Abstract
Genome-wide association studies (GWASs) have shown that pleiotropy is widespread, with the same genetic variants affecting multiple traits, and that complex traits are polygenic, affected by many genetic variants with very small effect sizes. However, despite the growing number of GWASs, the possible contribution of gene-environment correlations (rGE) to pleiotropy and polygenicity has been mostly ignored. rGE can lead to an environmentally mediated pleiotropy, as the same genetically influenced environment can affect multiple traits/disorders. By leading to correlations with additional, mediated, genetic variants, this also contributes to polygenicity. This process can be termed “gene-environment-trait correlations” (rGET). For example, both socioeconomic status (SES) and stressful life events are genetically influenced and have been associated with a myriad of physiological and mental health disorders, possibly leading to finding the genetic correlates of SES and stressful life events in GWASs of disorders. Consequently, some of the genetic associations with physiological and mental health disorders may be modified by public policy that affects SES and the experience of stressful life events. Thus, other than shedding light on findings from GWASs, the understanding of rGET processes may have important implications for public health.
Keywords: Gene-environment correlation (rGE), pleiotropy, single nucleotide polymorphism (SNP), genome-wide association study, polygenic risk scores, mediated pleiotropy, environment
Nearly two decades ago Turkheimer (2000) proposed three laws of behavior genetics, the first being that “All human behavioral traits are heritable”. This assertion relied on family designs, which consistently showed that the heritability of various psychological traits is different from zero. Family designs, such as twin and adoption studies, have been the major source of heritability estimations, taking advantage of varying degrees of relatedness to partition phenotypic variation into genetic\heritable, shared environment, and non-shard environment components (Plomin, DeFries, McClearn, & McGuffin, 2008). A recent meta-analysis of all twin studies found that, on average, the heritability of traits, from anthropometric measurements to mental disorders, is 49% (Polderman et al., 2015).
As genetic methods became more sophisticated and less costly, genome wide association studies (GWASs) became more prevalent. GWASs are hypothesis-free analyses that assess associations between a phenotype of interest and a large number of common genetic variations (hundreds of thousands to millions of single nucleotide polymorphisms; SNPs), and they have been used to try and uncover the specific genetic markers that account for quantitative heritability estimates. The findings from GWASs have added another level of intricacy to our understanding of complex traits by showing that: 1) each trait is polygenic, influenced by many genetic loci with very small effect sizes, thus necessitating very large sample sizes to gain the statistical power needed to correctly assess the contribution of each variation to the selected trait. This has been suggested as the fourth law of behavior genetics (Chabris, Lee, Cesarini, Benjamin, & Laibson, 2015); and 2) many genetic variations are pleiotropic, affecting more than just a single trait (Boyle, Li, & Pritchard, 2017).
The Various Faces of Pleiotropy
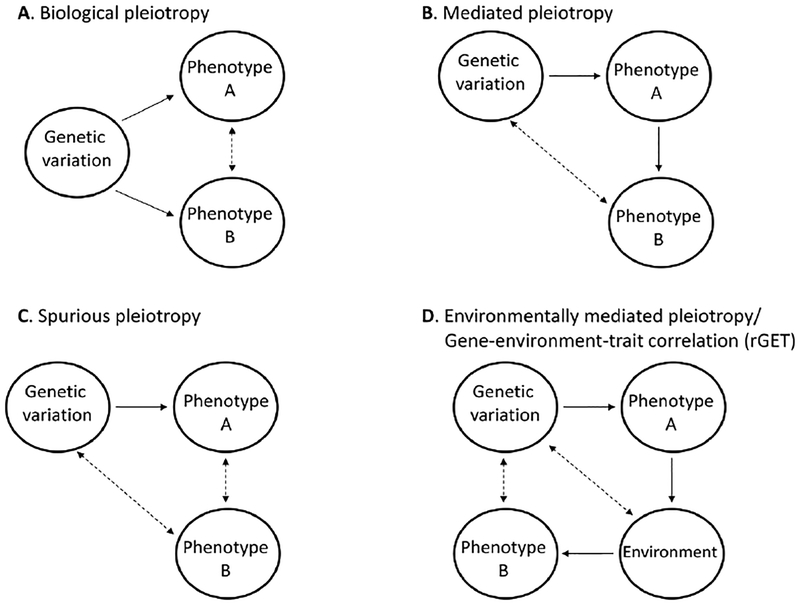
Accumulating evidence shows that genetic correlations between traits and diseases are widespread (Bulik-Sullivan et al., 2015). In other words, shared genetic etiology between phenotypes is common. Three different non-mutually exclusive processes have been suggested to underlie these cross-phenotype genetic associations (Solovieff, Cotsapas, Lee, Purcell, & Smoller, 2013): biological pleiotropy (figure 1A), where a genetic variant or gene directly affects more than one phenotype; mediated pleiotropy (figure 1B), where the genetic effect on one phenotype is mediated by an effect on another phenotype, meaning that controlling for the first phenotype will decrease the association with the second; and spurious pleiotropy (figure 1C), where a genetic variant is associated with multiple phenotypes due to bias originating in the study’s design and/or methods. For example, due to linkage disequilibrium, defined as the non-random correlation between genetic variants, one genetic variant may correlate with two phenotypes, but only be causally related to one.
Figure 1. Types of pleiotropy.
Biological pleiotropy (A), where a genetic variant or gene directly affects more than one phenotype; mediated pleiotropy (B), where the genetic effect on one phenotype is mediated by an effect on another phenotype; spurious pleiotropy (C), where a genetic variant is associated with multiple phenotypes due to bias originating in the study’s design and/or methods; environmentally mediated pleiotropy/gene-environment-trait correlation (D), where a genetically influenced phenotype shapes an environment that in turn affects an additional phenotype.
Note. Dashed double arrows depict correlations, while one directional arrows depict causal pathways.
Notably, when mediated pleiotropy is discussed, it is usually in the context of one trait that mediates a genetic effect on another trait, such as the association between a genetic locus on chromosome 15 and both nicotine dependence and lung cancer (genetic variant-->nicotine dependence-->lung cancer; e.g., Gage, Smith, Ware, Flint, & Munafò, 2016; Salinas et al., 2017; Solovieff et al., 2013). Another form of mediated pleiotropy, that has been largely overlooked, is environmentally mediated pleiotropy (figure 1D), where a genetic variation influences a trait that in turn affects another trait through a mediating environment, i.e., an individual’s surroundings (genetic variation→Strait A→environment→trait B). Environmentally mediated pleiotropy stems from the somewhat surprising find that environmental measures, such as parenting and characteristics of friends, are heritable (Kendler & Baker, 2007), due to processes that have been termed “gene-environment correlations”.
Gene-Environment Correlations (rGE)
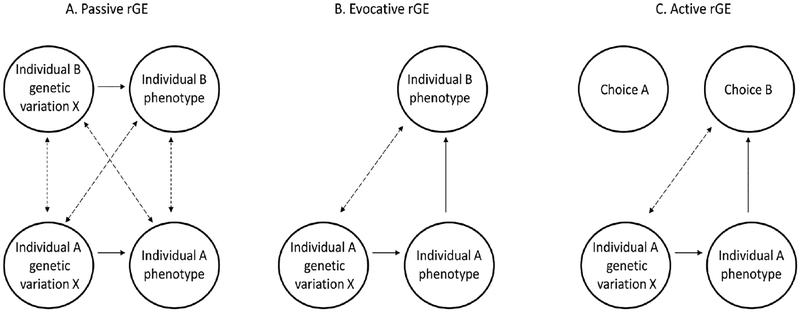
Gene-environment correlations (Plomin, DeFries, & Loehlin, 1977; Scarr & McCartney, 1983) are passive, evocative, and active processes that create associations between an individual’s genotype and the environment, consequently leading to nonrandom exposures to the environment. Passive rGE (figure 2A) can arise when two individuals share genetic variation (e.g., family members) and one provides an environment to the other. For example, the genes that affect parental depression can pass on to the child and affect their conduct problems. This will create a spurious correlation between the phenotypes, that does not depend on a causal effect of the environment (parental depression) on the child’s behavior (conduct problems) or vice versa. Evocative rGE (figure 2B) refers to the reactions an individual’s genetically influenced behavior can evoke from others. For example, genetic influences on a child’s self-control may correlate with parental warmth, because children with higher self-control may elicit higher warmth in parents. Active rGE (figure 2C) refers to instances in which individuals choose their environment (e.g. friends, activities) based on genetically influenced traits. Environmentally mediated pleiotropy - whereby genetic influences on a specific trait predispose an individual to a specific environment, which in turn affects an additional trait - can be viewed as an extension of rGE -- a gene-environment-trait correlation.
Figure 2. Types of gene-environment correlations (rGE).
Passive rGE (A), occurs when two individuals share genetic variation (e.g., family members) and one provides an environment to the other; evocative rGE (B), occurs when an individual’s genetically influenced behavior evokes certain responses from others; and active rGE (C), occurs when individuals choose their environment (e.g. friends, activities) based on genetically influenced traits.
Note. Dashed double arrows depict correlations, while one directional arrows depict causal pathways. Adapted from (Avinun & Knafo-Noam, 2017)
It has been theorized that during development, as individuals gain independence and agency, evocative and active rGE will increase (Scarr & McCartney, 1983). Put differently, it is likely that as individuals grow older they will become more able to shape and select their environment, so that it will be in accordance with their inherent genetic tendencies. This is supported by studies showing that heritability estimates of various traits increase with age (Briley & Tucker-Drob, 2013; Knafo & Plomin, 2006; Knafo, Zahn-Waxler, Van Hulle, Robinson, & Rhee, 2008). Indeed, evocative rGE has been shown to increase during development (Avinun & Knafo, 2014), and accordingly, a close inspection of the increase in the heritability of cognitive ability across development revealed that the underlying explanation was an amplification of early genetic influences, and not new genetic influences that arise with age (Briley & Tucker-Drob, 2013). Thus evocative and active rGE, and consequently gene-environment-trait correlations, are especially relevant during adulthood, the developmental period that is targeted by most GWASs.
Gene-Environment-Trait Correlations (rGET) May Be Widespread
Many environmental measures, that are thought to meaningfully contribute to individual differences in various physiological and psychological traits, have been shown to be genetically influenced (Kendler & Baker, 2007), including parenting (which is an environment for the child and a behavior of the parent; Avinun & Knafo, 2014), socioeconomic status (SES; Hill et al., 2016), and stressful life events (Power et al., 2013). For example, parenting has been associated with prosocial behavior (Carlo, Mestre, Samper, Tur, & Armenta, 2011), callous-unemotional traits (Waller et al., 2014), self-regulation (Eiden, Colder, Edwards, & Leonard, 2009), delinquent and antisocial behavior, and depression (Gershoff, 2002); SES has been associated with physiological diseases such as diabetes (Everson, Maty, Lynch, & Kaplan, 2002) and cardiovascular and respiratory diseases (Galobardes, Lynch, & Davey Smith, 2004), and with mental health disorders, such as depression (Everson et al., 2002) and schizophrenia (Werner, Malaspina, & Rabinowitz, 2007); stressful life events have been associated with cardiovascular diseases, diabetes, autoimmune disorders, depression, and anxiety (Goodwin & Stein, 2004; Mersky, Topitzes, & Reynolds, 2013). The pervasive impact of these genetically influenced environments may explain some of the observed genetic associations between traits and diseases.
SES as an Environment Mediating rGET
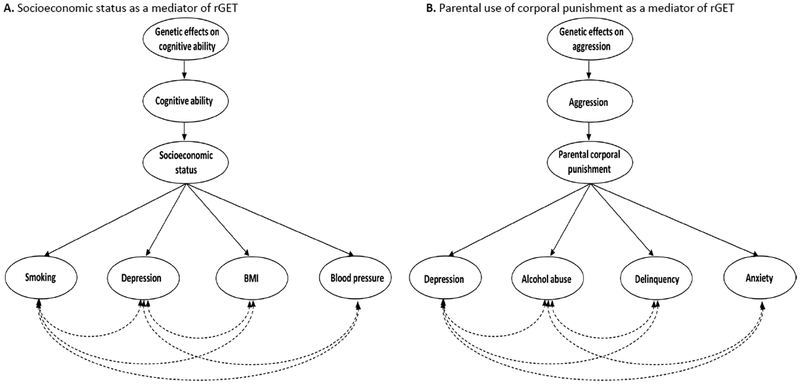
The above prediction is supported by a GWAS of SES, as assessed by the social deprivation index, which showed that it is genetically correlated with numerous traits such as coronary artery disease, systolic and diastolic blood pressure, smoking, obesity, depression, and schizophrenia (Hill et al., 2016). The genetic effects on SES likely originate in a genetically influenced phenotype such as intelligence (Hill et al., 2018) and/or self-control (Beaver, Wright, DeLisi, & Vaughn, 2008). In other words, there is a possible rGET, where cognitive ability and/or self-control affect SES, which in turn affects various disorders, traits, and behaviors (Figure 3A). Accordingly, cognitive ability has been shown to genetically correlate with several of the phenotypes that were shown to correlate with SES, including vascular-metabolic diseases and psychiatric disorders (Hagenaars et al., 2016).
Figure 3. Gene-Environment-Trait correlation, theoretical examples.
Note. The associations between the various outcomes, the first phenotype and the genetic influences on the first phenotype are not shown, to make the figure easier to follow. Dashed double arrows depict genetic correlations, while one directional arrows depict causal pathways.
Educational attainment, assessed as the number of completed schooling years, has been used in GWASs as a proxy for cognitive ability and intelligence, because it can be easily measured and it can be obtained for very large samples, the most recent being 1.1 million individuals (Lee et al., 2018). As mentioned above, a large samples is an advantage due to the polygenic nature of these traits. As more individuals are included, the likelier it is that most of the common genetic variation relevant to the trait will be correctly estimated. Indeed the large sample improved the findings, and currently an educational attainment polygenic score can explain 11% of the variance in educational attainment (Lee et al., 2018). Consequently, the educational attainment polygenic score is considered to be one of the most powerful polygenic scores in psychology, and it has been widely used in research, demonstrating associations between the genetic influences on educational attainment and other phenotypes, such as longevity of parents (Marioni et al., 2016), schizophrenia (both positive and negative associations were found; Bansal et al., 2018), and cardiovascular diseases (Hagenaars et al., 2016). When these associations are examined in light of both the phenotypic (Shavers, 2007) and genetic (Krapohl & Plomin, 2016) links between educational attainment and SES, they are rather unsurprising.
Corporal Punishment as an Environment Mediating rGET
Another example of a possible environment that mediates rGET is parental use of corporal punishment (figure 3B). The use of corporal punishment by parents has been associated with depression, aggression, delinquent and antisocial behavior, and alcohol abuse in children (Gershoff, 2002), and studies have shown that it is affected by children’s genetically influenced tendencies, i.e., that evocative rGE processes are at play (Avinun, Davidov, Mankuta, & Knafo-Noam, 2018; Jaffee et al., 2004). Specifically, parents may use corporal punishment more with aggressive children (Jaffee et al., 2004). Consequently, genetic variants linked to aggression may be found in GWASs of phenotypes that have been associated with corporal punishment, such as depression and alcohol abuse, and contribute to the genetic correlations between these phenotypes. Corporal punishment is relatively prevalent in the US and across Europe (Keyes et al., 2015; Schneider, MacKenzie, Waldfogel, & Brooks-Gunn, 2015), but laws against it have been proven useful (Durrant, 2000). As a result, it is possible that genetic correlations between psychiatric diseases will diminish once additional countries adopt and enforce corporal punishment bans.
rGET May Contribute to the General Psychopathology Factor
The co-occurrence, or comorbidity, of mental disorders is prevalent. About 40% of the individuals with one class of disorders (e.g., mood, anxiety, substance abuse) are likely to be diagnosed with another (Merikangas et al., 2010; Newman, Moffitt, Caspi, & Silva, 1998). These correlations between the various mental disorders were explained by higher order latent factors, including externalizing, internalizing and thought disorders, which also correlated, leading researchers to find a general psychopathology factor (Kotov et al., 2017). This shared variance across disorders, which is thought to mark the risk of an individual for developing psychopathologies, has been termed the p factor (Caspi et al., 2014). Interestingly, genetic correlations also emerged between the various psychiatric disorders (Selzam, Coleman, Caspi, Moffitt, & Plomin, 2018), indicating that they share a genetic etiology. Mediated pleiotropy was raised by Selzam and colleagues (2018) as a possible explanation for the genetic correlations between psychopathologies: “An alternative explanation is mediated pleiotropy, in which comorbidity occurs because DNA variants increase risk for one disorder, and then this disorder causes other disorders in turn” (p. 7). However, another possible mediation is through the environment, for example, through stressful life events. Similarly to the p factor, stressful life events have also been associated with internalizing, externalizing, and thought disorders (Carr, Martins, Stingel, Lemgruber, & Juruena, 2013). Consequently, the genetic variants that affect stressful life events could contribute to the genetic variance shared between psychopathologies.
Which genetically influenced traits can affect stressful life events?
The individual selects environments, makes choices, and evokes responses that correspond with his/her genetic tendencies (i.e., active and evocative rGE). Personality and socially undesirable traits may affect our experience of stressful life events. Indeed, personality has been found to partly mediate the genetic effects on stressful life events (Kandler, Bleidorn, Riemann, Angleitner, & Spinath, 2012). Personality can influence stressful life events by affecting an individual’s proactivity, risk seeking, openness to experience, and social circle. As personality has been shown to correlate with the p factor (Caspi et al., 2014), the following rGET is possible: genetic influences on personality affect the experience of stressful life events, which in turn affects multiple mental disorders, contributing to a shared variance between these disorders, i.e., the p factor (genetic influences on personality → personality → stressful life events → various mental disorders). Notably, mediated pleiotropy (i.e., one phenotype to another) and rGET are not mutually exclusive, and both may contribute to the observed association between personality and the p factor.
Stressful life events can also arise due to traits that evoke negative responses from the environment. For instance, as a consequence of weight-related stigma, obese and overweight individuals suffer from bullying, teasing, and shaming (Puhl & Latner, 2007). In accordance with the high heritability of obesity (Haberstick et al., 2010), an evocative rGE has been shown, where a genetic risk for high body mass index (BMI) predicted higher early life stress (Avinun & Hariri, 2019). Notably, obesity has been associated with various conditions including insulin resistance and type-2 diabetes (Kahn, Hull, & Utzschneider, 2006), cardiovascular diseases (Twig et al., 2016), and internalizing, externalizing and thought disorders (Ter Bogt et al., 2006). This suggest the possibility of an rGET in which genetic influences on BMI → BMI → early life stress → various mental disorders/p factor. Relatedly, Avinun and Hariri (2019) also showed that the genetic risk for higher BMI predicted higher depressive symptoms via elevated levels of early life stress. Thus, interventions that aim to reduce weight-related stigma may be able to attenuate the association between BMI and mental and physical health, and consequently also mitigate the genetic correlations between them.
The Studied Population
Distinguishing between mediated pleiotropy and rGET highlights the possible role of the environment in “spreading” genetic influences. As the above examples imply, genetic association may differ based on the culture, norms and context of the studied population. Family studies have shown that heritability estimations can change between populations, depending on whether the environment suppresses or nurtures the individual’s genetic tendencies (e.g., Boardman, 2009; Tucker-Drob & Bates, 2016), a gene-environment interaction. Yet, not only the magnitude of the heritability can change between populations, but also the genetic variants involved. For example, building on the BMI and bullying example above, in a society with no bias toward obese individuals, the genetic links between depression and obesity may be much smaller. Put differently, if the environment mediates some genetic influences and GWASs detect these indirect effects, then the specific genetic associations found in each GWAS and the magnitude of these associations will be population-dependent. This can affect replication attempts and hinder our etiological understanding of phenotypes.
The Complexity of Human Behavior
Family studies that enabled the partitioning of the phenotypic variance into genetic and environmental influences created the illusion that the two can be easily separated. However, as Turkheimer (2000) mentioned in his seminal paper on the three laws of behavior genetics: “Everything is interactive”. From the moment of conception genetic variants interact with other genetic variants and with the environment, so that main effects of specific genetic variations are highly unlikely. Gene-environment correlations, gene-environment-trait correlations, and gene-environment interactions, should be a constant reminder that genetic and environmental effects are intertwined.
Notably, there are additional levels of complexity. For example, the environment can moderate rGE. Child aggressive behavior may evoke the use of corporal punishment by parents, but whether or not it will do so depends on context, such as culture (Avinun et al., 2018). Genetic correlations between phenotypes can also be moderated by the environment ((rG)xE; Wedow et al., 2018). The genetic correlation (rG) between educational attainment and smoking has been shown to change based on year of birth (Wedow et al., 2018). Notably, even though the term “environment” in rGE is usually used to describe the social environment, genes may also affect environments such as the gut microbiome (Kurilshikov, Wijmenga, Fu, & Zhernakova, 2017) and the hostility of mosquitoes (Fernández-Grandon, Gezan, Armour, Pickett, & Logan, 2015), which in turn may also affect susceptibility to diseases. Additionally, the environment can also affect gene expression through epigenetic processes (i.e., biological changes that can affect gene expression without changes to the DNA sequence), such as méthylation (Avinun & Knafo-Noam, 2014). All of these create challenges for the estimation of genetic influences and for the correct identification of the directly involved genetic variants. The statistical methods that attempt to deal with these challenges have been discussed elsewhere (e.g., Avinun & Knafo-Noam, 2014; Gage et al., 2016; Hackinger & Zeggini, 2017; Pingault et al., 2018; Salinas et al., 2017).
Conclusions
Because measured phenotypes are the result of an intricate web of genetic and environmental influences, results from GWASs include both direct and indirect genetic effects and the distinction between genetic and environmental becomes blurred. As the sample sizes of GWASs increase and with it the power to identify single nucleotide polymorphisms (SNPs) with miniscule effect sizes, the detection of SNPs that relate to rGET processes becomes more likely. Identifying rGET can have at least four important implications: 1) advance our understanding of the etiology of phenotypes; 2) partly explain the prevalence of pleiotropy and polygenicity; 3) shed light on the context in which certain genetic associations are more likely to replicate; and 4) highlight specific environments as potential targets for interventions that can have broad effects on public health.
ACKNOWLEDGMENTS AND DISCLOSURE
I would like to thank Maxwell Elliott, Megan Cooke, and Salomon Israel for commenting on earlier versions of this manuscript. RA received partial support from US-National Institutes of Health grant R01AG049789 and from the Jerusalem Brain Community (JBC).
References
- Avinun R, Davidov M, Mankuta D, & Knafo-Noam A (2018). Predicting the use of corporal punishment: Child aggression, parent religiosity, and the BDNF gene. Aggressive behavior, 44(2), 165–175. [DOI] [PubMed] [Google Scholar]
- Avinun R, & Hariri AR (2019). A Polygenic Score for Body Mass Index is Associated with Depressive Symptoms via Early Life Stress: Evidence for Gene-Environment Correlation. bioRxiv. doi: 10.1101/536938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avinun R, & Knafo-Noam A (2014). Socialization, Genetics and their Interplay in Development In Grusec JE & Hastings PD (Eds.), Handbook of Socialization: Theory and research (2 ed., pp. 347–371): Guilford Press. [Google Scholar]
- Avinun R, & Knafo-Noam A (2017). Behavior genetics In Hopkins B, Geangu E& Linkenauger S(Eds.), The Cambridge Encyclopedia of Child Development (Second ed., pp. 758–763): Cambridge University Press. [Google Scholar]
- Avinun R, & Knafo A (2014). Parenting as a Reaction Evoked by Children’s Genotype A Meta-Analysis of Children-as-Twins Studies. Personality and Social Psychology Review, 18(1), 87–102. [DOI] [PubMed] [Google Scholar]
- Bansal V, Mitjans M, Burik CA, Linner RK, Okbay A, Rietveld CA, … de Vlaming R (2018). Genome-wide association study results for educational attainment aid in identifying genetic heterogeneity of schizophrenia. Nature Communications, 9. doi: 10.1038/s41467-018-05510-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver KM, Wright JP, DeLisi M, & Vaughn MG (2008). Genetic influences on the stability of low self-control: Results from a longitudinal sample of twins. Journal of criminal justice, 36(6), 478–485. [Google Scholar]
- Boardman JD (2009). State-level moderation of genetic tendencies to smoke. American Journal of Public Health, 99(3), 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle EA, Li YI, & Pritchard JK (2017). An expanded view of complex traits: from polygenic to omnigenic. Cell, 169(7), 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA, & Tucker-Drob EM (2013). Explaining the Increasing Heritability of Cognitive Ability Across Development A Meta-Analysis of Longitudinal Twin and Adoption Studies. Psychological Science, 24(9), 1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, … Robinson EB (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47(11), 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo G, Mestre MV, Samper P, Tur A, & Armenta BE (2011). The longitudinal relations among dimensions of parenting styles, sympathy, prosocial moral reasoning, and prosocial behaviors. International Journal of Behavioral Development, 35(2), 116–124. [Google Scholar]
- Carr CP, Martins CMS, Stingel AM, Lemgruber VB, & Juruena MF (2013). The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. The Journal of nervous and mental disease, 201(12), 1007–1020. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Poulton R (2014). The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2(2), 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris CF, Lee JJ, Cesarini D, Benjamin DJ, & Laibson DI (2015). The fourth law of behavior genetics. Current Directions in Psychological Science, 24(4), 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JE (2000). Trends in youth crime and well-being since the abolition of corporal punishment in Sweden. Youth & Society, 31(4), 437–455. [Google Scholar]
- Everson SA, Maty SC, Lynch JW, & Kaplan GA (2002). Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. Journal of Psychosomatic Research, 53(4), 891–895. [DOI] [PubMed] [Google Scholar]
- Eiden RD, Colder C, Edwards EP, & Leonard KE (2009). A longitudinal study of social competence among children of alcoholic and nonalcoholic parents: Role of parental psychopathology, parental warmth, and self-regulation. Psychology of Addictive Behaviors, 23(1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Grandon GM, Gezan SA, Armour JA, Pickett JA, & Logan JG (2015). Heritability of attractiveness to mosquitoes. PLoS ONE, 10(4), e0122716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Smith GD, Ware JJ, Flint J, & Munafò MR (2016). G=E: What GWAS can tell us about the environment. PLoS Genetics, 12(2), el005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, & Davey Smith G (2004). Childhood socioeconomic circumstances and cause-specific mortality in adulthood: systematic review and interpretation. Epidemiologic Reviews, 26(1), 7–21. [DOI] [PubMed] [Google Scholar]
- Gershoff ET (2002). Corporal punishment by parents and associated child behaviors and experiences: a meta-analytic and theoretical review. Psychological Bulletin, 128(4), 539–579. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, & Stein MB (2004). Association between childhood trauma and physical disorders among adults in the United States. Psychological Medicine, 34(3), 509–520. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, McQueen MB, Boardman JD, Hopfer CJ, Smolen A, & Hewitt JK (2010). Stable genes and changing environments: body mass index across adolescence and young adulthood. Behavior Genetics, 40(4), 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackinger S, & Zeggini E (2017). Statistical methods to detect pleiotropy in human complex traits. Open biology, 7(11), 170125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DC, Ritchie SJ, … Malik R (2016). Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N= 112 151) and 24 GWAS consortia. Molecular Psychiatry, 21(11), 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Arslan RC, Xia C, Luciano M, Amador C, Navarro P,… McIntosh AM (2018). Genomic analysis of family data reveals additional genetic effects on intelligence and personality. Molecular Psychiatry, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Hagenaars SP, Marioni RE, Harris SE, Liewald DC, Davies G, … Deary IJ (2016). Molecular genetic contributions to social deprivation and household income in UK Biobank. Current Biology, 26(22), 3083–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Polo-Tomas M, Price TS, & Taylor A (2004). The limits of child effects: Evidence for genetically mediated child effects on corporal punishment but not on physical maltreatment. Developmental Psychology, 40(6), 1047–1057. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, & Utzschneider KM (2006). Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 444(7121), 840. [DOI] [PubMed] [Google Scholar]
- Kandler C, Bleidorn W, Riemann R, Angleitner A, & Spinath FM (2012). Life events as environmental states and genetic traits and the role of personality: A longitudinal twin study. Behavior Genetics, 42(1), 57–72. [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Baker JH (2007). Genetic influences on measures of the environment: a systematic review. Psychological Medicine, 37(5), 615–626. [DOI] [PubMed] [Google Scholar]
- Keyes K, Leray E, Pez O, Bitfoi A, Koç C, Goelitz D, … Otten R (2015). Parental use of corporal punishment in Europe: intersection between public health and policy. PLoS ONE, 10(2), e0118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo A, & Plomin R (2006). Prosocial behavior from early to middle childhood: genetic and environmental influences on stability and change. Developmental Psychology, 42(5), 771–786. doi: 10.1037/0012-1649.42.5.771 [DOI] [PubMed] [Google Scholar]
- Knafo A, Zahn-Waxler C, Van Hulle C, Robinson JL, & Rhee SH (2008). The developmental origins of a disposition toward empathy: Genetic and environmental contributions. Emotion, 8(6), 737–752. doi: 10.1037/a0014179 [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger R, Watson D, Achenbach T, Althoff R, Bagby R, … Clark L (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126(4), 454. [DOI] [PubMed] [Google Scholar]
- Krapohl E, & Plomin R (2016). Genetic link between family socioeconomic status and children’s educational achievement estimated from genome-wide SNPs. Molecular Psychiatry, 21(3), 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilshikov A, Wijmenga C, Fu J, & Zhernakova A (2017). Host genetics and gut microbiome: challenges and perspectives. Trends in Immunology, 38(9), 633–647. [DOI] [PubMed] [Google Scholar]
- Lee J, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, … Karlsson LR (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics,50(8), 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Ritchie SJ, Joshi PK, Hagenaars SP, Okbay A, Fischer K, … Nagy R (2016). Genetic variants linked to education predict longevity. Proceedings of the National Academy of Sciences, 113(47), 13366–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J. p., Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersky JP, Topitzes J, & Reynolds AJ (2013). Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the US. Child Abuse and Neglect, 37(11), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman DL, Moffitt TE, Caspi A, & Silva PA (1998). Comorbid mental disorders: implications for treatment and sample selection. Journal of Abnormal Psychology, 107(2), 305. [DOI] [PubMed] [Google Scholar]
- Pingault J-B, O’reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, & Dudbridge F (2018). Using genetic data to strengthen causal inference in observational research. Nature Reviews Genetics, 1. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries J, McClearn G, & McGuffin P (2008). Behavioral genetics (Vol. 5). New York: Worth. [Google Scholar]
- Plomin R, DeFries JC, & Loehlin JC (1977). Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin, 84(2), 309–322. [PubMed] [Google Scholar]
- Polderman TJ, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM, & Posthuma D (2015). Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nature Genetics, 47(7), 702. [DOI] [PubMed] [Google Scholar]
- Power R, Wingenbach T, Cohen-Woods S, Uher R, Ng M, Butler A, … Korszun A (2013). Estimating the heritability of reporting stressful life events captured by common genetic variants. Psychological Medicine, 43(9), 1965–1971. [DOI] [PubMed] [Google Scholar]
- Puhl RM, & Latner JD (2007). Stigma, obesity, and the health of the nation’s children. Psychological Bulletin, 133(4), 557. [DOI] [PubMed] [Google Scholar]
- Salinas YD, Wang Z, & DeWan AT (2017). Statistical Analysis of Multiple Phenotypes in Genetic Epidemiologic Studies: From Cross-Phenotype Associations to Pleiotropy. American Journal of Epidemiology, 187(4), 855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, & McCartney K (1983). How people make their own environments: A theory of genotype environment effects. Child Development, 54(2), 424–435. [DOI] [PubMed] [Google Scholar]
- Schneider W, Mackenzie M, Waldfogel J, & Brooks-Gunn J (2015). Parent and child reporting of corporal punishment: New evidence from the fragile families and child wellbeing study. Child Indicators Research, 8(2), 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzam S, Coleman JR, Caspi A, Moffitt TE, & Plomin R (2018). A polygenic p factor for major psychiatric disorders. Translational Psychiatry, 8(1), 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers VL (2007). Measurement of socioeconomic status in health disparities research. Journal of the National Medical Association, 99(9), 1013. [PMC free article] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, & Smoller JW (2013). Pleiotropy in complex traits: challenges and strategies. Nature Reviews Genetics, 14(7), 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Bogt TF, van Dorsselaer SA, Monshouwer K, Verdurmen JE, Engels RC, & Vollebergh WA (2006). Body mass index and body weight perception as risk factors for internalizing and externalizing problem behavior among adolescents. Journal of Adolescent Health, 39(1), 27–34. [DOI] [PubMed] [Google Scholar]
- Tucker-Drob EM, & Bates TC (2016). Large cross-national differences in genex socioeconomic status interaction on intelligence. Psychological Science, 27(2), 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E (2000). Three laws of behavior genetics and what they mean. Current Directions in Psychological Science, 9(5), 160–164. [Google Scholar]
- Twig G, Yaniv G, Levine H, Leiba A, Goldberger N, Derazne E, … Shamiss A (2016). Body-mass index in 2.3 million adolescents and cardiovascular death in adulthood. New England Journal of Medicine, 374(25), 2430–2440. [DOI] [PubMed] [Google Scholar]
- Waller R, Gardner F, Viding E, Shaw DS, Dishion TJ, Wilson MN, & Hyde LW (2014). Bidirectional associations between parental warmth, callous unemotional behavior, and behavior problems in high-risk preschoolers. Journal of Abnormal Child Psychology, 42(8), 1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedow R, Zacher M, Huibregtse BM, Mullan Harris K, Domingue BW, & Boardman JD (2018). Education, smoking, and cohort change: Forwarding a multidimensional theory of the environmental moderation of genetic effects. American Sociological Review, 83(4), 802–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, Malaspina D, & Rabinowitz J (2007). Socioeconomic status at birth is associated with risk of schizophrenia: population-based multilevel study. Schizophrenia Bulletin, 33(6), 1373–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]