelle et al. 2015), NHEJ and HR competent chromatin states closely coupled with transcription status (Clouaire et al. 2018), and spatial positioning within the nucleus (Lemaitre et al. 2014, Fontana et al. 2019). Our target DSBs were similar with respect to these properties as we deliberately sought to minimize confounding local factors such as transcription or heterochromatinization.
Future perspectives
Our recent results were a provocative challenge to the entry assumption that NHEJ leading to chromosomal rearrangement would be strongly dependent on locus positions in the nucleus before DSBs were formed. In this review we attempted to think through ways that movement of broken alleles could minimize this seemingly obvious relationship, with the interest of stimulating further investigations. We emphasize that ours was only one experimental system with its limitations. More work with controlled DSBs with faster kinetics and direct monitoring of DSB positions and contacts throughout repair will be necessary to establish the universality of the phenomenon we observed. It will also be interesting to explore the extent to which our findings could be extended to humans, where an important consideration will be the actual nuclear distances involved since the yeast nucleus is smaller than most higher eukaryotes.
DSB movement is a widely observed phenomenon but important details may depend on repair pathways and other contexts. It is currently unknown what factors impact the mobility of unresected DSBs during NHEJ repair phases. One candidate could be Mec1, an upstream component of DNA damage response that enhances chromosome mobility during HR (Dion et al. 2012). The Smc5-Smc6 complex has also been suggested to regulate DSB relocalization independently of Rad51 and Rad52 (Torres-Rosell et al. 2007; Chiolo et al. 2011). Assembly of actin filaments in association with myosin directs the DSBs to nuclear periphery (Caridi et al. 2018) and several studies have reported impacts of chromatin remodelers such as Ino80 on DSB mobility (Neumann et al. 2012; Dion & Gasser, 2013). Whether these or other factors are critical to trans NHEJ remains to be established.
It will be equally important to understand the purpose of DSB movement in supporting repair by NHEJ. One possibility is that coordination of repair machinery at fewer sites may increase NHEJ efficiency and/or protect multiple simultaneous DSBs if repair is prolonged. DSB movement might also segregate damaged DNA from the rest of the genome to minimize recombination with undamaged DNA. It will be difficult to explore these important functional questions without better descriptions of early DSB movement and its associated mechanisms in the context of NHEJ in both yeast and human cells.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Agmon N, Liefshitz B, Zimmer C, Fabre E, & Kupiec M (2013) Effect of nuclear architecture on the efficiency of double-strand break repair. Nat Cell Biol 15(6):694–699. [DOI] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, & Kupiec M (2004) The CDK regulates repair of double-strand breaks by homologous recombination during the cell cylce. EMBO J. 23(24):4868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard F et al. (2017) Genome-wide mapping of long-range contacts unveils clustering of DNA double-strand breaks at damaged active genes. Nat Struct Mol Biol 24(4):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordelet H & Dubrana K (2019) Keep moving and stay in a good shape to find your homologous recombination partner. Curr Genet 65(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridi CP et al. (2018) Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature 559(7712):54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiolo I et al. (2011) Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair. Cell 144(5):732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NW, Dilley RL, Lampson MA, & Greenberg RA (2014) Interchromosomal homology searches drive directional ALT telomere movement and synapsis. Cell 159(1):108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouaire T et al. (2018) Comprehensive mapping of histone modifications at DNA double-strand breaks deciphers repair pathway chromatin signatures. Mol Cell 72(2):250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion V, Gasser SM (2013) Chromatin movement in the maintenance of genome stability. Cell 152(6):1355–64. [DOI] [PubMed] [Google Scholar]
- Dion V, Kalck V, Horigome C, Towbin BD, & Gasser SM (2012) Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat Cell Biol 14(5):502–509. [DOI] [PubMed] [Google Scholar]
- Falk M et al. (2019) Heterochromatin drives compartmentalization of inverted and conventional nuclei. Nature 570(7761):395–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana GA et al. (2019) Rif1 S-acylation mediates DNA double-strand break repair at the inner nuclear membrane. Nat Commun 10(1):2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Honey S, Futcher B, & Grollman AP (2016) The non-homologous end-joining pathway of S. cerevisiae works effectively in G1-phase cells, and religates cognate ends correctly and non-randomly. DNA Repair (Amst) 42:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE & Leung WY (1996) Lack of chromosome territoriality in yeast: promiscuous rejoining of broken chromosome ends. Proc Natl Acad Sci U S A 93(24):13949–13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP (2001) Detecting, signalling and repairing DNA double-strand breaks. Biochem Soc Trans 29(Pt 6):655–661. [DOI] [PubMed] [Google Scholar]
- Larson AG, Narlikar GJ (2018) The role of phase separation in heterochromatin formation, function and regulation. Biochemistry 57(17): 2540–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS et al. (2016) Chromosome position determines the success of double-strand break repair. Proc Natl Acad Sci U S A 113(2):E146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Zhang Y, & Lee SE (2008) Saccharomyces cerevisiae ATM orthologue suppresses break-induced chromosome translocations. Nature 454(7203):543–546. [DOI] [PubMed] [Google Scholar]
- Lemaitre C et al. (2014) Nuclear position dictates DNA repair pathway choice. Genes Dev 28(22):2450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby M, Mortensen UH, & Rothstein R (2003) Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol 5(6):572–577. [DOI] [PubMed] [Google Scholar]
- Lobrich M, Jeggo P (2017) A process of resection-dependent nonhomologous end joining involving the goddess Artemis. Trends Biochem Sci. 42(9):690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottersberger F, Karssemeijer RA, Dimitrova N, & de Lange T (2015) 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DNA Repair. Cell 163(4):880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine-Hattab J & Rothstein R (2012) Increased chromosome mobility facilitates homology search during recombination. Nat Cell Biol 14(5):510–517. [DOI] [PubMed] [Google Scholar]
- Mine-Hattab J & Rothstein R (2013) DNA in motion during double-strand break repair. Trends Cell Biol 23(11):529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjelle R et al. (2015) Cell cycle regulation of human DNA repair and chromatin remodeling genes. DNA Repair (Amst) 30:53–67. [DOI] [PubMed] [Google Scholar]
- Neumann FR et al. (2012) Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev 26(4):369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paques F & Haber JE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63(2):349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roukos V et al. (2013) Spatial dynamics of chromosome translocations in living cells. Science 341(6146):660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank BR et al. (2018) Nuclear ARP2/3 drives DNA break clustering for homology-directed repair. Nature 559(7712):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber A, Dion V, Gasser SM (2013) Checkpoint kinases and the INO80 nucleosome remodeling complex enhance global chromatin mobility in response to DNA damage. Genes Dev 27(18):1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunder S, Wilson TE (2019) Frequency of DNA end joining in trans is not determined by the predamage spatial proximity of double-strand breaks in yeast. Proc Natl Acad Sci U S A 116(19):9481–9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Rosell J et al. (2007) The Smc5-Smc6 complex and SUMO modification of Rad52 regulates recombinational repair at the ribosomal gene locus. Nat Cell Biol 9(8):923–31. [DOI] [PubMed] [Google Scholar]
- Wang RW, Lee CS, Haber JE (2017) Position effects influencing intrachromosomal repair of a double-strand break in budding yeast. PLoS One 12(7):e0180994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C & Fabre E (2019) Chromatin mobility upon DNA damage: state of the art and remaining questions. Curr Genet 65(1):1–9. [DOI] [PubMed] [Google Scholar]