Abstract
Studies have identified prior conditions associated with late-onset Alzheimer’s disease dementia (LOAD), but all prior diseases have rarely been screened simultaneously in the literature. Our objective in the present study was to identify prior conditions associated with LOAD and construct pathways for them. We conducted a population-based matched case-control study based on data collected in the National Health Insurance Research database of Taiwan and the Catastrophic Illness Certificate database for the years 1997–2013. Prior diseases definitions were based on the first three digits of the codes listed in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Inclusion criteria required that each ICD-code existed for at least 1 year and incurred at least 2 outpatient visits or inpatient diagnosis. The case group comprised 4,600 patients newly diagnosed with LOAD in 2007–2013. The LOAD patients were matched by sex and age to obtain 4,600 controls. Using stepwise multivariate logistic regression analysis, diseases were screened for 1, 2 …, 9 years prior to the first diagnosis of LOAD. Path analysis was used to construct pathways between prior diseases and LOAD. Our results revealed that the following conditions were positively associated with the incidence of LOAD: anxiety (ICD-code 300), functional digestive disorder (ICD code 564), psychopathology-specific symptoms (ICD-code 307), disorders of the vestibular system (ICD-code 386), concussion (ICD-code 850), disorders of the urethra and urinary tract (ICD-code 599), disorders of refraction and accommodation (ICD-code 367), and hearing loss (ICD-code 389). A number of the prior diseases have previously been described in the literature in a manner identical to that in the present study. Our study supports the assertion that mental, hearing, vestibular system, and functional digestive disorders may play an important role in the pathogenesis of LOAD.
Introduction
Dementia is a severely debilitating health condition that imposes burdensome social and economic costs, and rapidly aging populations have increased the prevalence of dementia worldwide [1]. Treatments for dementia can help only to relieve symptoms; i.e., there is no cure. Recognizing the risk of dementia is a critical issue for preventing dementia prevalent.
Late-onset Alzheimer’s disease dementia (LOAD) accounts for 60–80% of all cases of dementia [2]; however, the etiology of LOAD has not yet been verified. LOAD is currently regarded as a neurodegenerative disease, wherein nearly 70% of the risk factors have a genetic link [3, 4]. Nonetheless, epidemiological studies have demonstrated that many diseases are associated with the incidence of LOAD. Vascular risk factors [5–8], including diabetes, hypertension, heart disease, obesity, and neuropsychiatric symptoms (e.g., depression, anxiety, and stress), have been examined in the context of cognitive decline [9–13]. Structural brain abnormalities are commonly observed in people with affective disorders, including hippocampal atrophy in patients with a history of depression [10, 14]. Major depression may be a risk factor for the development of AD, and patients with lifelong depression have a two-fold higher likelihood of developing AD and exhibiting more AD-related neuropathological symptoms [12, 13]. Pathological manifestation in head injury have also been linked to the risk of LOAD, including increased amyloid-beta and tau pathology, neuroinflammation, and axonal and cytoskeletal changes in the brain [15–17]. Hearing loss has been reported as a risk factor for dementia [18–21], where even mild levels suggest a link [22, 23]. Patients with hearing loss may suffer from social isolation, depression, disability, and increased risk of dementia [24–27]. Moreover, recent studies in animals have demonstrated that the microbiome-gut-brain axis may be involved in the pathogenesis of Alzheimer’s disease [28–30].
Identifying potentially prior diseases is crucial to the prevention of LOAD epidemics. To our knowledge, there has been a dearth of research on associations among all diseases and LOAD simultaneously. LOAD is influenced by multiple factors, and it is essential to explore the relevant factors using different research methods. The present study aims to determine prior diseases associated with LOAD simultaneously, and construct pathways analysis for these diseases associated with LOAD.
Materials and methods
Ethics statement
Approval for this study was obtained from the Institutional Review Board (IRB) of National Taiwan Normal University (Protocol Number: 201712HM015). Written consent was exempted because the data was obtained from the National Health Insurance Research Database (NHIRD) of Taiwan, which contains de-identified secondary data released for research purposes.
Data sources
We used data files pertaining to outpatient care, inpatient care, ambulatory care, and details of prior medical conditions from the NHIRD in Taiwan for the years 1997–2013 [31]. We also used the Catastrophic Illness Certificate (CIC) database of Taiwan for the same period. The insurance system records all patients with 30 categories of catastrophic illness, including malignant neoplasm, uremia, and chronic psychotic disorders (e.g., dementia) with the Registry of Patients with Catastrophic Illness. The attending physician of any patient diagnosed with a catastrophic illness can submit relevant information to the CIC. A committee formally reviews the applications, and if approved, patients are then exempted from co-payment during the effective period in Taiwan. Thus, this data can be regarded as comprehensive.
Study design and population
In this population-based matched case-control study, LOAD cases were defined as CIC patients newly diagnosed with dementia (International Classification of Diseases, Ninth Revision, Clinical Modification, ICD-9-CM: 290.xx) in 2007–2013 and prescribed any acetylcholinesterase inhibitors (AChEIs) (Anatomical Therapeutic Chemical, [ATC] N06D) after the LOAD diagnosis date. We excluded the following patients: (1) those who did not use AChEIs or had used AChEIs before the LOAD diagnosis date, (2) those diagnosed with Parkinson's disease (PD, ICD-9 332.0, 332.1), (3) those diagnosed with Vascular dementia (VaD, ICD-9 290.40, 290.41, 290.42, 290.43), (4) those aged <65 years, and (5) those with a first diagnosis date of LOAD in the 1997–2006 period. The index date was defined as the first date on which the patient received a definitive diagnosis of LOAD (Fig 1). The case group contained 4,600 patients newly diagnosed with LOAD in 2007–2013. LOAD patients were matched by sex and age to obtain 4,600 controls.
Fig 1.
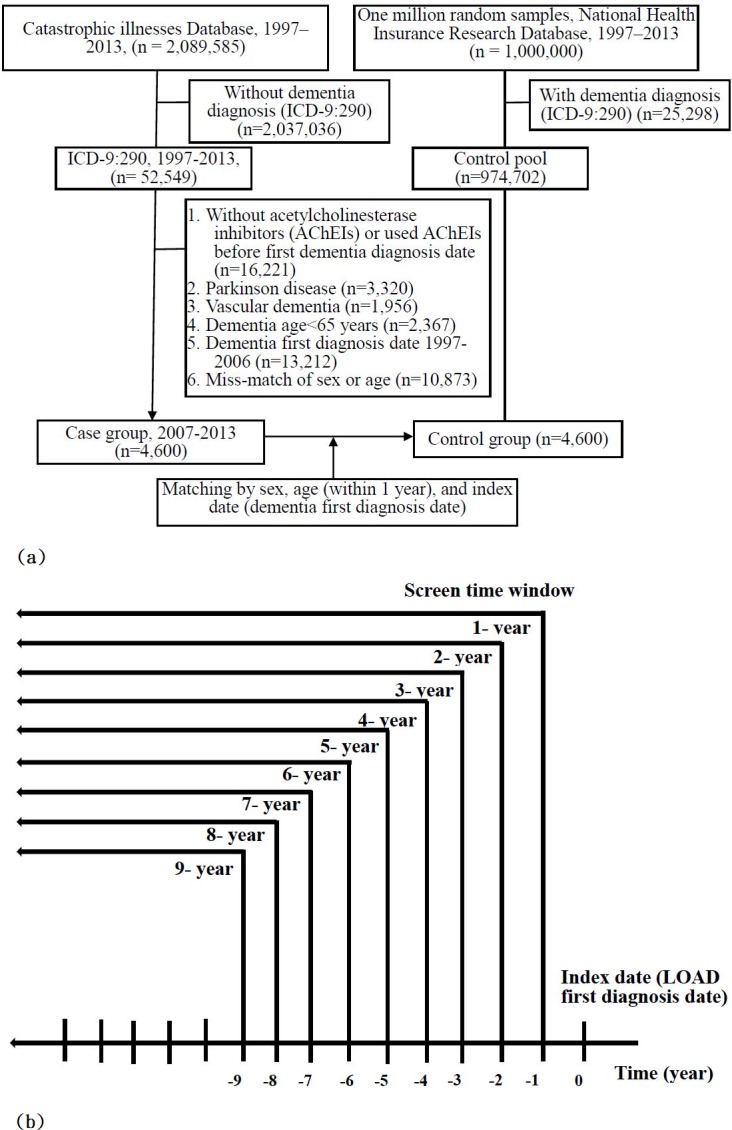
(a) Flowchart of subject selection process; (b) screen time windows used to identify prior diseases associated with LOAD.
Prior diseases definitions were based on the first three digits of the codes listed in the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Inclusion criteria required that each ICD-code existed for at least 1 year and incurred at least 2 outpatient visits or inpatient diagnosis. Prior diseases associated with LOAD were screened out for 1, 2, 3, …,9 years before the date of first diagnosis LOAD (Fig 1.). All medical claims between 1997 and 2013 containing this code were obtained from the NHIRD for further analysis. Finally, we constructed pathways related to the identified prior diseases and plotted the relationships among diseases.
Statistical analysis
The Chi-square test was used to compare the distributions of demographic factors of patients newly diagnosed with LOAD and controls. Associated diseases were identified using a conditional logistic regression model. Stepwise multivariate logistic regression was used to identify the diseases associated with LOAD and the adjusted odds ratio (OR). The 95% confidence was to set a P value of < 0.05. Two-sided data analysis was performed using the statistical package SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Path analysis correction was used with significant diseases during 4-year periods to construct pathways from diseases to LOAD. Five statistical tests were used to evaluate the overall goodness-of-fit of the correction model, including standardized root mean square residual (SRMR), root mean square error of approximation (RMSEA), comparative fit index (CFI), goodness-of-fit statistic (GFI), and normed-fit index (NFI) [32].
We fitted a hypothesized pathway model to the LOAD and control groups, and then executed a correction model to establish positive and negative associations via modification indices (MI). Finally, the positive and negative correction models were combined into one model using MIs for correction. The correction procedure modified the initial model one path at a time with the aim of improving the goodness-of-fit. Candidate paths where MI > 0 were then added to the correction model. The final step involved systematically trimming non-significant pathways based on coefficient estimates with a P value of > 0.05. In each step, interim evaluations of MI were conducted to identify relevant pathways that arose after simplifying the model. We included in the final correction model only the significant paths (P < 0.05) for which there was an acceptable overall goodness-of-fit. The overall process was stopped when no additional significant pathways were suggested by the MI. The direct and indirect effects of prior diseases on LOAD incidence were defined using the model constraint procedure and the maximum likelihood robust estimator. Total effects (determined as the sum of direct effects and indirect effects) were also calculated. Data analysis was performed using AMOS 21 (IBM Corp., 2012). Similar analysis was used in our previous research on diseases correlating with amyotrophic lateral sclerosis [33].
Results
Sample characteristics
The two groups comprised 4,600 patients newly diagnosed with LOAD (≥ 65 years of age) and 4,600 sex- and age-matched control groups (Table 1). Table 1 presents a comparison of the characteristics of the two groups. Most of the patients with LOAD were female (63.3%), the diagnosis age range was predominantly 65–79 years (68.43%), and half of the patients’ insurance premiums belonged to the fixed premium and dependent groups (49.33%). Patients who were insured under agriculture and fisheries categories had a lower incidence of LOAD.
Table 1. Characteristics of patients with and without LOAD.
| Characteristics | LOAD, (N = 4,600) |
Without LOAD, (N = 4,600) |
P value |
|---|---|---|---|
| Gender N (%) | |||
| Female | 2,912 (63.30) | 2,912 (63.30) | Exact match |
| Male | 1,688 (36.70) | 1,688 (36.70) | |
| Age of diagnosis (year) N (%) | |||
| 65–79 | 3,148 (68.43) | 3,154 (68.57) | Exact match |
| ≥ 80+ | 1,452 (31.57) | 1,446 (31.43) | |
| Insurance premiums, NT$ | |||
| Low income | 12 (0.26) | 10 (0.22) | <0.001 |
| Fixed premiums or dependent | 2,269 (49.33) | 1,867 (40.59) | |
| < 20,000 | 967 (21.02) | 1,070 (23.26) | |
| 20,000~39,999 | 1,333 (28.98) | 1,623 (35.28) | |
| ≥ 40,000 | 19 (0.41) | 30 (0.65) | |
| Job | |||
| Government employees | 27 (0.59) | 18 (0.39) | <0.001 |
| Employees of private enterprises | 68 (1.48) | 68 (1.48) | |
| Agriculture and fisheries | 1,267 (27.54) | 1,570 (34.13) | |
| Low-income with social welfare | 993 (21.59) | 1,082 (23.52) | |
| Unemployed | 2,245 (48.80) | 1,862 (40.48) | |
| Alzheimer drugs N (%) | |||
| No | 0 (0.00) | 4,600 (100.00) | |
| Yes | 4,600 (100.00) | 0 (0.00) |
Association between prior diseases and incidence of LOAD
We identified a total of 39 prior conditions that were significantly associated with the incidence of LOAD (1-year periods), which included 23 positive and 16 negative associations (Table 2). Conditions that occurred eight years prior to the first diagnosis included episodic mood disorders (ICD-code 296), concussion (ICD-code 850), fracture of radius and ulna (ICD-code 813), anxiety (ICD-code 300), local infections of skin and subcutaneous tissue (ICD-code 686), functional digestive disorders (ICD-code 564), vertiginous syndrome and disorders of vestibular system (ICD-code 386), and diabetes mellitus (ICD-code 250). Conditions with an early negative effect on LOAD incidence included asthma (ICD-code 493), disorders of synovium, tendon, and bursa (ICD-code 727), and ill-defined sprains and strains (ICD-code 848). General medical examination (ICD-code V70) and prophylactic vaccination (ICD-code V04) were also significant associated with LOAD and were therefore included in the path analysis; i.e., to determine whether they would have an impact on the model.
Table 2. Prior diseases associated with LOAD as a function of screen time windows prior to diagnosis.
| ICD-code | Disease | 1- to 9- years before LOAD first diagnosis ORa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1- | 2- | 3- | 4- | 5- | 6- | 7- | 8- | 9- | ||
| 331 | Other cerebral degenerations | 6.8 | 4.8 | |||||||
| 311 | Depressive disorder | 2.1 | 1.9 | 1.7 | 1.8 | |||||
| 296 | Episodic mood disorders | 2.1 | 1.9 | 1.9 | 1.7 | 1.9 | 1.9 | 1.9 | 2.1 | |
| 783 | Symptoms concerning nutrition, metabolism, and development | 1.6 | ||||||||
| 850 | Concussion | 1.5 | 1.4 | 1.6 | 1.6 | 1.5 | 1.7 | 1.7 | 1.7 | |
| 924 | Contusion of lower limb | 1.4 | 1.4 | 1.4 | 1.3 | 1.3 | 1.2 | 1.3 | ||
| 873 | Other open wound of head | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | ||||
| 813 | Fracture of radius and ulna | 1.4 | 1.7 | 1.7 | 2 | 1.8 | 1.8 | 1.8 | 1.9 | 1.9 |
| 389 | Hearing loss | 1.4 | 1.4 | 1.3 | 1.4 | 1.4 | 1.4 | |||
| 300 | Anxiety, dissociative and somatoform disorders | 1.4 | 1.4 | 1.3 | 1.4 | 1.3 | 1.3 | 1.3 | 1.2 | 1.3 |
| 686 | Other local infections of skin and subcutaneous tissue | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | 1.2 | 1.3 | 1.3 | 1.3 |
| 599 | Other disorders of urethra and urinary tract | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.2 | ||
| 578 | Gastrointestinal hemorrhage | 1.3 | 1.4 | 1.4 | ||||||
| 564 | Functional digestive disorders | 1.3 | 1.4 | 1.3 | 1.3 | 1.2 | 1.2 | 1.2 | 1.1 | |
| 486 | Pneumonia | 1.3 | 1.2 | |||||||
| 437 | Other and ill-defined cerebrovascular disease | 1.3 | 1.3 | 1.2 | 1.3 | 1.3 | 1.3 | 1.3 | ||
| 307 | Special symptoms or syndromes, not elsewhere classified | 1.3 | 1.3 | 1.2 | 1.2 | 1.2 | ||||
| V70 | General medical examination | 1.2 | 1.2 | 1.2 | 1.2 | 1.3 | 1.3 | 1.3 | 1.2 | 1.2 |
| V04 | Need for prophylactic vaccination and inoculation against certain diseases | 1.2 | 1.2 | 1.2 | 1.1 | 1.2 | 1.2 | 1.1 | 1.2 | 1.2 |
| 733 | Other disorders of bone and cartilage | 1.2 | 1.1 | 1.2 | 1.2 | |||||
| 682 | Other cellulitis and abscess | 1.2 | ||||||||
| 386 | Vertiginous syndromes and other disorders of vestibular system | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | ||
| 367 | Disorders of refraction and accommodation | 1.2 | 1.2 | 1.2 | 1.2 | 1.3 | ||||
| 692 | Contact dermatitis and other eczema | 1.1 | 1.2 | 1.1 | 1.1 | |||||
| 250 | Diabetes mellitus | 1.1 | 1.2 | 1.2 | 1.2 | 1.3 | ||||
| 729 | Other disorders of soft tissues | 0.9 | 0.9 | 0.9 | ||||||
| 571 | Chronic liver disease and cirrhosis | 0.9 | 0.9 | 0.9 | 0.9 | |||||
| 848 | Other and ill-defined sprains and strains | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 |
| 847 | Sprains and strains of other and unspecified parts of back | 0.8 | ||||||||
| 785 | Symptoms involving cardiovascular system | 0.8 | 0.8 | 0.8 | ||||||
| 726 | Peripheral enthesopathies and allied syndromes | 0.8 | 0.9 | |||||||
| 493 | Asthma | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| 477 | Allergic rhinitis | 0.8 | 0.9 | |||||||
| 413 | Angina pectoris | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | ||||
| 41 | Bacterial infection in conditions classified elsewhere and of unspecified site | 0.8 | ||||||||
| V45 | Other postprocedural states | 0.7 | 0.7 | |||||||
| 727 | Other disorders of synovium, tendon, and bursa | 0.7 | 0.7 | 0.7 | 0.6 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| 597 | Urethritis, not sexually transmitted, and urethral syndrome | 0.7 | 0.7 | |||||||
| 463 | Acute tonsillitis | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | ||||
| 518 | Other diseases of lung | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | ||||
| 273 | Disorders of plasma protein metabolism | 0.5 | ||||||||
| 217 | Benign neoplasm of breast | 0.5 | 0.6 | 0.5 | 0.5 | |||||
a Odds-ratios (OR) with P value < 0.05 are shown in this table.
Effects of prior diseases on LOAD incidence
The goodness-of-fit statistics revealed that the final correction models were acceptable (S1 Table). Table 3 presents the prior diseases with total, direct, and indirect effects on LOAD incidence for the 4-year period prior to diagnosis, after controlling for general medical examinations (ICD-code V70). Total effects exceeding 0.037 were identified in eight of the prior diseases, including anxiety, dissociative, and somatoform disorders (ICD-code 300); functional digestive disorders (ICD-code 564), special symptoms or syndromes not classified elsewhere (ICD-code 307); disorders of the vestibular system (ICD-code 386); concussion (ICD-code 850); disorders of the urethra and urinary tract (ICD-code 599); disorders of refraction and accommodation (ICD-code 367); and hearing loss (ICD-code 389). Two diseases with total negative effects were other diseases of the lungs (ICD-code 518) and other disorders of the synovium, tendon, and bursa (ICD-code 727).
Table 3. Total, direct, and indirect effects of significant prior diseases on LOAD incidence in the 4-year period prior to date of diagnosis.
| ICD-code | Disease | Total effecta | Direct effecta | Indirect effecta |
|---|---|---|---|---|
| 300 | Anxiety, dissociative and somatoform disorders | 0.089 | 0.038 | 0.051 |
| 564 | Functional digestive disorders | 0.060 | 0.032 | 0.028 |
| 307 | Special symptoms or syndromes, not elsewhere classified | 0.053 | 0.047 | 0.006 |
| 386 | Vertiginous syndromes and other disorders of vestibular system | 0.052 | 0.031 | 0.021 |
| 850 | Concussion | 0.050 | 0.046 | 0.004 |
| 599 | Other disorders of urethra and urinary tract | 0.044 | 0.030 | 0.014 |
| 367 | Disorders of refraction and accommodation | 0.042 | 0.040 | 0.001 |
| 389 | Hearing loss | 0.037 | 0.037 | 0.000 |
| 692 | Contact dermatitis and other eczema | 0.035 | 0.024 | 0.011 |
| 686 | Other local infections of skin and subcutaneous tissue | 0.035 | 0.036 | -0.001 |
| 437 | Other and ill-defined cerebrovascular disease | 0.033 | 0.023 | 0.010 |
| 813 | Fracture of radius and ulna | 0.028 | 0.028 | 0.000 |
| 311 | Depressive disorder | 0.027 | 0.026 | 0.001 |
| 413 | Angina pectoris | 0.009 | 0.000 | 0.009 |
| 463 | Acute tonsillitis | 0.009 | 0.000 | 0.009 |
| 493 | Asthma | 0.008 | 0.000 | 0.008 |
| 296 | Episodic mood disorders | 0.007 | 0.000 | 0.007 |
| 873 | Other open wound of head | 0.004 | 0.000 | 0.004 |
| 848 | Other and ill-defined sprains and strains | 0.002 | 0.000 | 0.002 |
| 924 | Contusion of lower limb and of other and unspecified sites | 0.000 | 0.000 | 0.000 |
| 727 | Other disorders of synovium, tendon, and bursa | -0.029 | -0.046 | 0.016 |
| 518 | Other diseases of lung | -0.030 | -0.030 | 0.000 |
| V70 | General medical examination | 0.051 | 0.037 | 0.014 |
a Total effect reflects an association between prior diseases and LOAD incidence via all paths in the model; an indirect effect reflects this association minus the direct effect of any path from a prior disease to LOAD incidence; and a direct effect is simply the total effect minus the total indirect effect.
Pathway between relevant prior diseases and incidence of LOAD
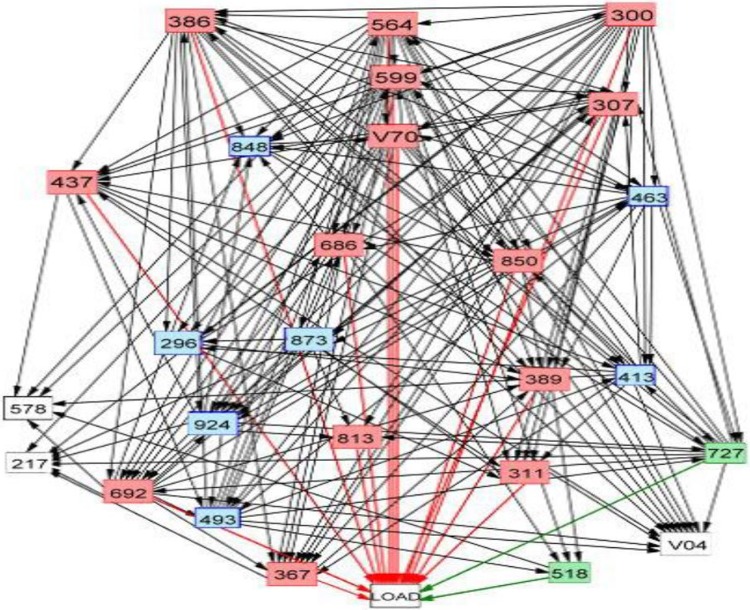
Fig 2 presents the final pathways model of total effects (merging positive and negative effects) on LOAD incidence for the 4-year period prior to the first diagnosis of LOAD (S1 Fig: positive final pathways model; S2 Fig: negative pathway model). Two features of the final model are noteworthy. First, five diseases (ICD-codes 300, 564, 599, 386, and 850) in upper positions in the final model have numerous direct pathways to other diseases in the model. Second, mental disorders (ICD-codes 300, 307, 311) play a predominant role in the model in terms of total positive effects on LOAD incidence.
Fig 2. Final path analysis model for diseases associated with LOAD in the 4 years prior to the first diagnosis of LOAD.
The red and blue lines respectively indicate direct positive and negative links between prior diseases and LOAD. Codes listed in the International Classification of Diseases, Ninth Revision, are displayed in the box.
Discussion
This is the first nationwide study in which multiple prior diseases linked to LOAD were simultaneously identified using stepwise multivariate logistic regression and path analysis. In the path analysis model, the following conditions had significant positive effects on LOAD incidence: anxiety (ICD-code 300), functional digestive disorders (ICD-code 564), special symptoms or syndromes not classified elsewhere (ICD-code 307), disorders of the vestibular system (ICD-code 386), concussion (ICD-code 850), disorders of the urethra and urinary tract (ICD-code 599), disorders of refraction and accommodation (ICD-code 367), and hearing loss (ICD-code 389).
Mental diseases have previously been identified as a risk factor for AD [7, 34], as a remote risk factor [35] and as a proximal prodromal feature of AD [36]. One Finnish study [36] similar to this work investigated the link between mental and behavioral disorders and AD. They found that depression and other mood disorders were associated with the risk of AD within a 5-year time window but not within a 10-year time window. The associations between mental/behavioral disorders and AD were modest and dependent on the time window near the onset. However, we found those mental diseases (ICD-codes 300, 311, 296) may reach associated with the risk of LOAD around 6- to 9- years period prior to the first diagnosis of LOAD. Our study differed from that study in two fundamental ways. The database used in this work links inpatient and outpatient medical records, was compiled more recently, and is more comprehensive. Furthermore, our model analyses each ICD-code as a variable rather than as a cluster of ICD-codes, thereby reducing the possibility of misdiagnosing mental disorders.
Our results revealed that vertiginous syndromes and disorders of the vestibular system (ICD-code 386) have a positive influence on LOAD incidence. It has been reported that bilateral vestibulopathy is due to a dysfunction of vestibular organs, nerves, or the brain [37, 38]. It has also been reported that cerebellar ischemia, chronic vertigo, acute dizziness, and balance disorders may be the first signs of severe neurological disorders related to the vestibular and ocular motor systems [39]. Previc [40] identified a few of the risk factors of AD that are also risk factors for vestibular disease: aging, cerebrovascular deficiencies, diabetes and other metabolic disorders, depression, and traumatic brain injury.
Hearing loss (ICD-code 389) is independently associated with lower scores on tests of memory and incidence of all-cause dementia or AD [21–23, 41, 42]. In a meta-analysis of prospective studies, Zheng et al. [18] found that people with hearing impairment faced a higher risk of developing AD than did those in a control group. Several possible mechanisms have been proposed. It has been suggested that hearing loss affects cortical processing by diverting cognitive resources away from cognitive processes, such as working memory toward auditory processing. It is also possible that social isolation due to hearing loss could contribute to the development of AD. There is also the strong possibility that both diseases have a common cause and that hearing loss is simply an early condition indicative of the underlying pathology [6, 43–46].
Permanent or sudden dysfunctions in the sensory system (e.g., sensorineural hearing loss) can lead to affective disorders, including depression, anxiety, and bipolar disorder [47]. The findings in the present study support this hypothesis. The long-term impairment of communication pathways can impose considerable strain on patients, undermining their quality of life, leading to social isolation, and compromising their ability to engage in pleasurable activities [48]. The risk intensity associated with affective disorders depends on the duration of sensory loss [49]. Vestibular dysfunction (a type of sensory loss) has been associated strongly with the concurrence of symptoms of affective disorders. This suggests the presence of vestibular cognitive affective syndrome [50]; however, vestibular dysfunction is related more directly to memory impairment. In the present study, mental disorders (ICD-codes 300, 307, 296, and 311) as well as hearing and vertiginous syndromes (ICD-codes 389 and 386) appeared simultaneously at least 4- to 9- years before the first diagnosis of LOAD.
Functional digestive disorders (FGIDs, ICD-code 564) exhibited a direct risk effect for LOAD development in this study. Irritable bowel syndrome (IBS) is a type of functional gastrointestinal disorder (FGID). Using the Taiwan NHIRD, Chen et al. [28] found that a new diagnosis of IBS had a positive effect on LOAD incidence and that this effect was obvious only in patients who were ≥ 50 years old. They suggested that the gut-brain axis (GBA) may play an important role in the association between IBS and AD. The GBA has recently been linked to cognitive performance, behavior, and emotions [51]. GBA is a bidirectional communication system comprising neural pathways encompassing the autonomic nervous system (ANS), enteric nervous system (ENS), immune system, and neuroendocrine system (such as acetylcholine, dopamine, 5 HT, and serotonin) [52], especially including the hypothalamic–pituitary–adrenal axis (HPA axis). The expansion of the GBA to include the functions of gut flora is referred to as the microbiome–gut–brain axis. Researchers have demonstrated that neuroinflammation can be triggered by pathogenic gut microbiota [53]. These, as well as a dysfunctional GBA, may promote cognitive impairment. It has been posited that interactions between dysfunctional GBA and the CNS can be attributed to changes in brain chemistry and neuro-endocrine systems involved in anxiety, depressive-like behaviors, stress response, and memory function [54]. In clinical practice, IBS is treated as a microbiome-GBA disorder [55]. The continual triggering of neuroinflammation by the gut has been shown to cause damage in various regions of the brain [56] and increase the risk of developing neurodegenerative disorders including AD [57].
Disorders of the urethra and urinary tract (ICD-code 599) include functional urogenital tract disorders, such as urethral hypermobility, urinary tract infection, and urethral instability. One review [58] proposed the existence of a “bladder–gut–brain axis (BGBA)” to explain the frequent co-occurrence of functional urological and gastrointestinal disorders. This interaction across organ systems could perhaps be attributed to an underlying central hypersensitivity manifesting as bodily distress. Within this schema, psychological and physical stress pathways could easily generate a range of false alarms and could arise the co-occurrence of functional disorders and mental conditions. In the present study, disorders of the urethra and urinary tract (ICD-code 599) as well as functional digestive disorders (ICD-code 564) were found to occur simultaneously at least 1 to 8 years prior to the first diagnosis of LOAD.
Limitation
One substantial limitation of this study (as is the case with other studies that use routine data) is the limited amount of information related to other potential confounders, such as body mass index, diet pattern, blood pressure, blood sugar, smoking, family history, mood disorders, and therapy for diabetes. It is possible that disease onset and diagnosis may differ according to the economic status and residence of patients, due to the fact that these variables can affect access to neurologists. Nonetheless, the association of some conditions (e.g., anxiety, depression, disorder of the vestibular system, concussion, and hearing loss) are consistent with the findings in many previous studies. Our use of path analysis also identified a number of relevant diseases (e.g., functional digestive disorders) that have scarcely been addressed in previous studies.
Conclusions
This study discovered that a number of conditions occurring prior to LOAD onset (e.g., mental conditions, hearing, vestibular system, and functional digestive disorder) may play an important role in the pathogenesis of LOAD. Moreover, our corrected pathways among neuropsychiatric conditions, the digestive system, and LOAD support the assertion that the gut–brain axis may play important roles in the pathogenesis of LOAD. Future studies will be needed to elucidate the mechanism underlying these associations.
Supporting information
Codes listed in the International Classification of Diseases, Ninth Revision, are displayed in the box.
(DOCX)
Codes listed in the International Classification of Diseases, Ninth Revision, are displayed in the box. The green box denotes the predictors that have negative effects on LOAD.
(DOCX)
(DOCX)
Data Availability
This study was approved by the Institutional Review Board (IRB) of National Taiwan Normal University (Protocol Number: 201712HM015) and based, in part, on the National Health Insurance Research Database provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. Academic researchers in Taiwan can assess all the data in this study by submitting an application to the Administration of National Health Insurance (website: https://dep.mohw.gov.tw/DOS/np-2497-113.html).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. The Lancet. 2009;374(9696):1196–208. 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's disease international. Available from: https://www.alz.co.uk/about-dementia
- 3.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377(9770):1019–31. Epub 2011/03/05. 10.1016/S0140-6736(10)61349-9 . [DOI] [PubMed] [Google Scholar]
- 4.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. The Lancet Neurology. 2006;5(3):228–34. 10.1016/S1474-4422(06)70355-6 [DOI] [PubMed] [Google Scholar]
- 5.Wen YH, Wu SS, Lin CH, Tsai JH, Yang P, Chang YP, et al. A Bayesian Approach to Identifying New Risk Factors for Dementia: A Nationwide Population-Based Study. Medicine (Baltimore). 2016;95(21):e3658 Epub 2016/05/27. 10.1097/md.0000000000003658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moonga I, Niccolini F, Wilson H, Pagano G, Politis M. Hypertension is associated with worse cognitive function and hippocampal hypometabolism in Alzheimer's disease. European Journal of Neurology. 2017;24(9):1173–82. 10.1111/ene.13374 [DOI] [PubMed] [Google Scholar]
- 7.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. The Lancet. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Fan YC, Hsu JL, Tung HY, Chou CC, Bai CH. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: a population-based cohort study. Alzheimers Res Ther. 2017;9(1):7 Epub 2017/02/07. 10.1186/s13195-017-0236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mah L, Szabuniewicz C, Fiocco AJ. Can anxiety damage the brain? Curr Opin Psychiatry. 2016;29(1):56–63. Epub 2015/12/10. 10.1097/YCO.0000000000000223 . [DOI] [PubMed] [Google Scholar]
- 10.Cole J, Costafreda SG, McGuffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134(1–3):483–7. Epub 2011/07/13. 10.1016/j.jad.2011.05.057 . [DOI] [PubMed] [Google Scholar]
- 11.Van der Mussele S, Fransen E, Struyfs H, Luyckx J, Marien P, Saerens J, et al. Depression in mild cognitive impairment is associated with progression to Alzheimer's disease: a longitudinal study. Journal of Alzheimer's disease: JAD. 2014;42(4):1239–50. Epub 2014/07/16. 10.3233/JAD-140405 . [DOI] [PubMed] [Google Scholar]
- 12.da Silva J, Goncalves-Pereira M, Xavier M, Mukaetova-Ladinska EB. Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry. 2013;202(3):177–86. Epub 2013/03/05. 10.1192/bjp.bp.111.101931 . [DOI] [PubMed] [Google Scholar]
- 13.Sierksma AS, van den Hove DL, Steinbusch HW, Prickaerts J. Major depression, cognitive dysfunction and Alzheimer's disease: is there a link? Eur J Pharmacol. 2010;626(1):72–82. Epub 2009/10/20. 10.1016/j.ejphar.2009.10.021 . [DOI] [PubMed] [Google Scholar]
- 14.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences. 1996;93(9):3908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tolppanen AM, Taipale H, Hartikainen S. Head or brain injuries and Alzheimer's disease: A nested case-control register study. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2017;13(12):1371–9. Epub 2017/06/10. 10.1016/j.jalz.2017.04.010 . [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, et al. Head Injury as a Risk Factor for Dementia and Alzheimer's Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PloS one. 2017;12(1):e0169650 Epub 2017/01/10. 10.1371/journal.pone.0169650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolppanen AM, Taipale H, Hartikainen S. Head or brain injuries and Alzheimer's disease: A nested case-control register study. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2017. Epub 2017/06/10. 10.1016/j.jalz.2017.04.010 . [DOI] [PubMed] [Google Scholar]
- 18.Zheng Y, Fan S, Liao W, Fang W, Xiao S, Liu J. Hearing impairment and risk of Alzheimer's disease: a meta-analysis of prospective cohort studies. Neurol Sci. 2017;38(2):233–9. Epub 2016/11/30. 10.1007/s10072-016-2779-3 . [DOI] [PubMed] [Google Scholar]
- 19.Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig Otolaryngol. 2017;2(2):69–79. Epub 2017/09/13. 10.1002/lio2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su P, Hsu CC, Lin HC, Huang WS, Yang TL, Hsu WT, et al. Age-related hearing loss and dementia: a 10-year national population-based study. Eur Arch Otorhinolaryngol. 2017;274(5):2327–34. Epub 2017/02/24. 10.1007/s00405-017-4471-5 . [DOI] [PubMed] [Google Scholar]
- 21.Deal JA, Betz J, Yaffe K, Harris T, Purchase-Helzner E, Satterfield S, et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. 2017;72(5):703–9. Epub 2016/04/14. 10.1093/gerona/glw069 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–70. Epub 2011/07/07. 10.1037/a0024238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214–20. Epub 2011/02/16. 10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthrie DM, Davidson JGS, Williams N, Campos J, Hunter K, Mick P, et al. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: Analysis of interRAI data for home care and long-term care recipients in Ontario. PloS one. 2018;13(2):e0192971 Epub 2018/02/16. 10.1371/journal.pone.0192971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes SE, Hutchings HA, Rapport FL, McMahon CM, Boisvert I. Social Connectedness and Perceived Listening Effort in Adult Cochlear Implant Users: A Grounded Theory to Establish Content Validity for a New Patient-Reported Outcome Measure. Ear Hear. 2018. Epub 2018/02/10. 10.1097/aud.0000000000000553 . [DOI] [PubMed] [Google Scholar]
- 26.Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H, Edmondson-Jones M, et al. Hearing loss and cognition: the role of hearing AIDS, social isolation and depression. PloS one. 2015;10(3):e0119616 Epub 2015/03/12. 10.1371/journal.pone.0119616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu CM, Lee CT. Association of Hearing Loss With Dementia. JAMA network open. 2019;2(7):e198112 Epub 2019/08/01. 10.1001/jamanetworkopen.2019.8112 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CH, Lin CL, Kao CH. Irritable Bowel Syndrome Is Associated with an Increased Risk of Dementia: A Nationwide Population-Based Study. PloS one. 2016;11(1):e0144589 Epub 2016/01/06. 10.1371/journal.pone.0144589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalski K, Mulak A. Brain-Gut-Microbiota Axis in Alzheimer's Disease. Journal of neurogastroenterology and motility. 2019;25(1):48–60. Epub 2019/01/17. 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szablewski L. Human Gut Microbiota in Health and Alzheimer's Disease. Journal of Alzheimer's disease: JAD. 2018;62(2):549–60. Epub 2018/02/27. 10.3233/JAD-170908 . [DOI] [PubMed] [Google Scholar]
- 31.Institutes. NHR. National Health Insurance Research Database. [cited 2017 Oct 2]. Available from: http://nhird.nhri.org.tw/date_01_en.html
- 32.Hooper D, Coughlan J, Mullen M. Structural equation modelling: Guidelines for determining model fit. Articles. 2008:2. [Google Scholar]
- 33.Tsai CP, Hu C, Lee CT. Finding diseases associated with amyotrophic lateral sclerosis: a total population-based case-control study. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2019;20(1–2):82–9. Epub 2018/11/14. 10.1080/21678421.2018.1522354 . [DOI] [PubMed] [Google Scholar]
- 34.Moon B, Kim S, Park YH, Lim JS, Youn YC, Kim S, et al. Depressive Symptoms are Associated with Progression to Dementia in Patients with Amyloid-Positive Mild Cognitive Impairment. Journal of Alzheimer's disease: JAD. 2017;58(4):1255–64. Epub 2017/05/28. 10.3233/JAD-170225 . [DOI] [PubMed] [Google Scholar]
- 35.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–8. Epub 2006/05/03. 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tapiainen V, Hartikainen S, Taipale H, Tiihonen J, Tolppanen AM. Hospital-treated mental and behavioral disorders and risk of Alzheimer's disease: A nationwide nested case-control study. Eur Psychiatry. 2017;43:92–8. Epub 2017/04/08. 10.1016/j.eurpsy.2017.02.486 . [DOI] [PubMed] [Google Scholar]
- 37.Lucieer F, Duijn S, Van Rompaey V, Perez Fornos A, Guinand N, Guyot JP, et al. Full Spectrum of Reported Symptoms of Bilateral Vestibulopathy Needs Further Investigation-A Systematic Review. Front Neurol. 2018;9:352 Epub 2018/06/20. 10.3389/fneur.2018.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucieer F, Vonk P, Guinand N, Stokroos R, Kingma H, van de Berg R. Bilateral Vestibular Hypofunction: Insights in Etiologies, Clinical Subtypes, and Diagnostics. Front Neurol. 2016;7:26 Epub 2016/03/15. 10.3389/fneur.2016.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strupp M. Challenges in neuro-otology. Front Neurol. 2010;1:121 Epub 2011/01/06. 10.3389/fneur.2010.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Previc FH. Vestibular loss as a contributor to Alzheimer's disease. Med Hypotheses. 2013;80(4):360–7. Epub 2013/02/05. 10.1016/j.mehy.2012.12.023 . [DOI] [PubMed] [Google Scholar]
- 41.Davies HR, Cadar D, Herbert A, Orrell M, Steptoe A. Hearing Impairment and Incident Dementia: Findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2017;65(9):2074–81. Epub 2017/07/25. 10.1111/jgs.14986 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Y, Ye B, Chen P, Wang Q, Fan C, Shu Y, et al. Cognitive Decline, Dementia, Alzheimer's Disease and Presbycusis: Examination of the Possible Molecular Mechanism. Front Neurosci. 2018;12:394 Epub 2018/06/26. 10.3389/fnins.2018.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Organization WH. International statistical classification of diseases and related health problems: World Health Organization; 2004. [Google Scholar]
- 44.Bate A. Guidance to reinforce the credibility of health care database studies and ensure their appropriate impact. Pharmacoepidemiol Drug Saf. 2017;26(9):1013–7. Epub 2017/09/16. 10.1002/pds.4305 . [DOI] [PubMed] [Google Scholar]
- 45.Martin S, Kelly S, Kuhn I, Cowan A, Brayne C. Disbility, dementia and fraility in later lofe: mid-life approaches to prevent or delay the ontset of these conditions: Cambridge Institute of Public Health, University of Cambridge; 2014. [cited 2017 Oct 7]. Available from: http://www.iph.cam.ac.uk [Google Scholar]
- 46.Lin F-C, Chuang Y-S, Hsieh H-M, Lee T-C, Chiu K-F, Liu C-K, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine. 2015;94(47). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell J, Sharma A. Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci. 2013;7:71 Epub 2014/01/31. 10.3389/fnsys.2013.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ronnberg J, Rudner M, Lunner T, Zekveld AA. When cognition kicks in: working memory and speech understanding in noise. Noise Health. 2010;12(49):263–9. Epub 2010/09/28. 10.4103/1463-1741.70505 . [DOI] [PubMed] [Google Scholar]
- 49.Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70(17):1534–42. Epub 2008/03/21. 10.1212/01.wnl.0000304345.14212.38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin FR, Yaffe K, Xia J, Xue QL, Harris TB, Purchase-Helzner E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–9. Epub 2013/01/23. 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith PA. The tantalizing links between gut microbes and the brain. Nature. 2015;526(7573):312–4. Epub 2015/10/16. 10.1038/526312a . [DOI] [PubMed] [Google Scholar]
- 52.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–12. Epub 2013/02/07. 10.1016/j.tins.2013.01.005 . [DOI] [PubMed] [Google Scholar]
- 53.Daulatzai MA. Chronic functional bowel syndrome enhances gut-brain axis dysfunction, neuroinflammation, cognitive impairment, and vulnerability to dementia. Neurochem Res. 2014;39(4):624–44. Epub 2014/03/05. 10.1007/s11064-014-1266-6 . [DOI] [PubMed] [Google Scholar]
- 54.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28(2):203–9. Epub 2015/04/02. [PMC free article] [PubMed] [Google Scholar]
- 55.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14(3):143–59. Epub 2017/01/18. 10.1038/nrgastro.2016.191 . [DOI] [PubMed] [Google Scholar]
- 56.Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20(2):145–55. Epub 2017/01/17. 10.1038/nn.4476 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bu XL, Yao XQ, Jiao SS, Zeng F, Liu YH, Xiang Y, et al. A study on the association between infectious burden and Alzheimer's disease. Eur J Neurol. 2015;22(12):1519–25. Epub 2014/06/10. 10.1111/ene.12477 . [DOI] [PubMed] [Google Scholar]
- 58.Leue C, Kruimel J, Vrijens D, Masclee A, van Os J, van Koeveringe G. Functional urological disorders: a sensitized defence response in the bladder-gut-brain axis. Nat Rev Urol. 2017;14(3):153–63. Epub 2016/12/07. 10.1038/nrurol.2016.227 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Codes listed in the International Classification of Diseases, Ninth Revision, are displayed in the box.
(DOCX)
Codes listed in the International Classification of Diseases, Ninth Revision, are displayed in the box. The green box denotes the predictors that have negative effects on LOAD.
(DOCX)
(DOCX)
Data Availability Statement
This study was approved by the Institutional Review Board (IRB) of National Taiwan Normal University (Protocol Number: 201712HM015) and based, in part, on the National Health Insurance Research Database provided by the Health and Welfare Data Science Center, Ministry of Health and Welfare, Taiwan. Academic researchers in Taiwan can assess all the data in this study by submitting an application to the Administration of National Health Insurance (website: https://dep.mohw.gov.tw/DOS/np-2497-113.html).