Abstract
World Health Organization recommends using dried blood spots (DBS) for HIV RNA viral load (VL) measurement whenever plasma processing is not convenient or feasible. DBS collected from 80 treatment-naïve HIV-infected patients presenting in three hospitals of two different regions of Thailand were shipped to a central laboratory along with corresponding plasma specimens. Viral load was measured in both DBS and plasma using the Abbott m2000 system. HIV RNA levels were strongly correlated (r = 0.94) with a mean of differences of 0.23 log10 copies/mL. Using the 1,000 copies/mL cut-off, the sensitivity of DBS was 97% (95%CI, 91–100%) and specificity was 75% (95%CI, 19–99%). DBS are useful to scale-up HIV RNA VL testing in settings with limited access to VL testing.
Introduction
In 2014, the UNAIDS set the 90-90-90 targets to reach by 2020 to end the AIDS epidemic [1]. In Thailand, it is estimated that about 450,000 people were living with HIV, over 270,000 individuals were receiving antiretroviral therapy (ART) in 2015 [2] and 211,000 (78% of those on ART) benefited HIV RNA viral load (VL) measurement [3]. Monitoring plasma HIV RNA VL is key to assess the response to ART. However, due to the limited access to plasma HIV RNA measurements in some settings, WHO has recommended using dried blood spots (DBS) as an alternative to plasma specimens [4]. Indeed, DBS present several advantages which make their use particularly attractive whenever access to VL testing is difficult: it is simple to prepare and convenient for blood collection and transportation (shipping by mail to a central testing laboratory) with low cost, and small volume of blood is needed. In addition, fingerpick blood specimen can be applied onto the DBS [5].
Previous studies have shown that DBS can be used to reliably measure HIV RNA VL with different platforms [6–9]. Routine monitoring of HIV RNA VL using DBS has been successfully implemented in resource-limited settings such as Angola, Tanzania, and Cameroon [10–12]. Several studies have evaluated the performances of the Abbott m2000 platform for the measurement of RNA in DBS versus plasma [5, 13–16]. Those studies were conducted among different populations (naïve or on ART) and used different DBS processing protocols. The sensitivity of DBS at the 1,000 cp/mL cut-off ranged between 76–100%, the specificity between 90–96% and the mean differences between Plasma-DBS were between 0.02–1.1 log10 cp/mL. These results indicate that the Abbott m2000 can perform well on DBS in different settings. In Thailand, HIV RNA are usually measured in plasma specimens and tests are mostly performed in centralized laboratories using commercial assays. In order to increase access to HIV RNA measurements for people living in remote areas, HIV RNA VL measurement in DBS was compared to measurement in plasma of treatment-naïve HIV-infected patients presenting at hospitals in two different regions of Thailand.
Materials and methods
Study setting and participants
Consenting HIV-infected individuals at least 18 years old, antiretroviral-naïve or having history of prevention of HIV mother-to-child transmission were enrolled at Sanpatong hospital (Chiangmai province) or Phayao hospital (Phayao province) in Northern region or Samut Sakorn hospital (Samut Sakorn province) in Central region of Thailand. Written informed consents were obtained from all participants for blood testing. Ethical approval was obtained from the Ethic committee of the Faculty of Associated Medical Sciences, Chiang Mai University (Reference no. AMSEC-58EX-038). Local ethical approval was also obtained from each hospital (The Ethic committees of Sanpatong hospital, of Phayao hospital, and of Samut Sakorn hospital).
Preparation of DBS and plasma
Six milliliters of whole blood were collected by venous puncture and transferred into an EDTA tube. Dried blood spots were prepared as previously described [17, 18]. Briefly, whole blood was dispensed in 5 spots of 50 μL each onto a Whatman 903® Protein Saver Card (Whatman, UK) and dried overnight at ambient temperature (25–30°C). Once dried, filter papers were stored in plastic zip-lock dispensing bags with a silica desiccant and kept at -20°C until shipment. The remaining EDTA whole blood specimens were centrifuged at 1,500 g for 15 minutes and plasma immediately transferred to sterile tubes and stored at -20°C until shipment. Both specimens were shipped with dry ice to the reference laboratory IRD UMI174-PHPT, Faculty of Associated Medical Sciences, Chiang Mai University, Chiangmai. All specimens were then stored at -80°C until testing.
HIV viral load measurement in DBS specimens
Blood was eluted from 2 spots of dried blood (equivalent to 100 μL of whole blood) placed in 2 mL of lysis buffer for 3 hours with gentle rotation. The eluted blood was then transferred to a new tube and centrifuged at 2,000 rpm (385 x g) for 10 minutes to remove the pieces of filter papers. The supernatant was further processed for automated isolation of nucleic acid using the Abbott m2000sp (Abbott Molecular Inc., USA) and the protocol “1 mL DBS HIV-1 RNA” according to the manufacturer’s instructions. Quantification was performed using the Abbott molecular m2000rt system (Abbott Molecular Inc., USA) with the detection threshold of 550 cp/mL. No manual data correction was required as there was an automatically software-embedded correction factor which took into account the input volumes used. HIV RNA VL obtained from DBS specimens was reported as copies per milliliter of plasma (cp/mL).
HIV viral load testing from plasma specimens
Plasma HIV RNA VL testing was performed using the Abbott m2000sp and Abbott molecular m2000rt system (Abbott Molecular Inc., USA) and the “plasma HIV-1 RNA protocol” according to the manufacturer’s instructions. The lower limit of detection (LOD) in plasma is 40 cp/mL. HIV RNA VL obtained from plasma specimens was reported as cp/mL.
Plasma HIV RNA VL testing was performed using the Abbott m2000sp and Abbott molecular m2000rt system (Abbott Molecular Inc., USA) and the “plasma HIV-1 RNA protocol” according to the manufacturer’s instructions.
Statistical analysis
The results of HIV RNA VL in DBS and plasma specimens are expressed as median and interquartile range (IQR). Undetectable HIV RNA VL results were considered as 1.30 log10 cp/mL (half of the limit of detection, LOD: 1.60 log10 cp/mL), detectable HIV RNA VL but lower than the LOD as 1.60 log10 cp/mL. Correlation between DBS HIV RNA VL and plasma VL was analyzed using Pearson’s correlation coefficient. The degree of agreement (concordance) between DBS HIV RNA VL and plasma HIV RNA VL results was assessed with Bland-Altman analysis. Specificity, sensitivity, and positive/negative predictive values of DBS HIV RNA VL testing versus plasma, as reference, were evaluated. All analyzes were performed using STATA™ version 14.1 software (Statacorp, College Station, TX). Statistical significance was considered when P-value was <0.05.
Results
Patient characteristics
Eighty patients were enrolled in this study. Sixty (75%) were male and 20 (25%) were female. The median age was 33.7 years old with an interquartile range (IQR) of 24.1–42.7. Seventy-six (95%) patients were antiretroviral-naïve and 4 (5%) patients had previously received antiretroviral drugs for prevention of mother-to-child transmission of HIV (Table 1).
Table 1. Baseline characteristics of study population.
| Baseline characteristics (N = 80) | Values (% or IQR) | |
|---|---|---|
| Gender | - Male | 60 (75%) |
| - Female | 20 (25%) | |
| DBS collected from | - Phayao hospital | 22 (27.5%) |
| - Samut Sakorn hospital | 20 (25%) | |
| - Sanpatong hospital | 38 (47.5%) | |
| Age, years old | Median (IQR) | 33.7 (24.1–42.7) |
| Antiretroviral drug received | - No | 76 (95%) |
| - Yes* | 4 (5%) | |
| Plasma HIV VL (log10 cp/mL)** | <1.60 | 1 (1.3%) |
| 1.60–1.99 | 0 (0%) | |
| 2.00–2.99 | 3 (3.8%) | |
| 3.00–3.99 | 5 (6.3%) | |
| 4.00–4.99 | 30 (37.5%) | |
| 5.00–5.99 | 35 (43.8%) | |
| 6.00–6.99 | 6 (7.5%) | |
| Median (IQR) | 5.05 (4.43–5.61) | |
*They had received antiretroviral drugs according to prevention of mother-to-child transmission of HIV
**Measured by the Abbott molecular m2000rt system
HIV-1 RNA measurement in plasma and DBS using Abbott molecular m2000 system
Median of plasma HIV RNA VL was 5.05 (IQR: 4.43–5.61) log10 copies/mL. Median HIV RNA VL in DBS was 4.80 (IQR: 4.13–5.39) log10 cp/mL. One patient (1.3%) had undetectable HIV RNA in both DBS and plasma specimens. Two (2.5%) patients had detectable HIV RNA VL (2.18 and 2.19 log10 cp/mL) in plasma specimens but undetectable in DBS. (Refer to the Supplementary data S1 Table)
Correlation and concordance between DBS and plasma viral load results
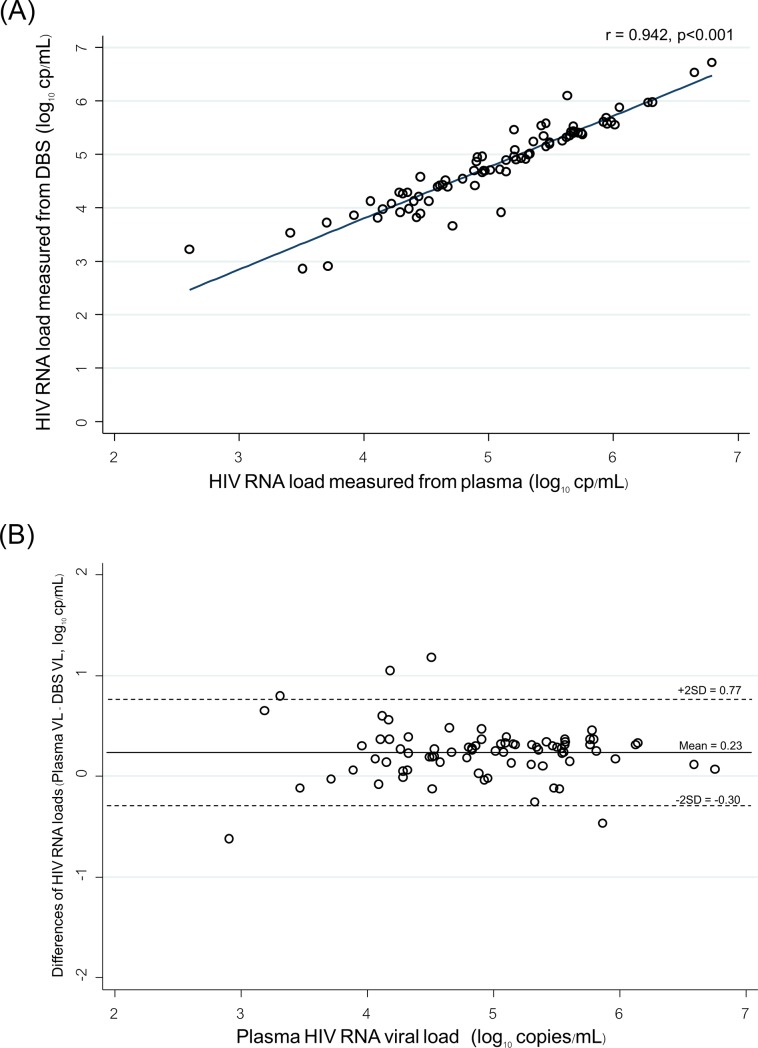
HIV RNA levels in plasma and in DBS were strongly correlated (correlation coefficient (r) of 0.94 and coefficient of determination (r2) of 0.89 (Fig 1A), excluding the values below the lower limit of quantitation. The concordance between plasma HIV RNA and DBS HIV RNA results is shown in the Bland-Altman plot (Fig 1B). The mean HIV RNA level was slightly higher in plasma than in DBS with the mean of differences of 0.23 log10 copies/mL and 2 standard deviation (2SD) of 0.53. Sixty (78%) specimens had a difference of plasma and DBS HIV RNA VL within a range of ±0.25 log10 copies/mL, and 72 (94%) and 77 (100%) were within a range of ±0.5 and of ±1.0 log10 copies/mL, respectively.
Fig 1.
(A) Regression analysis of HIV RNA levels in paired plasma and DBS specimens from 80 participants. (B) Bland-Altman analysis of agreement between HIV RNA levels in 80 paired plasma and DBS specimen. The horizontal lines represent the mean difference and +2 standard deviations (SD).
Among the 4 patients with plasma HIV RNA VL <1,000 copies/mL, 3 patients had also HIV RNA VL <1,000 copies/mL in DBS and 1 patient had HIV RNA VL ≥1,000 cp/mL in DBS (3.22 log10 cp/mL in DBS, 2.60 log10 in plasma). Of 76 patients with plasma HIV RNA VL ≥1,000 copies/mL, 74 had also HIV RNA VL ≥1,000 copies/mL in DBS. Sensitivity of DBS HIV RNA measurement was 97% (95%CI, 91–100%) and specificity was 75% (95%CI, 19–99%) as compared to plasma HIV RNA measurement. Positive predictive value of DBS VL was 99% (95%CI, 93–100%) and negative predictive value was 60% (95%CI, 26–87%). The accuracy was 96% (95%CI, 89–99%).
Discussion
In this study, HIV RNA VL results in DBS specimens were strongly correlated to VL results in plasma specimens, r = 0.94. HIV RNA levels measured in DBS were slightly lower than in plasma (0.23 log10 copies/mL). This could be due to the higher input volume of plasma (1mL) as compared to the DBS (0.1 mL of whole blood) [19] and possibly the higher rate of HIV RNA recovery in plasma than in DBS during the extraction. The higher level in plasma may also be due to the lack of hematocrit adjustment as reported in other studies [19, 20]. This slightly lower average HIV RNA levels in DBS than in plasma has also been observed in other studies with average differences ranging between 0.07–0.76 log10 copies/mL [5, 12, 16, 21, 22]. The smaller volume of blood tested in DBS might also explain that 2 DBS were undetectable while the corresponding plasma specimens had low HIV RNA VL (2.18 and 2.19 log10 cp/mL) which were below the threshold of detection in DBS.
We assessed the sensitivity of HIV RNA detection in DBS using the cut-off at 1,000 copies/mL of plasma, the usual cut-off to consider for treatment failure. We found similarly to studies conducted in other settings [5, 16, 23] a very high sensitivity of Abbott system to detect HIV RNA in DBS (97%) with a very high positive predictive value (99%). Also, the accuracy of HIV RNA measurement in DBS was very high (96%). All these results suggest that testing HIV RNA in DBS using the Abbott system will be useful to detect treatment failure in settings where plasma HIV RNA could not be easily performed. However, using DBS for monitoring the response to ART treatment have some drawbacks to consider: 1) false positive results due to the detection of cellular nucleic acids with the risk of diagnosing a virological failure in a patient who is actually controlling HIV replication or 2) false negative results due to the degradation of RNA in DBS stored in bad conditions with the risk of diagnosing viral suppression. In many resource-limited settings, the high humidity level in environment may impact on the preparation and processing of DBS. Accurate HIV RNA testing results require that DBS be completely dried before packing and shipped in plastic bag with desiccants.
Our study has some limitations. The correlation was done with DBS not shipped by regular post which may not reflect the real life. However, this study and other studies reported no or few losses of HIV RNA after shipping at various conditions, especially in whole blood specimens with high HIV RNA VL [24, 25]. Another limitation was that most of the specimens in this study were obtained from treatment-naïve patients who had a very high HIV RNA VL. Due to the low number of specimens (n = 4) with viral load below 1,000 copies/mL in plasma, the specificity was 75% with a wide confidence interval. In other studies conducted on high numbers of DBS with low HIV VL, specificity of Abbott system ranged between 95–96% [5, 16, 23].
In conclusion, our results suggest that DBS have a very good sensitivity at the threshold of 1,000 copies/mL and can be used for HIV RNA VL measurement in Thailand. Using DBS with Abbott system will increase the access to HIV RNA VL testing for HIV-infected patients who cannot benefit regular routine treatment monitoring. Scaling-up of HIV RNA VL will contribute to achieve the UNAIDS 90-90-90 targets to reach to end the AIDS epidemic.
Supporting information
(PDF)
Acknowledgments
The authors are grateful to all patients who participated in this study and the medical teams involved for their active and reliable commitment. The authors thank the Institut de Recherche pour le Développement (IRD) Unité Internationale 174-PHPT team for the help provided during this work.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the National Research University Project of Thailand Office of Higher Education Commission 2014, Chiang Mai University. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. There was no additional external funding received for this study.
References
- 1.The Joint United Nations Programme on HIV/AIDS. Fast-Track: ending the AIDS epidemic by 2030. Geneva, Switzerland: The Joint United Nations Programme on HIV/AIDS; 2014. [Google Scholar]
- 2.The Joint United Nations Programme on HIV/AIDS. 90-90-90 On the right track towards the global target Geneva, Switzerland: The Joint United Nations Programme on HIV/AIDS; 2016. [Google Scholar]
- 3.National Health Security Office. National Health Security Fund Management Manual Volume 2 Management of HIV / AIDS Service Accounts and Management of Tuberculosis Patients (in Thai). Bangkok, Thailand: National Health Security Office; 2016. [Google Scholar]
- 4.Smit PW, Sollis KA, Fiscus S, Ford N, Vitoria M, Essajee S, et al. Systematic review of the use of dried blood spots for monitoring HIV viral load and for early infant diagnosis. PLoS One. 2014;9(3):e86461 10.1371/journal.pone.0086461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Pahalawatta V, Frank A, Bagley Z, Viana R, Lampinen J, et al. HIV-1 viral load measurement in venous blood and fingerprick blood using Abbott RealTime HIV-1 DBS assay. J Clin Virol. 2017;92:56–61. 10.1016/j.jcv.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 6.Brambilla D, Jennings C, Aldrovandi G, Bremer J, Comeau AM, Cassol SA, et al. Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision, and RNA stability. J Clin Microbiol. 2003;41(5):1888–93. 10.1128/JCM.41.5.1888-1893.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marconi A, Balestrieri M, Comastri G, Pulvirenti FR, Gennari W, Tagliazucchi S, et al. Evaluation of the Abbott Real-Time HIV-1 quantitative assay with dried blood spot specimens. Clin Microbiol Infect. 2009;15(1):93–7. 10.1111/j.1469-0691.2008.02116.x [DOI] [PubMed] [Google Scholar]
- 8.Andreotti M, Pirillo M, Guidotti G, Ceffa S, Paturzo G, Germano P, et al. Correlation between HIV-1 viral load quantification in plasma, dried blood spots, and dried plasma spots using the Roche COBAS Taqman assay. J Clin Virol. 2010;47(1):4–7. 10.1016/j.jcv.2009.11.006 S1386-6532(09)00547-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Bertagnolio S, Parkin NT, Jordan M, Brooks J, Garcia-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: A review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12(4):195–208. [PubMed] [Google Scholar]
- 10.Garrido C, Zahonero N, Fernandes D, Serrano D, Silva AR, Ferraria N, et al. Subtype variability, virological response and drug resistance assessed on dried blood spots collected from HIV patients on antiretroviral therapy in Angola. J Antimicrob Chemother. 2008;61(3):694–8. 10.1093/jac/dkm515 [pii]. [DOI] [PubMed] [Google Scholar]
- 11.Johannessen A, Garrido C, Zahonero N, Sandvik L, Naman E, Kivuyo SL, et al. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin Infect Dis. 2009;49(6):976–81. 10.1086/605502 [DOI] [PubMed] [Google Scholar]
- 12.Mbida AD, Sosso S, Flori P, Saoudin H, Lawrence P, Monny-Lobe M, et al. Measure of viral load by using the Abbott Real-Time HIV-1 assay on dried blood and plasma spot specimens collected in 2 rural dispensaries in Cameroon. J Acquir Immune Defic Syndr. 2009;52(1):9–16. 10.1097/QAI.0b013e3181aeccbc [DOI] [PubMed] [Google Scholar]
- 13.Rutstein SE, Kamwendo D, Lugali L, Thengolose I, Tegha G, Fiscus SA, et al. Measures of viral load using Abbott RealTime HIV-1 Assay on venous and fingerstick dried blood spots from provider-collected specimens in Malawian District Hospitals. J Clin Virol. 2014;60(4):392–8. 10.1016/j.jcv.2014.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pannus P, Claus M, Gonzalez MM, Ford N, Fransen K. Sensitivity and specificity of dried blood spots for HIV-1 viral load quantification: A laboratory assessment of 3 commercial assays. Medicine (Baltimore). 2016;95(48):e5475 10.1097/MD.0000000000005475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeh C, Ndiege K, Inzaule S, Achieng R, Williamson J, Chih-Wei Chang J, et al. Evaluation of the performance of Abbott m2000 and Roche COBAS Ampliprep/COBAS Taqman assays for HIV-1 viral load determination using dried blood spots and dried plasma spots in Kenya. PLoS One. 2017;12(6):e0179316 10.1371/journal.pone.0179316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taieb F, Tran Hong T, Ho HT, Nguyen Thanh B, Pham Phuong T, Viet Ta D, et al. First field evaluation of the optimized CE marked Abbott protocol for HIV RNA testing on dried blood spot in a routine clinical setting in Vietnam. PLoS One. 2018;13(2):e0191920 10.1371/journal.pone.0191920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ngo-Giang-Huong N, Khamduang W, Leurent B, Collins I, Nantasen I, Leechanachai P, et al. Early HIV-1 diagnosis using in-house real-time PCR amplification on dried blood spots for infants in remote and resource-limited settings. J Acquir Immune Defic Syndr. 2008;49(5):465–71. 10.1097/QAI.0b013e31818e2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thepbundit V, Ngo-Giang-Huong N, Salvadori N, Laolue A, Kunyanone N, Sata-Un J, et al. Feasibility of using dried blood spots for HIV viral load testing among HIV-infected individuals in Thailand using QIAGEN QIAsymphony-artus HIV-1 platform. J Med Virol. 2019;91(9):1652–9. 10.1002/jmv.25506 [DOI] [PubMed] [Google Scholar]
- 19.Leelawiwat W, Young NL, Chaowanachan T, Ou CY, Culnane M, Vanprapa N, et al. Dried blood spots for the diagnosis and quantitation of HIV-1: stability studies and evaluation of sensitivity and specificity for the diagnosis of infant HIV-1 infection in Thailand. J Virol Methods. 2009;155(2):109–17. 10.1016/j.jviromet.2008.09.022 [DOI] [PubMed] [Google Scholar]
- 20.Nyagupe C, Shewade HD, Ade S, Timire C, Tweya H, Vere N, et al. HIV Viral Load Estimation Using Hematocrit Corrected Dried Blood Spot Results on a BioMerieux NucliSENS((R)) Platform. Diagnostics (Basel). 2019;9(3). 10.3390/diagnostics9030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrido C, Zahonero N, Corral A, Arredondo M, Soriano V, de Mendoza C. Correlation between human immunodeficiency virus type 1 (HIV-1) RNA measurements obtained with dried blood spots and those obtained with plasma by use of Nuclisens EasyQ HIV-1 and Abbott RealTime HIV load tests. J Clin Microbiol. 2009;47(4):1031–6. 10.1128/JCM.02099-08 JCM.02099-08 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vidya M, Saravanan S, Rifkin S, Solomon SS, Waldrop G, Mayer KH, et al. Dried blood spots versus plasma for the quantitation of HIV-1 RNA using a real-Time PCR, m2000rt assay. J Virol Methods. 2012;181(2):177–81. 10.1016/j.jviromet.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 23.Taieb F, Tram TH, Ho HT, Pham VA, Nguyen L, Pham BH, et al. Evaluation of Two Techniques for Viral Load Monitoring Using Dried Blood Spot in Routine Practice in Vietnam (French National Agency for AIDS and Hepatitis Research 12338). Open Forum Infect Dis. 2016;3(3):ofw142 10.1093/ofid/ofw142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez-Munoz MT, Zaragoza-Rodriguez S, Rojas-Montes O, Palacios-Saucedo G, Vazquez-Rosales G, Gomez-Delgado A, et al. High correlation of human immunodeficiency virus type-1 viral load measured in dried-blood spot samples and in plasma under different storage conditions. Arch Med Res. 2005;36(4):382–6. 10.1016/j.arcmed.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 25.Monleau M, Butel C, Delaporte E, Boillot F, Peeters M. Effect of storage conditions of dried plasma and blood spots on HIV-1 RNA quantification and PCR amplification for drug resistance genotyping. J Antimicrob Chemother. 2010;65(8):1562–6. 10.1093/jac/dkq205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.