Abstract
Purpose
The purpose of this study was to develop an effective treatment method using poloxamers to restore and maintain physiological hydration in postmortem porcine and human corneas during ex vivo experimentation, and to compare corneal inflation response with or without treatment.
Materials and Methods
Corneal buttons obtained from whole globes (n=30 porcine, n=8 human) were treated with various concentrations of poloxamer 188 (P188, a synthetic macromolecule surfactant) for 24 hrs to identify the concentration that would return the cornea to near-physiological hydration (i.e. H=3.2). Whole globes (n=12 porcine, n=16 human) were also used to monitor central corneal thickness (CCT) during deswelling treatment. Inflation testing from 5 to 30 mmHg was performed in the porcine globes and a subset of human globes to characterize the mechanical response of the cornea after treatment.
Results
Physiological hydration was obtained after 24 hrs immersion in 3.25% P188 for porcine corneas and 4.25% P188 treatment for human corneas. CCT was stabilized and returned to physiological levels after 24 hrs of treatment in 3.25% P188 in porcine (891±66 μm) and 4.25% P188 in human (574±34 μm) whole globes. Corneal axial strains at 30 mmHg were significantly larger at physiological hydration than in swollen cornea in both porcine (−6.42%±1.50% vs. −3.64%±1.05%, p=0.004) and human (−2.85%±0.09% in vs. −1.53%±0.27%, p=0.031) eyes.
Conclusions
Our results suggest that P188 treatment was effective in restoring and maintaining near physiological corneal hydration during ex vivo testing, and hydration appeared to significantly impact corneal inflation response in both porcine and human eyes.
Keywords: corneal hydration, poloxamers, corneal deswelling, inflation testing, corneal strains
1. Introduction
The hydration of corneal stroma is primarily regulated by corneal endothelium cells that actively remove water to keep the cornea relatively dehydrated and transparent (1). Corneas swell within hours postmortem because of the diminished pumping capability of the endothelial cells. Abnormal hydration is also found in certain clinical conditions, such as contact lens wear (2), cataract surgery (3), or Fuch’s endothelial dystrophy (4). Change in hydration state not only changes corneal thickness, but also alters its microstructural and biomechanical properties. Previous studies have shown the influence of corneal hydration on collagen fibril organization and spacing (5, 6) and both tensile (7–10) and compressive (11–13) mechanical properties. Returning the cornea to its physiological hydration and maintaining that hydration during ex vivo characterization are thus important for reflecting the normal in vivo condition, and generating consistent results across different samples using different mechanical testing methods.
Various solutions have been used to de-swell postmortem corneas towards physiological hydration to reach the expected osmotic pressure of in vivo corneas (9, 13, 14). The most frequently used method is immersion in dextran solutions at various concentrations (i.e. 8–25%) (8, 13–16). Dextran solutions at these concentrations are highly viscous, potentially interfering with experiments involving rapid fluid flow, tissue motion, or microscopic visualization of the cellular layers. Alternative treatment solutions include Optisol (13, 17, 18), polyethylene glycol (5, 19), hydroperoxyl methylcellulose (20, 21), and mineral oil (9, 22). These solutions reduce further swelling during aqueous immersion but are less effective in deswelling the cornea and returning it to the physiological hydration state. There remains a need to develop an efficient and widely adoptable method to restore postmortem corneas to their physiological hydration and maintain that hydration during several hours of ex vivo experimentation.
Poloxamers were proposed as an alternative to dextran for corneal preservation during eye banking due to their low toxicity to endothelial cells (23, 24). Poloxamers are surfactants that exert a strong osmotic colloidal pressure when in solution. Previous studies have shown that they produce a significant dehydrating effect on postmortem corneas, but are not suitable for eye banking because of excessive dehydration after days of storage (25). The use of poloxamers’ deswelling effects for ex vivo experimentation that typically lasts minutes to hours has not been reported. Poloxamer solutions are easy to prepare, not viscous, and can potentially be a convenient method adoptable in different types of ex vivo corneal experimentation. The purpose of this study was to develop a protocol using poloxamer immersion to return postmortem porcine and human corneas to physiological hydration and determine the optimal poloxamer concentrations and treatment duration. Poloxamer treatment of the corneas in whole globes was also tested and the inflation response of treated and untreated corneas were compared.
2. Methods
a. Corneal hydration after poloxamer treatment
Thirty porcine globes were obtained from a USDA-approved abattoir under HACCP guidelines (SiouxPreme Packing Co., Sioux City, IA) and were stored at 4°C in 0.9% saline during transport and before testing. Chemical-grade poloxamer 188 (P188, CAS #9003–11-6), which demonstrated high efficiency in dehydrating corneas (24), was obtained from Sigma-Aldrich (Kolliphor® P 188, St. Louis, MO). Cornea buttons with a scleral ring (approximately 1–2 mm) were excised from whole globes using a scalpel and surgical scissors. The thirty porcine corneal buttons were divided into five groups (n=6 per group) and treated with a range of P188 concentrations in order to identify the concentration that returns the cornea to physiological hydration within 24 hrs. In the four treatment groups (n=6 per group), corneal buttons were immersed for 24 hrs in 500 mL of one of the four concentrations of P188: 2.5%, 3.0%, 3.5%, or 4.0%. After the treatment period, a trephine was used to dissect out the central 9 mm of cornea (i.e. a corneal disk). In another group (n=6), corneal disks were trephinated without any treatment. Corneal disks were weighed using an electronic balance with 0.1 mg accuracy (Analytical Balance W3100-120, Accuris Instruments, Edison, NJ) immediately after trephination (the “wet” weight) and after drying in a 60° C oven for three days (the “dry” weight). Corneal hydration H was obtained using the following formula (26):
| (Eq. 1) |
Eight eyes from six human donors with no known corneal diseases were obtained from a local eye bank (Lions Eye Bank of West Central Ohio, Dayton, OH), transported at 4° C in a sealed moist container, and tested within 48 hrs postmortem. Human corneal buttons (8 in total) with scleral rings were obtained from two groups of whole globes treated in P188 solutions at concentrations of either 3.5% or 4.25% (n=4 in each treatment group). Corneal disks were then dissected as described above, except that a 7 mm trephine was used due to the smaller diameter of human cornea. Hydration was obtained and calculated as described above. All laboratory personnel completed blood-borne pathogen training and followed the laboratory safety and ethical guidelines for handling human and animal tissue.
b. Corneal thickness change in treated whole globes
Twelve porcine globes were divided into two groups to evaluate central corneal thickness (CCT) change with P188 treatment in whole globes. In the treated group (n=6), globes were placed in a custom-built holder using two spinal needles inserted at the equator of the eye. Two 20G needles were inserted into the anterior chamber, and the anterior chamber fluid was exchanged with 3.25% P188 solution. Globes were then pressurized to 15 mmHg via a column filled with 3.25% P188 solution and immersed in 3.25% P188 solution. The globes were placed in the refrigerator at 4° C during treatment. The cornea was imaged using a high-frequency ultrasound system (MS700 probe, Vevo 2100, FujiFilm VisualSonics Inc., Toronto) and CCT was measured with an ultrasound pachymeter (Pachette 2, DHG Technologies, Exton, PA) at 3 hr increments either from the onset of treatment or after overnight immersion. CCT was considered stable after consecutive measurements were within 10 μm. In the untreated group (n=6), porcine globes were immersed in and infused with 0.9% saline. CCT in porcine globes was monitored for 18 to 24 hrs from the onset of treatment, until consecutive measurements were within 10 μm.
Similar whole globe treatment was performed in 16 human donor globes (from 12 donors) with no known corneal diseases and tested within 48 hrs postmortem. Upon receipt, globes were secured to a custom-built holder and treated with either 3.5% P188 (n=8) or 4.25% P188 (n=8) solution at an IOP of 15 mmHg at 4° C until CCT stabilized as measured using ultrasound pachymetry. Human corneas were considered stable after consecutive CCT measurements were within 5 μm. CCT in human globes was monitored for 18 to 24 hrs from the onset of treatment, until consecutive measurements were within 5 μm.
c. Whole globe inflation tests
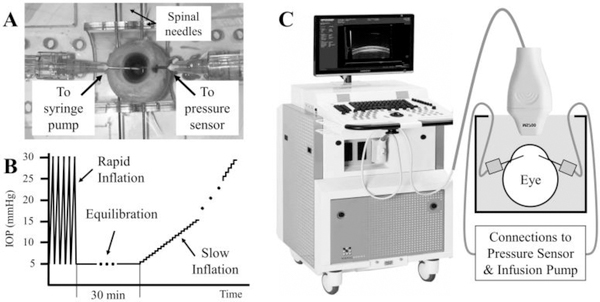
A subset of the P188-treated porcine and human donor globes with stabilized CCT were brought to room temperature for inflation testing (the rest were used for other experiments not included in the present study). The needles already inserted in the anterior chamber of the globes were connected to a programmable syringe pump (PhD Ultra, Harvard Apparatus, Boston, MA) and a pressure sensor (P75 venous pressure transducer, Harvard Apparatus) (Fig. 1A). Inflation tests at two different rates of pressure were performed in each eye: 1) a rapid inflation from 5 to 30 mmHg in 2 seconds; and 2) a slow inflation from 5 to 30 mmHg in about 1200 seconds in porcine eyes and 1500 seconds in human donor eyes. Specifically, the rapid inflation was achieved by automated infusion pump control of 10 cycles where IOP was continuously increased from 5 to 30 mmHg and then returned to 5 mmHg within 4 seconds (Fig. 1B). Corneal response at the last two cycles were acquired and analyzed without relaxation time between the cycles, since a previous study showed minimal preconditioning effects in planar tissues such as the cornea during inflation tests (27). Ultrasound radiofrequency data of B-mode frames of the central 9.73 mm of cornea were acquired during the last two cycles at a frame rate of 75 frames per second (Fig. 1C). The slow inflation was implemented as pressure increments of 0.5 mmHg at 30 second intervals from 5 to 20 mmHg, with the same increments from 20 to 30 mmHg for human eyes and 1 mmHg increments from 20 to 30 mmHg for porcine eyes. Ultrasound radiofrequency data from the same corneal cross-section were acquired at each pressure step at least 20 seconds after pressure change. Between the rapid and slow inflation tests, the globes were equilibrated for 15 minutes.
Figure 1.
A Pressure control and monitoring in a human donor globe. B Inflation protocol for whole globes, including five cycles of rapid inflation (data from the last 2 cycles were analyzed) and a slow inflation in the same eye with a 30-min equilibration between tests. C Experimental setup for ultrasound data acquisition.
Corneal strains were calculated using an ultrasound speckle tracking technique previously validated in our lab (28). Briefly, speckle tracking was performed using the radiofrequency data of ultrasound frames acquired at successive pressure levels. For rapid inflation tests, the pressure indexes of each frame were first identified and frames that are approximately 0.5 mmHg apart were used.
The radiofrequency data were sampled discretely with a pixel size of 1.5 μm vertically and 19 μm horizontally, where the vertical direction is the direction of ultrasound propagation and is oriented anatomically along the through-thickness direction of the cornea (i.e., from the anterior to posterior surface of the cornea), and the horizontal direction is perpendicular to ultrasound propagation and is oriented anatomically along the nasal-temporal meridian of the cornea.
A region of interest was defined in the frame acquired at 5 mmHg (i.e. the reference frame). Within this region, rectangular kernels of 51 × 41 pixels (axial × lateral, or approximately 75 μm × 780 μm) in size with a 50% overlap were defined. Each kernel was tracked individually between images acquired at consecutive IOP levels. A cross-correlation based algorithm was used to find the new location of each kernel in the next consecutive image (i.e. the deformed image) within a predefined search window, where the highest correlation coefficient was used as the new location of the kernel. Spline interpolation of the correlation coefficients was then applied to find the maximum correlation coefficient at sub-pixel resolution, thus achieving displacement identification at the sub-pixel level. To reduce noise in displacement data, outliers were identified as having a correlation coefficient below 0.8 or displacement differed by more than two standard deviation from the average of all kernels within the 5 × 5 neighborhood and these data points were replaced by the neighborhood average. Least squares strain estimations (28) in the axial (vertical) and lateral (horizontal) directions were used to obtain the respective strains for each kernel based on the displacement gradient in the 7 × 7 neighborhood of kernels. Strains averaged over all kernels within the region of interest were used for further analysis. The axial strain represents the gradient of displacements in the axial direction, while the lateral strain represents the gradient of the displacements in the lateral direction. Within the region of interest that is centered at the corneal apex, negative axial strain indicates through-thickness compression and positive lateral strain indicates in-plane stretch.
d. Statistical Analysis
Summary statistics, mean and standard deviation, were generated along with corresponding plots to demonstrate the changes in different groups at each test condition. Exploratory hypothesis testing was conducted using paired t-test for the same group under different test conditions and 2 sample t-tests for differences between groups.
3. Results
a. Corneal hydration after 24 hr treatment at different P188 concentrations
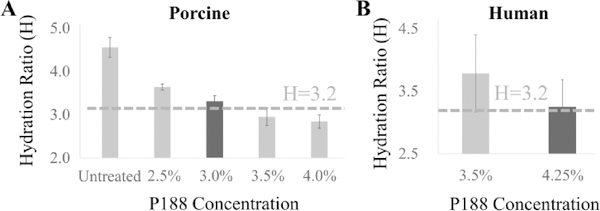
Hydration H measured in porcine corneal buttons after 24 hr treatment at different P188 concentrations are presented in Fig. 2A. H was 4.56±0.23 in untreated corneas and showed a larger decrease in corneas treated with higher P188 concentrations. A linear regression of hydration H vs. P188 concentration predicted that the porcine physiological hydration of H=3.2 (29) in porcine corneas would be obtained with P188 treatment at 3.25%, between the two tested concentrations of 3.0% and 3.5% and their respective H of 3.30±0.14 and 2.95±0.20.
Figure 2.
Hydration (H) obtained after P188 treatment of porcine (A, n=6 per group) and human (B, n=4 per group) corneal buttons.
Hydration H for human donor corneal buttons treated with two different P188 concentrations for 24 hrs is shown in Fig. 2B. The H values were 3.78±0.62 at 3.5% P188 treatment and 3.28±0.56 at 4.25% P188 treatment. The 4.25% P188 treatment appeared to return postmortem human corneas to near-physiological hydration of 3.2 (26, 29) (Fig. 2B).
b. CCT after P188 treatment in whole globes
P188 treatment in porcine and human donor globes (infusion into the anterior chamber and immersion of the whole globe) resulted in gradual decrease in CCT over time (Fig. 3). Both porcine and human donor globes showed an initial faster CCT decrease within the first 15 hrs (especially the first 6 hrs) of treatment, and a more stabilized CCT between 21 to 24 hrs. In some human eyes, the deswelling effect was diminished during the last 6 hours of treatment and the CCT started to increase slightly.
Figure 3.
CCT change over time during P188 treatment at cold in representative porcine and human whole globes.
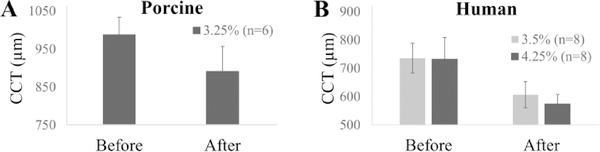
After 24 hr treatment with 3.25% P188 in porcine globes, CCT reduced from 987.8±45.9 μm (range: 903 to 1024 μm) to 891.8±65.6 μm (range: 799 to 956 μm) (Fig. 4A). The initial CCT in human donor eyes was 735.8±51.7 μm (range: 630 to 794 μm) in the 3.5% treatment group and 732.3±77.1 μm (range: 581 to 853 μm) in the 4.25% treatment group, and the CCT reduced to 606.7±37.6 μm (range: 549 to 697 μm) and 574.6±33.7 μm (range: 539 to 627 μm), respectively, after 24 hr treatment (Fig. 4B).
Figure 4.
Average CCT before and after 24 hr deswelling in P188 solutions in porcine (A) and human donor (B) globes.
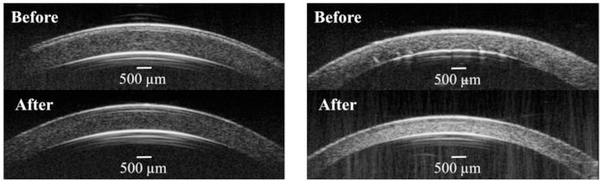
High-frequency ultrasound cross-sectional images of porcine and human corneas before and after treatment are shown in Figure 5. In human eyes, P188 treatment at both concentrations reduced and sometimes completely removed corneal posterior folds frequently observed in postmortem donor eyes.
Figure 5.
Representative B-mode ultrasound images before and after treatment in porcine (A) and human donor (B) globes. Note the reduction of posterior corneal folds in the human cornea after P188 treatment.
c. Whole globe inflation
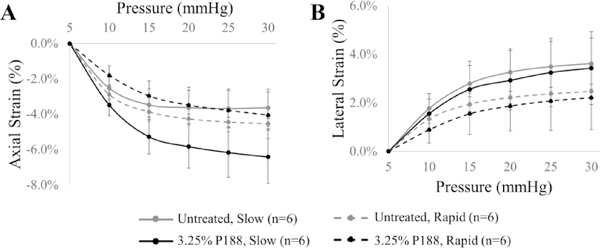
Corneal strains during slow and rapid inflation tests in porcine eyes are presented in Figure 6. Axial strains were compressive (negative) and significantly different between treated and untreated cornea at 10 and 15 mmHg during rapid inflation (p=0.004 and 0.035, respectively, paired t-tests, Fig. 6A), while strains were significantly different at all pressure levels during the slow inflation (all p’s<0.05, paired t-tests, Fig. 6A). For example, axial strain at 30 mmHg during slow inflation was −6.24±1.50% in the near-physiological hydration corneas and −3.64±1.05% in the untreated corneas (p=0.004). Lateral strains in porcine eyes were tensile (positive) and no significant differences were observed between treated and untreated corneas at all pressure levels during both slow and rapid inflation (all p’s>0.05, Fig. 6B). In P188-treated eyes, the axial strains were significantly different at all pressures between the two inflation speeds (all p’s<0.01), while lateral strains were not different. In the untreated eyes, the lateral strains were significantly different when comparing the two inflation speeds for pressures above 15 mmHg (all p’s<0.05), while the axial strains were not different (all p’s > 0.05).
Figure 6.
Axial (A) and lateral (B) corneal strains during slow and fast inflation testing in porcine globes with or without P188 treatment.
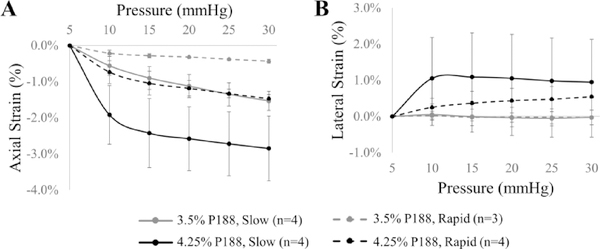
Corneal strains during slow and rapid inflation tests in human eyes are presented in Figure 7. A few tests in the 3.5% P188-treated group (n=2 in rapid inflation, n=1 in slow inflation) were excluded from further analysis due to poor speckle tracking (i.e. correlation coefficients were below 0.7 through entire tissue at multiple pressure steps), while all tests in the 4.25% P188 group had reliable speckle tracking for strain analysis. In the 4.25% P188-treated group, the compressive axial strains were about twice in magnitude of the tensile lateral strains in both rapid and slow inflation (Fig. 7). In the 3.5% P188-treated group, the lateral strains were minimal (Fig. 7B), and the axial strains were also significantly lower than those in the 4.25% P188-treated group (all p’s<0.05, two-sample t-tests, Fig. 7A). Due to a limited sample size, no statistical analyses were performed to compare the responses at different inflation rates, but the general observation was a larger corneal strain in slow inflation of donor eyes.
Figure 7.
Axial (A) and lateral (B) corneal strains during slow and fast inflation testing in human globes treated with solutions of different P188 concentrations.
It is noted that the outcome of the rapid inflation was not significantly different comparing the last two cycles, and the result from the last cycle (data not shown) appeared almost identical to that of the average of the last two cycles shown in Fig. 7, indicating that tissue response was stabilized after the first 8 cycles of inflation at the same rate and magnitude.
4. Discussion and Conclusions
In this study, we tested both corneal buttons and whole globes to identify P188 concentrations and treatment durations to return ex vivo corneas to physiological hydration. Our results showed that the concentrations for retuning the cornea to a hydration of H=3.2 within 24 hrs were achieved with 3.25% P188 treatment in porcine corneas and 4.25% P188 treatment in human corneas. Whole globe CCT measurements confirmed that the deswelling effect could be obtained with immersion of whole globe, with simultaneous infusion of P188 solutions into the anterior chamber. Inflation mechanical testing showed that the cornea’s mechanical response to IOP was significantly altered with changes in hydration. Poloxamers like P188 may provide a viable alternative for restoring and maintaining near physiological corneal hydration during ex vivo testing.
The average CCT in tested porcine and human globes was 891.8±65.6 μm and 574.6±33.7 μm, respectively, when returned to near-physiological hydration levels (i.e. H=3.2). These values fell within the reported ranges of in vivo porcine and human CCT. For example, porcine CCT was reported to range from 600 to 1000 μm, likely due to differences in age and breed of the measured samples (20, 30). Previous studies using ultrasound pachymetry to measure CCT in vivo in hundreds of human subjects reported an average value of 544 μm (31). This value is slightly smaller than that in our results, and the limited sample size in our study may have introduced some sampling effect.
Our results from whole globe inflation revealed interesting trends comparing physiological and overhydrated corneas. In the porcine eyes, the swollen corneas had a hydration around 4.5, and their inflation response was not substantially different from that of a normal hydration of 3.2 during rapid inflation (Fig. 5). In slow inflation, the axial responses between groups deviated from each other while the lateral response remained similar, indicating that the axial compression is more sensitive to hydration state. In human eyes, despite a small change in hydration state from 3.2 to 3.75, the inflation response appeared substantially altered, with reduction in both axial and lateral strains in the more swollen corneas. This trend was observed in both rapid and slow inflations.
Viscoelastic tissues often exhibit rate-dependent response, and tissue generally behaves stiffer at higher loading rates (32). Our results in the untreated (i.e. swollen) porcine corneas showed no significant difference in axial strains between rapid and slow inflations (Fig. 6A), while the lateral response followed the expected trend in this group (Fig. 6B). In addition, the treated group (at normal hydration) showed a strong rate dependence that followed the expected trend for both axial and lateral strains. These results suggested that corneal swelling might have a more pronounced effect on the viscoelastic response in the through-thickness direction, since swelling is in that direction.
The general trend we observed was that the axial strains were reduced in swollen corneas, suggesting a more structurally stiffer response to inflation in the anterior-posterior direction. This was consistent with a previous report showing an increased thickness reduction during ex vivo inflation in dextran-treated corneas (14) and a nano-indentation study showing a higher Young’s modulus in swollen corneas (13). However, this trend appears to contradict with other studies showing a reduced modulus or more compliant response in swollen corneas (7–10, 33–35). It is noted that inflation testing does not directly measure material properties (i.e. modulus) unless an adequate theoretical model is combined with the deformation data to inversely derive these properties. If all other parameters remain the same, an increase in CCT alone in overhydration would result in reduction in corneal strains. Therefore, our results may reflect the effect of CCT change or a combination in changes in corneal modulus and CCT. Several studies reported a decrease in corneal modulus in swollen corneas based on mechanical testing of dissected corneal strips or buttons (7–10, 12). The sample preparation and boundary conditions during these mechanical tests do not resemble inflation testing in intact whole globes; for example, fluid may exudate from the cut surfaces of the cornea when it is dissected. The biomechanical changes associated with corneal hydration is thus more complex than simple trends of being more compliant or stiff, and it is important to interpret the results within the context of the testing methods and the types of responses and properties measured.
There are a number of limitations in the current study. First, although our current results showed different inflation responses between groups of different hydration states, a detailed characterization of corneal biomechanical responses remains to be carried out to more directly verify whether poloxamer treatment can return swollen corneas to their physiological biomechanical responses. Future studies will aim to acquire fresh animal tissue samples prior to significant postmortem swelling and compare the responses in fresh tissue and that treated with P188. Second, the comparison of the inflation responses between the different groups in the current study may be confounded by the innate variance in biological samples. It is noted that the initial CCT between the two porcine groups and the two human donor groups was not significantly different. Using paired eyes may reduce such variance and should be investigated in the future.
In summary the current study has identified a protocol using P188 immersion to return postmortem porcine and human corneas to physiological hydration by determining suitable treatment concentrations and time scales. Our results in corneal buttons and whole globes confirmed that immersion in P188 can effectively deswell postmortem corneas to near-physiological hydration within 24 hrs, with a corresponding change in corneal mechanical response. Future studies are needed to test the poloxamers’ effect in maintaining corneal thickness in dissected corneas or other experimental conditions to evaluate its use as an economical and effective method for controlling corneal hydration during ex vivo testing.
Acknowledgments
Funding Source: This work was supported by the National Institutes of Health under grant number R01EY025358.
Footnotes
Disclosures: None.
References
- 1.Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung BK, Bonanno JA, Radke CJ. Oxygen-deficient metabolism and corneal edema. Prog Retin Eye Res. 2011;30:471–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Juan V, Herreras JM, Pérez I, Morejón Á, Río-Cristóbal A, Cristóbal AR, et al. Refractive Stabilization and Corneal Swelling After Cataract Surgery. Optom Vis Sci. 2013;90:31–6. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Patel DV. The pathophysiology of Fuchs’ endothelial dystrophy – A review of molecular and cellular insights. Exp Eye Res. 2015;130:97–105. [DOI] [PubMed] [Google Scholar]
- 5.Hayes S, White T, Boote C, Kamma-Lorger CS, Bell J, Sorenson T, et al. The structural response of the cornea to changes in stromal hydration. J R Soc Interface. 2017;14:20170062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Meek KM. Swelling Studies on the Cornea and Sclera: The Effects of pH and Ionic Strength. Biophys J. 1999;77:1655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatami-Marbini H, Rahimi A. Evaluation of hydration effects on tensile properties of bovine corneas. J Cataract Refract Surg. 2015;41:644–51. [DOI] [PubMed] [Google Scholar]
- 8.Seiler TG, Shao P, Frueh B, Yun S, Seiler T. The influence of hydration on different mechanical moduli of the cornea. Graefes Arch Clin Exp Ophthalmol. 2018;256:1653–60. [DOI] [PubMed] [Google Scholar]
- 9.Hatami-Marbini H, Rahimi A. Effects of bathing solution on tensile properties of the cornea. Exp Eye Res. 2014;120:103–8. [DOI] [PubMed] [Google Scholar]
- 10.Hatami-Marbini H, Rahimi A. The relation between hydration and mechanical behavior of bovine cornea in tension. J Mech Beh Biomed Materials. 2014;36:90–7. [DOI] [PubMed] [Google Scholar]
- 11.Ahearne M, Yang Y, Then K, Liu K. An Indentation Technique to Characterize the Mechanical and Viscoelastic Properties of Human and Porcine Corneas. Ann Biomed Eng. 2007;35:1608–16. [DOI] [PubMed] [Google Scholar]
- 12.Hatami-Marbini H, Etebu E. Hydration dependent biomechanical properties of the corneal stroma. Exp Eye Res. 2013;116:47–54. [DOI] [PubMed] [Google Scholar]
- 13.Dias J, Ziebarth N. Impact of Hydration Media on Ex Vivo Corneal Elasticity Measurements. Eye Contact Lens. 2015;41:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kling S, Marcos S. Effect of hydration state and storage media on corneal biomechanical response from in vitro inflation tests. J Refract Surg. 2013;29:490–7. [DOI] [PubMed] [Google Scholar]
- 15.Borja D, Manns F, Lamar P, Rosen A, Fernandez V, Parel J. Preparation and Hydration Control of Corneal Tissue Strips for Experimental Use. Cornea. 2004;23:61–6. [DOI] [PubMed] [Google Scholar]
- 16.Knox Cartwright NE, Tyrer JR, Marshall J. Age-related differences in the elasticity of the human cornea. Invest Ophthalmol Vis Sci. 2011;52:4324–9. [DOI] [PubMed] [Google Scholar]
- 17.Clayson K, Pavlatos E, Ma Y, Liu J. 3D Characterization of corneal deformation using ultrasound speckle tracking. J Innov Opt Health Sci. 2017;10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palko JR, Tang J, Cruz Perez B, Pan X, Liu J. Spatially heterogeneous corneal mechanical responses before and after riboflavin–ultraviolet-A crosslinking. J Cataract Refract Surg. 2014;40:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes S, Lewis P, Islam MM, Doutch J, Sorensen T, White T, et al. The structural and optical properties of type III human collagen biosynthetic corneal substitutes. Acta Biomater. 2015;25:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vetter JM, Brueckner S, Tubic-Grozdanis M, Voßmerbaumer U, Pfeiffer N, Kurz S. Modulation of central corneal thickness by various riboflavin eyedrop compositions in porcine corneas. J Cataract Refract Surg. 2012;38:525–32. [DOI] [PubMed] [Google Scholar]
- 21.Dick HB, Augustin AJ, Pfeiffer N. Osmolality of various viscoelastic substances: comparative study. J Cataract Refract Surg. 2000;26:1242–6. [DOI] [PubMed] [Google Scholar]
- 22.Boyce BL, Jones RE, Nguyen TD, Grazier JM. Stress-controlled viscoelastic tensile response of bovine cornea. J Biomech. 2006;40:2367–76. [DOI] [PubMed] [Google Scholar]
- 23.Thuret G, Manissolle C, Campos-Guyotat L, Guyotat D, Gain P. Animal Compound-Free Medium and Poloxamer for Human Corneal Organ Culture and Deswelling. Invest Ophthalmol Vis Sci. 2005;46:816–22. [DOI] [PubMed] [Google Scholar]
- 24.Zhao M, Thuret G, Piselli S, Pipparelli A, Acquart S, Peoc’h M, et al. Use of Poloxamers for Deswelling of Organ-Cultured Corneas. Invest Ophthalmol Vis Sci. 2008;49:550–9. [DOI] [PubMed] [Google Scholar]
- 25.Smith V, Johnson T. Identification and evaluation of a thinning agent compatible with MegaCell DCS, an animal product-free corneal storage medium. Graefes Arch Clin Exp Ophthalmol. 2012;250:1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ytteborg J, Dohlman CH. Corneal edema and intraocular pressure. II. Clinical Results. Arch Ophthalmol. 1965;74:477–84. [DOI] [PubMed] [Google Scholar]
- 27.Tonge TK, Murienne BJ, Coudrillier B, Alexander S, Rothkopf W, Nguyen TD. Minimal preconditioning effects observed for inflation tests of planar tissues. J Biomech Eng. 2013;135:114502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J, Liu J. Ultrasonic measurement of scleral cross-sectional strains during elevations of intraocular pressure: method validation and initial results in posterior porcine sclera. J Biomech Eng. 2012;134:091007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gyi TJ, Meek KM, Elliott GF. Collagen interfibrillar distances in corneal stroma using synchrotron X-ray diffraction: a species study. Int J Biol Macromol. 1988;10:265–9. [Google Scholar]
- 30.Sanchez I, Martin R, Ussa F, Fernandez-Bueno I. The parameters of the porcine eyeball. Graefes Arch Clin Exp Ophthalmol. 2011;249:475–82. [DOI] [PubMed] [Google Scholar]
- 31.Doughty MJ, Zaman ML. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-analysis Approach. Surv Ophthalmol. 2000;44:367–408. [DOI] [PubMed] [Google Scholar]
- 32.Elsheikh A, Wang D, Pye D. Determination of the modulus of elasticity of the human cornea. J Refract Surg. 2007;23:808–18. [DOI] [PubMed] [Google Scholar]
- 33.Shao P, Seiler TG, Eltony AM, Ramier A, Kwok SJJ, Scarcelli G, et al. Effects of Corneal Hydration on Brillouin Microscopy In Vivo. Invest Ophthalmol Vis Sci. 2018;59:3020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh M, Han Z, Li J, Vantipalli S, Aglyamov SR, Twa MD, et al. Quantifying the effects of hydration on corneal stiffness with noncontact optical coherence elastography. J Cataract Refract Surg. 2018;44:1023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackburn BJ, Gu S, Ford MR, de Stefano V, Jenkins MW, Dupps J, William J, et al. Noninvasive Assessment of Corneal Crosslinking With Phase-Decorrelation Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2019;60:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]