Abstract
Objective:
Direct activation of the hyperdirect (HD) pathway has been linked to therapeutic benefit from subthalamic deep brain stimulation (DBS) for the treatment of Parkinson’s disease (PD). We sought to quantify the axonal conduction biophysics of corticofugal axons directly stimulated by subthalamic DBS and reconcile those findings with short-latency cortical evoked potential (EP) results.
Methods:
We used a detailed computational model of human subthalamic DBS to quantify axonal activation and conduction. Signal propagation to cortex was evaluated for medium (5.7 μm), large (10.0 μm), and exceptionally large (15.0 μm) diameter corticofugal axons associated with either internal capsule (IC) fibers of passage or the HD pathway. We then compared the modeling results to human cortical EP measurements that have described an exceptionally fast component (EP0) occurring ~1 ms after the stimulus pulse, a fast component (EP1) at ~3 ms, and a slower component (EP2) at ~5 ms.
Results:
Subthalamic stimulation of the HD pathway with large and medium diameter axons propagated action potentials to cortex with timings that coincide with the EP1 and EP2 signals, respectively. Only direct activation of exceptionally large diameter fibers in the IC generated signals that could approach the EP0 timing. However, the action potential biophysics do not generally support the existence of a cortical EP less than 1.5 ms after DBS onset.
Conclusions:
The EP1 and EP2 signals can be biophysically linked to antidromic activation of the HD pathway.
Significance:
Theoretical reconstruction of cortical EPs from subthalamic DBS demonstrate a convergence of anatomical, biophysical, and electrophysiological results.
Keywords: Corticofugal Axon, Pyramidal Neuron, Subthalamic Nucleus, Hyperdirect Pathway
1. Introduction
Subthalamic deep brain stimulation (DBS) is an established treatment for the motor symptoms of advanced Parkinson’s disease (PD) [Limousin et al., 1998]. While the exact therapeutic mechanisms of subthalamic DBS remain unresolved, direct activation of the hyperdirect (HD) pathway is thought to play an important role [Li et al., 2014]. The HD pathway consists of a subset of corticofugal axons that originate in layer V of cortex, pass through the internal capsule and branch collaterals to the subthalamic nucleus (STN), while the corticofugal axon continues down to the brainstem [Haynes and Haber, 2013; Coude et al., 2018]. The HD pathway is distinct from typical internal capsule (IC) fibers of passage, which do not branch collaterals to the STN. So while stimulation of the HD pathway is believed to improve PD symptoms [Whitmer et al., 2012], direct activation of the IC pathway generates unwanted side effects such as muscle contractions [Tommasi et al., 2008]. Previously we have shown that recruitment of the HD pathway occurs at lower DBS amplitudes than the IC pathway [Gunalan et al., 2017]. However, stimulation of either pathway is capable of generating cortical evoked potentials (EPs) [e.g. Ashby et al., 2001; Baker et al., 2002; Walker et al., 2012; Miocinovic et al., 2018].
Clinical experiments measuring cortical EPs during subthalamic DBS have identified several different short-latency signals. Walker et al. [2012] observed three responses (R1, R2, and R3) in EEG recordings after each DBS pulse with peak latencies occurring at 1.0 ± 0.4 ms (R1), 5.7 ± 1.1 ms (R2), and 22.2 ± 1.8 ms (R3). They hypothesized that antidromic activation of the HD pathway generated the R1 signal, while the slower components were thought to be the result of intracortical synaptic action. More recently, Miocinovic et al. [2018] used ECoG to achieve a higher degree of specificity in the cortical EP recordings and found three short-latency signals (EP1, EP2, EP3). Specifically in M1, the peak of EP1 occurred 2.8 ± 0.3 ms, EP2 occurred 5.8 ± 1.0 ms, and EP3 occurred 7.7 ± 1.8 ms after onset of the DBS pulse. Therefore, we set out to explore the underlying biophysics that dictate these various EPs using a detailed computational model of human subthalamic DBS [Gunalan et al., 2017]. The model allowed us to quantify the arrival timings of individual action potentials (APs) in cortex following DBS of anatomically and electrically accurate representations of the IC and HD pathways. As such, we explored the role of stimulation parameter settings (monopolar, bipolar, and stimulus amplitude), as well as the influence of axon diameter, on the propagation of DBS-induced APs to cortex.
Understanding the biophysics that underlie the temporal characteristics of subthalamic DBS-induced cortical EPs is important for both mechanistic and engineering investigations. On the mechanistic side, attempts to define correlations between DBS, the generation of a specific EP signal, and improvements in clinical outcome variables necessitates an understanding of the pathway being stimulated [Walker et al., 2012; Miocinovic et al., 2018; Gunalan et al., 2018]. On the engineering side, if an EP signal can be directly related to therapeutic benefit, then it may become a candidate biomarker for use in closed-loop DBS control systems [Swann et al., 2018]. However, clinical implementation of such a closed-loop system would require precise definitions for the timing of the specific EP signals, as well as the range of variability that would need to be tolerated by the control system. The results of this study provide detailed estimates of DBS-induced signal propagation to cortex from both the IC and HD pathways, as well as their dependence on physiological variables.
2. Methods
This study analyzed activation of the IC and HD pathways by DBS electrodes implanted in the subthalamic region using a computational model (Figure 1). The technical details of the model system are described in their entirety in Gunalan et al. [2017], and briefly expanded upon below. The patient data used to construct the model was from a 67 year old, right handed, male, diagnosed with PD for ~11 years. The model explicitly represented the stimulus voltage distribution generated by the DBS electrode and then calculated the response of multi-compartment cable models of myelinated axons with anatomically realistic trajectories representing the IC and HD pathways. DBS-induced APs propagated from the subthalamic region to cortex and we measured the timing of their arrival as a function of the stimulation parameter settings and axon diameters.
Figure 1.
Deep brain stimulation of the IC (A) and HD (B) pathways. A1/B1) Axon trajectories displayed in a coronal view with the MRI (thalamus – yellow volume, STN – green volume). A2/B2) Sagittal view of the pathways. A3/B3) Zoomed in view of the pathways and DBS electrode. A4/B4) Voltage distribution generated by the DBS electrode interpolated onto the cable axon models. A5/B5) Axon models that were suprathreshold for the generation of propagating action potentials are displayed in red.
2.1. DBS voltage distribution
We used a finite element model, developed in COMSOL, to solve Laplace’s equation and estimate the voltage distribution generated by the DBS electrode. The model accounted for the tissue anisotropy and inhomogeneity of the human head by defining conductivities from diffusion-weighted images and tissue-type segmentation of soft tissue structures [Howell and McIntyre, 2017; Gunalan et al., 2017]. We then calculated the temporal characteristics of the voltage distribution using an equivalent electrical circuit model for voltage-controlled DBS, such that the modeled stimulus waveform matched the output of the Medtronic stimulator [Lempka et al., 2018].
2.2. Axon models
We constructed multi-compartment cable models of myelinated axons to represent the IC and HD pathways (Figure 1) [Gunalan et al., 2017]. We used probabilistic tractography (FSL – probtrackx; 1000 samples/ mm3) from a seed mask of the white matter near the STN to define the trajectory of the corticofugal axons. Of the 9,707 streamlines that were generated, we randomly sampled 1,000 to represent the IC pathway and 1,000 to represent the corticofugal axon of the HD pathway. We fit a smoothing spline to each tractography-generated streamline to ensure a smooth trajectory for use in the stimulation modeling [Gunalan et al., 2017]. The corticofugal streamlines between the cerebral peduncles and cortex were 89.0 ± 7.0 mm and 88.8 ± 6.6 mm in length for the IC and HD pathways, respectively (Figure 1).
Each axon collateral of the HD pathway was created by introducing a branch point at a node of Ranvier along the corticofugal axon that was near the dorsal boundary of the STN (Figure 1). The model was designed to have HD collaterals reach every voxel of the STN volume, and have some degree of variability in the dorsal-ventral location of the collateral branch point from the corticofugal axon to coincide with axonal tracing studies of HD collaterals [Haynes and Haber, 2014; Coude et al., 2018]. This anatomical variability in the model axons also helped avoid biasing the simulations to the arbitrary activation of a single group of HD collaterals. As such, each HD collateral had a single voxel within the STN selected as the termination point, and we generated a streamline arc connecting the branch point to the STN termination point, thereby defining the collateral trajectory [Gunalan et al., 2017].
The biophysical parameters for the axon simulations were defined from previously established models [McIntyre et al., 2002]. The myelinated axon was modeled with a double cable structure and the nodes of Ranvier contained active (i.e. voltage-gated fast Na+, persistent Na+, and slow K+ ion channel conductances) and passive (i.e. leak conductance and membrane capacitance) membrane properties. The hyperdirect axon collateral that projected into the STN had a diameter that was defined as a fraction (1/3) of the corticofugal axon diameter [Grill et al., 2008; Coude et al., 2018]. The response of each individual axon model to the DBS voltage distribution was calculated with NEURON [Hines and Carnevale, 2001]. The model system was simulated with a single DBS pulse and an axon was considered activated if an AP reached cortex.
The corticofugal axons within each pathway were modeled with either a medium (5.7 μm), large (10.0 μm), or exceptionally large (15.0 μm) diameter. We stimulated these axons with either monopolar or bipolar subthalamic DBS electrode configurations and a 60 μs pulse. However, the presented results concentrate on bipolar stimulation, as this mode of stimulation is typically required when attempting to experimentally record short-latency cortical EPs.
Results
The electrical stimulation threshold for AP initiation in an axon is directly proportional to its distance from the electrode, and inversely proportional to the diameter of the myelinated axon [McNeal, 1976]. In addition, the conduction velocity of a myelinated axon is directly proportional to its diameter [Boyd and Kalu, 1979]. As such, multiple factors work in concert to define the specific collection of axons that will be directly activated in response to a DBS pulse, and those APs propagate to cortex with a speed that is dictated by their axon morphology. The resulting short-latency cortical EPs are then produced from antidromic invasion of the layer V pyramidal neurons and transmembrane currents generated by their somatodendritic polarization [Li et al., 2012; Anderson et al., 2018].
Figure 2 provides example results on the activation and conduction of the HD and IC pathways in response to bipolar subthalamic DBS. The relative activation of the HD versus IC pathways differs as a function of the stimulus amplitude, as well as the corticofugal axon diameters being evaluated (Figure 2A1/B1). Given the axon diameter distribution of the IC [Graf von Keyserlingk and Schramm, 1984; Firmin et al., 2014], we hypothesize that focused evaluation on the large diameter axons (10.0 μm) provides the most clinically relevant example to highlight (Figure 2A2/B2). Our simulation results suggest that DBS activates a relatively small percentage of the IC pathway, compared to the HD pathway [Gunalan et al., 2017]. However, it should be noted that while our simulations used equal numbers of axons (1000) to represent each pathway, the percentage of pyramidal tract axons that have a hyperdirect collateral is approximated to be only ~5% [Kita and Kita, 2012]. As such, a more anatomically inspired variant of this model would have 1000 IC fibers of passage and only 50 HD axons. So if one wanted to compare raw totals of Figure 2 in that context, panels A2/B2 could be interpreted as an activation ratio of 100 “10 μm IC units” to 23 (460 * 0.05) “10 μm HD units” that would send antidromic APs up to cortex in response to the 4 V 60 μs stimulus.
Figure 2.
Bipolar DBS and AP conduction of the IC (A) and HD (B) pathways. A1/B1) Recruitment curves for the pathways as a function of the DBS stimulus amplitude for medium, large, and exceptionally large axon diameters. A2/B2) Conduction time to cortex for each activated axon (10μm diameter) from a 4V 60μs stimulus. A3/B3) Histogram of cortical arrival times for medium, large, and exceptionally large diameter axons activated with a 4V 60μs stimulus.
The IC and HD pathways showed differences in the distribution of times that APs arrived at cortex (Figure 2A3/B3). This difference arose primary from the variable site of AP initiation in the HD axons compared to the IC axons. The IC axons would always initiate their APs in the capsule. However, most of the HD axons had their APs initiate in the subthalamic collateral. This provided both a longer overall conduction path, as well as a slower conduction speed because of the smaller diameter of the axon collateral. Alternatively, some of the HD axons did initiate their APs in the corticofugal axon, enabling that group of HD axons to reach cortex as fast as the IC axons. However, as the corticofugal axon diameters were reduced in size, the proportion of HD axons with AP initiation in the capsule reduced and the spread of arrival times increased (Figure 2A3/B3). The results presented in Figure 2 are from bipolar stimulation. However, we found that the general trends of Figure 2 were independent of the stimulation modality (monopolar vs. bipolar) and/or contact(s) selected for stimulation.
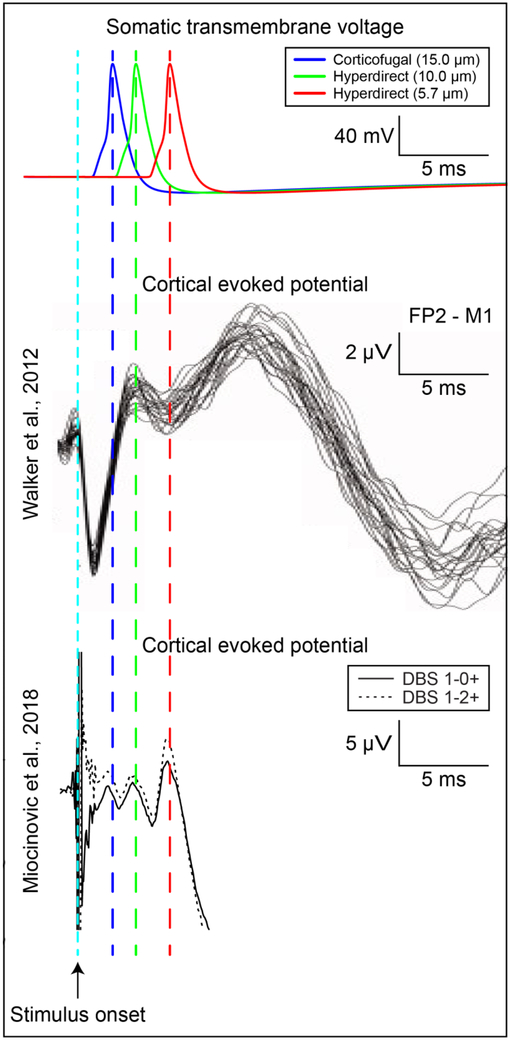
AP arrival in cortex is only the first step in generation of the electric field responsible for the experimentally recorded EPs. The synchronous polarization of the somatodendritic components of thousands of layer V pyramidal neurons, via antidromic AP invasion, are needed to create an electric field that is large enough to be recorded with EEG or ECoG. However, somatic APs and their propagation into the apical dendrites are slower and wider than axonal APs [Stuart and Sakmann, 1994]. As such, the timing associated with these additional biophysical processes needs to be considered when attempting to reconstruct the timing of the cortical EP. Figure 3 provides an overlay of simulated somatic APs from a layer V pyramidal neuron model [Mainen et al., 1995], initiated at the average arrival times for the different axonal populations (Figure 2). These somatic APs were then aligned with clinical examples of DBS-evoked cortical EPs [Walker et al., 2012; Miocinovic et al., 2018]. The results suggest that even the fastest conducting axons with exceptionally large diameters would not be able to generate a cortical EP at 1 ms. However, EPs observed at 2 ms, 3 ms, and 5 ms correspond well with DBS activation of exceptionally large IC axons, large HD axons, and medium HD axons, respectively (Figure 3).
Figure 3.
Cortical EPs. Top row, simulated layer V pyramidal neuron APs initiated at the average arrival time (0.9 ms, 1.9 ms, 3.8 ms) for DBS activation of different types of axons (15 μm corticofugal, 10 μm hyperdirect, 5.7 μm hyperdirect). Middle row, example EEG measurements from Walker et al. [2012]. Bottom row, example ECoG measurements from Miocinovic et al. [2018].
Discussion
Cortical EPs are valuable experimental measurements for addressing mechanistic questions on the effects of DBS in humans, and they might also provide useful control signals for future closed-loop DBS systems. However, even though short-latency (i.e. less than ~5 ms) subthalamic DBS-induced cortical EPs represent the most biophysically simple EP signal imaginable (i.e. directly activated antidromic activity), there are still numerous anatomical and electrical variables that can affect their interpretation. Therefore, we used a detailed computational model of human subthalamic DBS to simulate likely scenarios underlying their biophysical basis. Given current understanding of the HD pathway axonal anatomy [Coude et al., 2018], our results suggest that cortical EPs observed at ~3 ms (EP1) and ~5 ms (EP2) can be representative of activation of large and medium diameter components of the HD pathway, respectively (Figure 3). However, disentangling activation of IC fibers of passage from the HD pathway in the cortical EP is difficult. In addition, the raw number of activated axons from DBS EP experiments are likely to be biased toward the IC pathway (Figure 2). This is because of the relatively high stimulus amplitudes typically used to elicit cortical EPs in experimental studies, as well as the possible degeneration of the HD pathway in PD [Mathai et al., 2015]. Therefore, our results suggest that future clinical studies attempting to employ short-latency cortical EPs as subthalamic DBS biomarkers should concentrate on the EP1 signal and its generation at the lowest possible stimulus amplitudes where it is experimentally measurable. This maximizes the likelihood of monitoring a signal that is primarily derived from activation of the HD pathway, assuming the DBS electrode is well positioned in the subthalamic nucleus.
Short-latency cortical EPs generated by subthalamic DBS arise from axons that travel through the IC, and the primary determinates of the timing of each component of the overall EP waveform are the conduction velocities of the activated axons. The diameter of the axons, and their corresponding internodal spacing, dictate the conduction velocity [Boyd and Kalu, 1979]. Unfortunately, quantitative measurements on the distribution of myelinated axon diameters in the elderly, or parkinsonian, human do not exist. Firmin et al. [2014] performed extensive analyses associating the distribution of internal capsule axon diameters with the distribution of conduction velocities. The largest axon they found was only 13 μm in diameter, and axons approaching that size were very rare. However, that work was performed in the macaque brain, which when compared to the human, is expected to have results that are slightly skewed toward smaller diameter axons and slower conduction velocities. Nonetheless, some electron microscopy information is available on the axon diameters of pyramidal tract fibers in the adult human from postmortem specimens obtained after perfusion fixation [Graf von Keyserlingk and Schramm, 1984]. Those results show that the vast majority of fibers have diameters less than 4 μm, while large and exceptionally large diameter fibers account for only ~10% and ~1% of the total, respectively.
Electrical stimulation has its strongest effects the largest diameter axons because they have the largest internodal spacing. The large internodal spacing creates a large second spatial derivative of the extracellular voltage distribution at neighboring nodes of Ranvier that are close to the stimulating electrode, which is the primary determinate of membrane polarization from the DBS electric field [McNeal, 1976]. Nonetheless, typical DBS parameter settings directly activate a distributed collection of medium, large, and exceptionally large diameter axons, because axon proximity to the electrode is just as important as fiber diameter when quantifying axonal activation (Figure 2) [Gunalan et al., 2018]. As such, short-latency cortical EPs generated by DBS consist of multiple components [Walker et al., 2012; Miocinovic et al., 2018], which are generated by different groups of axons with different diameters and conduction velocities (Figures 2, 3). Our simulations used three groups of axons that have corticofugal conduction velocities ranging from 25 m/s (5.7 μm), to 55 m/s (10 μm), to 85 m/s (15 μm) [McIntyre et al., 2002]. Previous clinical studies have suggested that conduction velocities in the 20-40 m/s range would be sufficient to generate the short-latency cortical EPs seen with subthalamic DBS [Walker et al., 2012; Miocinovic et al., 2018]. However, what those previous estimates failed to consider are the components of time associated with initiating the AP in the subthalamic region, the tortuous path length of the corticofugal axon, and the AP invasion of the somatodendritic components of the pyramidal neuron [Anderson et al., 2018]. When accounting for all of these factors, the biophysics suggest that it is unlikely to generate a cortical EP less than 1.5 ms after stimulus onset, even with a corticofugal conduction velocity of 85 m/s. This is especially true when considering EPs generated via activation of axon collaterals in the STN that are associated with the HD pathway. The HD axon collaterals extend the path length and have smaller diameters, both of which add time to the conduction. Therefore, we propose that the best opportunity to observe a cortical EP that is primarily generated by the HD pathway is to concentrate on a signal that peaks at ~3 ms in motor cortex when using the lowest possible stimulus amplitude. The ~3 ms timing would be consistent with activating the largest diameter fibers (10 μm) expected to be associated with the human HD pathway, based on scaling up anatomical results from the rodent [Kita and Kita, 2012] and monkey [Coude et al., 2018]. Using a low stimulus amplitude would also promote AP initiation in the HD axon collaterals, and provide some degree of protection against contaminating activation of IC fibers of passage.
We propose that it is unlikely for the short-latency cortical EPs generated by subthalamic DBS (i.e. less than 5 ms after the stimulus) to be the result of synaptically generated neural activity. There does not appear to be enough time for AP propagation through IC, synaptic transmission in cortex, and subsequent AP spiking in a secondary group of neurons. However, the larger amplitude EP signals that occur in the ~10-20 ms time window are likely the result of the synaptic activation of the cortical microcircuitry from the DBS-induced antidromic inputs to the layer V pyramidal neurons [Kumaravelu et al., 2018]. These neurons have extensive intracortical axonal arbors that make wide spread synaptic connections throughout the cortical layers, and the timing of those synaptic activations correspond well with the long-latency cortical EPs [Anderson et al., 2018].
The model we used to perform our analyses represents the most technically detailed biophysical simulation of human DBS ever created. Unfortunately, while aspects of that model system, such as the DBS volume conductor or axon cable models, can be constrained and validated with experimental data [McIntyre et al., 2002; Miocinovic et al., 2009], the generalized output predictions of pathway activation percentages cannot be explicitly measured with current experimental techniques [Gunalan et al., 2017]. In addition, many of the model parameters (e.g. ion channel biophysics, axon myelination, HD collateral trajectories) were derived from results obtained in animal experiments that may not exactly represent the specific conditions in an individual patient participating in a particular research study. Further, the model was constructed as a generic example of a single PD subject with the DBS electrode well positioned in the STN.
Short-latency cortical EPs represent one of the most direct physiological measurements available from patients that we can use to evaluate the predictive capabilities of human DBS models (Figure 3). However, any attempt to use the model from this study, and the currently available EP experimental data, to try to “validate” that DBS of a given pathway is responsible for a given EP signal would be a circular argument. In addition, there remain many unanswered questions on the basic biophysical origins of the EP signals. For example, given the hypersynchrnous axonal activity and tight timing of the APs arriving at cortex less than 2 ms after the stimulus (Figure 2), it is conceivable that axonal currents could contribute to the very earliest signals, while the later potentials (2-3 ms) represent the somatodendritic polarization. It is also possible that an EP signal recorded at ~1 ms could be volume conducted from the STN [Maling et al., 2018], as robust large-amplitude potentials have been detected in the STN following a DBS pulse [Sinclair et al., 2018]. Therefore, we propose that coupled integration of prospective cortical EP recordings with patient-specific versions of the kind of DBS model used in this study represents a necessary next step to perform the validation studies needed to expand physiological understanding of electrical stimulation in the human brain.
HIGHLIGHTS.
Model of human subthalamic DBS to quantify corticofugal axonal activation and conduction to cortex.
Compared and contrasted activation of the internal capsule and hyperdirect pathways.
Cortical evoked potential reconstruction via merging of anatomical, biophysical, and electrophysiological results.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 NS085188 and R01 NS086100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
CCM is a paid consultant for Boston Scientific Neuromodulation, receives royalties from Neuros Medical, Hologram Consultants, Qr8 Health, and is a shareholder in the following companies: Hologram Consultants, Surgical Information Sciences, Cortics, Autonomic Technologies, Cardionomic, Enspire DBS.
References
- Anderson RW, Farokhniaee A, Gunalan K, Howell B, McIntyre CC. Action potential initiation, propagation, and cortical invasion in the hyperdirect pathway during subthalamic deep brain stimulation. Brain Stimul. 11(5):1140–1150, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby P, Paradiso G, Saint-Cyr JA, Chen R, Lang AE, Lozano AM. Potentials recorded at the scalp by stimulation near the human subthalamic nucleus. Clin Neurophysiol. 112(3):431–7, 2001. [DOI] [PubMed] [Google Scholar]
- Baker KB, Montgomery EB Jr, Rezai AR, Burgess R, Lüders HO. Subthalamic nucleus deep brain stimulus evoked potentials: physiological and therapeutic implications. Mov Disord. 17(5):969–83, 2002. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Kalu KU. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 289:277–97, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudé D, Parent A, Parent M. Single-axon tracing of the corticosubthalamic hyperdirect pathway in primates. Brain Struct Funct. 223(9):3959–3973, 2018. [DOI] [PubMed] [Google Scholar]
- Firmin L, Field P, Maier MA, Kraskov A, Kirkwood PA, Nakajima K, Lemon RN, Glickstein M. Axon diameters and conduction velocities in the macaque pyramidal tract. J Neurophysiol. 112(6):1229–40, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf von Keyserlingk D, Schramm U. Diameter of axons and thickness of myelin sheaths of the pyramidal tract fibres in the adult human medullary pyramid. Anat Anz. 157(2):97–111, 1984. [PubMed] [Google Scholar]
- Grill WM, Cantrell MB, Robertson MS. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J Comput Neurosci. 24(1):81–93, 2008. [DOI] [PubMed] [Google Scholar]
- Gunalan K, Chaturvedi A, Howell B, Duchin Y, Lempka SF, Patriat R, Sapiro G, Harel N, McIntyre CC. Creating and parameterizing patient-specific deep brain stimulation pathway-activation models using the hyperdirect pathway as an example. PLoS One. 12(4):e0176132, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunalan K, Howell B, McIntyre CC. Quantifying axonal responses in patient-specific models of subthalamic deep brain stimulation. NeuroImage. 172:263–277, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 33(11):4804–14, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. NEURON: a tool for neuroscientists. Neuroscientist. 7(2):123–35, 2001. [DOI] [PubMed] [Google Scholar]
- Howell B, McIntyre CC. Role of soft-tissue heterogeneity in computational models of deep brain stimulation. Brain Stimul. 10(1):46–50, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci. 32(17):5990–9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaravelu K, Oza CS, Behrend CE, Grill WM. Model-based deconstruction of cortical evoked potentials generated by subthalamic nucleus deep brain stimulation. J Neurophysiol. 120(2):662–680, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempka SF, Howell B, Gunalan K, Machado AG, McIntyre CC. Characterization of the stimulus waveforms generated by implantable pulse generators for deep brain stimulation. Clin Neurophysiol. 129(4):731–742, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Ke Y, Chan DC, Qian ZM, Yung KK, Ko H, Arbuthnott GW, Yung WH. Therapeutic deep brain stimulation in Parkinsonian rats directly influences motor cortex. Neuron. 76(5):1030–41, 2012. [DOI] [PubMed] [Google Scholar]
- Li Q, Qian ZM, Arbuthnott GW, Ke Y, Yung WH. Cortical effects of deep brain stimulation: implications for pathogenesis and treatment of Parkinson disease. JAMA Neurol. 71(1):100–3, 2014. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 339(16):1105–11, 1998. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Joerges J, Huguenard JR, Sejnowski TJ. A model of spike initiation in neocortical pyramidal neurons. Neuron. 15(6):1427–39, 1995. [DOI] [PubMed] [Google Scholar]
- Maling N, Lempka SF, Blumenfeld Z, Bronte-Stewart H, McIntyre CC. Biophysical basis of subthalamic local field potentials recorded from deep brain stimulation electrodes. J Neurophysiol. 120(4):1932–1944, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai A, Ma Y, Paré JF, Villalba RM, Wichmann T, Smith Y. Reduced cortical innervation of the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Brain. 138(Pt 4):946–62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol. 87(2):995–1006, 2002. [DOI] [PubMed] [Google Scholar]
- McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 23:329–337, 1976. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, Vitek JL, McIntyre CC. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Exp Neurol. 216(1):166–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, de Hemptinne C, Chen W, Isbaine F, Willie JT, Ostrem JL, Starr PA. Cortical Potentials Evoked by Subthalamic Stimulation Demonstrate a Short Latency Hyperdirect Pathway in Humans. J Neurosci. 38(43):9129–9141, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair NC, McDermott HJ, Bulluss KJ, Fallon JB, Perera T, Xu SS, Brown P, Thevathasan W. Subthalamic nucleus deep brain stimulation evokes resonant neural activity. Ann Neurol. 83(5):1027–1031, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 367(6458):69–72, 1994. [DOI] [PubMed] [Google Scholar]
- Swann NC, de Hemptinne C, Thompson MC, Miocinovic S, Miller AM, Gilron R, Ostrem JL, Chizeck HJ, Starr PA. Adaptive deep brain stimulation for Parkinson's disease using motor cortex sensing. J Neural Eng. 15(4):046006, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi G, Krack P, Fraix V, Le Bas JF, Chabardes S, Benabid AL, Pollak P. Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry. 79(7):813–9, 2008. [DOI] [PubMed] [Google Scholar]
- Walker HC, Huang H, Gonzalez CL, Bryant JE, Killen J, Cutter GR, Knowlton RC, Montgomery EB, Guthrie BL, Watts RL. Short latency activation of cortex during clinically effective subthalamic deep brain stimulation for Parkinson's disease. Mov Disord. 27(7):864–73, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson's disease. Front Hum Neurosci. 6:155, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]