Abstract
Background:
Primary antibody deficiencies (PAD) are the most prevalent primary immunodeficiencies. More severe forms of PAD, common variable immunodeficiency (CVID) and X-linked agammaglobulinemia (XLA), require immunoglobulin replacement therapy (IRT) and may have serious complications. Differentiating severe PAD from milder hypogammaglobulinemia not requiring IRT can involve prolonged evaluations and treatment discontinuation. Severe PAD is defined by plasma cell deficiency, but this requires a biopsy to establish. Serum B cell maturation antigen (sBCMA) is elevated in multiple myeloma, but levels are reduced among myeloma patients in remission who have hypogammaglobulinemia.
Objective:
We measured sBCMA levels in 165 subjects to determine whether it differentiates the severe PAD, CVID and XLA, from less severe forms not requiring IRT and those without PAD.
Methods:
sBCMA, B-cells, and tissue plasma cells were measured among subjects with and without PAD, and correlated to clinical and laboratory data.
Results:
Subjects with IgG < 600 mg/dL had reduced sBCMA levels compared with PAD with IgG ≥ 600 and non-PAD controls. sBCMA was lower in CVID and XLA compared to IgA or IgG deficiency and controls. sBCMA correlated with gastrointestinal plasma cells. sBCMA < 15 ng/mL had 97% positive predictive value for CVID or XLA, while 25 ng/mL or more had an 88% negative predictive value.
Conclusion:
sBCMA is profoundly reduced in severe PAD, including CVID, XLA and subjects with IgG < 600 mg/dL. sBCMA measurement has potential to augment clinical evaluation of PAD. Prospective studies are needed to evaluate sBCMA for new PAD diagnosis and determining necessity of IRT.
Keywords: B cell maturation antigen, common variable immunodeficiency, immunoglobulin replacement therapy, primary antibody deficiency, X-linked agammaglobulinemia
INTRODUCTION
The many forms of primary antibody deficiency (PAD) are the most prevalent primary immunodeficiencies.1 Severe forms of PAD, including common variable immunodeficiency (CVID) and X-linked agammaglobulinemia (XLA), are associated with chronic medical complications, such as bronchiectasis, and require immunoglobulin replacement therapy (IRT).2–4 However, more restricted defects of antibody production, or those associated with immaturity or medications, may not require lifelong IRT.5 Differentiating patients with PAD requiring IRT from those with these less profound immunoglobulin deficiencies requires evaluation of antibody responses; this can take weeks or more and may involve repeated vaccinations.6, 7 Moreover, such evaluation requires discontinuation of IRT in those for which such treatment is questionable and may expose the patient to a significant risk of infection.8 Rapid diagnostic tests to identify those with severe PAD that would not necessitate protracted vaccine challenges or stopping IRT could reduce diagnostic delay, diminish chronic pulmonary complications, and improve these patients’ compromised quality of life.9
The hallmark of XLA and most subjects with CVID is a lack of plasma cells in bone marrow or mucosal sites, but biopsy of these tissues is required to assess plasma cell abundance.10–13 B cell maturation antigen (BCMA) is a tumor necrosis factor receptor expressed largely on the surface of plasma cells and plasmablasts that promotes survival of these cells upon engagement with its ligands, a proliferation inducing ligand (APRIL) and B cell activating factor (BAFF).14 BCMA is cleaved endogenously by gamma secretase and can be quantified in a solubilized form in the blood.15 Serum B cell maturation antigen (sBCMA) is elevated among patients with multiple myeloma, a plasma cell malignancy where it both predicts patients’ survival, and can be used to monitor the course of disease.16 However, the utility of determining sBCMA in PAD to evaluate the plasma cell population has not yet been investigated.
In this study, our goal was to measure sBCMA in a large cohort of subjects with PAD to determine if this marker could distinguish more severe PAD (CVID and XLA) from milder forms of antibody loss. Combining sBCMA detection with measurement of peripheral B cell subsets, immunoglobulin levels, antibody production, and plasma cells in gastrointestinal biopsies, we found that CVID and XLA, as well as PAD subjects with IgG < 600 mg/dL, were characterized by decreased sBCMA, in correspondence with reduced plasma cells in mucosal tissues. sBCMA < 15 ng/mL had 97% positive predictive value (PPV) for CVID or XLA, while a cut-off of 25 ng/mL had an 88% negative predictive value (NPV). Our results demonstrate the potential of sBCMA measurement as an informative tool to aid diagnosis of the severe PADs, one that does not require stoppage of IRT or lengthy evaluation with vaccination challenges.
METHODS
Subjects and retrospective review of medical records
All subjects included in this study were patients at the Mount Sinai Clinical Immunology Faculty Practice. Patients with the International Classification of Diseases Ninth or Tenth Revision Code for CVID (279.06 or D83.9), selective IgA deficiency (IgAD, 279.01 or D80.2), IgA deficiency with IgG2 subclass deficiency (IgA/IgG2D, 279.03 or D80.3), IgG deficiency (IgGD, 279.01 or D80.1), and XLA (279.04 or D80.0) were enrolled. The diagnosis of CVID was confirmed in all subjects based on markedly low serum IgG and IgA and/or IgM levels (IgG < 400 mg/dl, IgA < 45 mg/dl, IgM < 35 mg/dl), poor response to at least one vaccine, and exclusion of other causes of hypogammaglobulinemia.17 Patient age, sex, laboratory values (IgG, IgA, IgM, CD 19+ B cells, isotype-switched memory B cells, and pneumococcal antibodies), and history of complications in CVID patients (autoimmunity, interstitial lung disease, enteropathy, bronchiectasis, or pneumonia) were derived from the medical records. This study was approved by the institutional review board of the Icahn School of Medicine at Mount Sinai and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
sBCMA measurement
Serum was diluted 1:50 in reagent diluent and levels of human sBCMA were determined using an ELISA-based assay with polyclonal anti-BCMA antibodies for capture and detection (R&D Systems, Minneapolis, MN) as done previously.16 Samples were analyzed using an EPOCH2 microplate reader (BioTek Instruments Inc.) set to 450 nm with Gen5 software for data collection.
Quantification of plasma cells in gastrointestinal biopsies
All gastrointestinal biopsies were conducted as part of routine clinical care. Plasma cells were quantified in 17 gastrointestinal biopsies from subjects with PAD in a blinded manner by two independent reviewers. Plasma cells were identified by CD138 staining via immunohistochemistry and/or by distinct morphology on hematoxylin and eosin staining if no tissue was available for immunohistochemistry.
Statistical Analysis
Analysis of two groups of continuous variables was calculated by Mann-Whitney test. Analysis of 3 or more groups of continuous variables was conducted using Kruskal-Wallis test followed by Dunn’s multiple comparisons. Comparison of categorical values was determined using Chi-square test for instances in which sample size was large and Fisher’s exact test for smaller sample size. Correlation was calculated using Spearman r. Calculations were made using Prism software (GraphPad). A P value less than 0.05 was considered significant.
RESULTS
sBCMA is reduced in severe PAD
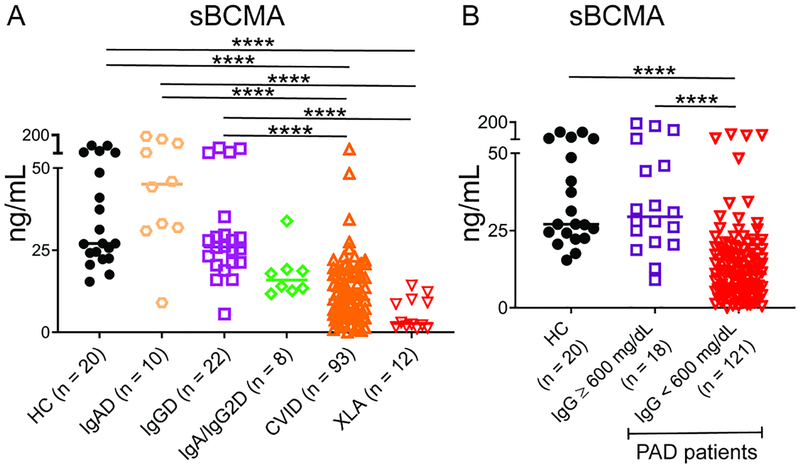
The level of sBCMA was determined in 145 patients with PAD who were evaluated at the Mount Sinai Clinical Immunology Practice as well as 20 controls without PAD. The PAD subjects (Table 1) had diagnoses that varied between IgAD (n = 10), IgA/IgG2D (n = 8), IgGD (n = 22), CVID (n = 93), and XLA (n = 12). We found sBCMA to be significantly reduced in CVID and XLA patients compared to those with IgGD, IgAD, and non-PAD controls (P < 0.0001, Figure 1A). sBCMA was generally lower in IgA/IgG2D subjects (median 16.0 ng/mL) compared to those with IgAD alone (45 ng/mL), IgGD (26 ng/mL), and non-PAD controls (27 ng/mL), but statistical significance was not reached. We noted that the median value for IgA/IgG2D (16.0 ng/mL) was not as low as that of either CVID (9.9 ng/mL) or XLA (2.7 ng/mL), suggesting more preserved plasma cell populations in these subjects, as would be expected. To determine whether sBCMA levels differed in those with varying degrees of hypogammaglobulinemia, we grouped subjects based upon whether or not they had a baseline serum IgG ≥ 600 mg/dL. This IgG cut-off was derived from consensus guidelines for the diagnosis of CVID, in which IgG levels should be at least two standard deviations below normal, or approximately 600 mg/dL.18, 19 As expected, sBCMA was lower in PAD patients with a serum IgG < 600 mg/dL (median 11.1 ng/mL) compared to those with IgG ≥ 600 mg/dL (29.5 ng/mL) as well as healthy controls (27 ng/mL) (P < 0.0001, Figure 1B). Together these results indicate that sBCMA levels are decreased in those with severe PAD, either defined by a clinical diagnosis of CVID or XLA or serum IgG < 600 mg/dL.
Table 1.
Characteristics of PAD subjects in the study.
| IgAD (n = 10) |
IgGD (n = 22) |
IgA/IgG2D (n = 8) |
CVID (n = 93) |
XLA (n = 12) |
P value | |
|---|---|---|---|---|---|---|
| Female (%) | 5 (50) | 15 (68) | 3 (38) | 52 (56) | 0 (0) | 0.609* |
| Median Age (95% CI) | 45 (28 – 60) | 69 (58 – 73) | 22 (16 – 41) | 50 (47 – 52) | 21 (11 – 36) | < 0.0001 |
| Median Lab Value (95% CI) | ||||||
| Diagnostic IgM [mg/dL] | 162 (61 – 485) | 81 (29 – 114) | 42 (18 – 67) | 15 (10 – 17) | 0 (0 – 0) | < 0.0001 |
| Diagnostic IgA [mg/dL] | 0 (0 – 63) | 114 (65 – 145) | 0(0 – 16) | 0 (0 – 6) | 0 (0 – 0) | < 0.0001 |
| Diagnostic IgG [mg/dL] | 1066 (779 – 2311) | 530(476 – 622) | 563 (508 – 752) | 145 (93 – 215) | N/A | < 0.0001 |
| Median sBCMA ng/mL (95% CI) | 45.1 (30.9 – 167.4) | 26.0 (21.2 – 29.7) | 16.0 (11.7 – 33.9) | 9.9 (6.8 – 12.1) | 2.7 (1.6 – 10.0) | <0.0001 |
CI, confidence interval; CVID, common variable immunodeficiency; IgAD, selective IgA deficiency; IgA/IgG2D, IgA and IgG2 deficiency; IgGD, selective IgG deficiency; sBCMA, serum B cell maturation antigen; XLA, X-linked agammaglobulinemia P value calculated by Chi-square for categorical values and Kruskal-Wallis test for continuous values.
P value calculation for female subjects excludes XLA
Figure 1.
sBCMA is reduced in severe PAD. (A) sBCMA is lower in CVID and XLA compared to IgAD, IgGD, and HC. (B) sBCMA is lower in PAD with IgG < 600 mg/dL compared to those ≥ 600 or healthy controls (HC). Five IgAD and IgA/IgG2D subjects were did not have baseline IgG levels. **** = P < 0.0001. Line denotes median.
Predictive value of sBCMA for severe PAD
We then determined the predictive value of sBCMA measurement for diagnosis of severe forms of PAD (CVID or XLA) within this study cohort. IgA/IgG2D subjects were excluded from this analysis as an unclear number of these patients may progress to CVID.20 The receiver operating characteristic (ROC) curve for sBCMA demonstrated excellent discrimination of severe forms of PAD (CVID and XLA) from other PAD and non-PAD subjects, with an area under the curve of 0.9448 (Supplementary Figure El). The fifth percentile for sBCMA in non-PAD controls was 15.6 ng/mL. Using a sBCMA value of less than 15 ng/mL for identifying CVID or XLA among PAD and non-PAD subjects, sensitivity was 73% (64 − 81, 95% confidence interval), specificity 96% (87 − 99), and PPV 97% (87 − 99) (Table 2). We did not find a notable improvement in specificity or PPV when a cut-off of 10 ng/mL sBCMA was used. Sensitivity and NPV did improve with increases in sBCMA, and a cut-off of 20 ng/mL had 88% sensitivity and 78% NPV, while 25 ng/mL (approximately the median of non-PAD controls) had 95% sensitivity and 88% NPV. Our findings suggest that sBCMA measurement has potential to aid in the evaluation of patients suspected of having CVID or XLA.
Table 2.
Predictive value of sBCMA for severe PAD.
| sBCMA (ng/mL) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
Likelihood Ratio |
|---|---|---|---|---|---|
| < 15 | 73% (64 – 81) | 96% (87 – 99) | 97% (87 – 99) | 64% (53 – 74) | 19.1 |
| < 20 | 88% (80 – 93) | 87% (75 – 93) | 93% (86 – 97) | 78% (65 – 86) | 6.5 |
| < 25 | 95% (89 – 98) | 69% (56 – 80) | 86% (79 – 91) | 88% (75 – 95) | 3.1 |
Severe PAD defined as CVID or XLA.
sBCMA, serum BCMA; CI, confidence interval; CVID, common variable immunodeficiency; NPV, negative predictive value; OR, odds ratio; PAD, primary antibody deficiency; PPV, positive predictive value; XLA, x-linked agammaglobulinemia.
sBCMA correlates poorly with conventional parameters used to evaluate PAD patients
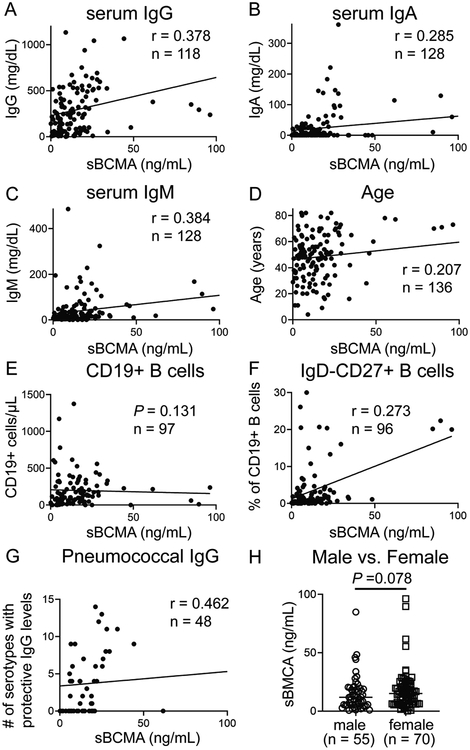
As there were significant differences in age and immunoglobulins among the distinct PAD diagnoses included (Table 1), we tested the correlation of sBCMA with these variables as well as other common clinical measurements from the medical records. Using data from all PAD subjects analyzed together, we calculated Spearman’s correlation coefficients (r) and for those that were significant (P < 0.05) used conventional interpretations for strength of correlation: r < 0.4 signifying poor correlation, r = 0.4 to 0.7 as moderate correlation, and r = 0.7 to 1.0 as strongly correlated.21, 22 sBCMA correlated poorly with serum IgG (r = 0.38), IgA (r = 0.285), IgM (r = 0.384), and age (r = 0.207) (Figure 2A − D). Thus, despite immunoglobulin levels and age, which were significantly variable amongst our subject groups, these characteristics were not correlated to sBCMA levels. We found that circulating B cell counts were also not correlated with sBCMA (P = 0.131) and levels of isotype-switched memory B cells in the blood were only poorly correlated (r = 0.273) (Figure 2E–F). Post-vaccination pneumococcal antibodies were tested in 48 PAD subjects and found to moderately correlated with sBCMA (r = 0.462) (Figure 2G). Female PAD subjects trended towards higher sBCMA levels but this difference was short of statistical significance (P = 0.078). Together these results indicate that sBCMA levels are not strongly associated with the typical clinical and laboratory parameters measured during PAD evaluation.
Figure 2.
sBCMA is independent of conventional parameters used in evaluation of PAD subjects with IgAD, IgA/IgG2D, IgGD, CVID, or XLA. Correlation of sBCMA with (A) pre-IRT IgG, (B) IgA, (C) IgM, (D) age, (E) CD19+ B cells, (F) isotype-switched memory B cells, and (G) pneumococcal serotypes with protective IgG. (H) sBCMA comparison between male and females (XLA excluded). Lines denote median.
sBCMA correlates with plasma cell counts in the gastrointestinal tract
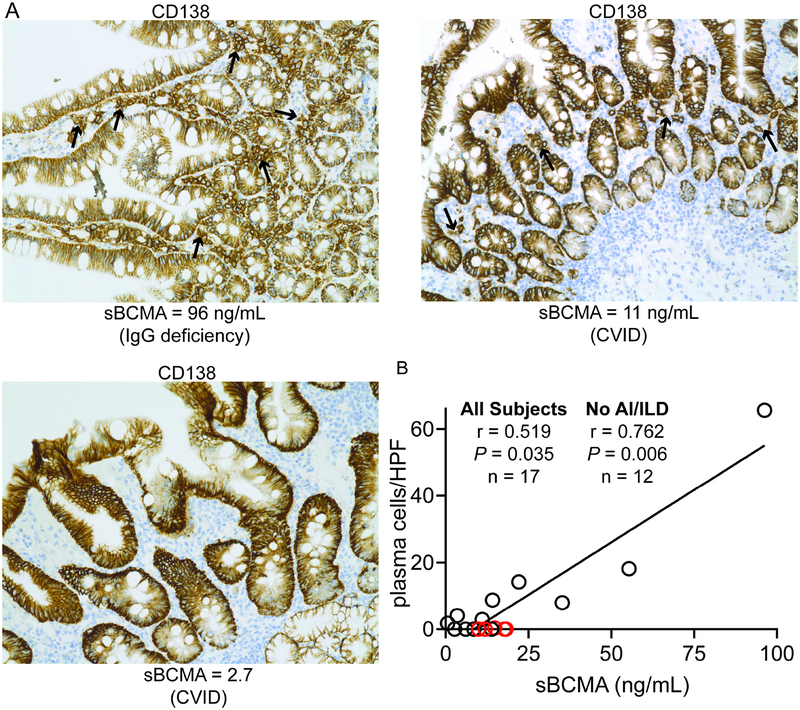
As BCMA is largely expressed by plasma cells, we correlated sBCMA measurement with quantification of plasma cells in the gastrointestinal tract mucosa, where these cells are typically numerous. Gastrointestinal biopsies from 17 PAD subjects performed as part of routine medical care were utilized. Plasma cells were quantified in the duodenum whenever possible (n = 14), the terminal ileum if no tissue from the duodenum was available (n = 1), and right colon in the instances where no tissue from the duodenum or terminal ileum was available (n = 2). Plasma cells are often profoundly reduced in CVID but maintained at near normal levels in those with less severe forms of PAD (Figure 3A).23 We found that that the level of sBCMA was correlated with the number of plasma cells per high-powered field (r = 0.519; Figure 3B). As we have shown that CVID subjects with autoimmunity and interstitial lung disease have IgM-producing plasmablasts (which may also produce BCMA), but still lack gastrointestinal plasma cells, we also evaluated correlation without the 5 subjects with this phenotype.24 Upon removing these subjects, we again analyzed the data and found even stronger correlation between sBCMA and gastrointestinal plasma cells (r = 0.762). Thus, sBCMA correlates with plasma cell count more strongly than with any of the conventional laboratory tests used during PAD evaluation.
Figure 3.
sBCMA correlates with plasma cell counts in the gastrointestinal tract. (A) Representative gastrointestinal biopsies from PAD patients. CD138+ plasma cells denoted by black arrows. (B) Correlation of sBCMA with number of plasma cells per high power field (HPF). Subjects with autoimmunity (AI) or interstitial lung disease (ILD) marked as red circles. Spearman r calculated with and without AI/ILD subjects.
sBCMA in CVID patients with or without history of complications
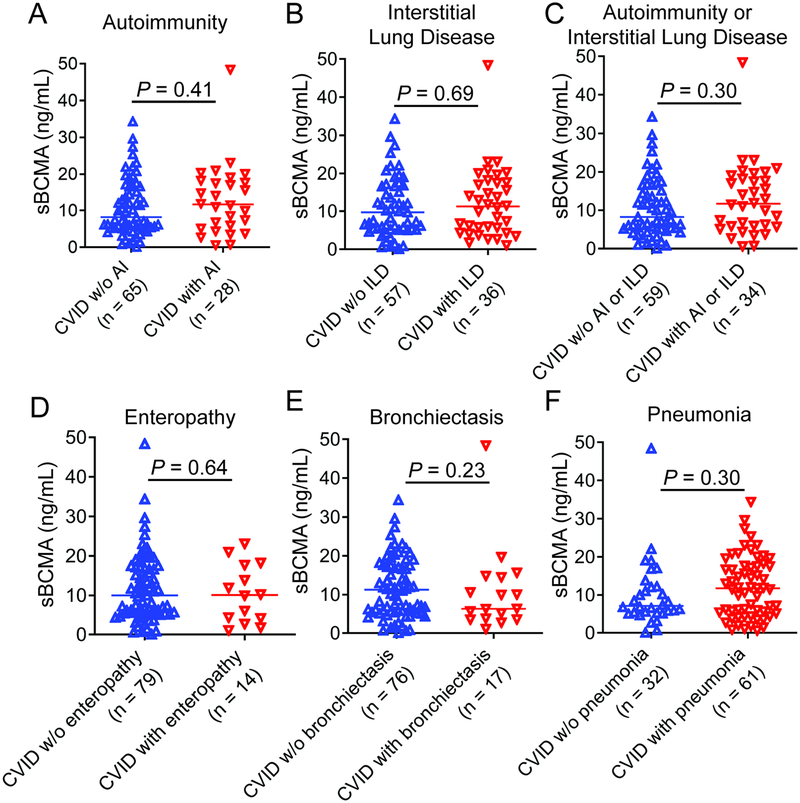
sBCMA levels were quite variable in CVID, not unexpected, as this PAD is noted for its heterogeneous clinical presentations.25 However, as BCMA exerts controls on autoimmunity in murine models,26–28 we investigated if these levels may be influenced by the presence of autoimmunity and/or interstitial lung disease in CVID. While the clinical and laboratory characteristics of the 93 CVID patients included in this study were similar to other cohorts (Table 3),29, 30 there was no statistical difference in sBCMA levels between those with or without histories of autoimmunity, interstitial lung disease, enteropathy, bronchiectasis, or pneumonia (Figure 4A–F).
Table 3.
Characteristics of CVID subjects in the study.
| CVID | |
|---|---|
| Number of Subjects | 93 |
| Female (%) | 51 (55) |
| Median Age (95% CI) | 50 (47 – 52) |
| Median Lab Value (95% CI) | |
| Diagnostic IgG [mg/dL] | 145 (93 – 215) |
| Diagnostic IgA [mg/dL] | 0 (0 – 6) |
| Diagnostic IgM [mg/dL] | 15 (10 – 17) |
| CD19+ B cells [#/μL] | 99.5 (76 – 140) |
| Isotype-switched memory B cells [% of CD19+] | 1.19 (0.75 – 1.55) |
| Complication History (%) | |
| Autoimmunity | 29 (31) |
| Interstitial Lung Disease | 27 (29) |
| Enteropathy | 14 (15) |
| Bronchiectasis | 17 (18) |
| Pneumonia | 60 (65) |
CI, confidence interval; CVID, common variable immunodeficiency
Figure 4.
sBCMA in CVID patients with or without history of complications. There is no significant difference in sBCMA between CVID patients with or without history of (A) autoimmunity (AI), (B) interstitial lung disease (ILD), (C) AI or ILD grouped together, (D) enteropathy, (E) bronchiectasis, and (F) pneumonia.
sBCMA and other characteristics of IgA/IgG2D and IgGD subjects receiving IRT
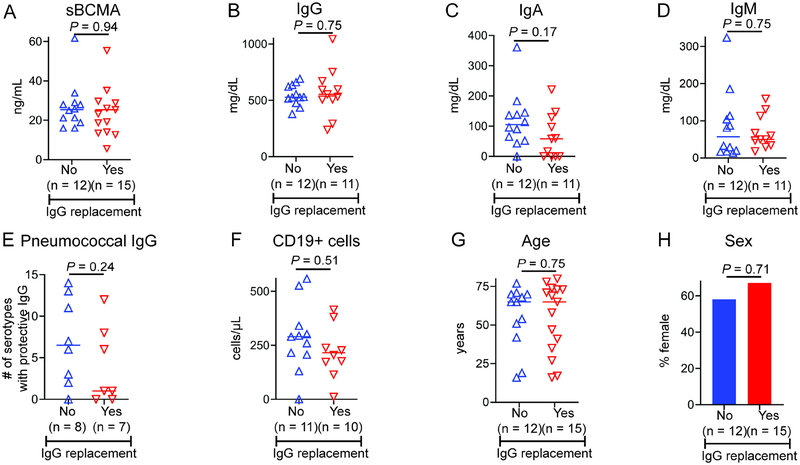
While IRT is standard of care for those with CVID or XLA, usage of this therapy is at the discretion of the treating physician in those with IgA/IgG2D or IgGD. We examined whether sBCMA levels, as well as other specific parameters, differed between the IgA/IgG2D and IgGD patients who were or were not prescribed IRT by their treating physician. There was no difference in sBCMA levels between IgA/IgG2D and IgGD patients on IRT compared to those not on IRT (Figure 5A). Likewise, there was no difference in pre-IRT IgG, IgA, IgM, pneumococcal antibodies, circulating CD19+ B cells, age, or sex between IgA/IgG2D and IgGD patients that were or were not prescribed this treatment (Figure 5B–H).
Figure 5.
sBCMA and other characteristics of IgA/IgG2D and IgGD subjects receiving IRT. (A) sBCMA, (B) diagnostic IgG, (C) IgA, (D) IgM, (E) pneumococcal IgG, (F) CD19+ B cells, (G) age, and (H) sex did not differ on the basis of IgG replacement. P by Mann-Whitney for continuous variables and Fisher’s exact test for categorical.
We also determined sBCMA in two subjects with persistent hypogammaglobulinemia as a consequence of rituximab given for autoimmunity more than 5 years ago. Both subjects had been prescribed IRT due to their severe antibody deficiency. Interestingly, the sBCMA levels for these subjects were 4.92 and 5.0 ng/mL, well below the 5th percentile (15 ng/mL) of non-PAD controls which had a PPV of 97%. These illustrative examples suggest the potential for utilizing sBCMA measurement to identify individuals requiring IRT due to persistent antibody deficiency from secondary causes.
DISCUSSION
BAFF receptor, transmembrane activator and CAML interactor, and BCMA comprise a group of tumor necrosis factor superfamily receptors that share ligands (BAFF and/or APRIL) to promote activation, maturation, and survival of B cells.14 These receptors are differentially expressed during B cell maturation, with BCMA largely restricted to plasma cells.31–34 sBCMA levels are elevated among patients with the plasma cell malignancy multiple myeloma; additionally, the RNA expression of the gene encoding BCMA is much reduced in the blood of CVID patients who show an absence of plasma cells, suggesting that sBCMA levels may be reduced among patients with severe forms of PAD.24 Despite its utility as a non-invasive means to monitor plasma cell abundance, sBCMA measurement has, to our knowledge, not been reported in the evaluation of PAD. In this study, we assessed sBCMA measurement for the identification of individuals with PAD, particularly those with more severe forms in which plasma cells are profoundly reduced or absent.
As patients with CVID and XLA have marked deficiency of plasma cells, many develop chronic respiratory complications and all require IRT. As expected, sBCMA was markedly reduced in CVID and XLA relative to subjects with IgAD and IgGD or normal controls. sBCMA was correlated more strongly with plasma cell abundance in gastrointestinal tissues than with immunoglobulin levels, age, CD 19+ and isotype-switched memory B cells in the blood, and antibody responses to pneumococcal vaccination. Notably, a sBCMA value at the 5th percentile (15 ng/mL) had a high PPV (97%) and the NPV of the median sBCMA in non-PAD controls (25 ng/mL) was 88%. Thus, sBCMA measurement has potential to be a useful diagnostic tool in the evaluation of PAD.
Consistent with its expression predominantly by plasma cells, we found sBCMA levels in the blood to be positively correlated with plasma cell abundance in tissues, as quantified in gastrointestinal biopsies. This approach was limited by the numbers of gastrointestinal tissue used for plasma cell quantification, as only biopsies done as part of regular medical care were available. Plasma cells were quantified in the duodenum preferentially, but the terminal ileum or right colon was used in a few instances. Also potentially affecting the correlation between sBCMA and gastrointestinal plasma cells is the likelihood that plasma cells in other tissues are a source of sBCMA. Plasma cells are abundant in the bone marrow, however biopsies of this tissue are infrequently obtained in PAD and were not available in these subjects. However, as 94% of CVID subjects have highly reduced or no plasma cells in the bone marrow, it is unlikely to be a significant source of sBCMA in these patients.11 Plasmablasts are another potential source of sBCMA and appear to be increased in CVID patients with autoimmunity and interstitial lung disease.24, 35 Indeed, upon exclusion of CVID patients with autoimmunity or interstitial lung disease, correlation between sBCMA and gastrointestinal plasma cells strengthened. Yet, we did not find significant differences in sBCMA between CVID patients on the basis of specific complications, including autoimmunity or interstitial lung disease. Thus, sBCMA levels may be influenced by factors we have yet to define, such as current state of autoimmune and interstitial lung disease activity or alternative sources of sBCMA, such as plasmacytoid dendritic cells.36, 37 Thus, while largely specific for plasma cells, sBCMA measurement may be complicated by its expression by short-lived plasmablasts in certain CVID patients as well as its shedding from other cell types.
While our results are encouraging, prospective studies are needed to validate the utility of sBCMA in PAD diagnosis. Moreover, we must determine whether sBCMA can help identify individuals requiring IRT, particularly those with immunoglobulin deficiency but do not have a diagnosis of CVID or XLA. This would include those in which evaluation is complicated by usage of immunosuppressive medications or malignancy. sBCMA measurement could be quicker and not have painful localized immunization reactions some experience as a consequence of vaccination challenges currently used for evaluation of PAD patients. sBCMA was very low in two subjects that had persistent hypogammaglobulinemia after receiving rituximab treatment that left them requiring IRT. These illustrative examples, though preliminary, indicate that larger studies of sBCMA measurement in patients for which the use of IRT is unclear may be informative.
BCMA has previously been shown to have utility as a biomarker and therapeutic target in plasma cell malignancy.16, 38–40 In addition, sBCMA levels are very low among multiple myeloma patients in complete remission with reduced nonmalignant plasma cells and associated hypogammaglobulinemia.41 Thus, we explored whether BCMA levels could be useful for the diagnosis of PAD in which plasma cells are deficient. Our work demonstrates that sBCMA is profoundly reduced in PAD patients with IgG < 600 mg/dL as well as those with diagnoses of CVID and XLA, specifically, relative to less severe forms PAD and those without PAD. These results indicate that sBCMA may help identify individuals with forms of PAD that require IRT and are associated with chronic complications; specific types of PAD in which earlier diagnosis is particularly crucial. Importantly, sBCMA measurement can help identify severe PAD without laborious vaccination challenges or the infection risks of stopping IRT in those for which its benefit is in question. Future studies are needed to validate the utility of sBCMA as a diagnostic tool for clinically-significant antibody deficiency and determine whether it can aid in the identification of patients for which IRT is most appropriate.
Supplementary Material
HIGHLIGHTS BOX.
What is already known about this topic?
Diagnosis of primary antibody deficiency requiring immunoglobulin replacement often necessitates laborious testing that can be impeded by medications.
What does this article add to our knowledge?
CVID and XLA patients have markedly reduced serum BCMA levels and its measurement may improve evaluation of PAD.
How does this study impact current management guidelines?
Low levels of serum BCMA can identify severe PAD, without requiring stoppage of immunoglobulin replacement or protracted functional antibody evaluation.
Funding:
This work was supported by National Institutes of Health grant AI137183 (to P.J.M.) and AI061093 (to C.C-R.).
ABBREVIATIONS
- APRIL
a proliferation inducing ligand
- BAFF
B cell activating factor
- BCMA
B cell maturation antigen
- CVID
common variable immunodeficiency
- IgAD
selective IgA deficiency
- IgA/IgG2D
IgA and IgG2 deficiency
- IgGD
IgG deficiency
- IRT
immunoglobulin replacement therapy
- NPV
negative predictive value
- PAD
primary antibody deficiencies
- PPV
positive predictive value
- sBCMA
serum BCMA
- XLA
X-linked agammaglobulinemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: JRB has an ownership interest and is an officer of OncoTracker. HC, ML and ES have ownership interests in OncoTracker. The authors declare no other conflicts of interest.
REFERENCES
- 1.Rosenberg E, Dent PB, Denburg JA. Primary Immune Deficiencies in the Adult: A Previously Underrecognized Common Condition. J Allergy Clin Immunol Pract 2016;4:1101–7. [DOI] [PubMed] [Google Scholar]
- 2.Wasserman RL. The Nuts and Bolts of Immunoglobulin Treatment for Antibody Deficiency. J Allergy Clin Immunol Pract 2016;4:1076–81.e3. [DOI] [PubMed] [Google Scholar]
- 3.Schussler E, Beasley MB, Maglione PJ. Lung Disease in Primary Antibody Deficiencies. J Allergy Clin Immunol Pract 2016;4:1039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filion CA, Taylor-Black S, Maglione PJ, Radigan L, Cunningham-Rundles C. Differentiation of Common Variable Immunodeficiency From IgG Deficiency. J Allergy Clin Immunol Pract 2019;7:1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SY, Carbone J, Jolles S. The Expanding Field of Secondary Antibody Deficiency: Causes, Diagnosis, and Management. Front Immunol 2019;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolles S, Chapel H, Litzman J. When to initiate immunoglobulin replacement therapy (IGRT) in antibody deficiency: a practical approach. Clin Exp Immunol 2017;188:333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson LA, Benson P, Sneller VP, Butler JC, Thompson RS, Chen RT, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. Jama 1999;281:243–8. [DOI] [PubMed] [Google Scholar]
- 8.Wijetilleka S, Jayne DR, Mukhtyar C, Ala A, Bright PD, Chinoy H, et al. Recommendations for the management of secondary hypogammaglobulinaemia due to B cell targeted therapies in autoimmune rheumatic diseases. Rheumatology (Oxford) 2018; [DOI] [PubMed] [Google Scholar]
- 9.Rider NL, Kutac C, Hajjar J, Scalchunes C, Seeborg FO, Boyle M, et al. Health-Related Quality of Life in Adult Patients with Common Variable Immunodeficiency Disorders and Impact of Treatment. J Clin Immunol 2017;37:461–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taubenheim N, von Hornung M, Durandy A, Warnatz K, Corcoran L, Peter HH, et al. Defined blocks in terminal plasma cell differentiation of common variable immunodeficiency patients. J Immunol 2005;175:5498–503. [DOI] [PubMed] [Google Scholar]
- 11.Ochtrop ML, Goldacker S, May AM, Rizzi M, Draeger R, Hauschke D, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood 2011;118:309–18. [DOI] [PubMed] [Google Scholar]
- 12.Chen M, Ko HM, Riffle ME, Andreae DA, Cunningham-Rundles C, Chehade M, et al. Eosinophilic esophagitis diagnosed in a patient with common variable immunodeficiency. J Allergy Clin Immunol Pract 2016;4:995–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maas A, Hendriks RW. Role of Bruton’s tyrosine kinase in B cell development. Dev Immunol 2001;8:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 2013;24:203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent SA, Hoffmann FS, Kuhn PH, Cheng Q, Chu Y, Schmidt-Supprian M, et al. gamma-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015;6:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez E, Li M, Kitto A, Li J, Wang CS, Kirk DT, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol 2012;158:727–38. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol 2012;129:1425–6.e3. [DOI] [PubMed] [Google Scholar]
- 18.Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract 2019; [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008;151:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghamohammadi A, Mohammadi J, Parvaneh N, Rezaei N, Moin M, Espanol T, et al. Progression of selective IgA deficiency to common variable immunodeficiency. Int Arch Allergy Immunol 2008;147:87–92. [DOI] [PubMed] [Google Scholar]
- 21.Chan YH. Biostatistics 104: correlational analysis. Singapore Med J 2003;44:614–9. [PubMed] [Google Scholar]
- 22.Akoglu H User’s guide to correlation coefficients. Turk J Emerg Med 2018;18:91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen SF, Reims HM, Frydenlund D, Holm K, Paulsen V, Michelsen AE, et al. A Cross-Sectional Study of the Prevalence of Gastrointestinal Symptoms and Pathology in Patients With Common Variable Immunodeficiency. Am J Gastroenterol 2016;111:1467–75. [DOI] [PubMed] [Google Scholar]
- 24.Maglione PJ, Gyimesi G, Cols M, Radigan L, Ko HM, Weinberger T, et al. BAFF-driven B cell hyperplasia underlies lung disease in common variable immunodeficiency. JCI Insight 2019;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maglione PJ. Autoimmune and Lymphoproliferative Complications of Common Variable Immunodeficiency. Curr Allergy Asthma Rep 2016;16:19. [DOI] [PubMed] [Google Scholar]
- 26.Luo J, Niu X, Zhang M, Zhang K, Chen M, Deng S. Inhibition of B lymphocyte-induced maturation protein-1 reduces the production of autoantibody and alleviates symptoms of systemic lupus erythematosus. Autoimmunity 2015;48:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Gross JA, Dillon SR, Min JK, Elkon KB. Increased BCMA expression in lupus marks activated B cells, and BCMA receptor engagement enhances the response to TLR9 stimulation. Autoimmunity 2011;44:69–81. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Loo WM, Greenley EJ, Tung KS, Erickson LD. B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus. J Immunol 2011;186:6136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gathmann B, Mahlaoui N, Gerard L, Oksenhendler E, Warnatz K, Schulze I, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol 2014;134:116–26. [DOI] [PubMed] [Google Scholar]
- 30.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood 2008;112:277–86. [DOI] [PubMed] [Google Scholar]
- 31.Naradikian MS, Perate AR, Cancro MP. BAFF receptors and ligands create independent homeostatic niches for B cell subsets. Curr Opin Immunol 2015;34:126–9. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Carmona Y, Cols M, Ting AT, Radigan L, Yuk FJ, Zhang L, et al. Differential induction of plasma cells by isoforms of human TACI. Blood 2015;125:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coquery CM, Erickson LD. Regulatory roles of the tumor necrosis factor receptor BCMA. Crit Rev Immunol 2012;32:287–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 2004;199:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flint SM, Gibson A, Lucas G, Nandigam R, Taylor L, Provan D, et al. A distinct plasmablast and naive B-cell phenotype in primary immune thrombocytopenia. Haematologica 2016;101:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuh E, Musumeci A, Thaler FS, Laurent S, Ellwart JW, Hohlfeld R, et al. Human Plasmacytoid Dendritic Cells Display and Shed B Cell Maturation Antigen upon TLR Engagement. J Immunol 2017;198:3081–8. [DOI] [PubMed] [Google Scholar]
- 37.Meinl E, Thaler FS, Lichtenthaler SF. Shedding of BAFF/APRIL Receptors Controls B Cells. Trends Immunol 2018;39:673–6. [DOI] [PubMed] [Google Scholar]
- 38.Ju S, Wang Y, Ni H, Wang X, Jiang P, Kong X, et al. Correlation of expression levels of BLyS and its receptors with multiple myeloma. Clin Biochem 2009;42:387–99. [DOI] [PubMed] [Google Scholar]
- 39.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014;123:3128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest 2019;130: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berenson JR. The role of serum BCMA in monitoring, predicting outcomes and immune deficiency of multiple myeloma. Presented at Third Annual Immuno-Therapy in Myeloma Scientific Workshop in Philadelphia, PA May 4–6, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.