Abstract
Abnormal tau hyperphosphorylation and its aggregation into neurofibrillary tangles are a hallmark of tauopathies, neurodegenerative disorders that include Alzheimer’s disease (AD). Active and passive Tau-immunotherapy has been proposed as a therapeutic approach to AD with mixed results. One of the limitations of active immunotherapy may be associated with the mediocre immunogenicity of vaccines that are not inducing therapeutically potent titers of antibodies. The aim of this study was to test the efficacy of an anti-tau vaccine, AV-1980R/A composed of N terminal peptide of this molecule fused with an immunogenic MultiTEP platform and formulated in a strong adjuvant, AdvaxCpG in a Tg4510 mouse model of tauopathy. Experimental mice were immunized with AV-1980R/A and a control group of mice were injected with adjuvant only. Nontransgenic and tetracycline transactivator (tTA) transgenic littermates were included as baseline controls to contrast with the tau phenotype. Active immunization with AV-1980R/A induced very strong anti-tau humoral immune responses in both nontransgenic and transgenic mice with evidence of IgG in brains of AV-1980R/A vaccinated mice. These experimental animals displayed an improvement in short-term memory during a novel object recognition test. However, impairments in other behavioral tasks were not prevented by AV-1980R/A vaccinations. At the same time, high titers of anti-tau antibodies reduced hyperphosphorylated pSer396 tau but did not lower the level of other phosphorylated tau species in the brains of AV-1980R/A vaccinated mice. These data indicate that active immunotherapy with an N-terminal Tau epitope was only partially effective in improving cognition and reducing pathology in the stringent Tg4510 mouse model of tauopathy.
1. Introduction
Alzheimer’s disease is the most common form of dementia, in which progressive accumulation of amyloid plaques and hyperphosphorylated tau aggregated into neurofibrillary tangles (NFTs) results in progressive cognitive impairments. Active and passive immunotherapy targeting Aβ has been successful in multiple AD animal models (reviewed in (Morgan et al., 2005, Wisniewski and Boutajangout, 2010, Agadjanyan et al., 2015) and has been tested in an increasing number of clinical trials without much success (Lobello et al., 2012; Lannfelt et al., 2014; Winblad et al., 2014; Wisniewski and Goni, 2014; Agadjanyan et al., 2015; Schilling et al., 2018; Selkoe, 2018; Bachmann et al., 2019; Panza et al., 2019). In the first active immunization trial with full-length fAβ42 some individuals developed a detrimental T cell- mediated inflammatory response (aseptic meningoencephalitis) leading to early termination of the trial (Orgogozo et al., 2003; Ferrer et al., 2004) indicating that activation of autoreactive T cells may infiltrate the brain and cause serious adverse events. In addition, we now understand that even though amyloid deposition occurs early in the disease, it doesn’t account for clinical symptoms by itself (Jack et al., 2009). Brain atrophy, neuronal and synaptic losses appear to be the key components of cognitive impairments in AD (DeKosky and Scheff, 1990; Terry et al., 1991; Fox et al., 1999), and are more likely to be caused by tau pathology.
Indeed, NFTs are observed early in the pathogenesis of AD and increase during aging (Braak and Braak, 1991). NFTs progression is correlated with cognitive deficits (Duyckaerts et al., 1997), supporting a pivotal role for tau pathology and spreading in AD-related memory impairments (Dujardin et al., 2015). As such, there is a possibility that targeting tau may represent a more effective method of treating AD than removing Aβ if a patient is already exhibiting clear signs of cognitive impairments. Therefore, development of safe and efficient immunotherapy targeting pathological tau could not only benefit AD patients, but also may become a useful tool against tauopathies in general.
However, as with Aβ immunotherapy, some studies reported increased neuroinflammation and encephalopathy following active immunization with full length tau (Rosenmann et al., 2006) or phospho-tau epitopes (Rozenstein-Tsalkovich et al., 2013). Furthermore, phosphorylation is essential for the regulation of tau’s normal physiological role in microtubule spatial organization. Therefore, one significant concern that is associated with active tau immunotherapy is that phospho-tau peptides may induce an immune response against physiological tau species (Kontsekova et al., 2014). Targeting non-phosphorylated epitopes or toxic tau conformation may be necessary. Changes in the conformations of tau are particularly important because they can directly affect the function of the protein and its toxic role in disease.
Recently, the phosphatase-activating domain (PAD) motif, a non-phosphorylated epitope, was identified in the extreme N-terminus of tau (Kanaan et al., 2011). This domain is normally hidden in a paperclip-like conformation of the native protein, but becomes exposed in aggregated, pathological tau (Jeganathan et al., 2006). Abnormal exposure of this motif has been linked to dysregulation of axonal transport and neuronal function (LaPointe et al., 2009; Kanaan et al., 2011; Ward et al., 2012). Immunohistochemical studies of human postmortem tissues in AD patients demonstrated that exposure of the N-terminal region of tau is an early event in AD that increases with progression of the disease (Kanaan et al., 2012; Ward et al., 2012). Therefore, this PAD motif is a reasonable target epitope for active immunotherapy. In fact, intracranial administration of monoclonal antibody targeting PAD motif reduced total tau and phosphorylated forms of tau in brains of Thy-Tau22 tau transgenic mice (Agadjanyan et al., 2017). In addition, we previously reported that a MultiTEP-based recombinant protein vaccine, AV-1980R/A targeting PAD was highly immunogenic in wildtype C57BL6 mice (Davtyan et al., 2016). Here we are reporting for the first time on immunogenicity of this vaccine in a mouse model of tauopathy and the efficacy of AV-1980R/A on pathology and cognitive impairments in Tg4510 mice.
2. Materials and methods
2.1. Animals
All animal testing procedures were approved by the Institutional Animal Care and Use Committee of the University of South Florida and were performed in accordance with the eighth edition “Guide for the Care and Use of Laboratory Animals,” published by the National Academy of Science, the National Academies Press, Washington, DC (2011).
Parental mutant tau and tetracycline-controlled transactivator (tTA; 129S6 background) protein transgenic mouse lines were maintained separately and bred to produce Tg4510 mice, tTA only mice, and nontransgenic littermates as described (Santacruz et al., 2005; Dickey et al., 2009). Mice were housed individually and maintained on a twelve-hour light/dark cycle. Food (Envigo 2018–18% protein- diet) and water were given ad libitum.
2.2. Epitope vaccines and purification of proteins
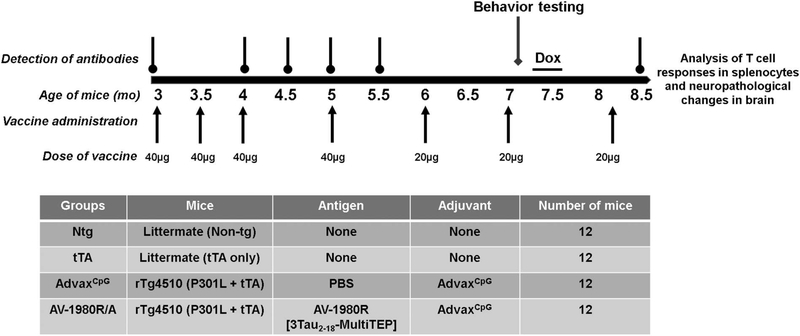
Recombinant protein was purified from E. coli BL21 (DE3) cells transformed with previously constructed pET24a/3Tau2–18-MultiTEP plasmids as described (Davtyan et al., 2016). The final recombinant protein was analyzed in 10% Bis-Tris gel electrophoresis (NuPAGE Novex Gel, Invitrogen, CA). Protein bands were visualized by Coomassie dye and specificity of the bands was confirmed by Western Blot (WB) with anti-tau2–18 1C9 monoclonal antibodies (Davtyan et al., 2016). The level of endotoxin was measured using E-TOXATE kits, as recommended by the manufacturer (Sigma, St Louis, MO). For vaccine preparation the AV-1980R protein was mixed with AdvaxCpG adjuvant. Mice were immunized with 40 μg or 20 μg protein (per injection) formulated in 1 mg adjuvant (Fig. 1).
Fig. 1.
Design of the Vaccine Study Conducted in Tg4510 (tetO-MAPT*P301L) Transgenic Mice. Starting at 3 months of age, 3 groups of Tg4510 mice received a total of 7 immunizations with either adjuvant AdvaxCpG (n = 12) or tau vaccine, AV1980R/A (n = 12). Intramuscular injections 1,2,3 were performed every other week at age 3 months, 3.5 months and 4 months. Injection 4,5,6 were performed every other month at 5 months, 6 months and 7 months of age. A final dose was given at 8 months, one week before euthanization. Due to high immune response, vaccine dose was decreased from 40 micrograms to 20 micrograms after injection 4. Additional groups of non-transgenic and tTA littermates were used as control for behavior and vaccine titers. Non-transgenic and tTA littermates were not injected with either adjuvant or antigen. A battery of behavior tests was performed 15 days after injection 6 (end timepoint, at 7 months of age). To assess the efficacy of the vaccine while stopping transcription of tau, doxycycline was given to the mice in the water during 8 days at a concentration of 200 mg/kg at the end of the last battery of behavior tests. An additional reversal testing was performed after treatment with doxycycline. Mice were euthanized at 8.5 months of age, 2 weeks after the seventh injection.
2.3. Experimental design
Tg4510 mice aged 3 months old received a total of 7 immunizations with either AdvaxCpG adjuvant (n = 12) or the tau vaccine, AV-1980R/A (n = 12). Mice were injected with 50 μl of indicated antigen intramuscularly (into right hind legs tibialis anterior muscle) using insulin syringes (3/10 ml/cc) with the BD Ultra-Fine needle (30G). Injection 1, 2, and 3 were performed at biweekly intervals starting at 3 mo. of age (Fig. 1). Injections 4, 5, and 6 were performed at 5 mo., 6 mo. and 7 mo. of age. A final dose was given after 8 mo, one week prior to tissue collection. Due to the strong antibody response (see below), the vaccine dose was decreased from 40 micrograms to 20 micrograms after injection 4. Additional groups of non-transgenic and tTA littermates were used as controls for behavior and vaccine titers. A battery of behavioral tests was performed 15 days after injection 6 (at 7 mo. of age). To assess the efficacy of the vaccine while stopping transcription of tau, doxycycline was given to the Tg4510 mice via drinking water for 8 days at a concentration of 200 mg/kg at the end of the second battery of behavior tests. An additional reversal testing in the radial arm water maze was performed after treatment with doxycycline. Mice of both sexes were included in each treatment condition.
2.4. Behavioral testing
All behavioral testing was performed in groups of mice balanced for gender by an observer unaware of the treatment/genotype of the mice. The open field was used as a standard test of general activity. Animals were monitored for 15 min in a 40 cm square open field with video tracking software (ANY-Maze, Stoelting, IL), under moderate lighting. General activity levels were evaluated by measurements of horizontal and vertical activity.
Each animal was placed in a walled Y-maze for a single 5 min trial. The sequence of arm entries and total number of arm choices were recorded. Spontaneous alternation (entering all three arms sequentially without repetition) was expressed as a percentage, as calculated according to the method of (Anisman, 1975).
Short term memory was evaluated by the novel object recognition test (NOR) which consists of a 40 × 40 cm arena monitored and quantified by video tracking (ANY-Maze, Stoelting, IL). Two objects similar in scale to the mouse were placed along the center line of the arena approximately 3–5 cm from the outside wall. Each animal was given three acclimation trials of 5 min each with a 5 min inter-trial interval. After each trial, the arena and object cues were cleaned with 70% ethanol to minimize olfactory cues. After the acclimation trials, one of the acclimated objects was replaced with a novel object. Animals were given a 5 min exploratory trial during which object exploration was monitored by video recording. Working memory was evaluated by the discrimination ratio, which is defined as the time exploring the novel object minus the time spent with familiar and divided by combined time spent exploring both novel and familiar objects.
A detailed description of radial arm water maze (RAWM) has been published previously (Alamed et al., 2006). For fear conditioning, a 0.5 mA mild foot shock was paired with an auditory conditioned stimulus within a novel environment. Freezing on the training day in response to the foot shock was used as an estimate of learning during the acquisition trial. Animals were placed in the fear conditioning apparatus for 3 min, and then a 30 s noise at 70 dB was delivered with a 0.5-mA shock applied to the floor grid during the last 2 s of the conditioned stimulus. Training consisted of two mild shocks paired with two conditioned stimuli with a 2 min interval between each shock. For contextual memory, the mice were placed in the acquisition chamber and monitored for freezing to the context 24 h after training (no shocks or auditory cue given) and tested for 3 min. Immediately after the contextual test, mice were placed in a novel environment, consisting of a chamber with different shape, floor and olfactory cues from the training chamber. Mice were allowed to explore it for 3 min (cued no tone) and exposed to the conditioned stimulus for 3 min (cued tone). Learning was assessed by measuring freezing behavior (i.e. motionless position) every second.
2.5. Tissue collection
To assess antibody titers over the course of the experiments, blood samples were collected from the submandibular vein before (pre-bleed) and after immunizations 2, 3, 4 and by intracardiac puncture at euthanasia. Blood was collected without anti-coagulant, incubated one hour at room temperature and then kept overnight at 4 °C. The next day samples were centrifuged for 10 min at 4000 rpm at room temperature (RT), in a bench-top centrifuge. The serum (upper phase) was carefully collected and centrifuged for 10 min at 7000 rpm. The supernatant was collected and kept at −80 °C for antibody titer measurements.
One week after the last injection (injection 7), mice were euthanized at 8.5 months of age with a solution containing pentobarbital and phenytoin, then transcardially perfused with 25 ml of 0.9% normal saline solution. Before perfusion, spleens were collected and single cell suspension was prepared as described in (Davtyan et al., 2014a, 2014b) using RBC (Red Blood Cells) Lysing Buffer (Sigma-Aldrich).
Brains were collected immediately following perfusion. The brain was trimmed to exclude olfactory bulbs or spinal cord and weighed. One hemisphere was dissected and frozen for western blot analysis and the second hemisphere was immersion fixed in freshly-prepared 4% phosphate-buffered paraformaldehyde for 24 h. The fixed hemispheres were cryoprotected in successive incubations of 10%, 20% and 30% sucrose solutions for 24 h each. Subsequently, brains were frozen on a cold stage and sectioned in the horizontal plane (25 μm thickness) on a sliding microtome and stored in Dulbecco’s phosphate buffered saline with 10 mM sodium azide solution at 4 °C.
2.6. Detection of splenocytes producing cytokines
Cytokine CBA (Cytometric bead array) assay. Concentrations (pg/ml) of Th1 [IFN-γ, IL-2 and THF-α] and Th2 [IL-4 and IL-5] cytokines were detected using Mouse Th1/Th2 Cytokine CBA kit by flow cytometry according to manufacturer’s instructions. Splenocytes were re-stimulated in vitro with a cocktail of 12 Th epitope peptides (2 μg/ml each) for 24 h and supernatant was collected for assay.
ELISpot assay. Analysis of IFN-γ producing T cells was performed in splenocyte cultures from immunized mice by ELISpot assay (BD Biosciences, CA), as previously described (Cribbs et al., 2003, Petrushina et al., 2007, Davtyan et al., 2013, Davtyan et al., 2014a, 2014b). Cultures of splenocytes were re-stimulated in vitro with a cocktail of 12 peptides representing the Th epitopes in the MultiTEP vaccine (Davtyan et al., 2014a, 2014b) (2 μg/ml of each peptide), and several individual peptides [PADRE, P23, P30, P17, P28] incorporated in MultiTEP platform, as well as Tau2–18 peptides at concentration of 10 μg/ml for 20 h. The numbers of IFN-γ spot-forming cells (SFC) per 106 splenocytes stimulated by different peptides were then counted.
2.7. Detection of B cells producing tau-specific antibodies
Antibody-secreting B cells specific to tau were detected in splenocytes by ELISpot (Mabtech Inc., Cincinnati, OH) as described in Davtyan et al. (Davtyan et al., 2016). Briefly, splenocytes from experimental and control mice were incubated for 24 h in 96-well plates coated with tau2–18 peptides. After incubation the assay was performed as recommended by the manufacturer.
2.8. Detection of tau-specific antibodies
The concentrations of anti-tau antibodies in serum was determined by ELISA, as described previously (Davtyan et al., 2016). To measure anti-tau antibody concentration plates were coated with 1 μg/per well with tau2–18 peptide (GenScript, NJ). Anti-tau antibody concentration was calculated using a calibration curve generated with 1C9 mAb (generated at The Institute for Molecular Medicine, Huntington Beach, CA). HRP-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, ME) was used as secondary antibody.
2.9. Histopathology
Stereological principles were employed to select evenly spaced sections to be stained for each marker. Immunohistochemical procedural methods were as described previously (Gordon et al., 2002). Sections from all animals were placed in a multi-sample staining tray and endogenous peroxidase was blocked (10% methanol, 3% H2O2 in phosphate buffered saline, 10 mM NaPO4, 154 NaCl, pH 7.4, PBS;30 min). Tissue samples were permeabilized with 0.2% lysine, 1%Triton X-100 in PBS solution and incubated overnight in anti-tau phosphorylated at Ser396 (rabbit polyclonal, Anaspec), total tau H150 (rabbit polyclonal, SantaCruz Biotechnology), anti-tau phosphorylated at AT8 (Thermo scientific, Waltham, MA), Iba-1 (Wako), GFAP (Dako), CD86 (Novus biologicals, Littleton CO). Additionally, we examined the staining for mouse IgG with biotinylated horse anti-mouse IgG specific secondary antibody (Vector Laboratories, Burlingame, CA, USA) to detect whether the systemically injected antibodies may have bound to brain tissue. Sections were washed in PBS, and then incubated in biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). The tissue was again washed after 2 h and incubated with Vectastain® Elite® ABC kit (Vector Laboratories, Burlingame, CA) for enzyme conjugation. Finally, sections were stained using 0.05% diaminobenzidine, 0.5% nickel ammonium sulfate and 0.03% H2O2. Tissue sections were mounted onto slides, dehydrated, cover slipped and scanned for analysis using a digital scanning microscope Axio Scan Z.1 (Zeiss Inc). Digitized sections were annotated for regions of interest and positively stained area was quantified with Nearcyte software. Values for all sections from the same mouse were averaged to represent a single value for that region in subsequent statistical analysis.
2.10. Biochemical analyses
Tissues for Western blot analysis were prepared as previously described (Brownlow et al., 2014). Dissected hippocampi (HPC) and posterior cortex (PCX) were homogenized then sonicated in RIPA buffer containing protease inhibitor cocktail (Sigma Aldrich) and phosphatase inhibitor cocktails I and II (Sigma Aldrich) and centrifuged at 40, 000 × sg for 30 min at 4 °C. The supernatant was collected (RIPA soluble fraction) and protein concentrations were determined by the BCA protein assay kit (Pierce, Rockford, IL). The remaining pellet was digested with 70% formic acid according to the wet tissue weight, and then neutralized with NaOH to analyze RIPA-insoluble proteins (RIPA insoluble fraction). Equal amounts of proteins according to BCA (5 μg/well for soluble fraction, 1 μg/well for insoluble fraction) were loaded in each well of a 4–12% Bistris gels and transferred to a 0.2 μm pore size nitrocellulose membrane and immunoblotted with H150 (Santacruz biotechnology, Dallas, TX), pSer396-tau (Anaspec, Fremont, CA), N-term Tau 12 (biolegend, San Diego, CA) and actin (Sigma Aldrich, St Louis, MO) at 1:1000-fold dilution. Fluorescently tagged secondary antibodies (IRDye 800CW, LI-COR Biosciences) were used at a dilution of 1:10,000. Western Blot results were quantified by scanning with a LI-COR Odyssey fluorescent scanner. Band intensities were quantified by densitometric analysis using the Odyssey imaging system (LI-COR Biosciences) and normalized to the band intensity of β-actin (for RIPA soluble fraction) or to total protein (RIPA insoluble fraction). Total protein was detected using REVERT reagent (LI-COR) according to the manufacturer’s instructions.
In addition, concentrations of human total and phosphorylated tau in soluble brain extracts were determined by Tau (total) Human ELISA kit, Tau [pS396] Human ELISA Kit, Tau [pS199] Human ELISA Kit, Tau [pT181] Human ELISA Kit, and Tau [pT231] Human ELISA Kit (all from ThermoFisher Scientific, MA), according to the manufacturer’s instructions.
2.11. Statistical methods
Data were analyzed by ANOVA or repeated measures ANOVA followed by Fisher’s protected least significant difference post hoc tests (statview) or ANCOVA. In all tests, a P-value < 0.05 was considered significant. All data are presented as mean values ± standard error of the mean (SEM) unless specified otherwise.
3. Results
3.1. Immunogenic efficacy of AD vaccine in Tg4510 mice
Recently we demonstrated that MultiTEP-based recombinant protein vaccine AV-1980R/A (tau vaccine) is highly immunogenic in wildtype C57BL6 mice (Davtyan et al., 2016). To test the immunogenic and therapeutic efficacy of tau vaccination in tau transgenic mice, 3-month-old Tg4510 mice were immunized with AV-1980R/A, while control groups injected with adjuvant (AdvaxCpG). Mice received seven injections at the time points shown in Fig. 1.
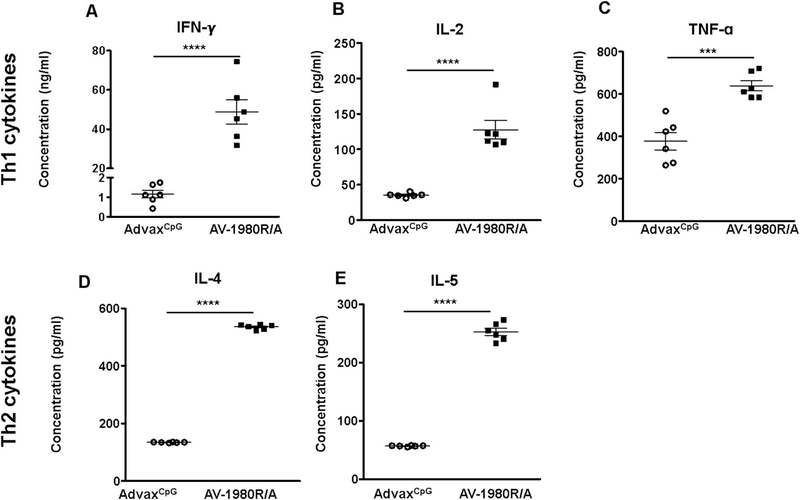
Cellular immune responses were measured in splenocytes of vaccinated mice by detection of production of Th1/Th2 cytokines (Fig. 2). As expected, AV-1980R/A vaccine generated a strong cellular immune response specific to the Th cell epitopes incorporated in the MultiTEP platform. Next, we tested Th cell immune responses specific to individual epitopes incorporated into the MultiTEP vaccine platform.
Fig. 2.
AV-1980R/A formulated in AdvaxCpG adjuvant generated both Th1 and Th2 immune responses in Tg4510 mice. Concentrations (pg/ml) of Th1 cytokines (A, B, C) and Th2 cytokines (D, E) in response to AdvaxCpG adjuvant (open circle) and AV-1980R/A tau vaccine (black square) in Tg4510 mice. [IFN-γ: Interferon-gamma, IL-2: Interleukin 2, TNF-α: Tumor necrosis factor alpha, IL-4: Interleukin 4, IL-5: Interleukin 5]. Cytokines were detected using Mouse Th1/Th2 Cytokine CBA kit by flow cytometry. Splenocytes were re-stimulated in vitro with a cocktail of 12 Th epitope peptides (2 μg/ml each) for 24 h and supernatant was collected for assay. Bars represent mean ± SEM (n = 6 mice/per group). Statistical significance was calculated against AdvaxCpG group using unpaired t-test. (*P < 0.05, ***P < 0.001 and ****P < 0.0001).
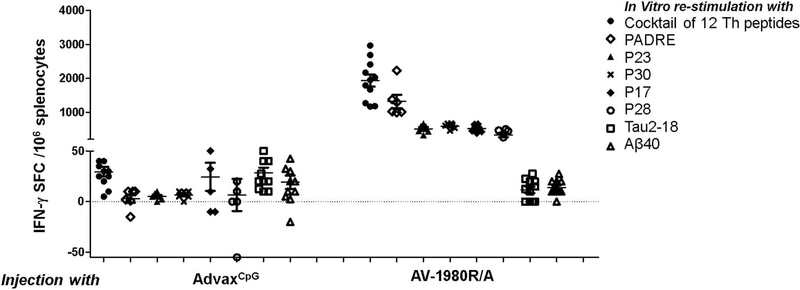
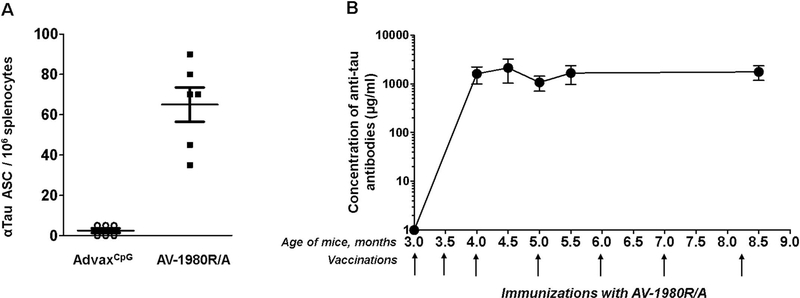
ELISpot data presented in Fig. 3 demonstrated that vaccination with AV-1980R/A stimulated Th cells specific to epitopes PADRE, P23, P30, P17 and P28 in this mouse strain possessing an H-2b/q immune haplotype. Interestingly, this repertoire was slightly different in C57BL6 (H-2b) and in THY-Tau22 strain, which was created on H-2b/k background and was backcrossed with C57BL6 mice (Schindowski et al., 2006). Of note, we detected significantly higher numbers of Th cells specific to PADRE, which is a promiscuous universal synthetic epitope, compared with P23, P30, P17 and P28 peptides in immune splenocytes isolated from tau vaccinated mice. High numbers of activated Th cells producing both Th1 and Th2 types of cytokines indicated that tau vaccine induced mixed Th1/Th2 types of immune responses. Importantly, no activation of potentially harmful autoreactive Th cells was detected after re-stimulation of immune splenocytes with tau self-epitope by ELISpot assay (Fig. 3). As might be expected, strong cellular immune responses specific to the MultiTEP vaccine platform should stimulate B cells to produce antibodies specific to tau2–18 epitope incorporated into AV-1980R/A vaccines. The ELISpot data confirms this assumption and demonstrates that vaccination generated a large numbers of anti-tau antibody-secreting B cells (ASC) after immunization with AV-1980R/A (Fig. 4A). No cross-reactive antibody-producing B cells were detected in splenocytes isolated from immunized mice (Fig. 4A). More importantly, these tau specific B cells activated by vaccination generated extremely strong humoral immune responses after only two immunizations with average concentrations of antibodies close to 2 mg/ml, > 10% of total serum IgG. This level of antibodies was maintained throughout the entire study (Fig. 4B).
Fig. 3.
AV-1980R/A induced strong cellular response specific to Th epitopes incorporated in the MultiTEP platform without activating potentially harmful auto-reactive Th cells specific to tau. Splenocytes from individual mice were re-stimulated in vitro with a cocktail of 12T-helper epitope peptides (2 μg/ml each) and several individual peptides [PADRE, P23, P30, P17, P28] incorporated in MultiTEP platform, as well as Tau2–18 peptide (10 μg/ml). IFNγ spot-forming colonies (SFC) were detected by ELISPOT assay and error bars indicate mean ± SEM (n = 11 per group).
Fig. 4.
AV-1980R/A induced specific B cells producing very high concentrations of antibodies.
Evaluation of humoral immune responses generated in Tg4510 transgenic mice after immunizations with AV-1980R vaccine formulated with AdvaxCpG adjuvant. (A) Detection of anti-tau antibody-secreting cells (ASC), visualized as spots, was done in splenocyte cultures obtained from experimental and control mice using ELISpot assay. (B) Concentrations of anti-tau antibodies in mouse sera were determined by ELISA. Plates were coated with Tau2–18 peptide (Genscript). Anti-tau antibody concentrations were calculated using a calibration curve generated with 1C9 anti-tau2–18 monoclonal antibody. Bars represent average ± SD (n = 11/per group).
Finally, to characterize the type of humoral immune responses, we measured the production of IgG1, IgG2ab, IgG2b, and IgM isotypes in mice immunized with AV-1980R/A vaccine. Mice from both groups generated mostly IgG antibodies (IgG1 > IgG2b > IgG2ab), while only negligible titers of IgM antibodies were detected in the sera of immunized mice (data not shown). Of note, IgG1/IgG2ab is equal to 4 for AV-1980R/A indicating that the immune response is biased towards Th2 type.
3.2. Impact of vaccination on cognitive impairments
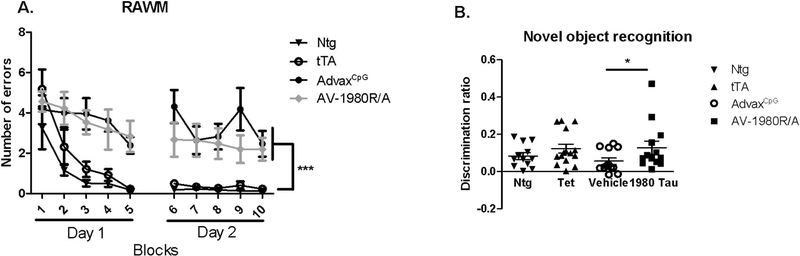
Spatial memory deficits were assessed by the 2-day radial arm water maze test. As previously shown, repeated measure ANOVA revealed a genotype effect: non-transgenic and tTA control mice learned the location of the platform by the end of day 1 and made few errors on day 2. Tg4510 mice treated with adjuvant were not able to remember the location of the platform as they made significantly more errors on day 1 and day 2 when compared to non-transgenic or tTA control mice (Fig. 5A). There was no significant improvement in the number of errors in Tg4510 vaccinated with AV-1980R/A compared to adjuvant treated Tg4510 mice. All Tg4510 mice performed worse than the non-transgenic and tTA mice, indicating no effect of the vaccine on spatial navigation memory.
Fig. 5.
Vaccination with AV-1980R/A did not improve spatial memory during radial arm water maze testing but rescued the short-term memory deficit observed in Tg4510 during the novel object recognition test.
(A) Tg4510 mice made significantly more errors regardless of treatment with AdvaxCpG adjuvant or AV-1980R/A when attempting to locate a hidden platform during the 2-day radial arm water maze (RAWM) test when compared to non-transgenic (Ntg) and tTA littermates. Data are presented as mean ± S.E.M., n = 12/group. ***p < 0.001. (B) Tg4510 mice treated with AdvaxCpG adjuvant spent less time with novel object compared to non-transgenic (Ntg) and tTA littermates as they displayed a lower discrimination ratio (which is defined as the time exploring the novel object minus the time spent with familiar and divided by combined time spent exploring both novel and familiar objects), indicating short term memory deficit. Tg4510 mice vaccinated with AV-1980R/A displayed a significant increase in discrimination ratio compared to control Tg4510 mice treated with AdvaxCpG adjuvant indicating an improvement in short memory. Data are presented as mean ± S.E.M., n = 12/group. *p < 0.05. 2-way ANOVA test was used for RAWM with Tukey’s LSD means comparisons for significant overall effects. A t-test was performed to compare the two Tg4510 groups for NOR.
Conversely, we observed an improvement in short term memory assessed by novel object recognition (NOR) in mice vaccinated against tau. As expected, two-way ANOVA revealed a genotype effect as non-transgenic and tTA control mice spent more time with the novel object than did the adjuvant Tg4510 mice (Fig. 5B). However, Tg4510 mice vaccinated with AV-1980R/A displayed a significant increase in time attending to the novel object when compared to Tg4510 adjuvant treated mice. In addition, AV-1980R/A vaccinated mice achieved the same discrimination ratio as tTA and non-transgenic control mice. A battery of other cognitive tests was performed (Table 1). As previously shown, we observed a genotype effect in total distance travelled during open field, number of entries in Y maze, contextual fear conditioning and nesting behavior. Vaccination with AV-1980R/A had not a significant influence on these cognitive deficits.
Table 1.
Summary of behavior tests performances in non-transgenic (Ntg), tet only (tTA) and Tg4510 mice treated with AdvaxCpG adjuvant or AV-1980R/A. Open field total distance travelled in meters (TDT), Ymaze entries and alternations, Radial arm water maze (RAWM) total errors, Fear conditioning (FC) percentage time freezing and nesting behavior.
| Ntg | Tta | AdvaxCpG | AV-1980R/A | |
|---|---|---|---|---|
| Open field (TDT) | 36.7 +/− 2.8a,b | 46.9 +/− 2.5a,b | 150.3 +/− 35.3 | 122.9 +/− 32.2 |
| Ymaze entries | 32.8 +/− 1.9a,b | 34.3 +/− 1.3a,b | 57.8 +/− 9 | 59.5 +/− 7.9 |
| Ymaze alternation | 60.7 +/− 3.2 | 59.9 ± 1.6 | 65.1 +/− 2.3 | 68.2 +/− 4.8 |
| RAWM reversal total errors | 25 +/− 4.7 a,b,c | 40.1 +/− 2.7a,b | 92.8 +/− 13.3 | 93.4 +/− 14.6 |
| RAWM post dox reversal total errors | 29.5 +/− 5.4a,b | 52.9 +/− 8.3 | 81.9 +/− 14.3 | 70.5 +/− 7.8 |
| FC context | 25.6 +/− 5.4a,b | 20.7 +/− 4.7a,b | 6.6 +/− 2.3 | 2.6 +/− 1.2 |
| FC cued (tone) | 42.1 +/− 7 | 26.4 +/− 5.1 | 27.7 +/− 8 | 33.6 +/− 6.7 |
| Nesting behavior | 3.8 +/− 0.3a,b | 3.9 +/− 0.2a,b | 1.1 +/− 0.5 | 0.9 +/− 0.4 |
p < 0.05 when compared to AdvaxCpG adjuvant.
p < 0.05 when compared to AV-1980R/A.
p < 0.05 when compared to tTA. As expected, Tg4510 treated with AdvaxCpG adjuvant displayed hyperactivity and cognitive impairments when compared to non-transgenic or tTA littermates. Immunization with AV-1980R/A did not affect these phenotypes.
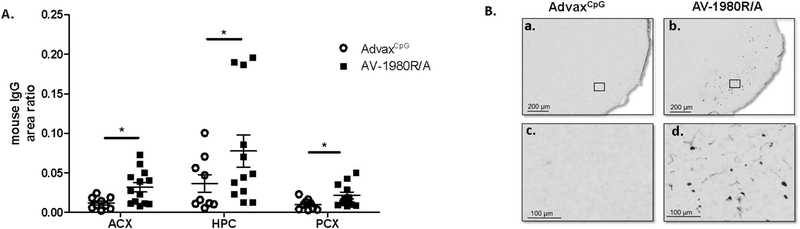
3.3. Vaccination with Tau epitope induced increased levels of mouse IgG in the brain
As shown in Fig. 4, very high concentrations of antibodies were produced after vaccination with AV-1980R/A. To investigate whether the antibodies produced during vaccination were reaching the brain, we incubated brain sections with an anti-mouse IgG specific secondary antibody. Quantification of positive area stained with anti-mouse IgG was performed in anterior cortex (ACX), posterior cortex (PCX), and hippocampus (HPC) calculated from digitized images is shown in Fig. 6A. Tg4510 mice vaccinated with tau epitope exhibited significantly higher levels of IgG in the ACX, HPC and PCX when compared to Tg4510 mice treated with adjuvant. When the parenchymal staining in the posterior cortex was viewed at higher magnification, there was staining of the neuropil only in Tg4510 mice vaccinated with AV-1980R/A (Fig. 6B, panels b, d). No positive staining was detected in Tg4510 mice treated with AdvaxCpG adjuvant (Fig. 6B, panels a, c). Mouse IgG staining was also absent in non-transgenic and tTA control mice (data not shown).
Fig. 6.
Tg4510 mice vaccinated with Tau vaccine AV-1980R/A displayed increased levels of mouse IgG in the brain compared to AdvaxCpG
(A) Quantification of positive area stained for mouse IgG in anterior cortex (ACX), hippocampus (HPC) and posterior cortex (PCX) in Tg4510 treated with AdvaxCpG adjuvant or with AV-1980R/A mice. Non-transgenic and tTA littermates displayed similar levels to Tg4510 treated with AdvaxCpG adjuvant (data not shown). Immunostaining quantification was performed utilizing Mirax software (Zeiss Inc.). (B) Micrographic representation of Mouse IgG immunostaining in the posterior cortex of Tg4510 mice treated with AdvaxCpG adjuvant (a, c) or AV-1980R/A (b, d). c and d are magnification of square area in a, b, respectively. Tg4510 mice immunized with AV-1980R/A seemed to display positive staining in cell bodies particularly in the posterior cortex. Data are presented as mean ± S.E.M., n = 12/group. Statistical significance was calculated against AdvaxCpG group using unpaired t-test *p < 0.05.
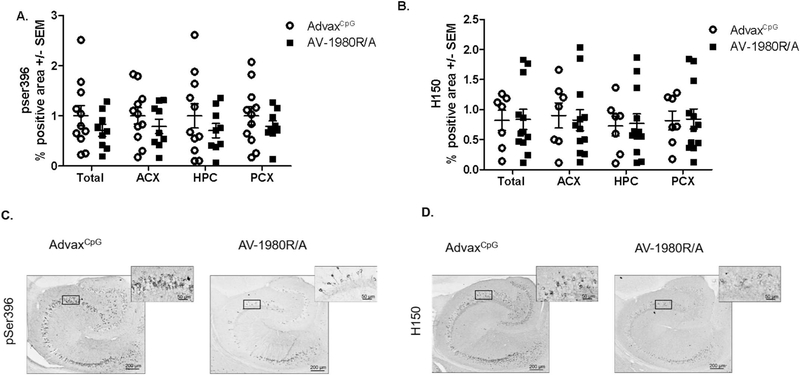
3.4. Tau pathology in Tg4510 vaccinated mice
We then evaluated the impact of the different vaccines on tau pathology. Quantification of positive immunostaining for pSer396-tau (phosphorylated tau) and H150 (total tau) in the entire hemisection (total), anterior cortex (ACX), hippocampus (HPC) and posterior cortex (PCX) are shown in Fig. 7. There was a trend towards decrease in total area as well as ACX and PCX in AV-1980R/A vaccinated mice when compared to AdvaxCpG adjuvant-treated mice, but the difference did not reach significance (Fig. 7A). A similar trend was observed in AT8 or Gallyas silver stained sections (Table 2). No difference was observed in total tau levels detected by immunohistochemistry as shown in Fig. 7B, D. As expected, no positive staining was detected in non-transgenic animals for pSer396-tau or H150 (data not shown). The adjuvant treated Tg4510 mice had values similar to historical values for untreated Tg4510 mice of the same age (Fig. S3).
Fig. 7.
Vaccination with AV-1980R/A did not affect levels of pathology in Tg4510 mice when assessed by immunohistochemistry.
Quantification of positive area stained for pSer396 (A) and H150 (B) in total area (total), anterior cortex (ACX), hippocampus (HPC) and posterior cortex (PCX) in Tg4510 mice treated with AdvaxCpG adjuvant or with AV-1980R. Immunostaining quantification was performed utilizing Mirax software (Zeiss Inc.). Micrographic representation of pSer396 (C) and H150 (D) immunostaining in the hippocampus of Tg4510 mice treated with AdvaxCpG adjuvant (C, D left panel), and AV-1980R (C, D right panel). Data are presented as mean ± S.E.M., n = 12/group. Statistical significance was calculated against AdvaxCpG group using unpaired t-test *p < 0.05.
Table 2.
Brain weight at euthanasia (in mg).
| Ntg | tTA | AdvaxCpG | AV-1980R/A | ||
|---|---|---|---|---|---|
| Brain weight (mg) | 503.9 +/− 6.1a,b,c | 474.6 +/− 6.5a,b,c | 427.9 +/− 13 | 426 +/− 11.6 | |
| Gallyas stain | – | – | 1.0 ± 0.21 | 0.92 ± 0.12 | |
| Immunohistochemistry | AT8 | – | – | 1.0 ± 0.16 | 0.84 ± 0.12 |
| gfap | 0.54 ± 0.05a,b | 0.56 ± 0.05a,b | 1.0 ± 0.10 | 1.13 ± 0.06 | |
| iba-1 | 0.77 ± 0.04a,b | 0.76 ± 0.05a,b | 1.0 ± 0.04 | 0.96 ± 0.07 | |
| cd86 total | 1.04 ± 0.11 | 0.81 ± 0.09b | 1.0 ± 0.08 | 1.12 ± 0.09 |
Positive total area stained with Gallyas, or for AT8 (phospho Tau Ser202/Thr205), Glial fibrillary acidic protein (GFAP), Ionized calcium binding adaptor molecule 1 (Iba-1) and cluster of differentiation 86 (CD86). As expected, Tg4510 treated with AdvaxCpG adjuvant displayed decreased brain weight and increased positive area ratio for GFAP, Iba-1 and CD86 when compared to non-transgenic or tTA littermates. Immunization with AV-1980R/A did not affect these phenotypes nor did it affect levels of phospho tau (pSer396), and total tau (H150) assessed by western blot analysis in hippocampus in RIPA soluble fraction, or positive area ratio for AT8. Statistical significance was calculated against using unpaired t-test or ANOVA.
p < 0.05 when compared to AdvaxCpG adjuvant.
p < 0.05 when compared to AV-1980R/A.
p < 0.05 when compared to tTA.
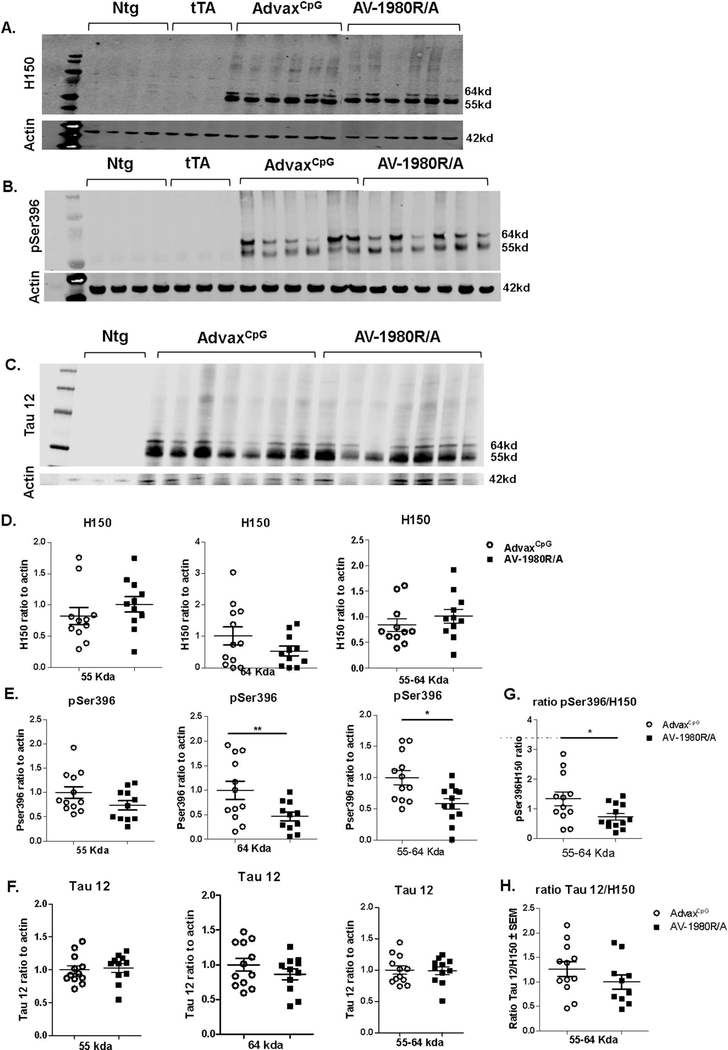
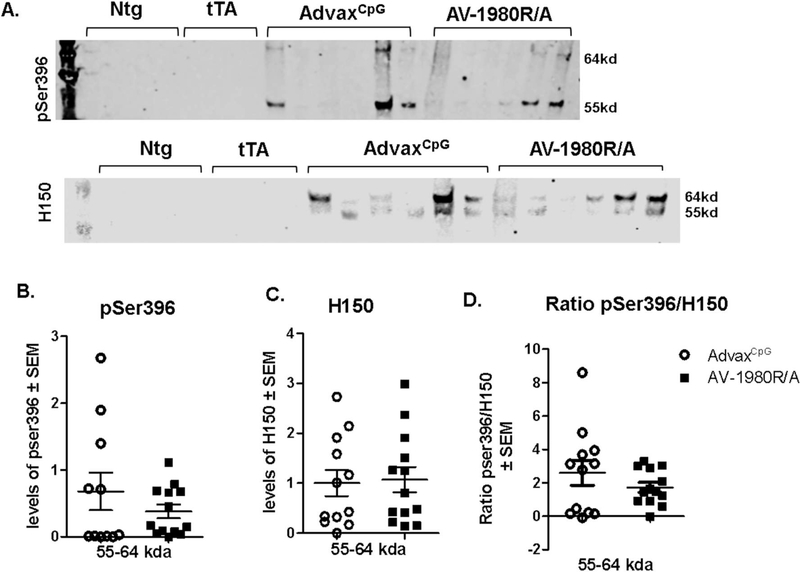
Western blot analysis revealed a decrease in pSer396-tau levels (Fig. 8B,E) but not H150 (Fig. 8A,D) or Tau12 (anti Nterm tau aa6–18; Fig. 8C,F) in the RIPA- soluble fraction of the posterior cortex in AV-1980R/A vaccinated mice compared to AdvaxCpG adjuvant treated mice (see Fig. S1 for western immunoblot of all samples). This was the case whether normalized to actin (Fig. 8) or when the phospho-epitopes were normalized to total tau (Fig. 8G,H and Fig. S2). There were no differences in the levels of these markers in PCX RIPA insoluble fraction (data not shown). In the hippocampus RIPA insoluble fraction, there was a trend towards decrease in levels of pSer396-tau in AV-1980R/A vaccinated mice compared to AdvaxCpG adjuvant treated mice that did not reach significance (Fig. 9).
Fig. 8.
Tg4510 mice vaccinated with tau vaccine AV-1980R/A display decreased levels of phospho tau in the posterior cortex soluble fraction when compared to AdvaxCpG adjuvant treated mice.
Phospho tau (pSer396), total tau (H150) and Nterm tau (Tau12) were assessed by western blot analysis (A, B, C representative WB images) in posterior cortex in RIPA soluble fraction.55 kda, 64 kda and combination of 55–64kda band intensity were quantified for H150 (D), pSer396 (E) and Tau12 (F) in Tg4510 mice treated with AdvaxCpG adjuvant or with AV-1980R. All groups were normalized to the adjuvant group and to actin. No bands were detected in non-transgenic (Ntg) and tet only (tTA) mice, as shown in image A, B, C. Ratio of Pser396 and Tau12 to H150 was calculated for bands 55–64 (G, H) and for bands 55 and 64 separately (Fig. S2). Data are presented as mean ± S.E.M., n = 12/group. Statistical significance was calculated against AdvaxCpG group using unpaired t-test *p < 0.05.
Fig. 9.
Tg4510 mice vaccinated with tau vaccine AV-1980R/A display a trend towards decrease of phospho Tau in the hippocampus insoluble fraction when compared to AdvaxCpG.
Phospho Tau (pSer396) and total Tau (H150) was assessed by western blot analysis (A, representative WB images) in hippocampus in RIPA insoluble fraction. Briefly, after high centrifugation, pellet was treated with formic acid and neutralized with NaOH. Band intensity was quantified for H150 (B) and pSer396 (C) as well as ratio of pSer396 over H150 (D) in Tg4510 mice treated with AdvaxCpG adjuvant, or AV-1980R/A. All groups were normalized to the adjuvant group. No bands were detected in non-transgenic (Ntg) and tet only (tTA) mice, as shown in image A. Data are presented as mean ± S.E.M., n = 12/group. Statistical significance was calculated against AdvaxCpG group using non parametric Mann Whitney test.
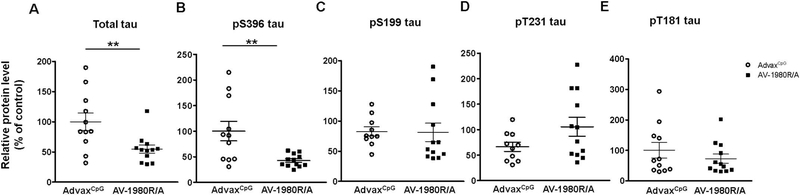
ELISA assay performed for total tau and several phosphorylated tau forms in soluble extractions revealed a significant decrease in pSer396-tau and total tau but not in pSer199-, pT181- and pT231-tau, in AV-1980R/A vaccinated mice when compared to AdvaxCpG adjuvant treated mice (Fig. 10).
Fig. 10.
Tg4510 mice vaccinated with AV-1980R/A display decreased levels of phosphorylated tau and total tau in the posterior cortex soluble fraction when compared to AdvaxCpG adjuvant treated mice when analyzed by ELISA.
Level of total (A) and several phosphorylated tau (pSer396 (B), pSer199 (C), pT181(D), pT231(E), in brain soluble extractions of posterior cortex from Tg4510 mice treated with AdvaxCpG adjuvant or AV-1980R/A, were analyzed by ELISA. Error bars represent average ± SEM (n = 11–12) Statistical significance was calculated against AdvaxCpG group using unpaired t-test. **p < 0.01.
Inflammation markers and brain weight, which have been previously shown to be affected by tau pathology in Tg4510 (Wes et al., 2014; Joly-Amado et al., 2016) were also analyzed (Table 2). Immunohistochemistry for Iba-1 and CD86 (microglia) as well as GFAP (astrocytes) and brain weight showed genotype effects but no treatment effect.
4. Discussion
The “amyloid cascade hypothesis” considers pathological Aβ as a triggering toxic event in AD and most clinical immunotherapy approaches have been aimed at reducing Aβ deposits. However, accumulating evidence show that pathological tau correlates with the onset of symptoms and the degree of dementia better than Aβ deposition. It is important to note that the lessons from clinical trials of active and passive immunization targeting Aβ suggest that anti-Aβ immunotherapy may be effective in the early/preclinical stages of AD, whereas in the later stages, clearing of Aβ alone may not be sufficient to stop/delay the disease. Therefore, anti-tau immunotherapy (passive and active immunizations) was proposed as an additional therapeutic approach.
The first attempts at tau immunotherapy involved the active immunization of wild type mice with full-length recombinant human tau protein (Rosenmann et al., 2006), followed by experiments using a variety of immunogens of tau and adjuvants in many different animal models of tauopathy (reviewed in (Schroeder et al., 2016). In general, active immunization was efficient at reducing tau pathology in mice as indicated by decreased phosphorylated tau (Boutajangout et al., 2010; Troquier et al., 2012; Selenica et al., 2014) and NFTs (Boimel et al., 2010). Some studies also demonstrated cognitive improvements (Boutajangout et al., 2010; Benhamron et al., 2018) or improved motor performance (Asuni et al., 2007). Passive immunotherapy studies in animal models also showed promising results when administered early in the disease process. Passive immunization with PHF1 or MC-1 antibody decreased tau pathology in JNPL3 (Boutajangout et al., 2011; Chai et al., 2011; d’Abramo et al., 2013) and P301S (PS19) mice (Chai et al., 2011). Targeting oligomeric tau was efficient in JNPL3 mice (Castillo-Carranza et al., 2014) but not in Tg4510 (Schroeder et al., 2017).
More recent work has identified some intriguing abilities of tau immunotherapy to clear not only tau deposits, but also Aβ deposits in mice. In 3xTg mice, Dai et al. (Dai et al., 2017) found that an N-terminal anti-tau antibody was more effective than a mid-domain antibody in reducing tau, but especially in reducing amyloid deposits also in these mice. Both antibodies rescued behavioral deficits. In follow up work, Dai et al. (Dai et al., 2018) also demonstrated the N-terminal antibody could block tau propagation in 3xtg mice caused by injecting AD-tau seeds. Rajamohamedsait H et al. (Rajamohamedsait et al., 2017) found that active immunization of 3xTg mice partially reduced tau deposition but considerably blocked amyloid deposition.Benhamron et al. (Benhamron et al., 2018) found that a phospho-tau vaccine was capable of reducing Aβ and improving memory in an APP/PS1 mouse model known to have minimal tau pathology. In a different comparison of N-terminal and mid-domain antibodies, Albert et al. (Albert et al., 2019) found the mid-domain antibodies more effective in blocking tau seeding and propagation. Thus, it seems likely that the properties of the antibody other than its domain specificity may have more influence on efficacy. However, it seems quite feasible from the mouse studies that anti-tau immunotherapy could reduce both tau and amyloid pathologies in cases of AD.
Two active vaccines (Godyn et al., 2016; Novak et al., 2019) are currently being tested in clinical trials with AD patients. Although these trials are in the early stages and are intended for safety studies, some preliminary efficacy data were published from AADVac1 trial, which reported that a trend towards slower cognitive decline and lower hippocampal atrophy rate was observed in patients with higher antibody titers (Novak et al., 2018).
There are advantages and disadvantages of both active and passive immunotherapy. Active immunotherapy has the advantage of requiring fewer doses and is more cost effective than passive immunotherapy. The disadvantages are that the antibody response is variable both quantitatively and qualitatively (especially in older adults) and adverse effects are challenging to reverse. Passive immunotherapy has the advantage of known dosing with an antibody with known characteristics, and if adverse events ensue, the antibody can be discontinued. Disadvantages include a high frequency of dosing (typically monthly by infusion) and the considerable costs associated with antibody production and delivery.
In this study, we tested the immunogenicity and therapeutic efficacy of AV-1980R/A MultiTEP platform based active vaccine targeting PAD (Tau2–18) region of tau in Tg4510 mice. MultiTEP platform composed of 12 foreign Th cell epitopes, was specifically designed to enhance immune responses in elderly patients with immunosenescence and provide a broad coverage of human MHC class II polymorphism utilizing the wide array of tetanus toxin, hepatitis B and influenza Th epitopes incorporated into the MultiTEP platform.
In Tg4510 mice, the vaccine induced strong cellular responses specific to foreign Th epitopes that provided help to B cells to be activated and produce very high titers of antibodies against tau, achieving 2 mg/ml or approximately 10% of the IgG concentration in mice. Moreover, no tau specific T cell response was detected indicating that the likelihood that this vaccine would produce autoreactive inflammatory T cell responses against tau in brain is very low.
The Tg4510 mice developed the expected behavioral phenotype, with impaired performance in open field, contextual fear conditioning, novel object recognition, radial arm water maze and nesting behavior. There was a significant rescue of the novel object recognition behavior back to levels similar to nontransgenic mice. However, there was no indication of improvements in any of the other behavioral tests. These results are consistent with our prior work. In Brownlow et al. (2014), we found that caloric restriction in Tg4510 mice caused significant improvements in the novel object task but not in other measures of the behavioral phenotype. This occurred with modest trends in reversal of the tau pathology, none of which were statistically significant (Brownlow et al., 2014). These results would suggest that the relatively simple novel object recognition task is easier for mice to perform. Hence even small changes in the tau deposition are capable of rescuing this deficit, but not the deficits associated with more complex behaviors.
Histological analysis indicated the presence of mouse IgG only in the tau mice vaccinated against tau. This suggested that these antibodies were most likely retained by the CNS in tau deposits that developed in these mice.
This could explain the reduction of phopho-tau epitopes at pSer396 observed in vaccinated mice. There was no effect on Gallyas stained tau deposits or brain atrophy with the tau epitope vaccine. We also observed reductions in pSer396-tau on western blots from dissected brain regions and with ELISA measurements. Thus, by several measures, vaccination against the AD domain of tau caused modest reduction in phospho-tau.
The Tg4510 mouse is a very aggressive model and manipulations aimed at rescuing the phenotype, short of turning off the transgene (Santacruz et al., 2005), have failed. In prior work with a tau oligomer antibody, we found little impact on the tau phenotype in this mouse (Schroeder et al., 2017). To our knowledge, only one immunotherapy has resulted in reduction of tau pathology in this mouse model. Sankaranarayan et al. (Sankaranarayanan et al., 2015) observed 20% reductions in tau deposition using a monoclonal antibody. However, in the same paper they reported much larger reductions in the phenotype of the P301S-tau model PS19. The Tg4510 mouse has 13-fold over expression of tau, while the PS19 mouse has 5-fold over expression (Roberson, 2012). Recently it has been identified that some of the Tg4510 phenotype may be related to insertional disruption of the Fgf14 gene by the transgene (Gamache et al., 2019). Thus, this contribution to the phenotype could not be readily reversed by preventing tau deposition. We conclude that the modest reductions in the phenotype seen here may reflect the Tg4510 model and perhaps not the overall efficacy of the vaccine or its intended target.
5. Conclusions
Altogether our results show that a highly immunogenic tau vaccine using the phosphatase-activating domain (PAD) of tau protein as immunogen was efficient at causing high titers of anti-tau antibodies with little risk of developing auto-reactive T cells. The vaccine was effective at modestly reducing some aspects of tau pathology and improving some components of cognitive performance in an aggressive model of tauopathy, the Tg4510 mouse.
Supplementary Material
Acknowledgments
This work was supported by funding from NIH (R01-NS050895, R01-AG020241, U01-AG060965, R21-NS101504, R01-AG061895, R01-AG055524, R01-NS076308) and Byrd Alzheimer’s Institute. Development of AdvaxCpG adjuvant was supported by funding from NIAID/NIH (Contract No. HHS-N272201400053C, HHS-N272200800039C and U01-AI061142). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of Competing Interest
MGA and AG are co-founders of Capo Therapeutics that licensed MultiTEP vaccine platform technology from the Institute for Molecular Medicine. The remaining authors declare no financial, commercial and non-financial conflict of interests.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2019.104636.
References
- Agadjanyan MG, Petrovsky N, Ghochikyan A, 2015. A fresh perspective from immunologists and vaccine researchers: active vaccination strategies to prevent and reverse Alzheimer’s disease. Alzheimers Dement. 11 (10), 1246–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agadjanyan MG, Zagorski K, Petrushina I, Davtyan H, Kazarian K, Antonenko M, Davis J, Bon C, Blurton-Jones M, Cribbs DH, Ghochikyan A, 2017. Humanized monoclonal antibody armanezumab specific to N-terminus of pathological tau: characterization and therapeutic potency. Mol. Neurodegener 12 (1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D, 2006. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat. Protoc 1 (4), 1671–1679. [DOI] [PubMed] [Google Scholar]
- Albert M, Mairet-Coello G, Danis C, Lieger S, Caillierez R, Carrier S, Skrobala E, Landrieu I, Michel A, Schmitt M, Citron M, Downey P, Courade JP, Buee L, Colin M, 2019. Prevention of tau seeding and propagation by immunotherapy with a central tau epitope antibody. Brain 142 (6), 1736–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, 1975. Time-dependent variations in aversively motivated behaviors: nonassociative effects of cholinergic and catecholaminergic activity. Psychol. Rev 82 (5), 359–385. [PubMed] [Google Scholar]
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM, 2007. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J. Neurosci 27 (34), 9115–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Jennings GT, Vogel M, 2019. A vaccine against Alzheimer’s disease: anything left but faith? Expert. Opin. Biol. Ther 19 (1), 73–78. [DOI] [PubMed] [Google Scholar]
- Benhamron S, Rozenstein-Tsalkovich L, Nitzan K, Abramsky O, Rosenmann H, 2018. Phos-tau peptide immunization of amyloid-tg-mice reduced non-mutant phos-tau pathology, improved cognition and reduced amyloid plaques. Exp. Neurol 303, 48–58. [DOI] [PubMed] [Google Scholar]
- Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H, 2010. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol 224 (2), 472–485. [DOI] [PubMed] [Google Scholar]
- Boutajangout A, Quartermain D, Sigurdsson EM, 2010. Immunotherapy Targeting Pathological Tau Prevents Cognitive Decline in a New Tangle Mouse Model. 30 (49). pp. 16559–16566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM, 2011. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem 118 (4), 658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82 (4), 239–259. [DOI] [PubMed] [Google Scholar]
- Brownlow ML, Joly-Amado A, Azam S, Elza M, Selenica ML, Pappas C, Small B, Engelman R, Gordon MN, Morgan D, 2014. Partial rescue of memory deficits induced by calorie restriction in a mouse model of tau deposition. Behav. Brain Res 271, 79–88. [DOI] [PubMed] [Google Scholar]
- Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Munoz MJ, Lasagna-Reeves CA, Kayed R, 2014. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J. Alzheimers Dis 40 (Suppl. 1), S97–s111. [DOI] [PubMed] [Google Scholar]
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M, 2011. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J. Biol. Chem 286 (39), 34457–34467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, Babikyan D, Kesslak P, Kieber-Emmons T, Cotman CW, Agadjanyan MG, 2003. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol 15 (4), 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Abramo C, Acker CM, Jimenez HT, Davies P, 2013. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS One 8 (4), e62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CL, Tung YC, Liu F, Gong CX, Iqbal K, 2017. Tau passive immunization inhibits not only tau but also Abeta pathology. Alzheimers Res. Ther 9 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai CL, Hu W, Tung YC, Liu F, Gong CX, Iqbal K, 2018. Tau passive immunization blocks seeding and spread of Alzheimer hyperphosphorylated Tau-induced pathology in 3 x Tg-AD mice. Alzheimers Res. Ther 10 (1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Poghosyan A, Marleau AM, Movsesyan N, Kiyatkin A, Rasool S, Larsen AK, Madsen PJ, Wegener KM, Ditlevsen DK, Cribbs DH, Pedersen LO, Agadjanyan MG, 2013. Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer’s disease: prelude to a clinical trial. J. Neurosci 33 (11), 4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Ghochikyan A, Petrushina I, Hovakimyan A, Davtyan A, Cribbs DH, Agadjanyan MG, 2014a. The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 10 (3), 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davtyan H, Petrushina I, Ghochikyan A, 2014b. Immunotherapy for Alzheimer’s disease: DNA- and protein-based epitope vaccines. Methods Mol. Biol 1143, 259–281. [DOI] [PubMed] [Google Scholar]
- Davtyan H, Zagorski K, Rajapaksha H, Hovakimyan A, Davtyan A, Petrushina I, Kazarian K, Cribbs DH, Petrovsky N, Agadjanyan MG, Ghochikyan A, 2016. Alzheimer’s disease Advax(CpG)- adjuvanted MultiTEP-based dual and single vaccines induce high-titer antibodies against various forms of tau and Abeta pathological molecules. Sci. Rep. 6, 28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW, 1990. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann. Neurol 27 (5), 457–464. [DOI] [PubMed] [Google Scholar]
- Dickey C, Kraft C, Jinwal U, Koren J, Johnson A, Anderson L, Lebson L, Lee D, Dickson D, de Silva R, Binder LI, Morgan D, Lewis J, 2009. Aging analysis reveals slowed tau turnover and enhanced stress response in a mouse model of tauopathy. Am. J. Pathol 174 (1), 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujardin S, Colin M, Buee L, 2015. Invited review: animal models of tauopathies and their implications for research/translation into the clinic. Neuropathol. Appl. Neurobiol 41 (1), 59–80. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Bennecib M, Grignon Y, Uchihara T, He Y, Piette F, Hauw JJ, 1997. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol. Aging 18 (3), 267–273. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F, 2004. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 14 (1), 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Scahill RI, Crum WR, Rossor MN, 1999. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 52 (8), 1687–1689. [DOI] [PubMed] [Google Scholar]
- Gamache J, Benzow K, Forster C, Kemper L, Hlynialuk C, Furrow E, Ashe KH, Koob MD, 2019. Factors other than hTau overexpression that contribute to tauopathy-like phenotype in rTg4510 mice. Nat. Commun 10 (1), 2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godyn J, Jonczyk J, Panek D, Malawska B, 2016. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol. Rep 68 (1), 127–138. [DOI] [PubMed] [Google Scholar]
- Gordon MN, Holcomb LA, Jantzen PT, DiCarlo G, Wilcock D, Boyett KL, Connor K, Melachrino JO, O’Callaghan JP, Morgan D, 2002. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp. Neurol 173, 183–195. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC, Alzheimer’s Disease Neuroimaging I, 2009. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 132 (Pt 5), 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E, 2006. Global hairpin folding of tau in solution. Biochemistry 45 (7), 2283–2293. [DOI] [PubMed] [Google Scholar]
- Joly-Amado A, Serraneau KS, Brownlow M, Marin de Evsikova C, Speakman JR, Gordon MN, Morgan D, 2016. Metabolic changes over the course of aging in a mouse model of tau deposition. Neurobiol. Aging 44, 62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI, 2011. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J. Neurosci. 31 (27), 9858–9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini G, Pigino G, LaPointe NE, Andreadis A, Song Y, Leitman E, Binder LI, Brady ST, 2012. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol. Aging 33 (4) 826.e815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontsekova E, Zilka N, Kovacech B, Skrabana R, Novak M, 2014. Identification of structural determinants on tau protein essential for its pathological function: novel therapeutic target for tau immunotherapy in Alzheimer’s disease. Alzheimers Res. Ther 6 (4), 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L, Relkin NR, Siemers ER, 2014. Amyloid-ss-directed immunotherapy for Alzheimer’s disease. J. Intern. Med 275 (3), 284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST, 2009. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J. Neurosci. Res 87 (2), 440–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobello K, Ryan JM, Liu E, Rippon G, Black R, 2012. Targeting Beta amyloid: a clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int. J. Alzheimers Dis 2012, 628070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM, 2005. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J. Neuropathol. Exp. Neurol 64 (9), 743–753. [DOI] [PubMed] [Google Scholar]
- Novak P, Kontsekova E, Zilka N, Novak M, 2018. Ten years of Tau-targeted immunotherapy: the path walked and the roads ahead. Front. Neurosci 12, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P, Zilka N, Zilkova M, Kovacech B, Skrabana R, Ondrus M, Fialova L, Kontsekova E, Otto M, Novak M, 2019. AADvac1, an active immunotherapy for Alzheimer’s disease and non Alzheimer tauopathies: an overview of preclinical and clinical development. J. Prev. Alzheimers Dis 6 (1), 63–69. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C, 2003. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61 (1), 46–54. [DOI] [PubMed] [Google Scholar]
- Panza F, Lozupone M, Seripa D, Imbimbo BP, 2019. Amyloid-beta immunotherapy for alzheimer disease: is it now a long shot? Ann. Neurol 85 (3), 303–315. [DOI] [PubMed] [Google Scholar]
- Petrushina I, Ghochikyan A, Mktrichyan M, Mamikonyan G, Movsesyan N, Davtyan H, Patel A, Head E, Cribbs DH, Agadjanyan MG, 2007. Alzheimer’s disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric Abeta species in amyloid precursor protein transgenic mice. J. Neurosci 27 (46), 12721–12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohamedsait H, Rasool S, Rajamohamedsait W, Lin Y, Sigurdsson EM, 2017. Prophylactic active tau immunization leads to sustained reduction in both tau and amyloid-beta pathologies in 3xTg mice. Sci. Rep 7 (1), 17034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, 2012. Mouse models of frontotemporal dementia. Ann. Neurol 72 (6), 837–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Karussis D, Boimel M, Touloumi O, Ovadia H, Abramsky O, 2006. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal tau protein. Arch. Neurol 63 (10), 1459–1467. [DOI] [PubMed] [Google Scholar]
- Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, Nousiopoulou E, Kassis I, Abramsky O, Karussis D, Rosenmann H, 2013. Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation. Exp. Neurol 248, 451–456. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, Cao Y, Snyder B, Zhang B, Nitla M, Hirschfeld G, Barrezueta N, Polson C, Wes P, Rangan VS, Cacace A, Albright CF, Meredith J Jr., Trojanowski JQ, Lee VM, Brunden KR, Ahlijanian M, 2015. Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models. PLoS One 10 (5), e0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH, 2005. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309 (5733), 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling S, Rahfeld JU, Lues I, Lemere CA, 2018. Passive Abeta immunotherapy: current achievements and future perspectives. Molecules 23 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L, 2006. Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am. J. Pathol 169 (2), 599–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder SK, Joly-Amado A, Gordon MN, Morgan D, 2016. Tau-directed immunotherapy: a promising strategy for treating Alzheimer’s disease and other tauopathies. J. Neuroimmune Pharmacol 11 (1), 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder S, Joly-Amado A, Soliman A, Sengupta U, Kayed R, Gordon MN, Morgan D, 2017. Oligomeric tau-targeted immunotherapy in Tg4510 mice. Alzheimers Res. Ther 9 (1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenica M-L, Davtyan H, Housley SB, Blair L, Gillies A, Nordhues BA, Zhang B, Liu J, Gestwicki JE, Lee D, Gordon MN, Morgan D, Dickey C, 2014. Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, 2018. Light at the end of the Amyloid TunnelPublished as part of the biochemistry series “biochemistry to bedside”. Biochemistry 57 (41), 5921–5922. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R, 1991. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol 30 (4), 572–580. [DOI] [PubMed] [Google Scholar]
- Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean ME, Zommer N, Sergeant N, Schraen-Maschke S, Blum D, Buee L, 2012. Targeting phospho- Ser422 by active tau immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr. Alzheimer Res 9 (4), 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Himmelstein DS, Lancia JK, Binder LI, 2012. Tau oligomers and tau toxicity in neurodegenerative disease. Biochem. Soc. Trans 40 (4), 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Easton A, Corradi J, Barten DM, Devidze N, DeCarr LB, Truong A, He A, Barrezueta NX, Polson C, Bourin C, Flynn ME, Keenan S, Lidge R, Meredith J, Natale J, Sankaranarayanan S, Cadelina GW, Albright CF, Cacace AM, 2014. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PLoS One 9 (8), e106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Graf A, Riviere ME, Andreasen N, Ryan JM, 2014. Active immunotherapy options for Alzheimer’s disease. Alzheimers Res. Ther 6 (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Boutajangout A, 2010. Immunotherapeutic approaches for Alzheimer’s disease in transgenic mouse models. Brain Struct. Funct 214 (2–3), 201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goni F, 2014. Immunotherapy for Alzheimer’s disease. Biochem. Pharmacol 88 (4), 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.