Abstract
The search for diagnostic and prognostic biomarkers for neurodegenerative conditions is of high importance, since these disorders may present difficulties in differential diagnosis, Biomarkers with high sensitivity and specificity are required. Neurofilament light chain (NfL) is a unique biomarker related to axonal damage and neural cell death, which is elevated in a number of neurological disorders, and can be detected in cerebrospinal fluid (CSF), as well as blood, serum or plasma samples. Although the NfL concentration in CSF is higher than that in blood, blood measurement may be easier in practice due to its lesser-invasiveness, reproducibility and convenience. Many studies have investigated NfL in both CSF and serum/plasma as a potential biomarker of neurodegenerative disorders. Neuroimaging biomarkers can also potentially improve detection of CNS-related disorders at an early stage. Magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) are sensitive techniques to visualize neuroaxonal loss. Therefore, investigating the combination of NfL levels with indices extracted from MRI and DTI scans, could potentially improve diagnosis of CNS-related disorders. This review summarizes the evidence for NfL being a reliable biomarker in the early detection and disease management in several CNS-related disorders. Moreover, we highlight the correlation between MRI and NfL, and ask whether they can be combined.
Keywords: Neurofilament light chain, Biomarker, Neurodegenerative disorders, Magnetic resonance imaging, Diffusion tensor imaging
1. Introduction
The past decade has seen impressive efforts in the search for biomarkers for neurodegenerative diseases. Based on the National Institutes of Health (NIH) definition, a “biomarker” is a characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention [1]. Depending on their applications, biomarkers can be categorized either as imaging biomarkers or molecular biomarkers. They can also be classified based on their chief application including, diagnostic, staging, prognosis, or monitoring treatment response [2].
Recently, it has been recognized that some common molecular mechanisms including protein aggregation and inclusion body formation are shared between almost all CNS-related disorders, that had been previously considered unrelated and biologically distinct [3]. Each type of neurodegenerative disease is identified by one specific protein that accumulates, often in an aggregated form. Since post-mortem pathology has been classically used for unambiguous diagnosis of such diseases, obtaining a biopsy from the brain of a living individual is fraught with difficulty. Therefore, biochemical and molecular imaging biomarkers have suggested that will allow pathological changes to be detected in the brain even without biopsy [4,5]. These developments have helped clinicians to apply accessible, simple and practical methods for early diagnosis, differential diagnosis, follow-up, and treatment assessment of CNS-related disorders [6]. Biochemical biomarkers can be obtained from tissue, cerebrospinal fluid (CSF) or blood samples. However, because of difficulty in obtaining tissue-based biopsies for diagnostic goals or for longitudinal studies, the use of biological fluids, including blood and CSF biomarkers has received the majority of attention in CNS-related disorders [7]. This review is focused on the use of neurofilament light chain (NfL), a neuron-specific protein component released after axonal damage, as a potential biomarker of CNS-related disorders. Elevated NfL concentrations in CSF and serum/plasma have been shown to serve as a potential biomarker for axonal injury in several neurological disorders [8]. Many studies have investigated the potential role of this protein in diagnosis, prognosis and monitoring of CNS-related disorders. In addition to this fluid-based biomarker, MRI indices have also been considered as imaging biomarkers, and the correlation between these two types of biomarkers has been considered.
2. Neurofilament light chain as a biomarker for CNS related disorders
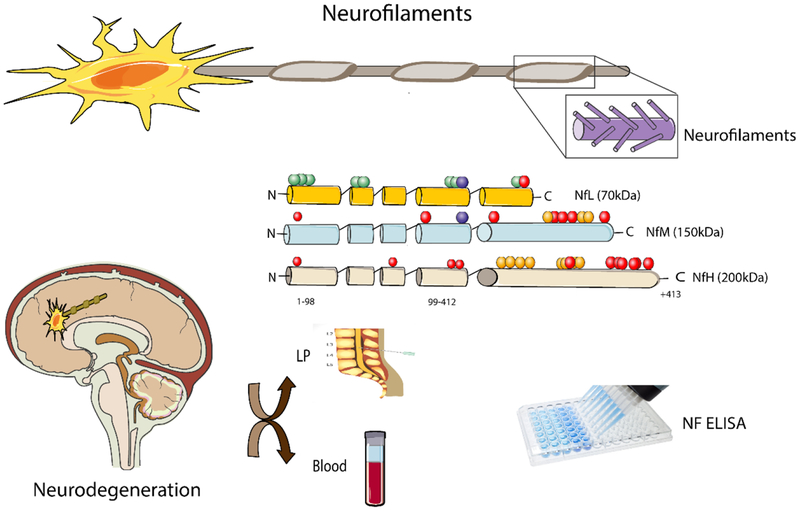
Neurofilaments (NFs) are the most important cytoskeletal proteins in myelinated subcortical axons [9–12]. NFs principally consist of four subunits: NF light (NfL), NF medium (NfM) and NF heavy (NfH) chains together with alpha-internexin [10].
In contrast to other ubiquitous cytoskeletal proteins such as actin, NFs are specific and abundant in the neuro-axonal compartments [10]. The NfL polypeptide is the most abundant intermediate filament in neurons and axons and plays a substantial role in the assembly and maintenance of the axonal cytoskeleton.
Disruption of the axonal membrane releases NFs into the interstitial fluid, and ultimately into both CSF and blood. NfL has the lowest molecular weight and the highest solubility compared to other subunits, therefore it diffuses more easily from the parenchyma into the CSF after axonal degeneration or neuronal death or disruption [13].
An abnormal increase of NF proteins in the CSF (including NfL and phosphorylated Nf heavy chain, pNfH) have been shown to be associated with neuronal death and axonal degeneration in a variety of disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS) (Table 1) [14].
Table 1.
Reports of alteration of NfL concentration in various brain disorders.
| Diseases | Material | Detection Method | Cut-Off or Control (pg/mL) | Disease (pg/mL) | Model | Sample | Citation |
|---|---|---|---|---|---|---|---|
| ALS | CSF | ELISA | 3819 | 9427 | Human | 220 | [22] |
| Serum | Single Molecule Array | 49 | 125 | Human | 44 | [23] | |
| Serum | ELISA | 159 | 179 | Human | 149 | [24] | |
| CSF | ELISA | 1838 | 4700 | Human | 190 | [25] | |
| CSF, Serum | ELISA | 6802, 255 | 2,300, 128 | Human | 135 | [26] | |
| CSF | ELISA | 1843.52 | 5741.51 | Human | 94 | [27] | |
| Blood | ELISA | 63 | 424 | Human | 125 | [28] | |
| CSF | ELISA | 1431 | 2521 | Human | 150 | [29] | |
| CSF | ECL | 201.8 | 7388 | Human | 76 | [30] | |
| CSF, Serum | ELISA | 663, 30 | 7118, 97 | Human | 65 | [31] | |
| CSF, Serum | ECL | 466.5, 22 | 7304, 90 | Human | 56 | [32] | |
| CSF | ELISA | 2140 | 8160 | Human | 37 | [33] | |
| CSF | ELISA | 2399 | 9285 | Human | 37 | [34] | |
| CSF | ELISA | 2200 | >2200 | Human | 242 | [35] | |
| MS | Serum | ELISA | 36.26 | 45.33 | Human | 74 | [36] |
| CSF, Serum | ELISA | 205, 10.5 | 925, 19.1 | Human | 286 | [37] | |
| CSF | ELISA | 1287 | 1900 | Human | 59 | [38] | |
| CSF | ELISA | 364 | 1468 | Human | 43 | [39] | |
| CSF | ELISA | 212 | 895 | Human | 41 | [40] | |
| CSF | ELISA | 29.6 | 43.4 | Human | 142 | [41] | |
| CSF, Serum | ECL | NA,1.3 | 9, 811 | Human | 31 | [42] | |
| Serum | ECL | 7.9 | 24.1 | Human | 100 | [43] | |
| CSF | ELISA | 10 | 26 | Human | 63 | [44] | |
| CSF | ELISA | 128.3 | 435.2 | Human | 47 | [46] | |
| CSF | ELISA | 1500 | 2500 | Human | 47 | [47] | |
| CSF | ELISA | 125 | 265 | Human | 60 | [48] | |
| CSF | ELISA | 125 | 1727 | Human | 66 | [49] | |
| CSF | ELISA | 350 | 1300 | Human | 92 | [50] | |
| CSF, Serum | Single Molecule Array | 341, 8.2 | 1475, 17 | Human | 39 | [51] | |
| AD | Plasma | ECL | 34.7 | 43 | Human | 187 | [45] |
| Plasma | ELISA | 25.7 | 49.1 | Human | 58 | [52] | |
| CSF | ELISA | 1296 | 10255 | In vivo (Mouse) | - | [53] | |
| CSF | ELISA | - | 12–24 fold | Human | 30 | [54] | |
| CSF | ELISA | 77.7 | 228.9 | Human | 93 | [55] | |
| Plasma | Single Molecule Array | 17.8 | 32.9 | Human | 119 | [56] | |
| serum | Ultrasensitive immunoassay | 6.1 | 23.2 | Human | 48 | [57] | |
| Dementia | CSF | ELISA | 981 | 2557 | Human | 41 | [58] |
| CSF | ELISA | 1197.1 | 5966.9 | Human | 103 | [59] | |
| CSF | ELISA | 250 | 844 | Human | 34 | [60] | |
| CSF | ELISA | 860 | 1380 | Human | 103 | [61] | |
| CSF | ELISA | 125 | 410 | Human | 24 | [62] | |
| CSF | ELISA | 250 | 780 | Human | 53 | [63] | |
| CSF | ELISA | 5 | 16.9 | Human | 46 | [64] | |
| CSF | ELISA | 390 | 510 | Human | 17 | [65] | |
| CSF | RT-QuIC | 1632 | 8908 | Human | 103 | [66] | |
| CSF and serum | ELISA | 804 | 6762 | Human | 126 | [67] | |
| Serum | ELISA | 19.6 | 57.8 | Human | 34 | [68] | |
| Serum and CSF | ECL | 17, NA | 56, 2948 | Human | 74 | [69] | |
| CSF | ELISA | 974 | 3168 | Human | 361 | [70] | |
| Serum | Single Molecule Array | 14.5 | 296 | Human | 45 | [71 | |
| CSF | ELISA, Western Blotting | 1167 | 1857 | Human | 77 | [72] | |
| CSF | ELISA | 686.5 | 2473 | Human | 16 | [73] | |
| PD | Plasma | Single Molecule Array | 17.8 | 23.3 | Human | 23 | [74] |
| CSF | Liquid chromatography-mass spectrometry | Not mentioned | Not mentioned | Human | 26 | [75] | |
| CSF and serum | ELISA | 887 | 896 | Human | 244 | [76] | |
| CSF | ELISA | 619 | 915 | Human | 32 | [77] | |
| CSF | ELISA | 5.2/33.4 | 1503 | Human | 31 | [78] | |
| Stroke | Serum | ELISA | 100> | <200 | Human | 196 | [79] |
| Serum | ECL | 16 | 211.2 | Human | 615 | [80] | |
| Serum | ECL | 34.59 | 73.45 | Human | 79 | [81] | |
| Blood | ECL | 46.3 | 108.9 | Human | 49 | [82] | |
| HD | CSF, Blood | ECL | 17.04, 13.79 | 491.1, 121.3 | In vivo (Mouse) | - | [83] |
| Spinal cord injury | Serum | ECL | 5 | 21 | Human | 23 | [84] |
| Traumatic brain injury | Serum | ELISA | 13 | 196 | Human | 72 | [85] |
| Repeated serum sampling and ventricular CSF | ELISA | 31 | 400 | Human | 182 | [86] | |
| Serum | ELISA | 9 | 22 | Human | 49 | [87] | |
| Charcot-Marie-Tooth | Plasma | Single Molecule Array | 14 | 26 | Human | 75 | [88] |
| DNA | Western Blotting, RT-PCR | - | In vivo (Mouse) | - | [89] | ||
| Motor neurons | PCR, GFP fluorescence, Cofractionation assay, immunoprecipitation assay and western blot | - | In vivo (Mouse) | - | [90] | ||
| SW13Vim− SW13Vim+ cell lines* | Western blotting, Indirect immunofluorescence microscopy | - | - | Human | - | [91] | |
| SW13Vim− SW13Vim+ cell lines* | Western blotting, Indirect immunofluorescence microscopy | - | - | Human | - | [92] | |
| Bipolar disorder | CSF | ELISA | 359 | 480 | Human | 133 | [93] |
| CSF | ELISA | 232> | 395 | Human | 77 | [94] | |
| CSF | ELISA | 254 | 485 | Human | 82 | [95] |
SW13 has two subtypes including SW13(vim-) which expresses neither BRG1 nor Brm, SW13(vim+) which does express both.
Autoantibodies against neuronal and axonal antigens are not only related to neurodegenerative disorders, but also are found in other CNS diseases [15]. In these diseases, tissue disruption occurs through various mechanisms, including activation of oligodendrocytes, complement activation, or physiological impairments [16]. It is has been shown that, when these antibodies against proteins of the cytoskeleton interact with neurons, cytoskeleton functions are impaired leading to neurodegeneration [17]. The deleterious effects of these anti-NF antibodies has been established in several experimental investigations. Immunization with NfL increased anti-NfL antibodies in mice [17]. In the immunized animals, axonal injuries and neurological symptoms were triggered. Anti-neurofilament antibodies seem to show damaging effects, particularly in the intra-neuronal compartments, but the possible existence of favorable effects should not be neglected. Prior studies of anti-NfH antibodies in the CSF and serum showed their presence, not only in subjects with different neurological disorders, but also to some extent in normal individuals [18,19]. It has been suggested that their presence might play a role in maintaining cognitive function. Talja et al., also showed that, in Down’s syndrome patients with mild to moderate disability, there was greater prevalence of serum antibodies against the NfH protein in comparison with patients with more severe disabilities [20].
Since obtaining CSF is considered too invasive, and CSF is not suitable for repetitive sampling or long-term follow-up, sequential blood samples would be a preferable alternative for measuring a biomarker. Therefore, blood Nf levels could be useful as a noninvasive, reproducible and rapid biomarker for both predicting and monitoring disease progression, and for assessing the efficiency or toxicity of future neuroprotective treatment approaches (Figure 1) [21].
Figure 1. A schematic of different types of Nfs and their detection in CNS related disorders.
Nfs act as neurotransmitter. These neurotransmitters are categorized based on their molecular weight. Different types of Nfs could be released in the CSF, serum and plasma. When occurred an injury an increased levels of Nfs will release in various body fluids including CSF, serum and plasma which could be detected by various techniques such as ELISA. NfL: NF light; NfM: NF medium; NfH: NF heavy; ELISA: Enzyme-linked immunosorbent assay
2.1. NfL Levels in Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS, also known as motor neuron disease) is an inexorable progressive disorder affecting the spinal cord and cerebellum resulting in motor neurodegeneration and ataxia (Figure 2) [14]. ALS may be challenging to diagnose, particularly in the early stages of the disease, because of its resemblance to other neurodegenerative diseases. Since the mean time to diagnosis is 16–19 months from the appearance of the first symptom, misdiagnosis is common. Although there is no effective therapy, early treatment with riluzole seems to decelerate the disease progression and extend survival. Therefore, establishing a sensitive biomarker for diagnosis, prognostic stratification and assessment of disease severity is required [21].
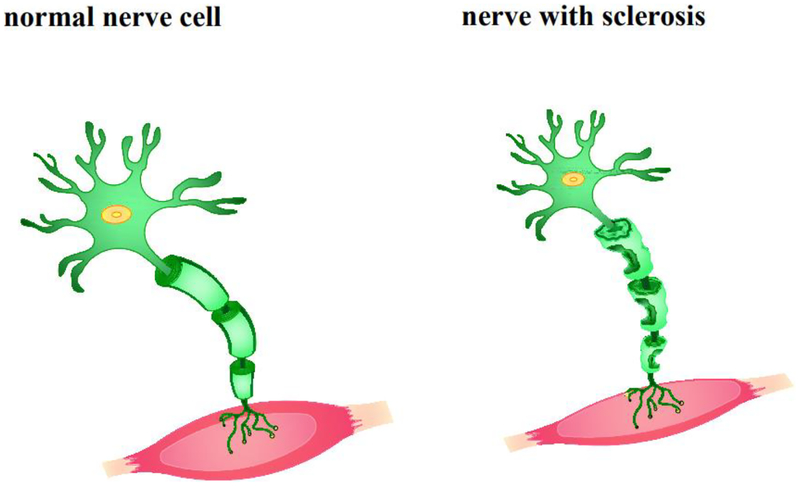
Figure 2.
A schematic representation of normal and ALS nerve cell. Injured motor neurons in the spinal cord and brain are ALS pathogenesis characteristic. The degenerated neurons are not capable of sending the impulses crucial for movement to the muscle fibers.
Many studies have shown that the levels of NfL in CSF provide both diagnostic and prognostic information for ALS [10,96–99]. These studies all showed that NfL levels in CSF and blood-based sample were higher in ALS patients compared to control groups without any signs of CNS structural disruption [10,96–99]. Moreover, the sensitivity and specificity have been evaluated in many of these studies using receiver operating curve (ROC) analysis, suggesting that NfL concentration may reflect the neurodegenerative process in ALS [14]. The increased amounts of CSF-NfL found in ALS could be due to the higher concentration of axonal proteins in motor neurons compared to other neuronal populations. In addition, the large myelinated axons in the motor neurons which are damaged could result in the release of more Nfs in the CSF [96]. High CSF-NfL concentrations in ALS are a predictor of a short TTG (time to generalization). These results confirm the role of NfL as a prognostic biomarker in ALS [100].
Using ROC analysis, Tortelli showed that an optimal NfL cut-off value of 1981 ng/L discriminated between ALS patients and controls. The correlation between CSF-NfL levels and the incidence of ALS was confirmed by multivariate logistic regression. CSF-NfL negatively correlated with the diagnostic delay, and positively correlated with the progression rate. The significant relationship between CSF-NfL levels and disease progression suggests that NfL may be a suitable biomarker for disease activity and progression in ALS [96].
Considering that ALS is known to be a heterogeneous disease, and that Nfs are markers of the general process of neurodegeneration and axonal damage [96], researchers have attempted to evaluate NfL levels in similar diseases such as AD and Guillain-Barré syndrome (GBS) [10]. CSF-NfL concentrations in ALS were generally higher than in AD and GBS. Moreover, CSF and serum NfL levels correlated with age in GBS and ALS. After correction for age, a significant difference remained between GBS and ALS, but not for AD versus controls. A similar study by Gianni [99] on frontotemporal dementia (FTD) and ALS patients, found that an NfL cut-off of 1843.52 pg/mL provided optimal discrimination between patients with ALS and other patients at a sensitivity of 81.9% and a specificity of 80.5%. A lower cut-off of 1380.48 pg/mL was most appropriate for differentiation between patients with ALS and controls at a sensitivity of 88.7% and a specificity of 89.4%. Conversely, a higher cut-off of 3113.03 pg/mL was optimal for differentiating between ALS and FTD, or motor neuropathies (MNs) with a sensitivity of 70.2% and a specificity of 86.8%. [99].
Studies in which both CSF and blood-based samples were evaluated as biomarkers in ALS, have confirmed the good correlation between CSF and serum/plasma levels [10,98,97]. For example Lu et al [98] showed that CSF, serum, and plasma NfL levels were higher in ALS patients, and could differentiate them from healthy controls with high sensitivity and specificity (cut-off: 1781 pg/mL, 36 pg/mL, and 36.2 pg/mL respectively). CSF NfL levels showed a significant correlation with NfL levels in serum. In this study, blood NfL levels of patients were robust, independent predictors of survival.
2.2. Parkinson’s Disease
Parkinson’s disease (PD) is the second most frequent neurodegenerative disease after AD [101]. PD is a movement disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra. The etiology of the disease is unknown but mutations in α-synnuclein have been found in rare cases of familial PD [102]. The prevalence of the disease is about 1% of the population older than 60 years of age, and the mean age of onset is estimated to be the early to mid 60s. The clinical manifestation of PD is principally motor dysfunction including involuntary tremor, bradykinesia, and rigidity [103].
It has been found that the symptoms and motor signs of PD are due to the degeneration of large parts of the substantia nigra (SN). PD is associated with the degeneration of multiple neurotransmitter systems (e.g. serotonin, noradrenaline, acetylcholine) [104] which are linked to non-motor diseases and can also impact cognitive function [105].
The most important challenge is differential diagnosis between PD and atypical Parkinsonian disorders (APD), including multiple system atrophy (MSA), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD). Reliable differentiation of PD from APD is often problematic due to the overlapping symptoms, especially during the early stages of the disease development [106,107].
Although there is no established biomarker for PD diagnosis [101], NfL protein in CSF may be a promising biomarker to distinguish PD from other similar diseases. The increased NfL levels in CSF could reflect the degree of neuronal cell apoptosis partly due to microglia-mediated inflammation [108]. It has been shown that the CSF concentration of NfL is increased in APD compared to PD [109,101], and that NfL levels in CSF can distinguish PD from MSA with a high degree of diagnostic accuracy, for instance, a 17.5 ng/L cut-off point gave high sensitivity (76–94%) and specificity (83–97%) [110].
The relation between PD disease severity and CSF-NfL levels is still controversial. While one study demonstrated that higher levels of NfL in CSF were associated with more severe PD, [111], a similar study claimed that although the NfL concentration was elevated in PD patients when compared to the normal group, there was no correlation between NfL levels and disease severity in PD patients [112]. Furthermore, there was no specificity with respect to the increase of NfL concentration. Interestingly, in that study, a positive correlation was found between CSF-NfL levels and VEGF [112].
In addition to CSF-NfL, blood-based NfL levels can be used to discriminate PD from APD. Several studies have demonstrated that, comparing PD and other diseases with healthy controls, blood-based NfL concentrations were increased in PSP, MSA, and CBD patients [110,109]. For example, Hansson et al. showed that blood NfL levels discriminated PD from APD in patients during the onset phase with 80% specificity and 70% sensitivity [109]. Therefore, blood-based NfL assays might be considered as a diagnostic biomarker in PD patients receiving primary care as well as in dedicated clinics [109].
2.3. Multiple Sclerosis (MS)
MS is a demyelinating autoimmune disease usually characterized by relapsing periods of neurological dysfunction. MS is the major cause of neurological disability among young adults. The onset of MS is usually followed several years later by progressive and permanent deterioration [113–117].
Axonal loss is one pathological element responsible for the progressive disability in both primary-progressive and relapsing-remitting MS, so finding a diagnostic biomarker to detect and quantify MS is of great importance [118]. NfL protein released into both CSF and blood in patients with MS could be a promising biomarker of disease activity, as well as other neurodegenerative diseases [118,116,119].
Researchers have demonstrated that CSF-NfL is increased in both relapsing-remitting and primary progressive MS, and CSF levels could be considered as a predictive biomarker of long-term disease outcome [120,121,117,116,119]. It is important to bear in mind that a cut-off value of CSF-NfL was considered 900 ng/L based on several previous studies [122–124]. These studies indicate that the CSF-NfL concentration is related to continuing axonal injury and mirrors the severity of the process. Therefore, CSF-NfL could be a promising biomarker for disease severity and progression, as well as for treatment assessment [125].
In addition to CSF-NfL, the potential value of serum NfL has been demonstrated as a biomarker of neuroaxonal injury in early MS [121,126]. Kuhle found that there was a relationship between baseline serum NfL, and whole brain atrophy, disability, and cognitive impairment [126] which supports the idea of the value of serum NfL as a noninvasive prognostic biomarker of the overall outcome of brain damage and continuing disease activity in early MS. The correlation between CSF-NfL and serum NfL has been investigated in several studies [121,119]. Novakova et al. found that the correlation between serum and CSF NfL levels was 0.62 in MS patients. For CSF-NfL, they found 75% specificity and 67% sensitivity, while serum NfL had 80% specificity and 45% sensitivity. In this study, serum NfL concentrations were considerably higher in patients with relapsing-remitting MS (16.9 ng/L) and in patients with progressive MS (23 ng/L) when compared to the healthy control group (10.5 ng/L) [119]. The calculated cut-off value of serum NfL in this study was 18.2 ng/L. While there is no common agreement on the relationship between CSF-NfL levels and age or gender [127–129], some studies have shown significant associations with age in both controls or patients [130,131].
As an overall conclusion, NfL levels in CSF or serum appear to be a sensitive and specific biomarker for white matter axonal damage in the CNS of MS patients with a significant relationship to disease severity and progression [121].
2.4. Alzheimer’s disease
AD is a common neurodegenerative disease that affects the cerebral cortex. The incidence increases with age, and about 30% of AD patients are above 85 years old [132]. The etiology of AD is not completely clear. Extracellular deposition of aggregated Aβ into amyloid plaques, and intraneuronal accumulation of neurofibrillary tau protein tangles are the considered to be the canonical pathophysiological hallmarks of AD. It is commonly believed that neurons that are injured by neurofibrillary tangles, amyloid plaques, or other causes, undergo breaks in the interneuronal connections. These synaptic breaks contribute to damage in the brain regions associated with cognition and memory (Figure 3) [133]. The abnormality of the cytoskeletal proteins observed in AD patients, is closely connected to the pathology of AD [132]. In addition to loss of cortical and hippocampal neurons and gray matter degeneration which are the principal features, progressive disconnection of cortical and subcortical regions due to disruption of white matter (WM) may be found in AD patients. WM atrophy can be demonstrated in tracts which are composed of large-caliber myelinated axons such as the corpus callosum, and in key regions such as the cingulum, which are rich in Nfs [134].
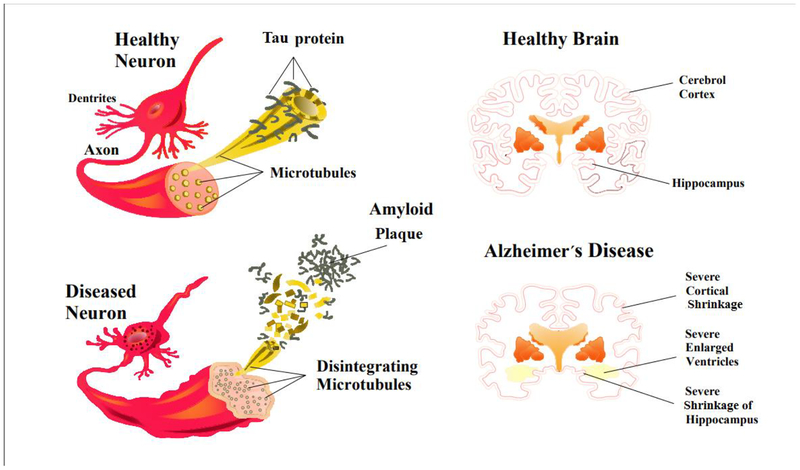
Figure 3.
A schematic showing different events in the pathogenesis of Alzheimer’s disease. An alteration in tau protein leads to microtubule breakdown in brain cells. A healthy neuron and an affected neuron are shown (A, B). Tau phosphorylation contributes to the formation of neurofibrillary tangles in Alzheimer’s disease. In patients with Alzheimer’s disease, hyperphosphorylation of specific amino acids in the tau protein leads to the proteins dissociating from the microtubules, and forming tau tangles. At the same time extracellular amyloid plaque disturbs the transport structure and leads to starvation of neurons, and ultimately induces cell death.
The brain changes in AD commence decades before the diagnosis of any disease [135]. Therefore, finding a noninvasive prognostic biomarker and testing potential early treatment options to prevent Alzheimer disease prior to the onset of symptoms is of great importance [136]. Several studies have assessed the level of CSF-NfL in AD patients [136,134,137]. Zetterberg et al. found that there was a significant correlation between CSF-NfL concentration and cognitive decline in AD patients. It was shown that CSF-NfL levels were higher in a AD dementia group as well as in a stable mild cognitive impairment (MCI) group, and in a progressive MCI group compared to a healthy control group. They also demostrated that CSF-NfL level was higher in AD dementia patients (mean 1479; 1134–1842 pg/mL), compared with the stable MCI group (mean 1182; 923–1687 pg/mL) and progressive MCI group (mean 1336; 1061–1693 pg/mL). A higher CSF NfL concentration was shown to be correlated with more rapid progression of brain atrophy over time, and it was associated with changes in the volume of the whole-brain, ventricular and hippocampal regions [134].
In a similar sudy, it was shown that CSF-NfL, could potentially discriminate AD pathophysiology-positive patients from a healthy control group, while the ability to segregate tau-positive patients from the healthy control group was only fair. It was also shown that CSF-NfL was not a satisfactory marker to distinguish AD pathophysiology-positive patients from FTD patients [138].
Blood based NfL levels have also been evaluated in AD [135,136]. It was confirmed that concentrations of plasma NfL in patients with AD, could be distinguished from healthy controls at a cut-off value of 25.7 pg/mL [135]. The values of absolute sensitivity, specificity, and accuracy were 84%, 78%, and 82%, respectively.
Similarly, serum NfL levels have been analysed in a study to distinct familial AD (FAD) from healthy controls. This study found that the serum NfL levels were amplified in FAD patients before symptom onset, and correlated with disease stage and severity [136].
To summarize, fluid based NfL assays may be a useful biomarker of AD-related neurodegeneration prior to symptom onset.
2.5. Frontotemporal dementia (FTD)
FTD describes a diverse spectrum of neurodegenerative disorders primarily affecting the frontal and temporal lobes [139]. The major causes of genetic FTD are mutations in the genes that encode microtubule-associated protein tau (MAPT), progranulin (GRN), or on chromosome 9 open reading frame 72 (C9orf72). Mutations in the C9orf72, GRN, and MAPT genes have been detected in 60% of familial FTD patients. C9orf72 mutations account for 25% of these, and are the most prevalent. Rarer mutations (<5%) take place in other genes including TBP, TBK1, ITM2B, FUS, TARDP, CHMP2B, and VPC [139–141].
Behavioral variant FTD (bvFTD), semantic dementia (SD) and progressive non-fluent aphasia (PNFA) are the principal clinical subtypes of FTD [142,143]. The clinicopathologic characteristics of corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP) overlap with those of FTD.
The main challenge in the diagnosis of FTD is determining the precise neuropathological subtype, based on clinical features alone. Although fluid biomarkers might aid in diagnosing disease onset and assessing disease-modifying treatments for FTD, few have as yet been investigated. Among CSF biomarkers for FTD, NfL has been recently assessed in several studies [139–141,13,144]. While several early studies showed variability in CSF-NfL concentrations in FTD, [145–147], others have claimed that CSF-NfL levels correlated with disease severity [144]. NfL levels in the CSF have been demonstrated to be increased in FTD (and in other neurodegenerative disorders like ALS, AD, PD and MS) [145–147,35,148, 149]. By contrast, a small series of presymptomatic individuals carrying FTD-causing mutations, have been found to have low levels of CSF-NfL [144]. In one study, the patients with neuropathologically verified FTD (although a limited number of cases) had NfL values that were significantly higher in the tau-negative cases (median 1620 ng/L) compared with the tau-positive cases (median 665ng/L). This study found no association between the levels of NfL and the severity of cortical degeneration [140]. Some studies have reported higher CSF-NfL levels in FTD patients, compared with both early-onset AD patients and a healthy control group [13]. Furthermore, it has been shown that the higher levels of NfL were correlated to shorter survival times in patients [140,141,150]. For example, higher CSF-NfL levels could distinguish patients from controls, using a cut-off level of 2165 pg/mL with sensitivity of 84% and specificity of 100% [141]. Similar to other degenerative diseases mentioned above, serum NfL levels have been investigated in several studies looking at biomarkers for FTD [141,150]. Serum NfL concentrations were shown to be correlated with CSF-NfL levels and were higher in FTD patients (mean 77.9 pg/mL) compared to controls (19.6 pg/mL). In addition, serum NfL levels were considerably higher in FTD patients (57.8 pg/mL) and in both the non-fluent and semantic variants of PPA (82.5 and 95.9 pg/mL, respectively) compared to normal controls. Moreover, serum NfL concentrations were higher in patients with semantic variant of PPA compared to the logopenic variant of PPA [150]. Similar to the previous study, levels of serum NFL were significantly higher in the C9orf72 mutation as well as the MAPT subgroups (79.2 pg/mL and 40.5 pg/mL respectively). Compared to these two latter subgroups, the GRN-mutated group had even higher concentrations of serum NfL (138.5 pg/mL) [141].
Overall, both CSF and serum NfL levels, could potentially serve as a valuable biomarker for clinical disease diagnosis and have a predictive value in genetic FTD [141,150].
2.6. Stroke
Elevated Nf levels in CSF after subarachnoid haemorrhage, represent the most convincing evidence that NfL analysis could be useful in stroke. Investigations have revealed that both NfL and NfH concentrations are increased in individuals with aneurysmal subarachnoid haemorrhage compared to patients with no neurological disease or to healthy controls [131,151,152]. In the absence of related focal injuries, the main mechanisms of Nf liberation in subarachnoid haemorrhage (ischemia due to vasospasm or parenchymal haematoma) are not completely obvious, but they could be connected to diffuse neuroaxonal damage or could possible be iatrogenic, for instance caused by placement of an external ventricular drain. A recent report suggested that raised Nf concentrations could be related to the extent and severity of morphological brain injury in stroke [151]. The evaluation of NfL levels in stroke by utilizing fourth-generation immunoassays to analyze NfL concentrations in blood samples, has been reported; however, there is usually no clinical indication for a lumbar puncture, so CSF analysis is not common. NfL serum levels were greater in spontaneous cervical artery dissection patients undergoing an ischemic stroke, than among transient ischemic attack subjects or those with isolated local symptoms [153]. Likewise, serum levels of NfL were found to be increased in affected individuals with a single, recent, small subcortical infarct in comparison to the levels in sex-matched and age-matched healthy subjects [154]. Furthermore, temporal dynamic evaluation of NfL levels at 3 and 15 months post-stroke showed particularly high concentrations in patients with new, clinically silent brain injuries associated with small vessel disease, that has been detected using magnetic resonance imaging (MRI) during follow-up. This observation suggests that increased levels of NfL are indicative of active small vessel disease. Also, during the first few days after stroke onset, serum levels of NfL were increased and remained elevated at a 3-month-follow-up assessment. Other investigations have documented similar findings of changes in Nf dynamics [121,155]. After acute neuronal injury, prolonged NfL release into the blood may be caused by persistent breakdown of the blood–brain barrier, but continuing post-ischemic inflammatory or immunological cascades could also be responsible.
2.7. Huntington’s disease (HD)
HD is a progressive neurodegenerative disorder, caused by repeated expression of CAG segments in the HTT gene, resulting in formation of mutant huntingtin protein.
HD commonly causes psychiatric, cognitive and movement disorders with a wide variety of signs and symptoms [156]. Recent investigations have proposed that in HD, the main pathogenic factor is the production of ubiquitinated aggregates of the N-terminal fragment of the mutated huntingtin protein. This aggregation is supposed to take place because of increased cleavage of the polyglutamine rich part of the mutant huntingtin N-terminus [157]. Aggregates of the mutant protein have been found in the striatum, hippocampus, pyramidal neurons, subiculum, entorhinal cortex, and the neocortex, especially in the case of juvenile and advanced onset HD patients. Neuropathological studies have shown that HD brain abnormalities probably commence well prior to the emergence of symptoms, and finally progress to affect the whole brain to a greater or lesser extent, leading to an approximately 25% loss of total brain weight in advanced HD [158]. However, gross atrophy occurring within the striatal part of the basal ganglia, accompanied by astrogliosis and extensive neuronal loss, is the most noticeable neuropathological finding. These changes become more severe as the disease progresses, with the atrophy resulting in excessive enlargement of the lateral ventricles [159].
Studies in biomarkers for HD, have suggested that changes in glutamic acid decarboxylase (GAD) or receptors for neuropeptides can be found in striatal neurons and their terminals. For instance, Grade 0 HD has been characterized by loss of adenosine receptor (A2a), dopamine receptor (D2) and cannabinoid receptor in the striatum, as well as large elevation in γ-amino-butyric acid A (GABAA) binding in external pallidal segments (GPe) [160]. These findings are consistent with the preferential loss of enkephalin-containing (ENK+) input to the GPe in Grade 0 HD. The absence of any decrease in D1 receptor binding in the internal pallidal segments (GPi) or the striatum in Grade O HD suggests that striatal substance P-containing (SP+) neurons, especially those projecting to the GPi are mostly unaffected in pre-symptomatic HD [160].
No proven disease-modifying therapies for HD have yet been discovered [161]. In HD the insidious and slow development of symptoms has made it challenging to detect disease-associated alterations in Nf proteins levels in blood [162]. However, elevated CSF concentrations of NfL have been reported in HD patients (control vs disease: 51.6 pg/mL, and 432.4 pg/mL respectively) [163,164], and fourth-generation technology has shown a good correlation between NfL plasma levels and HD onset, and the neurodegeneration progression (cut-off: 31·7 pg/mL) [161]. Therefore, to determine its usefulness as a diagnostic or prognostic biomarker in HD, NfL levels in blood should be considered in future investigations and clinical trials.
2.8. Bipolar disorder
Bipolar disorder (BD) is a chronic psychiatric disorder characterized by mood swings between manic and depressive states. BD affects 1–3% of the population and entails high costs for society, and is associated with great personal suffering, functional impairment, premature mortality, and a higher risk for other psychiatric and medical disorders [165]. It has been suggested that neuroaxonal injury and neurodegeneration could be related to BD [166]. Although neuroaxonal injury is not considered to be typical of BD, CSF concentrations of NfL were elevated in patients with BD (control vs disease: 359 pg/mL, 480 pg/mL)[165]. However, no significant correlation was shown between clinical outcomes and NfL concentrations, including suicide attempts, hypomanic or manic and depressive episodes, inpatient care or psychotic symptoms (cut-off: <395 pg/mL) [167]. Although the available data that neuroaxonal damage could be assessed in BD by Nf assays are limited, the current evidence warrants longitudinal investigations of well-diagnosed patients to test how Nf levels are altered in relation to disease phase (mania and depression), as well as whether Nfs are influenced by the adverse effects of therapies such as long-term lithium administration [168].
2.9. Spinal Cord Injury
Acute spinal cord injuries (SCI) are some of the most devastating accidents affecting young and active individuals. Mechanical injury of the spinal cord results in damage to neurons, axons, and glia at the area of impact [155]. Much effort has been put into the evaluation of SCI severity and prediction of recovery potential in affected individuals. Interventions designed to encourage the recovery of function following SCI include a combination of pharmacological, surgical and rehabilitation approaches. The benefits of these interventions, however, have been somewhat equivocal in clinical trials. It is assumed that patients with more severe SCIs respond differently to neuroprotective interventions than do patients with less severe SCIs. An accurate assessment of the initial extent of damage to the spinal cord that could differentiate between different severities of SCI may help physicians to choose a conventional treatment or explore experimental neuroprotective intervention in the acute phase [169]. Serum NfL may represent a useful indicator of SCI severity and the long-term outcome of neuronal injury, especially in cases where accurate clinical assessment is not possible. Further studies are warranted to assess the evidence for NfL as a treatment response marker in SCI [155].
2.10. Charcot-Marie-Tooth
Charcot-Marie-Tooth (CMT) disease is genetically and clinically heterogeneous. A large number of disease-causing mutations in several genes have been described. CMT is a neuromuscular disease characterized by length-dependent and progressive degeneration of peripheral nerves, manifesting with muscle wasting and weakness in the hands, feet and distal limbs. Its clinical severity ranges from mild to severe, and its onset can vary from childhood to adulthood in affected subjects. The neuropathological and neurophysiological impairments in the sensory and motor nerves contribute to the sensory deficits, wheelchair dependence, walking disabilities, and foot deformities. Recently, genetic and clinical investigations have suggested that CMT is quite heterogeneous. In the 1970s, a classification was suggested dividing the most prevalent CMT variants, as hereditary, sensory, and motor neuropathies. In CMT1 the Schwann cells are affected, while in CMT2 the axons undergo degeneration. Besides these two inherited autosomal dominant CMT sub-types, axonal as well as X-linked and recessive demyelinating subtypes of CMT have been recognized and are included in the mentioned classification [170]. Based on the severity of the sensory or motor deficits, other CMT subtypes have been categorized into hereditary sensory and autonomic neuropathies, and distal hereditary motor neuropathies [170]. Overlaps in both genetic analysis and clinical symptoms have been recently shown between hereditary spastic paraplegias and CMT neuropathies. Furthermore, some patients have more complex clinical phenotypes involving other tissues, including bone and skin [171,172].
Six pathogenic missense mutations and one 3-bp in-frame deletion in the NfL gene have been found in 323 patients with different CMT phenotypes. Mutations predominantly resulting in demyelination may cause concomitant axonal loss, and mutations primarily leading to axonal loss may be associated with demyelination. Mutations in the NfL gene also results in CMT neuropathies with variable clinical and electrophysiological characteristics. NfL mutations should be considered in the evaluation of patients with CMT or related neuropathy [173]. Nonsense NfL mutations probably cause a recessive phenotype, in contrast to missense mutations that cause a dominant phenotype in patients with CMT [12].
In one study, Sandelius et al., measured the plasma level of NfL in 142 subject (75 CMT subjects and 67 healthy subjects) and its relationship with disease severity [88]. Their results showed that the plasma levels of NfL were significantly higher in CMT subjects (median 26.0 pg/mL) compared to healthy subjects (median 14.6 pg/mL). Moreover, the plasma levels of NfL were correlated with neuropathy scores and disease severity. They reported that the subjects with different genetic subtypes including SPTLC1, CMT1A, and GJB1 had significantly higher levels of NfL compared to healthy subjects [88].
3. Assays to detect soluble neurofilaments
The sensitivity of immunoassay technologies has increased remarkably during the past three decades. Likewise, the detection of neurofilament biomarkers has also advanced, moving towards more clinically relevant applications (Figure 4). First-generation immunoassays were only semi-quantitative in nature. Methods such as immunoblots, which are based on electrophoretic protein separation, or dot blots, were able to show only the presence of neurofilament isoforms in the blood and CSF of subjects suffering from a range of diseases [174]. Second-generation sandwich ELISA technologies created the first trustworthy quantitative assays that enabled evaluation of the diagnostic and prognostic value of NfL and NfH determinations in the CSF of patients [175,176]. Human body fluids were analyzed with this method, expanded to include the vitreous fluid, amniotic fluid, plasma and serum, togther with extracellular or interstitial fluid [177,178].
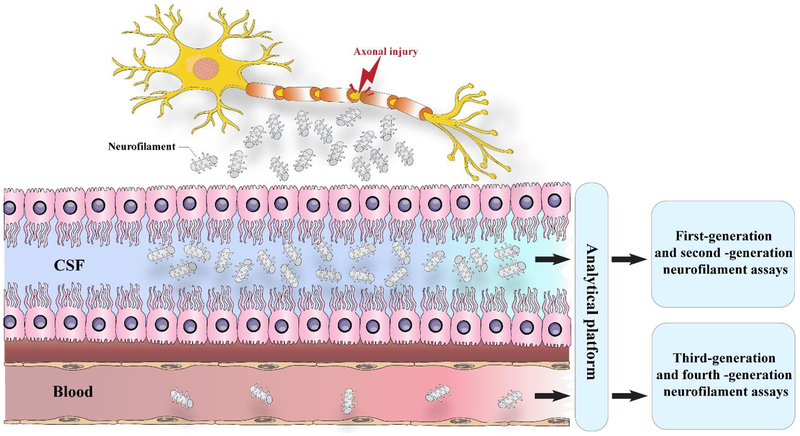
Figure 4. The release of Nf after axonal injury.
When an axon is injured, cytoskeletal proteins, such as neurofilaments, are released into the cerebrospinal fluid and, at lower levels, into the blood. First-generation (immunoblots) and second-generation (ELISA) immunoassays can measure neurofilaments in the CSF but have low sensitivity for detecting in the blood. Third-generation (electrochemiluminescence) and fourth-generation (single-molecule array) methods can detect blood concentrations of neurofilament light and measure subtle longitudinal alterations in healthy controls and in pathological cases.
International studies for validation and meta-analyses have found that high accuracy could be achieved in expert laboratories, but they also emphasized the requirement for standardization of the assays [179]. A considerable improvement in analytical sensitivity was achieved in third-generation ECL technology. ECL-based assays are recognized to require only a low sample volume, can have a broad dynamic range, and be highly sensitive. However, fourth-generation bead-based technology (SiMoA) is even more sensitive; 25-fold more sensitive than ECL, and 126-fold more than ELISA methods [180]. Therefore, the analytical sensitivity has steadily improved over time, leading to the reliable quantification of NfL levels in blood becoming possible for the wide range of levels found in both physiological conditions and in diseases [181]. The cutting-edge SiMoA technique is based on the simultaneous counting of individual microscopic beads with capture antibodies (2.7 μm diameter), and single-molecule arrays that work via sandwich antibody technology (one antigen recognized by two antibodies). The analytical sensitivity is many fold higher than that achieved using the same antibodies in an ELISA format, and can reliably measure the low NfL levels that are present in young healthy subjects, so that minor changes in concentrations of NfL that occur during normal aging or after mild injury could be detected [182]. A close relationship between concentrations of NfL in the CSF, and concentrations in the plasma or serum, has been established in several studies on individuals with different neurological disorders. This relationship permits measurements to be made on blood samples without any requirement to obtain CSF using lumbar puncture [183]. Studies of NfM have been sparse, but commercial SiMoA kits for the measurement of phosphorylated NfH as well as NfL are now available [184].
4. Magnetic resonance imaging and CNS-related disorders
The prognosis and diagnosis of CNS-related disorders can be achieved by combining both biomarkers and a neuroimaging approach [185]. Although the early diagnosis of CNS-related disorders is possible with the aid of various neuroimaging methods, there is need for gold standard in this context. Accordingly, it may be possible to screen individuals at high risk for neurological disorders and to test novel neuroprotective agents in such populations, aiming at the management of disease progression and the discovery of new disease-modifying interventions [186].
Magnetic resonance imaging (MRI) is a noninvasive neuroimaging approach to provide indices useful in the diagnosis and prognosis of CNS-related disorders [97]. Visual scoring, local morphometry and volumetric analyses are the principles behind structural MRI evaluation of different CNS-related disorders. The available guidelines are not only non-specific for accurately differentiating neurodegenerative diseases, but also require experienced clinical expertise and include a certain degree of subjective assessment. Automatic image quantification and computerized support for decisions are able to provide more information, than simple examination of images with the human eye, in order to differentiate the target disease from other neurodegenerative diseases, and may offer support for inexperienced clinicians [186].
Besides structural (anatomical) MRI, diffusion tensor imaging (DTI-MRI) can assess diffusivity (reflecting microstructural damage) and fractional anisotropy (reflecting white matter tract integrity), and may provide a higher degree of sensitivity and specificity. DTI is based on the measurement of “the random motion of water molecules in fluid water” [187]. Diffusion of water molecules is not identical in different structures of the brain. For example, water molecules move more easily along axonal bundles of white matter, resulting in anisotropic diffusion. In contrast, diffusion usually is isotropic in the gray matter because it does not possess a clear structural arrangement. This diffusion property of water can be used as a quantitative tool to interpret the brain anatomy [188]. It has many advantages to study the integrity and orientation of white matter in both normal and pathological states [189]. Mean diffusivity (MD) and fractional anisotropy are two fundamental metrics in DTI. MD illustrates the diffusion of water molecules in a biological tissue [190]. Also, values of fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AxD) are characteristic of white matter alteration in the brain. While decreased FA is a frequent observation in brain tissue injury, RD is a measure of diffusion perpendicular to the axons, and is therefore commonly associated with the microstructure of myelin [189].
Various studies have established that NfL and MRI indices might be considered as promising biomarkers for diagnosis or prediction of disease development in different neurological disorders. Several studies have been carried out to relate these two biomarkers directly in many common neurodegenerative diseases. It will be a major improvement if scientists can combine NfL with MRI measures, and identify the type and location of neurodegeneration before any clinical presentation of symptoms.
5. Correlation of MRI parameters and NFL
5.1. Amyotrophic lateral sclerosis
Combining biological biomarkers with neuroimaging biomarkers is critical in diagnosing and monitoring neurodegenerative disease [191,192]. Researchers have put much effort into investigating the correlation of CSF and/or blood based biomarkers such as NfL, with DTI imaging in ALS. In one study, CSF and serum NfL concentrations combined with DTI using a 3T MRI machine with 12 head coils was analyzed. It was found that NfL levels had a negative correlation with FA and a positive correlation with RD in the DTI of the corticospinal tract (CST) of ALS patients when compared to controls. The positive correlation between RD and NfL levels could be explained by the sensitivity of RD to Wallerian-type myelin degeneration found in ALS. In contrast, no association between NfL levels and AxD was observed [191].
Degeneration of the CST in ALS results in alteration of NfL levels and DTI markers. Therefore, it can be concluded that longitudinal DTI analysis of white matter changes is a noninvasive and quantitative biomarker that can be combined with NfL levels as a more robust method to monitor neurodegeneration, which correlates with clinical progression in ALS [191,192].
One study reported the correlation of CSF-NfL with FA and different DTI metrics (MD, RD). The changes in FA and RD were sensitive to loss of axonal integrity, demyelination and Wallerian-type myelin degeneration, especially in chronic diseases with pronounced axonal damage [193]. Evidence suggests a relationship between MD alterations and changes in cellularity, probably due to myelinated axonal loss [194,195]. The findings should be interpreted cautiously, because of the uncertain biological basis of the differences in DTI variables, particularly for pathological comorbidities, including axonal damage, demyelination and inflammation [193]. These results are consistent with studies that reported the relationship between higher CSF-NfL levels and altered DTI metrics in ALS [196,197]. However they are inconsistent with another ALS study that found no relationship between DTI CST integrity and CSF-NfL [196]. Steinacker et al. scanned patients using two different MRI systems with two different field strengths (two thirds with a 1.5T MRI, and one third with a 3T MRI). Their results showed comparable data from the two systems, therefore all the DTI values were combined in a single analysis. The 1.5T field strength gave a signal-to-noise ratio lower than the 3T field strength, potentially masking the relationship between FA values and NfL levels [196]. Menke et al. [31] found a relationship between NfL levels and both FA and RD values in ALS patients using data from a single 3T scanner with a protocol similar to the Steinacker study. This field strength may be able to increase the study sensitivity to detect the effects masked by noise.
Contradictory results were reported by another study, which found no relationship between serum NFL levels and ALS pathological stage using DTI for assessing the integrity of brain white matter tracts [198]. There are reports on the relationship between CSF-Nfs and damage caused to upper or lower motor neurons in different parts of the body, as well as between CSF-NfL levels and MRI markers of corticospinal tract degeneration [22,31].
5.2. Multiple sclerosis (MS)
The CSF or serum NfL levels have been investigated as biomarkers along with MRI of lesions to assess relapses, neurological disability and treatment methods in MS [199].
The annual serum NfL levels were measured to predict 10-year clinical progress and MRI findings in MS patients, using a brain MRI acquisition protocol on a 3T unit with three sagittal sequences of 3D T1-weighted gradient echo, 3D T2 spin echo, and 3D T2-FLAIR. The evaluation of the relationship between NfL levels and 10 years of measurement of brain parenchymal fraction (BPF) exhibited an inverse relationship between year 5 NfL levels and year 10 BPF. There were statistically significant relationships between averaged annual NfL values and year 10 BPF. The evaluation of relationship between NfL levels and year 10 T2LV revealed a positive relationship between years 1–4 with T2LV, highlighting the relationship between higher NfL levels and more severe brain lesions. According to the results from this study, there was a relationship between both early and averaged annual serum NfL levels, and 10-year MRI findings and worsening fatigue. The relationship between early NfL levels and long-term progression suggests the necessity for predictive models to detect patients at risk for more severe disease [199].
One study examined baseline and annual brain MRI scans (T1w MPRAGE and T2w lesion) on patients using a 1.5 T magnetic resonance scanner [200]. The relationship between serum NfL levels and brain MRI findings was evaluated to compare disease activity and normalized brain volume. The results showed that the serum NfL was enhanced with increasing lesion size and was related to all the MRI metrics. The multivariable model exhibited a relationship between each contrast-enhancing lesion and increased serum NfL levels. There was a relationship between smaller normalized brain volume and higher serum NfL levels. However, the multivariable analysis showed no relationship between NfL and T2 lesion volume. They suggested the increased serum NfL levels were caused by focal active inflammation, which was measured by brain contrast enhancing lesions and enlarging T2 lesions. There was a strong relationship between the serum NfL levels and the number of T2 lesions, which are a more comprehensive marker of brain lesion burden, suggesting the serum NfL could be used to detect the extent of brain injury in individual patients. The findings also underlined the relationship between serum NfL levels and the normalized brain volume at the time of sampling. They concluded that the serum NfL levels could be used as a quantitative marker for the extent of neuronal loss in the CNS [200].
5.3. AD and FTD
In one study, Weston et al., carried out MRI in AD patients on the same 3T Siemens scanner, while also taking blood samples, they used sagittal 3D magnetization-prepared rapid gradient echo (mparage) T1-weighted volumetric MRI that allowed calculation of whole brain, ventricular, and hippocampal volumes [57]. They also computed the annual rate of changes in brain, ventricular, and hippocampal volumes over the interscan intervals, as well as exploring a relationship between NfL and MRI findings to measure AD-related neurodegeneration. Their results revealed a relationship between serum NfL levels and MRI findings for cross-sectional volume loss and atrophy, indicating a relationship between serum NfL levels and AD severity or progression rate [57].
A similar study carried out 3D-SPGR T1-WI cortical reconstruction and volumetric segmentation on a 3.0T MRI scanner, and measured the cortical thickness of AD and FTD patients in different anatomical regions in comparison with controls [201]. They studied the hippocampus, parietal, and frontal lobes in the AD group, and superior frontal, orbito-frontal, caudal middle frontal, rostral middle frontal, inferior and superior parietal lobes in the FTD group. The differences in cortical thickness between patients and controls were calculated in accordance with an equation. The assumption was a priori, so that the cortical thickness was lower in the patients compared to the control. Their results revealed a marginal inverse correlation between NfL levels and left orbito-frontal cortical thickness in the FTD group. This biological finding, although not statistically significant, confirmed the relationship between elevated NfL levels and increased left frontal lobe atrophy in the patients suffering from semantic variant primary progressive aphasia (svPPA) and nonfluent variant primary progressive aphasia (nfvPPA) but not from lvPPA23. According to studies conducted on genetic FTD, NfL levels showed an inverse correlation with whole brain volume and with the volume of frontal, temporal, parietal, insular and cingulate cortices [201].
In another study, Rohrer et al., carried out volumetric T1 brain MRI on a 3T scanner in patients with FTD on the same day as serum sampling, over a maximum of 6 months. They measured whole-brain and individual lobar cortical volumes [68]. The difference in volume between the baseline and follow-up scans was used to compute annualized lobar atrophy. The relationship between serum NfL levels and the cognitive and imaging parameters was assessed by Pearson correlation coefficient. No significant correlation was found with baseline brain volumes. There was a correlation between the serum NfL levels and rates of whole brain, frontal lobe, and parietal lobe atrophy, but not between the serum NfL levels and other lobar atrophy rates. The correlation with frontal lobe atrophy was corrected for multiple comparisons. According to their results, the serum NfL levels showed a relationship between the extent of subsequent brain atrophy, but not with the baseline brain volumes. The brain atrophy parameters are probably more suitable for the detection of disease severity when compared with the cross-sectional index of the whole-brain or lobar volumes, reflecting disease duration and illness severity. There was a relationship between serum NfL levels and baseline indices of executive function, but not between serum NfL levels and longitudinal indices [68].
White matter damage in FTD was investigated using DTI-MRI analysis in several studies. Decreased FA and increased MD in the white matter was a common finding in these studies in FTD patients [202–204]. One study found that FA was increased and MD was decreased within the body of the corpus callosum, bilateral cingulum bundle, and bilateral uncinate fasciculus [205]. NfL levels in CSF or serum has been investigated as a potential biomarker in predicting neurodegeneration in FTD [145–147,35,148]. Up to now, we have found only one study comparing white matter damage in FTD using NfL measures, and DTI indices in which FA, MD, AD and RD were measured [206]. In that study, twenty white matter tracts were analyzed based on tract-specific correlation. In the fronto-posterior tracts, a positive correlation was found between NfL measures, and MD, AD and particularly with RD, and a negative relationship with FA was observed in frontal regions. There were no correlations found in C9orf72 carriers [206]. It was concluded that combining NfL levels and DTI measures together resulted better monitoring of white matter damage in FTD [206].
5.4. Stroke
Studies have investigated correlations between NfL levels and cerebral small vessel disease (SVD), which is a leading cause of stroke and vascular cognitive dysfunction. One study examined patients with SVD using a single 3T/1.5T MRI scanner with a standardized protocol, consisting of 3D-T1, FLAIR, T2, and DTI sequences [207]. White matter hyperintensity (WMH), lacunae, brain volumes and the number of cerebral microbleeds were measured in this study, followed by evaluation of relationships between the serum NfL levels and established MRI markers for SVD, corrected for age and sex. According to a simple linear regression model, there was a significant relationship between serum NfL levels and all the MRI markers, as well as age in the patients with CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), and sporadic SVD. The most significant correlation was with mean diffusivity in SVD patients. A significant relationship was found after controlling for age. There was a relationship between the serum NfL levels and MRI, as well as with clinical features in hereditary and sporadic SVD, thereby verifying the use of MRI to assess SVD severity [207].
Another study investigated the temporal characteristics of serum NfL levels and its relationship with MRI findings of neuroaxonal injury, and with clinical outcomes [79]. There was a relationship between six-month post-stroke serum NfL levels and recurrent ischemic lesions in six-month post-stroke brain MRI scans. Recurrent lesions increased the serum NfL levels during the follow-up period. It should be noted that there was also a relationship between the six-month post-stroke serum NfL levels and MRI findings of secondary neurodegeneration. Moreover, the results showed a relationship between increased serum NfL levels and the shift of MD toward higher levels in the main white matter tracts on the same side of the body as the infarct [79].
5.5. Huntington’s disease (HD)
In one study, they evaluated the levels of mutant huntington (mHTT) and NfL proteins in CSF and blood, as well as the clinical findings and MRI scans in HD mutation carriers, both before and after symptoms emerged [208]. Annual MRI scans were performed in the HD mutation carriers using standardized 3-T T1 volumetric MRI. The demographic, clinical, and biochemical profiles of the patients were the same as those that did not receive MRI scans. Moreover, the patients underwent CSF mHTT, CSF-NfL and plasma NfL measurement. There was a relationship between the CSF-NfL levels and pre-specified MRI volume measurements of the whole brain, white matter, gray matter, and caudate regions, which all were computed as tissue lesion volume (TIV) and age-related percentages. The age and the number of CAG repeats was correlated with MRI findings in the gray matter and caudate. The serum NfL levels showed a relationship with whole brain, gray matter and caudate volumes. At any given time point, the serum NfL levels showed a relationship with clinical and MRI measures. According to the results from the simultaneous assessment of CSF-mHTT, CSF-NfL, and serum NfL in the HD-CSF cohort, a significant relationship was found between the serum NfL levels and clinical severity, while only NfL levels were related to MRI brain volume [208].
5.6. Traumatic brain injury (TBI)
One of leading causes of death in patients with TBI is diffuse axonal injury (DAI). No easy and reliable approaches are available for early diagnosis and prognosis of long-term outcome in DAI patients [209]. Analysis was performed to assess the relationships between the serum NfL levels and MRI in the acute stage, as well as to follow clinical outcome and MR-DTI parameters over 12 months. The results showed a 30-fold increase in the mean NfL levels in the patients when comparing with the controls, as well as a significant difference in the serum NfL levels between the patients and the controls. There was also a relationship between serum NFL and MR-DTI indices. Moreover, higher NfL levels were found in patients with higher trace and lower FA, suggesting the importance of the serum NfL levels as a blood biomarker for DAI intensity in the TBI patients [209].
6. Conclusions
The models for CNS and PNS diseases propose that complex pathophysiological events occur though a sequence of multiple mechanisms (i.e., neurodegeneration and axonal damage). Following injury to the axons of the PNS or CNS, Nfs are detectable in CSF and also in the circulation. Therefore, increased levels of Nfs could be a new biomarker for diagnosis of neurodegenerative disorders. Among different Nfs, evidence suggests that there are significant correlations between the concentrations of NfL in serum, plasma and CSF with the progression of CNS-related disorders. One of crucial aspects of using NfL as a diagnostic biomarker, is to develop new generation assays for detecting this protein in CSF, serum and plasma. Nowadays, there are different assays for the detection of NfL. It has been shown that the use of immunoblots as a first-generation assay, and ELISA as a second-generation assays for detection of Nfs are associated with limited sensitivity. Recently, several studies showed that electrochemiluminescence as a third-generation assay, and the single-molecule array as a fourth-generation assay are able to the reliably measure Nfs in a wide range of biological samples. Along with biochemical biomarkers, MRI is an approved technique for diagnosis of CNS-related disorders. There is a relationship between the average yearly serum NfL concentrations and early MRI findings, and worsening fatigue rates. The correlation of short-term and long-term outcomes with early NfL concentrations, suggests a predictive model, which can identify patients at risk for more severe disease and who require more aggressive therapy. Further studies will unveil the impacts of different therapies on the NfL concentrations. More investigation is needed to confirm these results, and to search for additional predictors of short-term and long-term disease progression correlated with MRI findings using machine learning and multivariate models. Furthermore, there is a positive association between NfL and T1, T2 relaxation times in white matter and cortical gray matter of normal appearance, which reinforces the utility of serum NfL to help diagnose diffuse white matter pathologies, although these associations require to be validated in larger studies. Taken together, the correlation between MRI metrics and NfL concentrations could be used as a powerful predictor for detection and monitoring of CNS-related disorders.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Strimbu K, Tavel JA (2010) What are biomarkers? Current opinion in HIV and AIDS 5 (6):463–466. doi: 10.1097/COH.0b013e32833ed177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Califf RM (2018) Biomarker definitions and their applications. Experimental biology and medicine (Maywood, NJ) 243 (3):213–221. doi: 10.1177/1535370217750088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim J, Yue Z (2015) Neuronal aggregates: formation, clearance, and spreading. Developmental cell 32 (4):491–501. doi: 10.1016/j.devcel.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeromin A, Bowser R (2017) Biomarkers in Neurodegenerative Diseases. Adv Neurobiol 15:491–528. doi: 10.1007/978-3-319-57193-5_20. [DOI] [PubMed] [Google Scholar]
- 5.Di Battista AP, Buonora JE, Rhind SG, Hutchison MG, Baker AJ, Rizoli SB, Diaz-Arrastia R, Mueller GP (2015) Blood biomarkers in moderate-to-severe traumatic brain injury: potential utility of a multi-marker approach in characterizing outcome. Frontiers in neurology 6:110. doi: 10.3389/fneur.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henley SM, Bates GP, Tabrizi SJ (2005) Biomarkers for neurodegenerative diseases. Current opinion in neurology 18 (6):698–705 [DOI] [PubMed] [Google Scholar]
- 7.Herrmann W, Obeid R (2011) Homocysteine: a biomarker in neurodegenerative diseases. Clinical chemistry and laboratory medicine 49 (3):435–441. doi: 10.1515/CCLM.2011.084 [DOI] [PubMed] [Google Scholar]
- 8.Soylu-Kucharz R, Sandelius Å, Sjögren M, Blennow K, Wild EJ, Zetterberg H, Björkqvist M (2017) Neurofilament light protein in CSF and blood is associated with neurodegeneration and disease severity in Huntington’s disease R6/2 mice. Scientific reports 7 (1):14114. doi: 10.1038/s41598-017-14179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs E, Cleveland DW (1998) A structural scaffolding of intermediate filaments in health and disease. Science 279 (5350):514–519 [DOI] [PubMed] [Google Scholar]
- 10.Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL (2013) Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PloS one 8 (9):e75091. doi: 10.1371/journal.pone.0075091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petzold A, Thompson EJ, Keir G, Quinn N, Holmberg B, Dizdar N, Wenning GK, Rascol O, Tolosa E, Rosengren L (2009) Longitudinal one-year study of levels and stoichiometry of neurofilament heavy and light chain concentrations in CSF in patients with multiple system atrophy. Journal of the neurological sciences 279 (1–2):76–79. doi: 10.1016/j.jns.2008. [DOI] [PubMed] [Google Scholar]
- 12.Abe A, Numakura C, Saito K, Koide H, Oka N, Honma A, Kishikawa Y, Hayasaka K (2009) Neurofilament light chain polypeptide gene mutations in Charcot–Marie–Tooth disease: nonsense mutation probably causes a recessive phenotype. Journal of human genetics 54 (2):94–7. doi: 10.1038/jhg.2008.13. [DOI] [PubMed] [Google Scholar]
- 13.Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL (2014) Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Annals of neurology 75 (1):116–126. doi: 10.1002/ana.24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zetterberg H, Jacobsson J, Rosengren L, Blennow K, Andersen P (2007) Cerebrospinal fluid neurofilament light levels in amyotrophic lateral sclerosis: impact of SOD1 genotype. European journal of neurology 14 (12):1329–1333 [DOI] [PubMed] [Google Scholar]
- 15.Sadaba MC, Tzartos J, Paino C, Garcia-Villanueva M, Alvarez-Cermeno JC, Villar LM, Esiri MM (2012) Axonal and oligodendrocyte-localized IgM and IgG deposits in MS lesions. Journal of neuroimmunology 247 (1–2):86–94. doi: 10.1016/j.jneuroim.2012.03.020 [DOI] [PubMed] [Google Scholar]
- 16.Huizinga R, Linington C, Amor S (2008) Resistance is futile: antineuronal autoimmunity in multiple sclerosis. Trends in immunology 29 (2):54–60. doi: 10.1016/j.it.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 17.Huizinga R, Heijmans N, Schubert P, Gschmeissner S, t Hart BA, Herrmann H, Amor S (2007) Immunization with neurofilament light protein induces spastic paresis and axonal degeneration in Biozzi ABH mice. Journal of neuropathology and experimental neurology 66 (4):295–304. doi: 10.1097/nen.0b013e318040ad5c [DOI] [PubMed] [Google Scholar]
- 18.Silber E, Semra YK, Gregson NA, Sharief MK (2002) Patients with progressive multiple sclerosis have elevated antibodies to neurofilament subunit. Neurology 58 (9):1372–1381. doi: 10.1212/wnl.58.9.1372 [DOI] [PubMed] [Google Scholar]
- 19.Soussan L, Tchernakov K, Bachar-Lavi O, Yuvan T, Wertman E, Michaelson DM (1994) Antibodies to different isoforms of the heavy neurofilament protein (NF-H) in normal aging and Alzheimer’s disease. Molecular neurobiology 9 (1–3):83–91. doi: 10.1007/bf02816107 [DOI] [PubMed] [Google Scholar]
- 20.Talja I, Reimand T, Uibo O, Reimand K, Aun S, Talvik T, Janmey PA, Uibo R (2009) Antibodies to neurofilaments. Annals of the New York Academy of Sciences 1173 (1):130–136. doi: 10.1111/j.1749-6632.2009.04624.x [DOI] [PubMed] [Google Scholar]
- 21.Turner MR, Kiernan MC, Leigh PN, Talbot K (2009) Biomarkers in amyotrophic lateral sclerosis. The Lancet Neurology 8 (1):94–109. doi: 10.1016/S1474-4422(08)70293-X [DOI] [PubMed] [Google Scholar]
- 22.Poesen K, De Schaepdryver M, Stubendorff B, Gille B, Muckova P, Wendler S, Prell T, Ringer TM, Rhode H, Stevens O, Claeys KG, Couwelier G, D’Hondt A, Lamaire N, Tilkin P, Van Reijen D, Gourmaud S, Fedtke N, Heiling B, Rumpel M, Rodiger A, Gunkel A, Witte OW, Paquet C, Vandenberghe R, Grosskreutz J, Van Damme P (2017) Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 88 (24):2302–2309. doi: 10.1212/wnl.0000000000004029 [DOI] [PubMed] [Google Scholar]
- 23.Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S, Tumani H, von Arnim CAF, Dorst J, Feneberg E, Mayer B, Muller HP, Gorges M, Rosenbohm A, Volk AE, Silani V, Ludolph AC, Otto M (2018) Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. Journal of neurology, neurosurgery, and psychiatry. doi: 10.1136/jnnp-2018-318704 [DOI] [PubMed] [Google Scholar]
- 24.Gille B, De Schaepdryver M, Goossens J, Dedeene L, De Vocht J, Oldoni E, Goris A, Van Den Bosch L, Depreitere B, Claeys KG, Tournoy J, Van Damme P, Poesen K (2018) Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathology and applied neurobiology. doi: 10.1111/nan.12511 [DOI] [PubMed] [Google Scholar]
- 25.Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, La Bella V (2018) CSF neurofilament proteins as diagnostic and prognostic biomarkers for amyotrophic lateral sclerosis. 265 (3):510–521. doi: 10.1007/s00415-017-8730-6 [DOI] [PubMed] [Google Scholar]
- 26.Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P, Gray E, Grosskreutz J, Jardel C, Kuhle J, Koerner S, Lamari F, Amador MDM, Mayer B, Morelli C, Muckova P, Petri S, Poesen K, Raaphorst J, Salachas F, Silani V, Stubendorff B, Turner MR, Verbeek MM, Weishaupt JH, Weydt P, Ludolph AC, Otto M (2018) Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology 90 (1):e22–e30. doi: 10.1212/wnl.0000000000004761 [DOI] [PubMed] [Google Scholar]
- 27.Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E, Soraru G (2017) Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis: Neurofilament Light Chain Levels in Definite Subtypes of Disease. JAMA neurology 74 (5):525–532. doi: 10.1001/jamaneurol.2016.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinacker P, Huss A, Mayer B, Grehl T, Grosskreutz J, Borck G, Kuhle J, Lule D, Meyer T, Oeckl P, Petri S, Weishaupt J, Ludolph AC, Otto M (2017) Diagnostic and prognostic significance of neurofilament light chain NF-L, but not progranulin and S100B, in the course of amyotrophic lateral sclerosis: Data from the German MND-net. Amyotrophic lateral sclerosis & frontotemporal degeneration 18 (1–2):112–119. doi: 10.1080/21678421.2016.1241279 [DOI] [PubMed] [Google Scholar]
- 29.Oeckl P, Jardel C, Salachas F, Lamari F, Andersen PM, Bowser R, de Carvalho M, Costa J, van Damme P, Gray E, Grosskreutz J, Hernandez-Barral M, Herukka SK, Huss A, Jeromin A, Kirby J, Kuzma-Kozakiewicz M, Amador Mdel M, Mora JS, Morelli C, Muckova P, Petri S, Poesen K, Rhode H, Rikardsson AK, Robberecht W, Rodriguez Mahillo AI, Shaw P, Silani V, Steinacker P, Turner MR, Tuzun E, Yetimler B, Ludolph AC, Otto M (2016) Multicenter validation of CSF neurofilaments as diagnostic biomarkers for ALS. Amyotrophic lateral sclerosis & frontotemporal degeneration 17 (5–6):404–413. doi: 10.3109/21678421.2016.1167913 [DOI] [PubMed] [Google Scholar]
- 30.Weydt P, Oeckl P, Huss A, Muller K, Volk AE, Kuhle J, Knehr A, Andersen PM, Prudlo J, Steinacker P, Weishaupt JH, Ludolph AC, Otto M (2016) Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann Neurol 79 (1):152–158. doi: 10.1002/ana.24552 [DOI] [PubMed] [Google Scholar]
- 31.Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Annals of clinical and translational neurology 2 (7):748–755. doi: 10.1002/acn3.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu CH, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M, Orrell R, Howard R, Talbot K, Greensmith L, Kuhle J, Turner MR, Malaspina A (2015) Neurofilament light chain: A prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84 (22):2247–2257. doi: 10.1212/wnl.0000000000001642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tortelli R, Copetti M, Ruggieri M, Cortese R, Capozzo R, Leo A, D’Errico E, Mastrapasqua M, Zoccolella S, Pellegrini F, Simone IL, Logroscino G (2015) Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. Eur J Neurol 22 (1):215–218. doi: 10.1111/ene.12421 [DOI] [PubMed] [Google Scholar]
- 34.Tortelli R, Ruggieri M, Cortese R, D’Errico E, Capozzo R, Leo A, Mastrapasqua M, Zoccolella S, Leante R, Livrea P, Logroscino G, Simone IL (2012) Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. Eur J Neurol 19 (12):1561–1567. doi: 10.1111/j.1468-1331.2012.03777.x [DOI] [PubMed] [Google Scholar]
- 35.Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, von Arnim CA, Böhm S, Kassubek J, Kubisch C (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. Journal of neurology, neurosurgery, and psychiatry 87 (1):12–20 [DOI] [PubMed] [Google Scholar]
- 36.Siller N, Kuhle J, Muthuraman M, Barro C, Uphaus T, Groppa S, Kappos L, Zipp F, Bittner S (2018) Serum neurofilament light chain is a biomarker of acute and chronic neuronal damage in early multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England): 10.1177/1352458518765666 [DOI] [PubMed] [Google Scholar]
- 37.Novakova L, Zetterberg H, Sundstrom P, Axelsson M, Khademi M, Gunnarsson M, Malmestrom C, Svenningsson A, Olsson T, Piehl F, Blennow K, Lycke J (2017) Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89 (22):2230–2237. doi: 10.1212/wnl.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmestrom C, Piehl F, Olsson T, Lycke J (2017) Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. Journal of neurochemistry 141 (2):296–304. doi: 10.1111/jnc.13881 [DOI] [PubMed] [Google Scholar]
- 39.Novakova L, Axelsson M, Khademi M, Zetterberg H, Blennow K, Malmestrom C, Piehl F, Olsson T, Lycke J (2017) Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 23 (1):62–71. doi: 10.1177/1352458516639384 [DOI] [PubMed] [Google Scholar]
- 40.Hakansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, Dahle C, Vrethem M, Ernerudh J (2017) Neurofilament light chain in cerebrospinal fluid and prediction of disease activity in clinically isolated syndrome and relapsing-remitting multiple sclerosis. Eur J Neurol 24 (5):703–712. doi: 10.1111/ene.13274 [DOI] [PubMed] [Google Scholar]
- 41.Disanto G, Barro C, Benkert P, Naegelin Y, Schadelin S, Giardiello A, Zecca C, Blennow K, Zetterberg H, Leppert D, Kappos L, Gobbi C, Kuhle J (2017) Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 81 (6):857–870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, Yaldizli O, Regeniter A, Derfuss T, Canales M, Schluep M, Du Pasquier R, Krueger G, Granziera C (2016) Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Multiple sclerosis (Houndmills, Basingstoke, England) 22 (12):1550–1559. doi: 10.1177/1352458515623365 [DOI] [PubMed] [Google Scholar]
- 43.Disanto G, Adiutori R, Dobson R, Martinelli V, Dalla Costa G, Runia T, Evdoshenko E, Thouvenot E, Trojano M, Norgren N, Teunissen C, Kappos L, Giovannoni G, Kuhle J (2016) Serum neurofilament light chain levels are increased in patients with a clinically isolated syndrome. Journal of neurology, neurosurgery, and psychiatry 87 (2):126–129. doi: 10.1136/jnnp-2014-309690 [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Li X, Qiao J (2007) [Neurofilament protein light in multiple sclerosis]. Zhonghua yi xue za zhi 87 (39):2745–2749 [PubMed] [Google Scholar]
- 45.Zhou W, Zhang J, Ye F, Xu G, Su H, Su Y, Zhang X (2017) Plasma neurofilament light chain levels in Alzheimer’s disease. Neuroscience letters 650:60–64. doi: 10.1016/j.neulet.2017.04.027 [DOI] [PubMed] [Google Scholar]
- 46.Haghighi S, Andersen O, Oden A, Rosengren L (2004) Cerebrospinal fluid markers in MS patients and their healthy siblings. Acta neurologica Scandinavica 109 (2):97–99 [DOI] [PubMed] [Google Scholar]
- 47.Norgren N, Rosengren L, Stigbrand T (2003) Elevated neurofilament levels in neurological diseases. Brain research 987 (1):25–31 [DOI] [PubMed] [Google Scholar]
- 48.Lycke J, Karlsson J, Andersen O, Rosengren L (1998) Neurofilament protein in cerebrospinal fluid: a potential marker of activity in multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 64 (3):402–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malmeström C, Haghighi S, Rosengren L, Andersen O, Lycke J (2003) Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61 (12):1720–1725 [DOI] [PubMed] [Google Scholar]
- 50.Gunnarsson M, Malmeström C, Axelsson M, Sundström P, Dahle C, Vrethem M, Olsson T, Piehl F, Norgren N, Rosengren L (2011) Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Annals of neurology 69 (1):83–9. doi: 10.1002/ana.22247. [DOI] [PubMed] [Google Scholar]
- 51.Piehl F, Kockum I, Khademi M, Blennow K, Lycke J, Zetterberg H, Olsson T (2018) Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Multiple Sclerosis Journal 24 (8):1046–1054. doi: 10.1177/1352458517715132. [DOI] [PubMed] [Google Scholar]
- 52.Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H (2018) Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s research & therapy 10 (1):71. doi: 10.1186/s13195-018-0404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clement A, Mitchelmore C, Andersson DR, Asuni AA (2017) Cerebrospinal fluid neurofilament light chain as a biomarker of neurodegeneration in the Tg4510 and MitoPark mouse models. Neuroscience 354:101–109. doi: 10.1016/j.neuroscience.2017.04.030 [DOI] [PubMed] [Google Scholar]
- 54.Hu YY, He SS, Wang XC, Duan QH, Khatoon S, Iqbal K, Grundke-Iqbal I, Wang JZ (2002) Elevated levels of phosphorylated neurofilament proteins in cerebrospinal fluid of Alzheimer disease patients. Neuroscience letters 320 (3):156–160 [DOI] [PubMed] [Google Scholar]
- 55.Mattsson N, Insel PS, Palmqvist S, Portelius E, Zetterberg H, Weiner M, Blennow K, Hansson O (2016) Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO molecular medicine 8 (10):1184–1196. doi: 10.15252/emmm.201606540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin YS, Lee WJ, Wang SJ, Fuh JL (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 8 (1):17368. doi: 10.1038/s41598-018-35766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weston PSJ, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H, Klimova J, Mead S, Blennow K, Rossor MN, Schott JM, Zetterberg H, Fox NC (2017) Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 89 (21):2167–2175. doi: 10.1212/wnl.0000000000004667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilke C, Preische O, Deuschle C, Roeben B, Apel A, Barro C, Maia L, Maetzler W, Kuhle J, Synofzik M (2016) Neurofilament light chain in FTD is elevated not only in cerebrospinal fluid, but also in serum. Journal of neurology, neurosurgery, and psychiatry 87 (11):1270–1272. doi: 10.1136/jnnp-2015-312972 [DOI] [PubMed] [Google Scholar]
- 59.Scherling CS, Hall T, Berisha F, Klepac K, Karydas A, Coppola G, Kramer JH, Rabinovici G, Ahlijanian M, Miller BL, Seeley W, Grinberg LT, Rosen H, Meredith J Jr., Boxer AL (2014) Cerebrospinal fluid neurofilament concentration reflects disease severity in frontotemporal degeneration. Ann Neurol 75 (1):116–126. doi: 10.1002/ana.24052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landqvist Waldo M, Frizell Santillo A, Passant U, Zetterberg H, Rosengren L, Nilsson C, Englund E (2013) Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC neurology 13:54. doi: 10.1186/1471-2377-13-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall S, Ohrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Hakan W, Decraemer H, Nagga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Archives of neurology 69 (11):1445–1452. doi: 10.1001/archneurol.2012.1654 [DOI] [PubMed] [Google Scholar]
- 62.Mattsson N, Ruetschi U, Pijnenburg YA, Blankenstein MA, Podust VN, Li S, Fagerberg I, Rosengren L, Blennow K, Zetterberg H (2008) Novel cerebrospinal fluid biomarkers of axonal degeneration in frontotemporal dementia. Molecular medicine reports 1 (5):757–761. doi: 10.3892/mmr_00000025 [DOI] [PubMed] [Google Scholar]
- 63.Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M (2007) Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology 69 (15):1536–1541. doi: 10.1212/01.wnl.0000277635.05973.55 [DOI] [PubMed] [Google Scholar]
- 64.de Jong D, Jansen RW, Pijnenburg YA, van Geel WJ, Borm GF, Kremer HP, Verbeek MM (2007) CSF neurofilament proteins in the differential diagnosis of dementia. Journal of neurology, neurosurgery, and psychiatry 78 (9):936–938. doi: 10.1136/jnnp.2006.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pijnenburg YA, Janssen JC, Schoonenboom NS, Petzold A, Mulder C, Stigbrand T, Norgren N, Heijst H, Hack CE, Scheltens P, Teunissen CE (2007) CSF neurofilaments in frontotemporal dementia compared with early onset Alzheimer’s disease and controls. Dementia and geriatric cognitive disorders 23 (4):225–230. doi: 10.1159/000099473 [DOI] [PubMed] [Google Scholar]
- 66.Steinacker P, Blennow K, Halbgebauer S, Shi S, Ruf V, Oeckl P, Giese A, Kuhle J, Slivarichova D, Zetterberg H, Otto M (2016) Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci Rep 6:38737. doi: 10.1038/srep38737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meeter LH, Dopper EG, Jiskoot LC, Sanchez-Valle R, Graff C, Benussi L, Ghidoni R, Pijnenburg YA, Borroni B, Galimberti D, Laforce RJ, Masellis M, Vandenberghe R, Ber IL, Otto M, van Minkelen R, Papma JM, Rombouts SA, Balasa M, Oijerstedt L, Jelic V, Dick KM, Cash DM, Harding SR, Jorge Cardoso M, Ourselin S, Rossor MN, Padovani A, Scarpini E, Fenoglio C, Tartaglia MC, Lamari F, Barro C, Kuhle J, Rohrer JD, Teunissen CE, van Swieten JC (2016) Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Annals of clinical and translational neurology 3 (8):623–636. doi: 10.1002/acn3.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso MJ, Ourselin S, Nicholas JM, Norgren N, Mead S, Andreasson U, Blennow K, Schott JM, Fox NC, Warren JD, Zetterberg H (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87 (13):1329–1336. doi: 10.1212/wnl.0000000000003154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinacker P, Anderl-Straub S, Diehl-Schmid J, Semler E, Uttner I, von Arnim CAF, Barthel H, Danek A, Fassbender K, Fliessbach K, Foerstl H, Grimmer T, Huppertz HJ, Jahn H, Kassubek J, Kornhuber J, Landwehrmeyer B, Lauer M, Maler JM, Mayer B, Oeckl P, Prudlo J, Schneider A, Volk AE, Wiltfang J, Schroeter ML, Ludolph AC, Otto M (2018) Serum neurofilament light chain in behavioral variant frontotemporal dementia. Neurology 91 (15):e1390–e1401. doi: 10.1212/wnl.0000000000006318 [DOI] [PubMed] [Google Scholar]
- 70.Meeter LHH, Vijverberg EG, Del Campo M, Rozemuller AJM, Donker Kaat L, de Jong FJ, van der Flier WM, Teunissen CE, van Swieten JC, Pijnenburg YAL (2018) Clinical value of neurofilament and phospho-tau/tau ratio in the frontotemporal dementia spectrum. Neurology 90 (14):e1231–e1239. doi: 10.1212/wnl.0000000000005261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson AGB, Luk C, Heslegrave AJ, Zetterberg H, Mead SH, Collinge J, Jackson GS (2018) Neurofilament light chain and tau concentrations are markedly increased in the serum of patients with sporadic Creutzfeldt-Jakob disease, and tau correlates with rate of disease progression. Journal of neurology, neurosurgery, and psychiatry 89 (9):955–961. doi: 10.1136/jnnp-2017-317793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, Polischi B, Martinelli P, Caroppo P, Ladogana A, Parchi P (2018) The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. 10 (1):3. doi: 10.1186/s13195-017-0331-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rostgaard N, Roos P, Portelius E, Blennow K, Zetterberg H, Simonsen AH, Nielsen JE (2018) CSF neurofilament light concentration is increased in presymptomatic CHMP2B mutation carriers. Neurology 90 (2):e157–e163. doi: 10.1212/wnl.0000000000004799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin YS, Lee WJ, Wang SJ, Fuh JL (2018) Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. 8 (1):17368. doi: 10.1038/s41598-018-35766-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magdalinou NK, Noyce AJ, Pinto R, Lindstrom E, Holmen-Larsson J, Holtta M, Blennow K, Morris HR, Skillback T, Warner TT, Lees AJ, Pike I, Ward M, Zetterberg H, Gobom J (2017) Identification of candidate cerebrospinal fluid biomarkers in parkinsonism using quantitative proteomics. Parkinsonism & related disorders 37:65–71. doi: 10.1016/j.parkreldis.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 76.Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Zetterberg H, Blennow K (2017) Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88 (10):930–937. doi: 10.1212/wnl.0000000000003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herbert MK, Aerts MB, Beenes M, Norgren N, Esselink RA, Bloem BR, Kuiperij HB, Verbeek MM (2015) CSF Neurofilament Light Chain but not FLT3 Ligand Discriminates Parkinsonian Disorders. Front Neurol 6:91. doi: 10.3389/fneur.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdo WF, Bloem BR, Van Geel WJ, Esselink RA, Verbeek MM (2007) CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiology of aging 28 (5):742–747. doi: 10.1016/j.neurobiolaging.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 79.Tiedt S, Duering M, Barro C, Kaya AG, Boeck J, Bode FJ, Klein M, Dorn F, Gesierich B, Kellert L, Ertl-Wagner B, Goertler MW, Petzold GC, Kuhle J, Wollenweber FA, Peters N, Dichgans M (2018) Serum neurofilament light: A biomarker of neuroaxonal injury after ischemic stroke. Neurology 91 (14):e1338–e1347. doi: 10.1212/wnl.0000000000006282 [DOI] [PubMed] [Google Scholar]
- 80.De Marchis GM, Katan M, Barro C, Fladt J, Traenka C, Seiffge DJ, Hert L, Gensicke H, Disanto G, Sutter R, Peters N, Sarikaya H, Goeggel-Simonetti B, El-Koussy M, Engelter S, Lyrer PA, Christ-Crain M, Arnold M, Kuhle J, Bonati LH (2018) Serum neurofilament light chain in patients with acute cerebrovascular events. Eur J Neurol 25 (3):562–568. doi: 10.1111/ene.13554 [DOI] [PubMed] [Google Scholar]
- 81.Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Grobke S, Voortman M, Pirpamer L, Hofer E, Ropele S, Schmidt R, Kuhle J, Fazekas F, Khalil M (2017) Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89 (20):2108–2114. doi: 10.1212/wnl.0000000000004645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Traenka C, Disanto G, Seiffge DJ, Gensicke H, Hert L, Grond-Ginsbach C, Peters N, Regeniter A, Kloss M, De Marchis GM, Bonati LH, Lyrer PA, Kuhle J, Engelter ST (2015) Serum Neurofilament Light Chain Levels Are Associated with Clinical Characteristics and Outcome in Patients with Cervical Artery Dissection. Cerebrovascular diseases (Basel, Switzerland) 40 (5–6):222–227. doi: 10.1159/000440774 [DOI] [PubMed] [Google Scholar]
- 83.Soylu-Kucharz R, Sandelius A, Sjogren M, Blennow K, Wild EJ (2017) Neurofilament light protein in CSF and blood is associated with neurodegeneration and disease severity in Huntington’s disease R6/2 mice. 7 (1):14114. doi: 10.1038/s41598-017-14179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, Lu CH, Dobson R, Disanto G, Norgren N, Nissim A, Kappos L, Hurlbert J, Yong VW, Giovannoni G, Casha S (2015) Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. Journal of neurology, neurosurgery, and psychiatry 86 (3):273–279. doi: 10.1136/jnnp-2013-307454 [DOI] [PubMed] [Google Scholar]
- 85.Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, Mattsson N, Andreasen N, Öst M, Zetterberg H (2016) Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Scientific reports 6:36791. doi: 10.1038/srep36791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al Nimer F, Thelin E, Nyström H, Dring AM, Svenningsson A, Piehl F, Nelson DW, Bellander B-M (2015) Comparative assessment of the prognostic value of biomarkers in traumatic brain injury reveals an independent role for serum levels of neurofilament light. PloS one 10 (7):e0132177. doi: 10.1371/journal.pone.0132177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shahim P, Zetterberg H, Tegner Y, Blennow K (2017) Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology: 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sandelius A, Zetterberg H, Blennow K, Adiutori R, Malaspina A, Laura M, Reilly MM, Rossor AM (2018) Plasma neurofilament light chain concentration in the inherited peripheral neuropathies. Neurology 90 (6):e518–e524. doi: 10.1212/wnl.0000000000004932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goryunov D, Nightingale A, Bornfleth L, Leung C, Liem RK (2008) Multiple disease-linked myotubularin mutations cause NFL assembly defects in cultured cells and disrupt myotubularin dimerization. Journal of neurochemistry 104 (6):1536–1552. doi: 10.1111/j.1471-4159.2007.05103.x [DOI] [PubMed] [Google Scholar]
- 90.Zhai J, Lin H, Julien JP, Schlaepfer WW (2007) Disruption of neurofilament network with aggregation of light neurofilament protein: a common pathway leading to motor neuron degeneration due to Charcot-Marie-Tooth disease-linked mutations in NFL and HSPB1. Human molecular genetics 16 (24):3103–3116. doi: 10.1093/hmg/ddm272 [DOI] [PubMed] [Google Scholar]
- 91.Perez-Olle R, Jones ST, Liem RK (2004) Phenotypic analysis of neurofilament light gene mutations linked to Charcot-Marie-Tooth disease in cell culture models. Human molecular genetics 13 (19):2207–2220. doi: 10.1093/hmg/ddh236 [DOI] [PubMed] [Google Scholar]
- 92.Perez-Olle R, Leung CL, Liem RK (2002) Effects of Charcot-Marie-Tooth-linked mutations of the neurofilament light subunit on intermediate filament formation. Journal of cell science 115 (Pt 24):4937–4946 [DOI] [PubMed] [Google Scholar]
- 93.Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, Blennow K, Landen M (2014) Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39 (10):2349–2356. doi: 10.1038/npp.2014.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Isgren A, Sellgren C, Ekman CJ, Holmen-Larsson J, Blennow K, Zetterberg H, Jakobsson J, Landen M (2017) Markers of neuroinflammation and neuronal injury in bipolar disorder: Relation to prospective clinical outcomes. Brain, behavior, and immunity 65:195–201. doi: 10.1016/j.bbi.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 95.Rolstad S, Jakobsson J, Sellgren C, Ekman CJ, Blennow K, Zetterberg H, Palsson E, Landen M (2015) Cognitive performance and cerebrospinal fluid biomarkers of neurodegeneration: a study of patients with bipolar disorder and healthy controls. PLoS One 10 (5):e0127100. doi: 10.1371/journal.pone.0127100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tortelli R, Ruggieri M, Cortese R, D’errico E, Capozzo R, Leo A, Mastrapasqua M, Zoccolella S, Leante R, Livrea P (2012) Elevated cerebrospinal fluid neurofilament light levels in patients with amyotrophic lateral sclerosis: a possible marker of disease severity and progression. European journal of neurology 19 (12):1561–1567. doi: 10.1111/j.1468-1331.2012.03777.x [DOI] [PubMed] [Google Scholar]
- 97.Menke RA, Gray E, Lu CH, Kuhle J, Talbot K, Malaspina A, Turner MR (2015) CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Annals of clinical and translational neurology 2 (7):748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu C-H, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, Giovannoni G, Fratta P, Sidle K, Fish M (2015) Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84 (22):2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, Toffanin E, Cagnin A, Briani C, Pegoraro E (2017) Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA neurology 74 (5):525–532. doi: 10.1001/jamaneurol.2016.5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tortelli R, Copetti M, Ruggieri M, Cortese R, Capozzo R, Leo A, D’errico E, Mastrapasqua M, Zoccolella S, Pellegrini F (2015) Cerebrospinal fluid neurofilament light chain levels: marker of progression to generalized amyotrophic lateral sclerosis. European journal of neurology 22 (1):215–218. doi: 10.1111/ene.12421. [DOI] [PubMed] [Google Scholar]
- 101.Miller DB, O’Callaghan JP (2015) Biomarkers of Parkinson’s disease: present and future. Metabolism 64 (3):S40–S6. doi: 10.1016/j.metabol.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. science 276 (5321):2045–2047 [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Xiao Y, Chen S, Zhong C, Luo M, Luo H (2012) Creatine for Parkinson’s disease. Cochrane Database Syst Rev (2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Halliday GM, Leverenz JB, Schneider JS, Adler CH (2014) The neurobiological basis of cognitive impairment in Parkinson’s disease. Movement Disorders 29 (5):634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yarnall AJ, Rochester L, Burn DJ (2013) Mild cognitive impairment in Parkinson’s disease. Age and ageing 42 (5):567–576 [DOI] [PubMed] [Google Scholar]
- 106.Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery & Psychiatry 55 (3):181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tolosa E, Wenning G, Poewe W (2006) The diagnosis of Parkinson’s disease. The Lancet Neurology 5 (1):75–86 [DOI] [PubMed] [Google Scholar]
- 108.Sako W, Murakami N, Izumi Y, Kaji R (2015) Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis. Journal of the neurological sciences 352 (1–2):84–87. doi: 10.1016/j.jns.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 109.Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R (2017) Blood-based NfL A biomarker for differential diagnosis of parkinsonian disorder. Neurology 88 (10):930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abdo WF, Bloem BR, Van Geel WJ, Esselink RA, Verbeek MM (2007) CSF neurofilament light chain and tau differentiate multiple system atrophy from Parkinson’s disease. Neurobiology of aging 28 (5):742–747 [DOI] [PubMed] [Google Scholar]
- 111.Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Widner H, Decraemer H, Nägga K (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Archives of neurology 69 (11):1445–52 [DOI] [PubMed] [Google Scholar]
- 112.Constantinescu R, Mondello S (2013) Cerebrospinal fluid biomarker candidates for parkinsonian disorders. Frontiers in neurology 3:187. doi: 10.3389/fneur.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Steinman L (2001) Multiple sclerosis: a two-stage disease. Nature immunology 2 (9):762. [DOI] [PubMed] [Google Scholar]
- 114.Munemoto M, Otaki Y, Kasama S, Nanami M, Tokuyama M, Yahiro M, Hasuike Y, Kuragano T, Yoshikawa H, Nonoguchi H (2011) Therapeutic efficacy of double filtration plasmapheresis in patients with anti-aquaporin-4 antibody-positive multiple sclerosis. Journal of Clinical Neuroscience 18 (4):478–80. doi: 10.1016/j.jocn.2010.07.141. [DOI] [PubMed] [Google Scholar]
- 115.Carson MJ (2002) Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40 (2):218–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, Yaldizli Ö, Regeniter A, Derfuss T, Canales M (2016) Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Multiple Sclerosis Journal 22 (12):1550–1559 [DOI] [PubMed] [Google Scholar]
- 117.Cai L, Huang J (2018) Neurofilament light chain as a biological marker for multiple sclerosis: a meta-analysis study. Neuropsychiatric disease and treatment 14:2241. doi: 10.2147/NDT.S173280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khalil M, Salzer J (2016) CSF neurofilament light A universal risk biomarker in multiple sclerosis? Neurology, 87(11):1068–9. doi: 10.1212/WNL.0000000000003107. [DOI] [PubMed] [Google Scholar]
- 119.Novakova L, Zetterberg H, Sundström P, Axelsson M, Khademi M, Gunnarsson M, Malmeström C, Svenningsson A, Olsson T, Piehl F (2017) Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89 (22):2230–2237. doi: 10.1212/WNL.0000000000004683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arrambide G, Espejo C, Eixarch H, Villar LM, Alvarez-Cermeño JC, Picón C, Kuhle J, Disanto G, Kappos L, Sastre-Garriga J (2016) Neurofilament light chain level is a weak risk factor for the development of MS. Neurology 87 (11):1076–84. doi: 10.1212/WNL.0000000000003085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, Bergenheim T, Svenningsson A (2016) Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurology-Neuroimmunology Neuroinflammation 3 (5):e271. doi: 10.1212/NXI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Masjuan J, Alvarez-Cermeno J, Garcia-Barragan N, Diaz-Sanchez M, Espiño M, Sadaba M, Gonzalez-Porque P, San Millán JM, Villar L (2006) Clinically isolated syndromes A new oligoclonal band test accurately predicts conversion to MS. Neurology 66 (4):576–578 [DOI] [PubMed] [Google Scholar]
- 123.Petzold A, Keir G, Green A, Giovannoni G, Thompson E (2003) A specific ELISA for measuring neurofilament heavy chain phosphoforms. Journal of immunological methods 278 (1–2):179–190 [DOI] [PubMed] [Google Scholar]
- 124.Williams A, Piaton G, Aigrot M-S, Belhadi A, Théaudin M, Petermann F, Thomas J-L, Zalc B, Lubetzki C (2007) Semaphorin 3A and 3F: key players in myelin repair in multiple sclerosis? Brain 130 (10):2554–2565 [DOI] [PubMed] [Google Scholar]
- 125.Teunissen CE, Khalil M (2012) Neurofilaments as biomarkers in multiple sclerosis. Multiple sclerosis journal 18 (5):552–556. 10.1177/1352458512443092. [DOI] [PubMed] [Google Scholar]
- 126.Kuhle J, Nourbakhsh B, Grant D, Morant S, Barro C, Yaldizli Ö, Pelletier D, Giovannoni G, Waubant E, Gnanapavan S (2017) Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 88(9):826–831. doi: 10.1212/WNL.0000000000003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Burman J, Zetterberg H, Fransson M, Loskog AS, Raininko R, Fagius J (2014) Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta neurologica Scandinavica 130 (2):81–89. doi: 10.1111/ane.12239. [DOI] [PubMed] [Google Scholar]
- 128.Kuhle J, Plattner K, Bestwick JP, Lindberg RL, Ramagopalan SV, Norgren N, Nissim A, Malaspina A, Leppert D, Giovannoni G (2013) A comparative study of CSF neurofilament light and heavy chain protein in MS. Multiple Sclerosis Journal 19 (12):1597–1603. doi: 10.1177/1352458513482374 [DOI] [PubMed] [Google Scholar]
- 129.Villar L, Picon C, Costa-Frossard L, Alenda R, García-Caldentey J, Espiño M, Muriel A, Álvarez-Cermeño J (2015) Cerebrospinal fluid immunological biomarkers associated with axonal damage in multiple sclerosis. European journal of neurology 22 (8):1169–1175. doi: 10.1111/ene.12579. [DOI] [PubMed] [Google Scholar]
- 130.Comabella M, Fernández M, Martin R, Rivera-Vallve S, Borrás E, Chiva C, Julià E, Rovira A, Cantó E, Alvarez-Cermeño JC (2010) Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 133 (4):1082–93. doi: 10.1093/brain/awq035. [DOI] [PubMed] [Google Scholar]
- 131.Nylen K, Csajbok L, Öst M, Rashid A, Karlsson J-E, Blennow K, Nellgård B, Rosengren L (2006) CSF–neurofilament correlates with outcome after aneurysmal subarachnoid hemorrhage. Neuroscience letters 404 (1–2):132–136 [DOI] [PubMed] [Google Scholar]
- 132.Burns A, Perry E, Holmes C, Francis P, Morris J, Howes M-JR, Chazot P, Lees G, Ballard C (2011) A double-blind placebo-controlled randomized trial of Melissa officinalis oil and donepezil for the treatment of agitation in Alzheimer’s disease. Dementia and geriatric cognitive disorders 31 (2):158–164. doi: 10.1159/000324438. [DOI] [PubMed] [Google Scholar]
- 133.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E (2012) National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & dementia 8 (1):1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zetterberg H, Skillbäck T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, Blennow K (2016) Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA neurology 73 (1):60–67. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, Maler JM, Kornhuber J, Blennow K, Zetterberg H (2018) Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s research & therapy 10 (1):71. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weston PS, Poole T, Ryan NS, Nair A, Liang Y, Macpherson K, Druyeh R, Malone IB, Ahsan RL, Pemberton H (2017) Serum neurofilament light in familial Alzheimer disease: A marker of early neurodegeneration. Neurology 89 (21):2167–2175. doi: 10.1212/WNL.0000000000004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hoy AR, Ly M, Carlsson CM, Okonkwo OC, Zetterberg H, Blennow K, Sager MA, Asthana S, Johnson SC, Alexander AL (2017) Microstructural white matter alterations in preclinical Alzheimer’s disease detected using free water elimination diffusion tensor imaging. PloS one 12 (3):e0173982. doi: 10.1371/journal.pone.0173982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lista S, Toschi N, Baldacci F, Zetterberg H, Blennow K, Kilimann I, Teipel SJ, Cavedo E, Dos Santos AM, Epelbaum S (2017) Diagnostic accuracy of CSF neurofilament light chain protein in the biomarker-guided classification system for Alzheimer’s disease. Neurochemistry international 108:355–360. doi: 10.1016/j.neuint.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 139.Pijnenburg YA, Verwey NA, van der Flier WM, Scheltens P, Teunissen CE (2015) Discriminative and prognostic potential of cerebrospinal fluid phosphoTau/tau ratio and neurofilaments for frontotemporal dementia subtypes. Alzheimers Dement (Amst) (4):505–12. doi: 10.1016/j.dadm.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Waldö ML, Santillo AF, Passant U, Zetterberg H, Rosengren L, Nilsson C, Englund E (2013) Cerebrospinal fluid neurofilament light chain protein levels in subtypes of frontotemporal dementia. BMC neurology 13 (1):54. doi: 10.1186/1471-2377-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meeter LH, Dopper EG, Jiskoot LC, Sanchez-Valle R, Graff C, Benussi L, Ghidoni R, Pijnenburg YA, Borroni B, Galimberti D (2016) Neurofilament light chain: a biomarker for genetic frontotemporal dementia. Annals of clinical and translational neurology 3 (8):623–636. doi: 10.1002/acn3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Van Swieten JC, Seelaar H, Dopper EG, Onyike CU (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134 (9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert P, Albert M (1998) Frontotemporal lobar degeneration A consensus on clinical diagnostic criteria. Neurology 51 (6):1546–1554 [DOI] [PubMed] [Google Scholar]
- 144.Skillbäck T, Farahmand B, Bartlett JW, Rosén C, Mattsson N, Nägga K, Kilander L, Religa D, Wimo A, Winblad B (2014) CSF neurofilament light differs in neurodegenerative diseases and predicts severity and survival. Neurology 83 (21):1945–1953. doi: 10.1212/WNL.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 145.Rosengren L, Karlsson J-E, Sjögren M, Blennow K, Wallin A (1999) Neurofilament protein levels in CSF are increased in dementia. Neurology 52 (5):1090–3. [DOI] [PubMed] [Google Scholar]
- 146.Sjögren M, Rosengren L, Minthon L, Davidsson P, Blennow K, Wallin A (2000) Cytoskeleton proteins in CSF distinguish frontotemporal dementia from AD. Neurology 54 (10):1960–1964 [DOI] [PubMed] [Google Scholar]
- 147.De Jong D, Jansen RW, Pijnenburg YA, van Geel WJ, Borm GF, Kremer BP, Verbeek MM (2007) CSF neurofilament proteins in the differential diagnosis of dementia. Journal of Neurology, Neurosurgery & Psychiatry 78(9):936–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuhle J, Disanto G, Lorscheider J, Stites T, Chen Y, Dahlke F, Francis G, Shrinivasan A, Radue E-W, Giovannoni G (2015) Fingolimod and CSF neurofilament light chain levels in relapsing-remitting multiple sclerosis. Neurology: 10.1212/WNL.0000000000001491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Weydt P, Oeckl P, Huss A, Müller K, Volk AE, Kuhle J, Knehr A, Andersen PM, Prudlo J, Steinacker P (2016) Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Annals of neurology 79 (1):152–8. doi: 10.1002/ana.24552. [DOI] [PubMed] [Google Scholar]
- 150.Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, Druyeh R, Cardoso MJ, Ourselin S (2016) Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology 87 (13):1329–36. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lewis SB, Wolper RA, Miralia L, Yang C, Shaw G (2008) Detection of phosphorylated NF-H in the cerebrospinal fluid and blood of aneurysmal subarachnoid hemorrhage patients. Journal of Cerebral Blood Flow & Metabolism 28 (6):1261–71. doi: 10.1038/jcbfm.2008.12. [DOI] [PubMed] [Google Scholar]
- 152.Zanier ER, Refai D, Zipfel GJ, Zoerle T, Longhi L, Esparza TJ, Spinner ML, Bateman RJ, Brody DL, Stocchetti N (2011) Neurofilament light chain levels in ventricular cerebrospinal fluid after acute aneurysmal subarachnoid haemorrhage. Journal of Neurology, Neurosurgery & Psychiatry 82 (2):157–19. doi: 10.1136/jnnp.2009.177667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Traenka C, Disanto G, Seiffge DJ, Gensicke H, Hert L, Grond-Ginsbach C, Peters N, Regeniter A, Kloss M, De Marchis GM (2015) Serum neurofilament light chain levels are associated with clinical characteristics and outcome in patients with cervical artery dissection. Cerebrovascular Diseases 40 (5–6):222–7. doi: 10.1159/000440774. [DOI] [PubMed] [Google Scholar]
- 154.Gattringer T, Pinter D, Enzinger C, Seifert-Held T, Kneihsl M, Fandler S, Pichler A, Barro C, Gröbke S, Voortman M (2017) Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology 89 (20):2108–2114. doi: 10.1212/WNL.0000000000004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, Lu C-H, Dobson R, Disanto G, Norgren N (2015) Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. Journal of neurology, neurosurgery, and psychiatry 86 (3):273–279. doi: 10.1136/jnnp-2013-307454. [DOI] [PubMed] [Google Scholar]
- 156.Nguyen HP, Kobbe P, Rahne H, Worpel T, Jager B, Stephan M, Pabst R, Holzmann C, Riess O, Korr H, Kantor O, Petrasch-Parwez E, Wetzel R, Osmand A, von Horsten S (2006) Behavioral abnormalities precede neuropathological markers in rats transgenic for Huntington’s disease. Human molecular genetics 15 (21):3177–3194. doi: 10.1093/hmg/ddl394 [DOI] [PubMed] [Google Scholar]
- 157.Vonsattel JP (2008) Huntington disease models and human neuropathology: similarities and differences. Acta neuropathologica 115 (1):55–69. doi: 10.1007/s00401-007-0306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sharp AH, Ross CA (1996) Neurobiology of Huntington’s disease. Neurobiology of disease 3 (1):3–15. doi: 10.1006/nbdi.1996.0002 [DOI] [PubMed] [Google Scholar]
- 159.Reiner A, Dragatsis I, Dietrich P (2011) Genetics and neuropathology of Huntington’s disease. International review of neurobiology 98:325–372. doi: 10.1016/b978-0-12-381328-2.00014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Glass M, Dragunow M, Faull RL (2000) The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 97 (3):505–519 [DOI] [PubMed] [Google Scholar]
- 161.Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RA, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D (2017) Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington’s disease: a retrospective cohort analysis. The Lancet Neurology 16 (8):601–609. doi: 10.1016/S1474-4422(17)30124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wild EJ, Petzold A, Keir G, Tabrizi SJ (2007) Plasma neurofilament heavy chain levels in Huntington’s disease. Neuroscience letters 417 (3):231–233 [DOI] [PubMed] [Google Scholar]
- 163.Constantinescu R, Romer M, Oakes D, Rosengren L, Kieburtz K (2009) Levels of the light subunit of neurofilament triplet protein in cerebrospinal fluid in Huntington’s disease. Parkinsonism & related disorders 15 (3):245–248. doi: 10.1016/j.parkreldis.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 164.Niemelä V, Landtblom A-M, Blennow K, Sundblom J (2017) Tau or neurofilament light—Which is the more suitable biomarker for Huntington’s disease? PloS one 12 (2):e0172762. doi: 10.1371/journal.pone.0172762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, Blennow K, Landén M (2014) Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients Neuropsychopharmacology 39(10):2349–56. doi: 10.1038/npp.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cousins DA, Grunze H (2012) Interpreting magnetic resonance imaging findings in bipolar disorder. CNS neuroscience & therapeutics 18 (3):201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Isgren A, Sellgren C, Ekman C-J, Holmén-Larsson J, Blennow K, Zetterberg H, Jakobsson J, Landén M (2017) Markers of neuroinflammation and neuronal injury in bipolar disorder: relation to prospective clinical outcomes. Brain, behavior, and immunity 65:195–201. doi: 10.1016/j.bbi.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 168.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14(10):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 169.Pouw M, Hosman A, Van Middendorp J, Verbeek M, Vos P, van de Meent H (2009) Biomarkers in spinal cord injury. Spinal cord 47 (7):519–25. doi: 10.1038/sc.2008.176. [DOI] [PubMed] [Google Scholar]
- 170.Timmerman V, Strickland AV, Zuchner S (2014) Genetics of Charcot-Marie-Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes 5 (1):13–32. doi: 10.3390/genes5010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Saporta MA, Shy ME (2013) Inherited peripheral neuropathies. Neurologic clinics 31 (2):597–619. doi: 10.1016/j.ncl.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Timmerman V, Clowes VE, Reid E (2013) Overlapping molecular pathological themes link Charcot-Marie-Tooth neuropathies and hereditary spastic paraplegias. Experimental neurology 246:14–25. doi: 10.1016/j.expneurol.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 173.Jordanova A, De Jonghe P, Boerkoel C, Takashima H, De Vriendt E, Ceuterick C, Martin JJ, Butler I, Mancias P, Papasozomenos SC (2003) Mutations in the neurofilament light chain gene (NEFL) cause early onset severe Charcot–Marie–Tooth disease. Brain 126 (3):590–597 [DOI] [PubMed] [Google Scholar]
- 174.Petzold A (2005) Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J Neurol Sci 233 (1–2):183–198. doi: 10.1016/j.jns.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 175.Petzold A, Keir G, Green AJ, Giovannoni G, Thompson EJ (2003) A specific ELISA for measuring neurofilament heavy chain phosphoforms. J Immunol Methods 278 (1–2):179–190 [DOI] [PubMed] [Google Scholar]
- 176.Rosengren LE, Karlsson JE, Karlsson JO, Persson LI, Wikkelso C (1996) Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. Journal of neurochemistry 67 (5):2013–2018 [DOI] [PubMed] [Google Scholar]
- 177.Zucchi E, Lu CH, Cho Y, Chang R, Adiutori R, Zubiri I, Ceroni M, Cereda C, Pansarasa O, Greensmith L, Malaspina A, Petzold A (2018) A motor neuron strategy to save time and energy in neurodegeneration: adaptive protein stoichiometry. 146 (5):631–641. doi: 10.1111/jnc.14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Petzold A, Tisdall MM, Girbes AR, Martinian L, Thom M, Kitchen N, Smith M (2011) In vivo monitoring of neuronal loss in traumatic brain injury: a microdialysis study. Brain 134 (Pt 2):464–483. doi: 10.1093/brain/awq360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Petzold A, Keir G, Warren J, Fox N, Rossor MN (2007) A systematic review and meta-analysis of CSF neurofilament protein levels as biomarkers in dementia. Neuro-degenerative diseases 4 (2–3):185–194. doi: 10.1159/000101843 [DOI] [PubMed] [Google Scholar]
- 180.Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, Liman V, Norgren N, Blennow K, Zetterberg H (2016) Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 54 (10):1655–1661. doi: 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 181.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, Song L, Piech T, Patel PP, Chang L, Rivnak AJ, Ferrell EP, Randall JD, Provuncher GK, Walt DR, Duffy DC (2010) Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nature biotechnology 28 (6):595–599. doi: 10.1038/nbt.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Norgren N, Karlsson JE, Rosengren L, Stigbrand T (2002) Monoclonal antibodies selective for low molecular weight neurofilaments. Hybridoma and hybridomics 21 (1):53–59. doi: 10.1089/15368590252917647 [DOI] [PubMed] [Google Scholar]
- 183.Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, Blennow K, Zetterberg H (2016) Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 3:135–140. doi: 10.1016/j.ebiom.2015.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Martinez-Morillo E, Childs C, Garcia BP, Alvarez Menendez FV, Romaschin AD, Cervellin G, Lippi G (2015) Neurofilament medium polypeptide (NFM) protein concentration is increased in CSF and serum samples from patients with brain injury. 53 (10):1575–1584. doi: 10.1515/cclm-2014-0908 [DOI] [PubMed] [Google Scholar]
- 185.Koikkalainen J, Rhodius-Meester H, Tolonen A, Barkhof F, Tijms B, Lemstra AW, Tong T, Guerrero R, Schuh A, Ledig C, Rueckert D, Soininen H, Remes AM, Waldemar G, Hasselbalch S, Mecocci P, van der Flier W, Lotjonen J (2016) Differential diagnosis of neurodegenerative diseases using structural MRI data. NeuroImage Clinical 11:435–449. doi: 10.1016/j.nicl.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stoessl AJ (2012) Neuroimaging in the early diagnosis of neurodegenerative disease. Translational neurodegeneration 1 (1):5. doi: 10.1186/2047-9158-1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Stieltjes B, Brunner RM, Fritzsche K, Laun F (2013) Diffusion tensor imaging: introduction and atlas. Springer Science & Business Media, [Google Scholar]
- 188.Kantarci K, Murray ME, Schwarz CG, Reid RI, Przybelski SA, Lesnick T, Zuk SM, Raman MR, Senjem ML, Gunter JL (2017) White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiology of aging 56:172–179. doi: 10.1016/j.neurobiolaging.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fornari E, Maeder P, Meuli R, Ghika J, Knyazeva MG (2012) Demyelination of superficial white matter in early Alzheimer’s disease: a magnetization transfer imaging study. Neurobiology of aging 33(2):428.e7–19. doi: 10.1016/j.neurobiolaging.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 190.Sykova E (2004) Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 129 (4):861–876 [DOI] [PubMed] [Google Scholar]
- 191.Baldaranov D, Khomenko A, Kobor I, Bogdahn U, Gorges M, Kassubek J, Müller H-P (2017) Longitudinal Diffusion Tensor Imaging-Based Assessment of Tract Alterations: An Application to Amyotrophic Lateral Sclerosis. Frontiers in human neuroscience 11:567. doi: 10.3389/fnhum.2017.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Foerster BR, Dwamena BA, Petrou M, Carlos RC, Callaghan BC, Churchill CL, Mohamed MA, Bartels C, Benatar M, Bonzano L (2013) Diagnostic accuracy of diffusion tensor imaging in amyotrophic lateral sclerosis: a systematic review and individual patient data meta-analysis. Academic radiology 20 (9):1099–1106. doi: 10.1016/j.acra.2013.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schreiber S, Spotorno N, Schreiber F, Acosta-Cabronero J, Kaufmann J, Machts J, Debska-Vielhaber G, Garz C, Bittner D, Hensiek N, Dengler R, Petri S, Nestor PJ, Vielhaber S (2018) Significance of CSF NfL and tau in ALS. Journal of neurology 265 (11):2633–2645. doi: 10.1007/s00415-018-9043-0 [DOI] [PubMed] [Google Scholar]
- 194.Acosta-Cabronero J, Nestor PJ (2014) Diffusion tensor imaging in Alzheimer’s disease: insights into the limbic-diencephalic network and methodological considerations. Frontiers in aging neuroscience 6:266. doi: 10.3389/fnagi.2014.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A (2018) Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know? Front Neurol 9:92. doi: 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Steinacker P, Feneberg E, Weishaupt J, Brettschneider J, Tumani H, Andersen PM, von Arnim CA, Bohm S, Kassubek J, Kubisch C, Lule D, Muller HP, Muche R, Pinkhardt E, Oeckl P, Rosenbohm A, Anderl-Straub S, Volk AE, Weydt P, Ludolph AC, Otto M (2016) Neurofilaments in the diagnosis of motoneuron diseases: a prospective study on 455 patients. Journal of neurology, neurosurgery, and psychiatry 87 (1):12–20. doi: 10.1136/jnnp-2015-311387 [DOI] [PubMed] [Google Scholar]
- 197.Grossman M, Elman L, McCluskey L, McMillan CT, Boller A, Powers J, Rascovsky K, Hu W, Shaw L, Irwin DJ, Lee VM, Trojanowski JQ (2014) Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA neurology 71 (4):442–448. doi: 10.1001/jamaneurol.2013.6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Verde F, Steinacker P, Weishaupt JH, Kassubek J, Oeckl P, Halbgebauer S, Tumani H, von Arnim CAF, Dorst J, Feneberg E, Mayer B, Muller HP, Gorges M, Rosenbohm A, Volk AE, Silani V, Ludolph AC, Otto M (2019) Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. Journal of neurology, neurosurgery, and psychiatry 90 (2):157–164. doi: 10.1136/jnnp-2018-318704 [DOI] [PubMed] [Google Scholar]
- 199.Chitnis T, Gonzalez C, Healy BC, Saxena S, Rosso M, Barro C, Michalak Z, Paul A, Kivisakk P, Diaz-Cruz C, Sattarnezhad N, Pierre IV, Glanz BI, Tomic D, Kropshofer H, Haring D, Leppert D, Kappos L, Bakshi R (2018) Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. 5 (12):1478–1491. doi: 10.1002/acn3.638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Barro C, Benkert P, Disanto G, Tsagkas C, Amann M, Naegelin Y, Leppert D, Gobbi C, Granziera C, Yaldizli O, Michalak Z, Wuerfel J, Kappos L, Parmar K, Kuhle J (2018) Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 201.Niikado M, Chrem-Mendez P, Barbieri-Kennedy M, Calandri I, Martinetto H, Serra M, Calvar J, Campos J, Russo MJ, Pertierra L, Allegri R, Sevlever G, Surace EI (2018) Evaluation of CSF neurofilament light chain levels as a routine biomarker in a memory clinic. The journals of gerontology Series A, Biological sciences and medical sciences. doi: 10.1093/gerona/gly179 [DOI] [PubMed] [Google Scholar]
- 202.Zhang Y, Schuff N, Du A-T, Rosen HJ, Kramer JH, Gorno-Tempini ML, Miller BL, Weiner MW (2009) White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain 132 (9):2579–2592. doi: 10.1093/brain/awp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Agosta F, Scola E, Canu E, Marcone A, Magnani G, Sarro L, Copetti M, Caso F, Cerami C, Comi G (2012) White matter damage in frontotemporal lobar degeneration spectrum. Cerebral cortex 22 (12):2705–2714. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- 204.Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012) Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PloS one 7 (8):e43993. doi: 10.1371/journal.pone.0043993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mahoney CJ, Simpson IJ, Nicholas JM, Fletcher PD, Downey LE, Golden HL, Clark CN, Schmitz N, Rohrer JD, Schott JM (2015) Longitudinal diffusion tensor imaging in frontotemporal dementia. Annals of neurology 77 (1):33–46. oi: 10.1002/ana.24296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Panman JL, van der Ende EL, Meeter LH, Bouts MJ, Dopper EG, Jiskoot LC, Kaat LD, van Minkelen R, Teunissen CE, Rombouts SA (2017) ARE NEUROFILAMENT LIGHT CHAIN AND WHITE MATTER INTEGRITY RELATED BIOMARKERS FOR FAMILIAL FRONTOTEMPORAL DEMENTIA? Alzheimer’s & Dementia 13 (7):P751–P752. 10.1016/j.jalz.2017.06.992 [DOI] [Google Scholar]
- 207.Duering M, Konieczny MJ, Tiedt S, Baykara E, Tuladhar AM, Leijsen EV, Lyrer P, Engelter ST, Gesierich B, Achmuller M, Barro C, Adam R, Ewers M, Dichgans M, Kuhle J, de Leeuw FE, Peters N (2018) Serum Neurofilament Light Chain Levels Are Related to Small Vessel Disease Burden. Journal of stroke 20 (2):228–238. doi: 10.5853/jos.2017.02565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Byrne LM, Rodrigues FB (2018) Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington’s disease. 10 (458). doi: 10.1126/scitranslmed.aat7108 [DOI] [PubMed] [Google Scholar]
- 209.Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T (2017) Serum Neurofilament Light Protein as a Marker for Diffuse Axonal Injury: Results from a Case Series Study. Journal of neurotrauma 34 (5):1124–1127. doi: 10.1089/neu.2016.4496 [DOI] [PubMed] [Google Scholar]