Abstract
Neuroimaging studies of aphasia recovery have linked treatment-related improvements in language processing to changes in functional brain activation in left hemisphere language regions and their right hemisphere homologues. Although there is some consensus that better behavioral outcomes are achieved when activation is restored to the left hemisphere, the circumstances that dictate how and why regions in both hemispheres respond to naming therapy are still unclear. In this study, an fMRI picture-naming task was used to examine 16 regions of interest in 26 patients with chronic aphasia before and after 12 weeks of semantic naming treatment. Ten control patients who did not receive treatment and 17 healthy controls were also scanned. Naming therapy resulted in a significant increase in cortical activation, an effect that was largely driven by patients who responded most favorably to treatment, as patients who responded less favorably (as well as those who did not receive treatment) had little change in activation over time. Relative to healthy controls, patients had higher pre-treatment activation in the bilateral inferior frontal gyri (IFG) and lower activation in the bilateral angular gyri; after treatment, they had higher activation in bilateral IFG, as well as in the right middle frontal gyrus. These results suggest that the predominant effect of beneficial naming treatment was an upregulation of traditional language areas and their right hemisphere homologues and, in particular, regions associated with phonological and semantic/executive semantic processing, as well as broader domain general functions. Additionally, in some left hemisphere regions, post-treatment changes in activation were greater when there was more damage than when there was less damage, indicating that spared tissue in otherwise highly damaged regions can be modulated by treatment.
Keywords: aphasia, language rehabilitation, picture naming, fMRI, region of interest (ROI) analysis
1. Introduction
Anomia, or impaired word retrieval, is a hallmark characteristic of post-stroke aphasia and a frequent target of language treatment. Treatments targeting semantic and/or phonological processes have been shown to improve word retrieval in persons with aphasia (PWA) (Boyle, 2004, 2010; Boyle & Coelho, 1995; Kiran & Bassetto, 2008; Kiran & Thompson, 2003; Leonard, Rochon, & Laird, 2008; Nickels, 2002; van Hees, Angwin, McMahon, & Copland, 2013; Wisenburn & Mahoney, 2009); however, our understanding of the mechanisms underlying recovery after rehabilitation is incomplete and continues to be the topic of considerable debate. Generally, this debate has focused on the respective roles of the left and right hemispheres and the potential ramifications structural damage has on behavior and functional laterality for language. Heiss and Thiel (2006) proposed a three-tiered hierarchy for different dynamics of neural compensation for lesions within the language network which states that: (1) optimal recovery of language abilities is associated with reactivation of minimally-damaged left hemisphere regions; (2) sub-optimal yet satisfactory improvement is linked with recruitment of ipsilateral, perilesional regions; and (3) least optimal and potentially unsatisfactory recovery is associated with recruitment of right hemisphere areas subsequent to extensive damage to essential components of the left hemisphere language network.
Heiss and Thiel’s (2006) hierarchy explains possible mechanisms of the natural course of recovery from stroke, but may also be applicable to treatment-induced changes in neural function associated with language recovery, as a number of studies have linked activation in the left hemisphere to favorable treatment outcomes; however, the right hemisphere has also been associated with treatment-related language improvement (see reviews and meta-analyses by Cappa, 2011; Crinion & Leff, 2007; Crosson et al., 2007; Price & Crinion, 2005; Thompson & den Ouden, 2008; Turkeltaub, Messing, Norise, & Hamilton, 2011). For example, in two of the largest naming treatment/imaging studies to date, Fridriksson and colleagues found that treatment-induced naming improvement was related to recruitment of spared left hemisphere regions that support naming in healthy adults (see Table I for an overview of traditional naming regions), as well as left hemisphere areas that are not traditionally associated with naming (Fridriksson, 2010; Fridriksson, Richardson, Fillmore, & Cai, 2012). In contrast, treatment-induced recruitment of right hemisphere homologues of traditional language regions and bilateral engagement of left and right hemisphere regions have also been reported. One recent example is a study by Nardo and colleagues (Nardo, Holland, Leff, Price, & Crinion, 2017), in which pars opercularis of the right inferior frontal gyrus (RIFGop), whose homologue in the left hemisphere has been linked to phonological and semantic processing (Indefrey, 2011; Indefrey & Levelt, 2004; Price, 2012; Vigneau et al., 2006), was modulated by treatment in a study of 18 PWA (see Table II for additional treatment studies and the left and right hemisphere regions they have implicated in naming improvement). Given the variability of these results, the notion that one hemisphere is always more critical to recovery than the other would seem to be an oversimplification.
Table I.
Selected left hemisphere language regions and associated functions for naming in healthy individuals.
Abbreviations: AG: angular gyrus; IFG: inferior frontal gyrus; MFG: middle frontal gyrus; MTG: middle temporal gyrus; PCG: precentral gyrus; SMG: supramarginal gyrus
Table II.
Neuroimaging studies that have implicated left hemisphere language regions and/or their right hemisphere homologues in treatment-related naming recovery. Included studies administered explicit naming treatment or provided general language treatment and utilized functional tasks intended to tap into naming or naming-related processes (e.g., semantic processing tasks). Checkmarks indicate that a region was implicated by a given study; annotations indicate that results were specific to a particular task, participant, or subsection of IFG (explanations are provided below the table). Gray shading highlights studies that primarily found reductions in activation after treatment or that found a relationship between decreased activation and behavioral improvement. Note that this table is not a comprehensive summary of all results from these studies; rather, it is intended to highlight findings associated with the ROIs investigated in the present study.
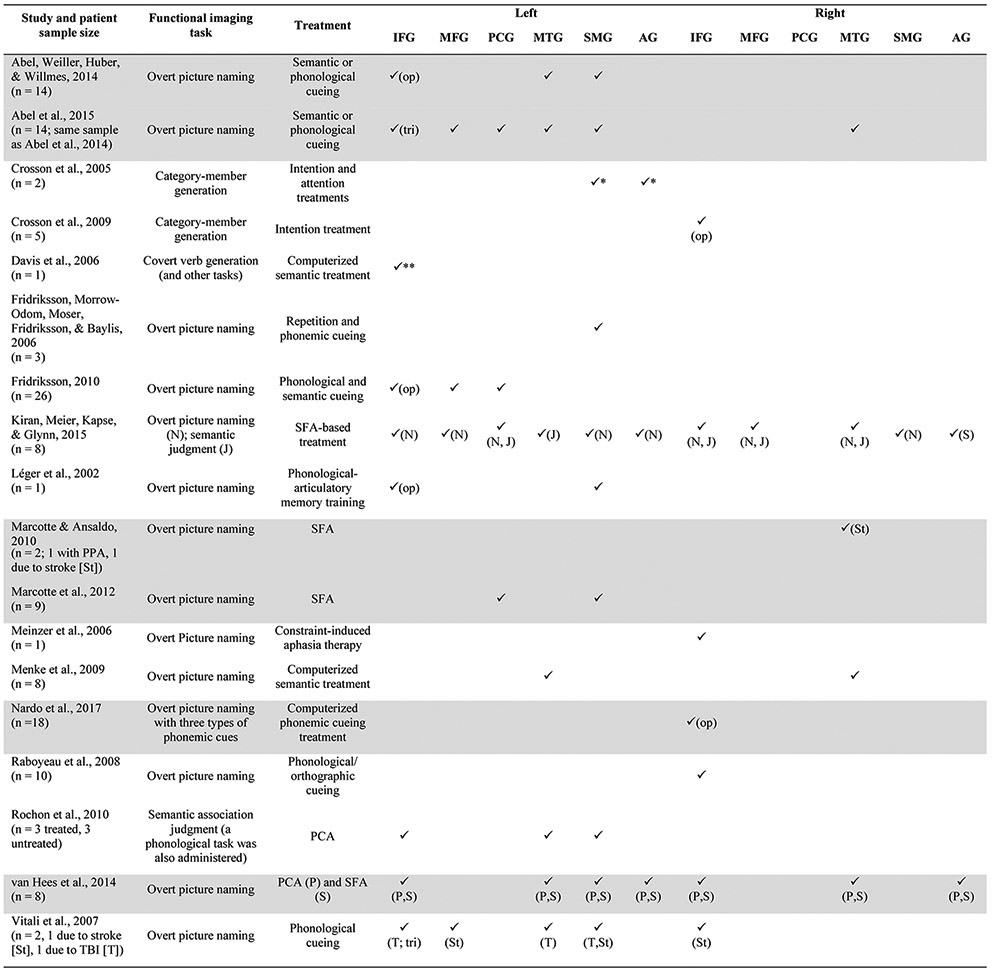 |
Annotations:
results for one of two participants
activation associated with an ROI including Brodmann areas 44-47; J: results associated with semantic judgment task; N: results associated with naming task; P: results associated with items trained via PCA; S: results associated with items trained via SFA; St: results for patient whose aphasia was caused by stroke; T: results for patient whose aphasia was caused by TBI.
Abbreviations: PCA: phonological components analysis; PPA: primary progressive aphasia; SFA: semantic feature analysis; TBI: traumatic brain injury; AG: angular gyrus; IFG: inferior frontal gyrus; op: pars opercularis; tri: pars triangularis; MFG: middle frontal gyrus; MTG: middle temporal gyrus; PCG: precentral gyrus; SMG: supramarginal gyrus
In addition to the issues described above, there are several open questions about the mechanism of neural changes associated with behavioral improvements after treatment. Specifically, many studies have associated behavioral improvement with increased activation (Cornelissen et al., 2003; Davis, Harrington, & Baynes, 2006; Fridriksson, 2010; Fridriksson et al., 2012; Léger et al., 2002; Meinzer et al., 2008; Vitali et al., 2007), but some recent studies have linked behavioral gains to decreased activation after treatment (Abel, Weiller, Huber, Willmes, & Specht, 2015; Marcotte et al., 2012; Nardo et al., 2017; van Hees, McMahon, Angwin, de Zubicaray, & Copland, 2014). These contrasting findings may be indicative of different mechanisms of recovery, but are also likely associated with variables such as the type of treatment and point in time in which it was introduced, the specific functional tasks and imaging analyses utilized, and patient-specific variables like lesion volume and location (for additional review of issues and recommendations associated with neuroimaging treatment studies in aphasia, see Kiran et al., 2013; Meinzer et al., 2013). In fact, lesion characteristics may be particularly critical as they have been linked not only to the nature and severity of patients’ behavioral deficits, but also to the location and extent of task-related functional activation in both hemispheres (Anglade, Thiel, & Ansaldo, 2014; Sebastian & Kiran, 2011; Sims et al., 2016; Skipper-Kallal, Lacey, Xing, & Turkeltaub, 2017; Turkeltaub et al., 2011).
The dynamics of treatment-related language recovery are clearly complex, and further research is critical to the continued advancement of clinical practice and rehabilitation. A better understanding of the interaction between neural function, language treatment, and behavioral performance will be essential to the identification of biomarkers for recovery, improved prognostic accuracy, and the development of novel and effective therapeutic strategies. To build upon existing research in this area, we administered semantic feature analysis-based therapy to a group of patients with chronic aphasia and performed a region-of-interest (ROI) analysis to examine changes in activation in left-hemisphere regions known to mediate aspects of picture naming and their right-hemisphere homologues. Given the nature of the treatment utilized in this study, which aims to improve lexical retrieval and production by strengthening semantic representations, the cortical ROIs examined were chosen a priori based on literature implicating them in semantic processing, word retrieval, and production in healthy controls and patients. These regions included bilateral middle frontal gyrus (MFG), inferior frontal gyrus pars orbitalis (IFGorb), pars triangularis (IFGtri), and pars opercularis (IFGop), precentral gyrus (PCG), middle temporal gyrus (MTG), supramarginal gyrus (SMG), and angular gyrus (AG) (refer to Tables I and II for the rationale and relevant references for the selection of these regions). Critically, we accounted for individual variability in lesion size and location by modifying each patient’s left hemisphere ROIs so that they comprised only tissue spared by their infarct. No prior group study of which we are aware has investigated treatment-related changes in specific ROIs while controlling for the lesion in this manner.
The aims of this study were:
(1) To compare neural activation during picture naming in the specified ROIs in healthy controls and PWA before and after 12 weeks of anomia therapy. Based on reviews and meta-analyses of the typical neural architecture for naming (Indefrey, 2011; Indefrey & Levelt, 2004; Price, 2012; Vigneau et al., 2006, 2011) and treatment studies in PWA, we hypothesized that healthy controls would recruit left hemisphere ROIs to a greater extent than patients before treatment, whereas patients were expected to exhibit greater right hemisphere activation than controls (Cao, Vikingstad, George, Johnson, & Welch, 1999; Fridriksson, Baker, & Moser, 2009; Heiss & Thiel, 2006; Postman-Caucheteux et al., 2010; Price & Crinion, 2005). Following treatment, we expected that patients would increase their recruitment of left hemisphere ROIs and reduce their reliance on the right hemisphere, bringing them into closer alignment with controls, consistent with findings from prior naming treatment studies (Abel et al., 2014, 2015; Davis et al., 2006; Fridriksson, 2010; Léger et al., 2002).
(2) To measure treatment-induced changes in activation within PWA and determine how such changes were related to the proportion of spared tissue within left hemisphere ROIs (as opposed to simply controlling for total lesion volume and location), as well as patients’ responsiveness to treatment. As in aim 1, we hypothesized that treatment would facilitate increased activation in left hemisphere ROIs and reduced reliance on at least some regions in the right hemisphere. We further posited that changes in activation would be larger when left hemisphere ROIs were more intact, on the basis of prior work demonstrating that increased recruitment of spared left hemisphere cortex was associated with better treatment outcomes (Fridriksson, 2010). Finally, we expected that patients who benefited most from treatment would demonstrate more dramatic increases in activation than those who did not respond as favorably, particularly in the left hemisphere (Fridriksson, 2010; Fridriksson et al., 2012; Léger et al., 2002; Meinzer et al., 2008; Vitali et al., 2007).
(3) To better understand the effect of treatment on neural functions in TxPWA by examining activation longitudinally in a group of untreated PWA. Given that all patient participants were in the chronic stage of aphasia recovery, no changes in functional activation were expected in this group.
2. Materials and methods
The present project was completed under the Center for the Neurobiology of Language Recovery (NIH/NIDCD 1P50DC012283; PI: Cynthia Thompson) (http://cnlr.northwestern.edu/). In the following sections, we report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
2.1. Participants
Thirty-five adults with chronic post-stroke aphasia were enrolled in this study from an initial screening pool of 96 potential participants. Sample size was determined by the number of eligible screened participants. Recruitment was conducted in the Boston and Chicago metropolitan areas. 1Inclusion was based on a history of left-hemisphere stroke at least six months before enrollment, premorbid proficiency in English, and anomia indicated by a study-specific naming battery. Exclusion criteria included major neurological or psychiatric disorders (other than stroke) and medical history incompatible with MRI. Of the 61 potential participants who were screened but not enrolled, 49 were ineligible based on the criteria outlined above, eight declined to participate, and four were lost to contact after initial screening.
The 35 patients who qualified and were enrolled in the study were assigned to either a treatment group (TxPWA) or an untreated group (unPWA) in a pseudo-randomized fashion, as follows. Every fourth patient was asked to consider enrolling in the untreated group but was given the option to enroll in the treatment group instead. Patients who completed all stages of the non-treatment arm of the study were given the option of subsequently enrolling in the treatment group. Based on these procedures, 12 patients were enrolled in the untreated group, though two patients voluntarily withdrew prior to completion of the study; thus, the untreated group in this study included a total of 10 patients.
Twenty-three patients were enrolled directly in the treatment group and another seven patients were enrolled in the treatment group after completing the non-treatment arm of the study. Of these 30 unique patients, four were excluded from the present study because they had incomplete data sets due to not completing treatment and post-treatment scanning (n=1) or fMRI acquisition errors (n=3). This resulted in a final treatment group that included 26 patients.
All patients (treated and untreated) were administered a cognitive-linguistic assessment battery that included: the Western Aphasia Battery – Revised (WAB; Kertesz, 2007), which provides an aphasia quotient (AQ) that reflects overall aphasia severity; the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001), a standardized measure of confrontation naming abilities; and the Pyramids and Palm Trees test (PPT; Howard & Patterson, 1992), which assesses non-verbal semantic processing skills. Please see Table III for assessment results and patient demographics, including time between aphasia onset and study enrollment.
Table III.
Patient demographics and standardized assessment data.
| Patient ID | Sex | Age (years) |
MPO | Handedness | Lesion Volume (mm3) |
WAB AQ (/100) |
BNT (/60) |
PPT (/52) |
|---|---|---|---|---|---|---|---|---|
| Treated Patients (TxPWA) | ||||||||
| BU03 | F | 63 | 62 | R | 175,378 | 52.00 | 10 | 46 |
| BU04 | M | 79 | 13 | R | 84,778 | 74.10 | 52 | 49 |
| BU06 | M | 49 | 113 | R | 298,967 | 66.60 | 44 | 48 |
| BU07 | M | 55 | 137 | R | 181,973 | 48.00 | 6 | 46 |
| BU08c01* | M | 49 | 57 | R | 87,587 | 82.80 | 51 | 48 |
| BU09 | F | 71 | 37 | R | 11,660 | 95.20 | 45 | 50 |
| BU10 | F | 53 | 12 | R | 76,553 | 80.40 | 37 | 49 |
| BU11 | M | 78 | 22 | R | 32,114 | 92.10 | 41 | 49 |
| BU12 | M | 68 | 104 | R | 186,845 | 40.00 | 1 | 46 |
| BU13 | M | 42 | 18 | L | 12,131 | 92.70 | 43 | 49 |
| BU14 | F | 64 | 24 | R | 96,932 | 64.40 | 41 | 49 |
| BU15 | F | 71 | 74 | R | 189,309 | 87.20 | 43 | 44 |
| BU16co5* | M | 50 | 71 | R | 317,071 | 33.60 | 1 | 41 |
| BU17 | M | 61 | 152 | R | 163,488 | 74.30 | 54 | 51 |
| BU18 | F | 70 | 152 | R | 69,643 | 78.00 | 24 | 50 |
| BU19c02* | M | 80 | 22 | R | 89,026 | 28.90 | 1 | 43 |
| BU20 | F | 48 | 14 | R | 164,327 | 13.00 | 0 | 40 |
| BU21 | M | 65 | 16 | R | 247,593 | 11.70 | 0 | 43 |
| BU22 | M | 62 | 12 | R | 100,019 | 65.40 | 1 | 37 |
| BU23 | M | 60 | 24 | R | 172,812 | 45.20 | 6 | 42 |
| BU24c06* | M | 69 | 170 | R | 183,449 | 40.40 | 3 | 49 |
| BU25 | F | 76 | 33 | R | 184,390 | 37.50 | 2 | 34 |
| BU26 | F | 64 | 115 | R | 127,704 | 58.00 | 15 | 36 |
| BU27c08* | M | 65 | 17 | L | 34,148 | 84.30 | 41 | 50 |
| BU28 | M | 63 | 15 | R | 76,654 | 56.00 | 21 | 51 |
| BU30c11* | M | 59 | 29 | R | 186,520 | 60.00 | 16 | 48 |
| Mean | 62.8 | 58.3 | 136,579.7 | 60.1 | 23.4 | 45.7 | ||
| SD | 10.2 | 51.8 | 81100.4 | 24.0 | 20.2 | 4.8 | ||
| Untreated Patients (unPWA) | ||||||||
| BUc01 | M | 49 | 49 | R | 87,587 | 85.50 | 53 | 49 |
| BUc02 | M | 79 | 10 | R | 89,026 | 26.90 | 3 | 47 |
| BUc05 | M | 49 | 67 | R | 317,071 | 32.30 | 3 | 44 |
| BUc06 | M | 69 | 164 | R | 183,449 | 39.30 | 5 | 48 |
| BUc07NU | M | 39 | 17 | R | 26,221 | 71.30 | 36 | 52 |
| BUc08 | M | 64 | 13 | L | 34,148 | 79.60 | - | 50 |
| BUc09 | M | 62 | 21 | L | 1,565 | 91.50 | 39 | 49 |
| BUc10NU | M | 68 | 21 | R | 80,283 | 78.60 | 31 | 49 |
| BUc11 | M | 58 | 23 | R | 186,520 | 61.80 | 10 | 51 |
| BUc12 | M | 53 | 467 | R | 120,817 | 91.20 | 51 | 49 |
| Mean | 59.0 | 85.2 | 112,668.7 | 65.8 | 25.7 | 48.8 | ||
| SD | 11.8 | 141.9 | 94,592.8 | 24.6 | 20.6 | 2.2 | ||
Asterisks indicate patients enrolled in the treatment group after first completing all stages of the study as untreated patients. Subject IDs of patients who crossed over are appended with their ID from the untreated group (e.g., the first untreated control patient, BUc01, was enrolled in the treatment group as BU08c01; the second untreated patient, BUc02, was enrolled in the treatment group as BU19c02; etc.).
Abbreviations: MPO: Months post-onset of aphasia; WAB AQ: Western Aphasia Battery Aphasia Quotient; BNT: Boston Naming Test; PPT: Pyramids and Palm Trees test
Seventeen healthy, right-handed older adults (11 males; age: M = 60.41 years, SD = 10.81 years) were recruited from the Boston area and scanned at a single time point to provide normative activation data for the fMRI task. Informed consent was obtained from all participants in accordance with Boston University and Massachusetts General Hospital or Northwestern University IRB protocols.
2.2. Stimuli
The stimuli used in this study were 180 items from four experimental categories (vegetables, birds, furniture, and clothing) and one control category (fruit). Stimuli were identified in prior studies by Kiran and colleagues (Kiran, 2008; Kiran et al., 2015; Kiran & Thompson, 2003) and consisted of nouns balanced for frequency (van der Wouden, 1990), familiarity, and concreteness (Coltheart, 1981). These items were compiled into a picture-naming battery that was administered to PWA three times before and after treatment (or after the equivalent no-treatment hold phase for unPWA) in order to determine baseline and post-treatment/post-hold phase naming accuracy. The battery was also administered three additional times roughly 12 weeks after the final treatment session to assess for maintenance effects. The battery was administered via laptop using E-Prime 2.0 (Psychology Software Tools, Inc., 2012) with items presented in random order at each administration.
2.3. Experimental Design
TxPWA were trained on items from two of the four experimental categories. Naming accuracy was measured before and after treatment and via weekly probes throughout treatment, which lasted up to 12 weeks. The control category, fruit, was only assessed before and after treatment. In order to balance analyses across groups, unPWA and controls were also assigned two of the experimental categories, though they did not receive any training as part of the study. For the sake of simplicity, when describing fMRI methods and results, the term pictures will be used to refer to items from participants’ assigned experimental categories (i.e., items on which TxPWA were trained and the comparable but untrained items assigned to unPWA and healthy controls).
2.4. Treatment
Treatment was administered to TxPWA via a laptop using E-Prime 2.0 with assistance from a speech-language pathologist or research assistant. Treatment tasks required participants to name target items and evaluate their semantic properties. Specific tasks included: sorting pictures of trained and untrained items into their respective superordinate categories; attempting to name a target item; indicating whether 20 written semantic features applied to the target; reviewing a list of features pertaining to the target; indicating whether 15 verbally presented features applied to the target; attempting to name the target a second time; and generative naming of items in the trained category. Treatment was administered for as many items as time allowed, and the order of items and features was randomized at every session.
Naming probes were administered every other session. Treatment continued for 12 weeks (24 sessions) or until the participant achieved 90% accuracy or higher in both trained categories on two consecutive probes.
2.5. fMRI Methods
2.5.1. Task and stimuli
Functional imaging data were collected on two runs per time point (i.e., pre- and post-treatment) of an overt picture-naming task using an event-related design with a randomized, jittered inter-stimulus interval of two or four seconds. This method accounts for motion artifacts associated with overt speech and has been used in a number of studies using overt-naming tasks (Birn, Cox, & Bandettini, 2004; Kiran et al., 2015; Meltzer, Postman-Caucheteux, McArdle, & Braun, 2009; Menke et al., 2009; Postman-Caucheteux et al., 2010). As depicted in Figure 1, stimuli were color photographs of trained items2 (n = 36), as well as the items from the control category (fruit, n = 36); an active baseline condition of scrambled pictures (n = 36) was also presented. Stimuli were presented in random order, with each trial lasting for four seconds. Between trials, a fixation cross on a white background was presented. Participants were instructed to say the names of pictures and “skip” for scrambled pictures. TxPWA and unPWA were scanned at two time points, the first being after all initial assessments were completed (i.e., scan 1/pre-treatment) and the second being after the cessation of up to 12 weeks of naming treatment (TxPWA) or after at least 12 weeks without naming treatment (unPWA) (i.e., scan 2/post-treatment). Healthy controls were scanned at a single time point.
Figure 1.
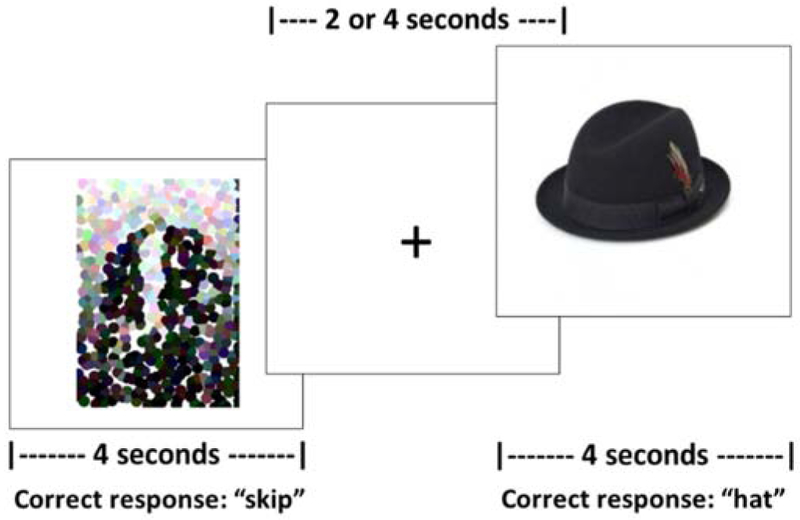
fMRI picture naming task.
2.5.2. Data acquisition
MRI data for all healthy controls and all PWA except BUc07NU and BUc10NU were acquired on a Siemens 3T Trio Tim scanner at the Athinoula A. Martinos Center for Biomedical Imaging in Charlestown, MA. Imaging data for BUc07NU and BUc10NU were acquired on a Siemens 3T Prisma Fit scanner at the Center for Translational Imaging in Chicago, IL3. For all participants, T1 structural images were acquired with the following parameters: 176 sagittal slices, 1mm3 voxels, 256 × 256mm matrix, FOV = 256 × 256mm, flip angle = 9°, TR = 2300ms, TE = 2.91ms. Blood oxygen level-dependent (BOLD) functional images were acquired with the following parameters: interleaved parallel acquisition, 40 axial slices, 2 × 2 × 3mm voxels, 0.3mm interslice gap, 80 × 78mm matrix, FOV = 220 × 220mm, flip angle = 90°, TR = 2570ms, TE = 30ms. For participants scanned in Charlestown, a Fibersound Fiber Optic microphone (Micro Optics Technologies, Cross Plains, WI) was used to record responses in the scanner. For BUc07NU and BUc10NU, responses were recorded using an Avotec audio/mic system (Avotec Incorporated, Stuart, FL) in conjunction with a custom algorithm to reduce scanner noise.
2.5.3. fMRI Data Analysis
2.5.3.1. Preprocessing
Preprocessing was performed with SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and included slice timing correction with reference to the middle slice to address differences in slice acquisition and realignment to correct for motion during scanning. Functional images were coregistered with the T1 structural scan, which was segmented into white matter, gray matter, and cerebrospinal fluid according to the tissue probability maps in SPM12 and warped to the ICBM European brain template. Normalization of structural and functional images to MNI space was performed via 4th-degree b-spline interpolation, and functional data were spatially smoothed using a 4mm-smoothing kernel. For all PWA, hand-drawn lesion masks were generated based on T1 images in MRIcron (Rorden & Brett, 2000). These masks, in which lesioned voxels were deleted, and corresponding lesion maps, in which only lesioned voxels were retained, were integrated into coregistration, segmentation, and normalization to ensure that lesions were adequately masked (Brett, Leff, Rorden, & Ashburner, 2001). All normalized structural and functional images were visually compared to the template using SPM12’s Check Reg function and images that were deemed to be insufficiently aligned to the template were manually corrected and/or preprocessed again after skull-stripping.
The Artifact Detection Toolbox for SPM (ART; https://www.nitrc.org/projects/artifact_detect) was used to identify functional volumes with excessive motion or deviation from the global mean signal (i.e., functional outliers) based on a linear motion threshold of 2mm, rotational motion threshold of .5 radians, or global signal deviation of more than three standard deviations from the mean image intensity.
2.5.3.2. Definition of ROIs and percentage of spared tissue.
For all participants, the anatomical regions identified previously were extracted from the AAL Atlas using the MarsBAR toolbox for SPM (Brett, Anton, Valabregue, & Poline, 2002). Patients’ normalized lesion maps were used to calculate total lesion volume and the percentage of spared tissue in each ROI. Specifically, any overlap between each patient’s lesion map and the atlas-based left hemisphere ROIs was deleted, creating a unique set of ROIs for each patient comprising only spared tissue (see Figure 2). The volume of each of the spared-tissue ROIs was extracted from MarsBAR, divided by the total volume of the region from the AAL atlas, and multiplied by 100, thereby providing the percentage of spared tissue in each ROI (Sims et al., 2016). Healthy controls’ ROIs and patients’ right hemisphere ROIs consisted of the intact, atlas-based regions. See Figure 2 for lesion overlap in each of the patient groups and Table IV for the percentage of spared tissue in each ROI for all PWA.
Figure 2.
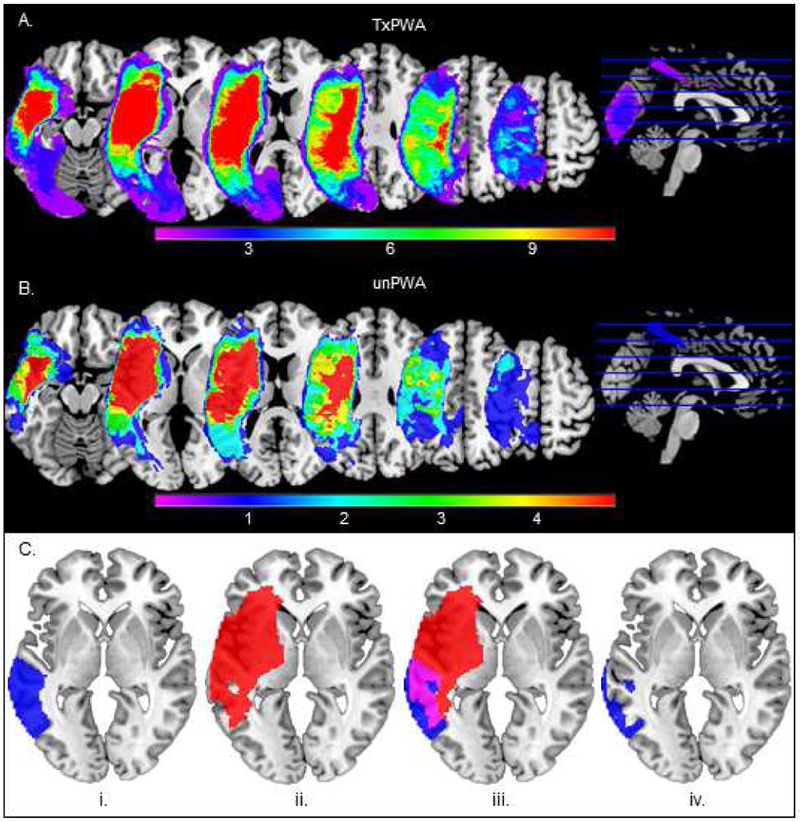
Lesion overlay for patients in the (A) treatment group (TxPWA) and (B) untreated group (unPWA). Warmer colors indicate areas of greater lesion overlap among patients and cooler colors indicate areas where fewer patients have lesions. C. The process for creating patient-specific lesioned ROIs using one ROI (LMTG) in one patient (BU17) as an example. The atlas-based ROI (i, blue) and patient’s lesion map (ii, red) are overlaid and their intersection (iii, violet) is deleted, leaving behind whatever portion of the ROI is intact (iv, blue). This process was performed for all ROIs in all PWA.
Table IV.
Percentage of spared tissue in left hemisphere ROIs for all PWA. Higher values reflect more spared tissue (i.e., less damage) and lower values reflect less spared tissue (i.e., more damage).
| Patient ID | IFGorb | IFGtri | IFGop | MFG | PCG | MTG | SMG | AG |
|---|---|---|---|---|---|---|---|---|
| Treated Patients (TxPWA) | ||||||||
| BU03 | 98.20 | 99.92 | 87.86 | 99.72 | 77.38 | 18.90 | 0.68 | 6.04 |
| BU04 | 99.92 | 100 | 99.77 | 100 | 100 | 20.80 | 68.24 | 58.39 |
| BU06 | 44.01 | 12.45 | 3.65 | 79.80 | 26.85 | 19.50 | 0.07 | 0.23 |
| BU07 | 94.14 | 94.47 | 78.12 | 96.55 | 85.31 | 81.16 | 9.13 | 25.59 |
| BU08c01 | 86.20 | 93.33 | 71.13 | 100 | 98.08 | 72.25 | 86.62 | 100 |
| BU09 | 99.99 | 100 | 100 | 100 | 100 | 95.20 | 100 | 100 |
| BU10 | 100 | 100 | 100 | 100 | 100 | 91.97 | 30.75 | 4.15 |
| BU11 | 100 | 100 | 100 | 100 | 100 | 99.65 | 100 | 100 |
| BU12 | 24.73 | 26.42 | 17.29 | 90.12 | 67.06 | 76.95 | 5.72 | 85.86 |
| BU13 | 99.83 | 99.89 | 99.74 | 100 | 99.42 | 99.99 | 99.71 | 99.31 |
| BU14 | 80.47 | 64.83 | 46.46 | 91.36 | 94.16 | 61.59 | 98.21 | 100 |
| BU15 | 99.77 | 84.12 | 54.49 | 60.05 | 44.26 | 99.77 | 96.18 | 49.67 |
| BU16c05 | 86.50 | 54.19 | 19.97 | 44.14 | 10.86 | 17.56 | 9.68 | 75.67 |
| BU17 | 74.09 | 57.08 | 28.55 | 97.23 | 91.53 | 46.27 | 84.52 | 94.82 |
| BU18 | 90.36 | 93.70 | 86.01 | 99.92 | 98.89 | 96.76 | 88.03 | 99.19 |
| BU19c02 | 54.72 | 66.74 | 48.98 | 98.67 | 85.38 | 99.99 | 52.55 | 90.66 |
| BU20 | 47.82 | 10.64 | 17.09 | 92.49 | 79.93 | 75.72 | 4.68 | 36.31 |
| BU21 | 30.20 | 12.01 | 2.00 | 73.75 | 32.00 | 80.25 | 0.79 | 66.58 |
| BU22 | 100 | 100 | 100 | 100 | 100 | 97.87 | 99.05 | 99.05 |
| BU23 | 93.71 | 64.99 | 11.61 | 55.67 | 48.20 | 85.88 | 17.32 | 91.99 |
| BU24c06 | 65.01 | 66.71 | 60.77 | 96.65 | 93.00 | 41.64 | 67.53 | 58.68 |
| BU25 | 86.01 | 87.64 | 78.91 | 93.15 | 93.24 | 64.74 | 80.62 | 81.83 |
| BU26 | 96.80 | 71.56 | 39.97 | 84.63 | 71.00 | 91.39 | 85.75 | 90.46 |
| BU27c08 | 99.79 | 97.84 | 99.46 | 99.72 | 99.93 | 100 | 100 | 100 |
| BU28 | 87.03 | 90.35 | 84.86 | 100 | 99.97 | 48.72 | 81.90 | 99.17 |
| BU30c11 | 24.84 | 16.14 | 6.29 | 69.45 | 47.18 | 87.88 | 41.41 | 100 |
| Mean | 79.39 | 71.73 | 59.34 | 89.35 | 78.60 | 72.02 | 58.04 | 73.60 |
| Untreated Patients (unPWA) | ||||||||
| BUc01 | 86.20 | 93.33 | 71.13 | 100 | 98.08 | 72.25 | 86.62 | 100 |
| BUc02 | 54.72 | 66.74 | 48.98 | 98.67 | 85.38 | 99.99 | 52.55 | 90.66 |
| BUc05 | 86.50 | 54.19 | 19.97 | 44.14 | 10.86 | 17.56 | 9.68 | 75.67 |
| BUc06 | 65.01 | 66.71 | 60.77 | 96.65 | 93.00 | 41.64 | 67.53 | 58.68 |
| BUc07NU | 100 | 99.78 | 100 | 100 | 100 | 99.71 | 99.70 | 98.21 |
| BUc08 | 99.79 | 97.84 | 99.46 | 99.72 | 99.93 | 100 | 100 | 100 |
| BUc09 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| BUc10NU | 61.92 | 6.49 | 14.34 | 63.15 | 75.61 | 100 | 100 | 100 |
| BUc11 | 24.84 | 16.14 | 6.29 | 69.45 | 47.18 | 87.88 | 41.41 | 100 |
| BUc12 | 82.64 | 42.27 | 6.35 | 93.55 | 63.26 | 76.48 | 35.55 | 99.14 |
| Mean | 76.16 | 64.35 | 52.73 | 86.53 | 77.33 | 79.55 | 69.30 | 92.24 |
Abbreviations: IFGorb: inferior frontal gyrus pars orbitalis; IFGtri: inferior frontal gyrus pars triangularis; IFGop: inferior frontal gyrus pars opercularis; MFG: middle frontal gyrus; PCG: precentral gyrus; MTG: middle temporal gyrus; SMG: supramarginal gyrus; AG: angular gyrus
2.5.3.3. First-level analysis
First-level analyses were conducted for each participant using the general linear model (GLM) in SPM12. Onset and duration of stimuli were convolved with the canonical hemodynamic response function and its temporal derivative, and motion parameters and functional outlier volumes were included as regressors in the GLM. Model estimation utilized a restricted maximum likelihood approach. Once first-level GLMs were complete, the MarsBAR toolbox was used to extract percent BOLD signal change (PSC) from each of the selected ROIs for each condition in order to obtain a measure of activation for the contrast of interest, pictures > fixation. This contrast was selected for the present investigation because it was expected to be sensitive to treatment-related changes in activation associated with picture naming while also controlling for within-subject variability in overall neural activation at scan 1 and scan 2.
2.6. Statistical analysis
Statistical analyses and visualizations were completed in RStudio (RStudio Team, 2015) with the packages lme4 (Bates, Mächler, Bolker, & Walker, 2015), lmertest (Kuznetsova, Brockhoff, & Christensen, 2016), emmeans (Lenth, 2018), effects (Fox, 2003), sjPlot (Lüdecke, 2018), and ggplot2 (Wickham, 2009). To determine the effect of treatment on naming performance, naming battery and scan task accuracy were analyzed via two-sample and paired t-tests (two-tailed), and individual treatment effect sizes were calculated according to Beeson and Robey (2006). To address the primary aims of the study, the following analyses of neuroimaging data were performed:
Aim 1) Pre-treatment TxPWA activation was compared to that of healthy controls via a linear mixed-effects regression model (LMEM). Percent BOLD signal change (PSC) for the contrast pictures > fixation was the dependent variable, group (TxPWA or control), ROI, and their interaction were fixed effects, and participant was a random effect to account for baseline variability in PSC. To compare the groups after treatment, this analysis was repeated using TxPWAs’ post-treatment PSC in the dependent variable.
Aim 2) Changes in activation from pre- to post-treatment in the full sample of TxPWA were initially analyzed via an LMEM predicting PSC from time (i.e., pre-treatment and post-treatment), ROI, and their interaction, with participant as a random effect. As explained in section 3.2.3 of the results, this analysis suggested there might be hemispheric differences in activation change; thus, a follow-up regression was conducted with PSC as the dependent variable, time, hemisphere (rather than specific ROI), and their interaction as fixed effects, and participants as a random effect.
To determine the extent to which longitudinal changes in left hemisphere ROIs might be associated with their structural integrity (i.e., the proportion of spared tissue in each ROI), another regression model was fit predicting PSC from time, ROI (left hemisphere ROIs only, since patients’ right hemisphere ROIs were intact), the percentage of spared tissue in each ROI, and all two- and three-way interactions, with a random effect for participants. Next, we investigated the association between patients’ responsiveness to treatment and changes in activation with an LMEM in which PSC was predicted from time, ROI, treatment response (i.e., responder vs. nonresponder), and their interactions, with participant as a random effect.
Aim 3) Finally, longitudinal changes in activation in PWA in the absence of treatment were examined with a mixed effects model predicting PSC in unPWA from time (i.e., scan 1 and scan 2, which were separated by a period of at least 12 weeks to mimic the duration of treatment in the TxPWA group), ROI, and their interaction, with participant as a random effect.
3. Results
3.1. Behavioral Results
Percent change in average naming battery accuracy from pre- to post-treatment and corresponding effect sizes for experimental and control categories are shown in Table V. For TxPWA, a paired-sample t-test indicated that there was no difference in naming accuracy between trained and control categories before treatment (mean (SD) trained = 28.65% (22.32), mean (SD) control = 26.37% (22.78); t25 = 1.046, p = .305). After treatment, accuracy was significantly higher for trained categories than the control category (mean (SD) trained = 54.91% (34.35), mean (SD) control = 28.81% (23.85); t25 = 8.646, p < .001). Additionally, percent change in accuracy (i.e., average post-treatment score minus average pre-treatment score) and effect sizes were significantly higher for trained than control categories (t25 = 8.298, p < .001, and t25 = 8.142, p < .001, respectively; percent change and effect sizes are reported in Table V).
Table V.
Response to treatment, as indicated by percent change in naming battery accuracy and effect sizes for trained/experimental and control stimuli, and corresponding treatment response categorization. Percent change on the naming battery reflects the difference in mean scores across 3-4 assessments at pre (i.e., pre-treatment/pre-hold phase) and post (i.e., post-treatment/post-hold phase). Effect sizes for trained pictures in TxPWA and corresponding untrained experimental pictures in unPWA reflect the average of participants’ two assigned experimental categories. Response indicates whether participants achieved an effect size ≥ 4.0 in at least one trained category (TxPWA only). R: Responder; N: Nonresponder.
| % Change in Naming Battery Accuracy (post – pre) |
Treatment Effect Size | ||||
|---|---|---|---|---|---|
| Patient ID | Trained/Experimental | Control | Trained/Experimental | Control | Response |
| Treated Patients (TxPWA) | |||||
| BU03 | 30.56 | 1.85 | 9.50 | 0.33 | R |
| BU04 | 50.00 | 6.48 | 8.70 | 0.56 | R |
| BU06 | 33.56 | 5.09 | 5.84 | 0.91 | R |
| BU07 | 19.44 | 7.41 | 4.27 | 1.88 | R |
| BU08c01 | 27.78 | −0.92 | 3.63 | 0.00 | R |
| BU09 | 46.30 | 0.00 | 8.87 | 0.00 | R |
| BU10 | 33.07 | 10.19 | 9.82 | 2.34 | R |
| BU13 | 52.25 | 4.63 | 11.04 | 1.32 | R |
| BU14 | 50.00 | 10.19 | 8.33 | 1.49 | R |
| BU15 | 15.74 | −3.70 | 3.66 | −1.15 | R |
| BU17 | 48.15 | 6.48 | 11.84 | 2.02 | R |
| BU18 | 37.96 | 0.00 | 5.47 | 0.24 | R |
| BU19c02 | 16.67 | 0.92 | 5.20 | 0.12 | R |
| BU20 | 41.67 | 3.70 | 5.08 | 1.29 | R |
| BU22 | 10.19 | 4.63 | 3.15 | 1.08 | R |
| BU27c08 | 28.70 | 3.70 | 4.59 | 0.61 | R |
| BU28 | 40.74 | −0.93 | 5.43 | 0.01 | R |
| BU11 | 19.44 | 1.85 | 2.10 | 0.38 | N |
| BU12 | 7.41 | −0.92 | 1.15 | −0.32 | N |
| BU16co5 | 7.41 | 6.48 | 2.31 | 0.00 | N |
| BU21 | 5.56 | 0.00 | 1.74 | 0.00 | N |
| BU23 | 3.70 | −2.78 | 0.67 | −0.87 | N |
| BU24c06 | 12.96 | 0.93 | 2.07 | 0.29 | N |
| BU25 | 5.56 | 0.00 | 0.72 | 0.00 | N |
| BU26 | 13.89 | −1.85 | 3.23 | −0.33 | N |
| BU30c11 | 24.07 | 0.00 | 2.29 | 0.00 | N |
| Mean | 26.26 | 2.44 | 5.02 | 0.47 | |
| SD | 15.95 | 3.83 | 3.30 | 0.86 | |
| Untreated Patients (unPWA) | |||||
| BUc01 | −3.7 | 4.86 | −0.70 | 0.97 | |
| BUc02 | 1.85 | −0.93 | 0.15 | −0.58 | |
| BUc05 | −7.41 | −2.78 | −1.16 | −0.91 | |
| BUc06 | 0.00 | 0.92 | 0.00 | 0.29 | |
| BUc07NU | 9.26 | 5.56 | 1.09 | 1.12 | |
| BUc08 | 4.63 | 2.78 | 0.55 | 0.87 | |
| BUc09 | 28.70 | 16.67 | 2.16 | 2.75 | |
| BUc10NU | −5.56 | 5.55 | −1.35 | 1.44 | |
| BUc11 | 14.58 | 3.47 | 3.28 | 0.39 | |
| BUc12 | 12.04 | 1.85 | 2.46 | 0.07 | |
| Mean | 5.44 | 3.80 | 0.65 | 0.64 | |
| SD | 11.03 | 5.29 | 1.58 | 1.05 | |
Like TxPWA, unPWA showed no difference in naming battery accuracy between experimental pictures and control pictures at their initial evaluation (mean (SD) trained = 31.41% (21.44), mean (SD) control = 30.65% (25.05); t9 = .184, p = .858); however, there remained no difference after the unPWA completed their untreated hold phase (mean (SD) trained = 36.85% (23.78), mean (SD) control = 34.44% (28.40); t9 = .767, p = .463), nor were there differences in percent change in naming battery accuracy (t9 = .644, p = .536) and effect size (t9 = .011, p = .991) (see Table V). Thus, treatment improved oral naming of trained pictures but not control pictures in TxPWA, while unPWA did not improve on either set between scans. More in-depth results pertaining to the efficacy of the treatment used here are reported in Gilmore, Meier, Johnson, and Kiran (2018), which includes comprehensive analyses of direct treatment effects and generalization to untrained items and tasks in the majority of patients included in the present study.
Additionally, while in-depth analyses of treatment maintenance and its associated neural correlates are beyond the scope of this study, the average naming accuracy for trained items in 25/26 treated patients4 approximately 12 weeks after treatment ended was 48.07% (SD = 32.34). This reflects a decline of 6.84 percentage points from the mean post-treatment score of 54.91%, but it also represents a gain of 19.42 percentage points over pre-treatment accuracy, suggesting that gains associated with trained items were partially maintained 12 weeks after the end of treatment.
Importantly, although treatment was largely beneficial among TxPWA, there was variability in participants’ response to treatment, as indicated by the results presented in Table V. Thus, as in Gilmore et al. (2018), TxPWA were sub-classified as having had a favorable response to treatment (i.e., responders) or a less satisfactory response (i.e., nonresponders), based on a cutoff of achieving at least a small effect size (i.e., ≥ 4.0, per Beeson & Robey, 2006) in at least one trained category. This classification was utilized in subsequent analyses, as described below.
3.2. fMRI results
3.2.1. Task accuracy
A two-sample Welch’s t-test of naming accuracy for pictures in the scanner indicated that healthy controls were significantly more accurate than TxPWA prior to treatment (control M = 73.86%, SD = 22.46%; TxPWA pre-treatment M = 24.83%, SD = 22.90%, t34.8 = 6.944, p < .001). After treatment, TxPWA accuracy improved significantly relative to pre-treatment (post-treatment M = 42.66%, SD = 36.67%, paired t25 = −4.936, p < .001), although it was still significantly lower than that of controls (two-sample t40.9 = 3.458, p = .001). No change in task accuracy was observed in unPWA between their first and second scans (paired t9 = .738, p = .479)
3.2.2. Differences in activation between controls and TxPWA
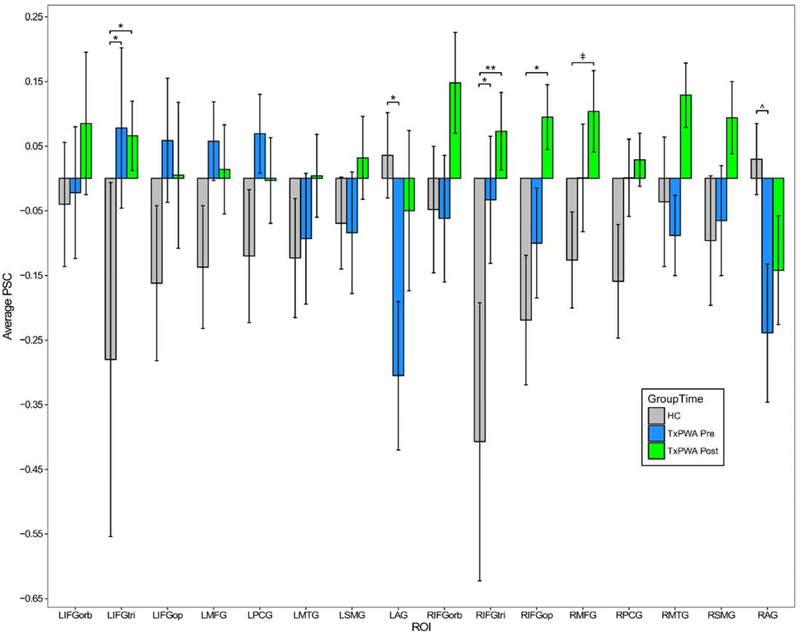
Mean activation in each ROI for TxPWA and healthy controls is shown in Figure 3. The regression model comparing controls and TxPWA at pre-treatment revealed a significant interaction of group and ROI (F15,643 = 3.450, p < .001), indicating there were group differences in activation that varied across ROIs. To more fully interpret this interaction, we conducted a post hoc test comparing estimated marginal means (EM means) between groups for each ROI. Results showed that TxPWA had significantly higher activation than controls in bilateral IFGtri (LIFGtri: t152 = −2.390, p = .018; RIFGtri: t152 = −2.490, p = .014) and significantly lower activation than controls in LAG (t154 = 2.237, p = .027), as well as lower activation in RAG which approached significance (t152 = 1.801, p = .074).
Figure 3.
Average activation by ROI in healthy controls (HC) and treated patients (TxPWA) before and after treatment. Error bars reflect standard error. Significance indicators are based on post hoc EM means tests. **p < .001; *p < .05; ^p = .074; ǂp = .089.
Abbreviations: HC: healthy controls; TxPWA: treated patients; PSC: percent signal change (pictures > fixation); L: left; R: right; IFGorb: inferior frontal gyrus pars orbitalis; IFGtri: IFG pars triangularis; IFGop: IFG pars opercularis; MFG: middle frontal gyrus; PCG: precentral gyrus; MTG: middle temporal gyrus; SMG: supramarginal gyrus; AG: angular gyrus
In the post-treatment regression model, the effects of group and group-by-ROI interaction were significant (F1, 43 = 4.651, p = .037 and F15, 643 = 1.873, p = .023, respectively). A post hoc EM means test examining the effect of group indicated that activation, averaged across all ROIs, was significantly higher for TxPWA after treatment than for controls (t45 = −2.106, p = .041). The group-by-ROI interaction was also investigated with a post hoc EM means test, in this case contrasting activation between the groups in each ROI. Results showed that TxPWA had significantly higher activation than controls in bilateral IFGtri (LIFGtri: t316 = −2.559, p = .011; RIFGtri: t316 = −3.552, p < .001) and RIFGop (t316 = −2.329, p = .021), and near-significantly higher activation in RMFG (t316 = −1.705, p = .089).
To summarize these results, prior to treatment, TxPWA had higher mean activation than controls in 11/16 ROIs (see Figure 3), with significantly higher activation in bilateral IFGtri, while controls had significantly higher activation (or nearly so) in bilateral AG. After treatment, TxPWA had higher activation than controls in 14/16 ROIs, with significantly higher activation in bilateral IFGtri, RIFGop, and, to a lesser extent, RMFG. Additionally, patients no longer had significantly lower activation than controls did in any of the ROIs.
3.2.3. The effect of treatment on activation in TxPWA
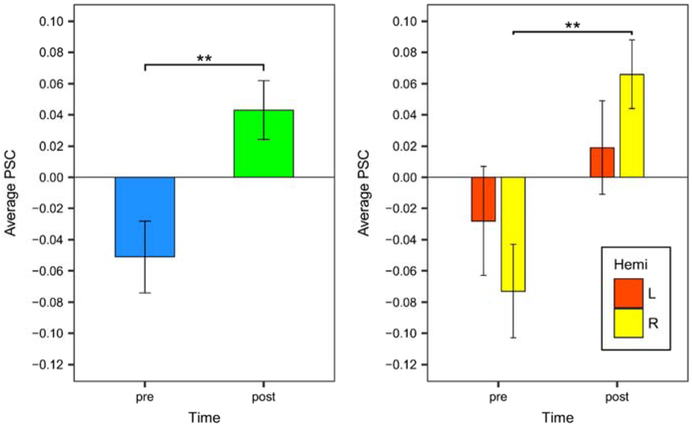
The LMEM predicting PSC within TxPWA revealed significant effects of ROI (F15, 802 = 2.355, p = .003) and time (F1, 802 = 14.968, p < .001); however, the time-by-ROI interaction was not significant. Because we were primarily interested in the effect of time (i.e., treatment), a post hoc EM means test of the main effect of time was performed to compare pre- and post-treatment activation averaged across the ROIs. This test revealed a significant increase in activation subsequent to treatment (t834 = −3.793, p < .001), as shown in Figure 4 (left panel).
Figure 4.
Pre- and post-treatment activation in TxPWA, averaged across all ROIs (left panel), and averaged by hemisphere (right panel). Error bars reflect standard error. Significance indicators are based on post hoc EM means tests, as described in the text. **p < .001
While we concluded from the previous analyses that the primary effect of treatment was a general increase in activation across ROIs, it was also apparent that average activation decreased in some ROIs, namely LIFGtri, LIFGop, LMFG, and LPCG (as shown in Figure 3). Based on this observation, a follow-up analysis was conducted to determine if activation changed over time when averaged among the ROIs within each hemisphere (i.e., left hemisphere ROIs vs. right hemisphere ROIs). An LMEM predicting PSC from time, hemisphere, and their interaction with a random effect for participants indicated that there was a significant effect of time (F1, 802 = 13.929, p < .001) and a nearly significant time-by-hemisphere interaction (F1, 802 = 3.338, p = .068). The effect of time was consistent with the prior analysis predicting PSC from time and ROI and therefore was not investigated further. A post hoc EM means test comparing change in PSC over time by hemisphere revealed a significant increase in activation in the right hemisphere (t805 = −3.933, p < .001) and a non-significant increase in the left hemisphere (p = .180) (Figure 4, right panel).
3.2.4. The relationship between spared tissue and activation changes in left hemisphere ROIs
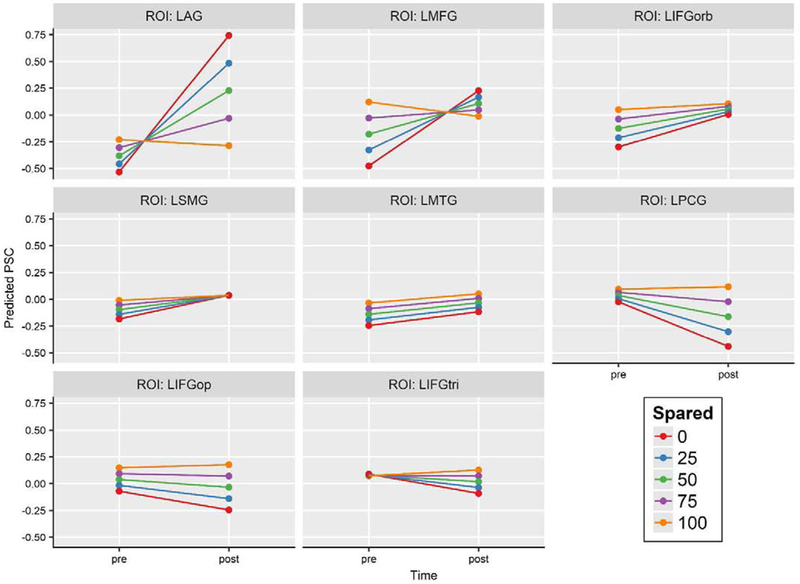
Next, we examined the association between the amount of spared tissue in left hemisphere ROIs and activation changes therein. This analysis revealed significant effects of time (F1, 386 = 3.940, p = .048), time-by-ROI interaction (F7, 386 = 3.437, p = .001), and time-by-ROI-by-spared tissue interaction (F7, 386 = 2.565, p = .014). Given that the focus of this analysis was on the effect of spared tissue, the three-way interaction of time, ROI, and spared tissue was examined by plotting estimated pre- and post-treatment activation in each left hemisphere ROI against proportional spared tissue, ranging from 0 to 100% intact. As shown in Figure 5, the estimated effect of spared tissue on PSC varied by ROI. In LAG, LMFG, LIFGorb, and, to a lesser extent, LSMG, less spared tissue (i.e., more damage) was associated with a larger increase in activation from pre- to post-treatment, while more spared tissue (i.e., less damage), was associated with a smaller increase in activation (or, in the case of LAG and LMFG, a slight decrease in activation). On the other hand, in LPCG, LIFGop, and LIFGtri, less spared tissue was associated with a larger decrease in activation after treatment, and more spared tissue was associated with a smaller decrease or minimal change. Finally, in LMTG, there was little association between change in activation and spared tissue, as the difference between pre- and post-treatment PSC was roughly constant across the range of spared tissue values. In summary, there was a relatively substantial association between the quantity of spared tissue and change in activation after treatment in some regions, and a negligible association in other regions.
Figure 5.
Predicted pre- and post-treatment activation in left hemisphere ROIs at various quantities of spared tissue, ranging from 0 to 100% spared in 25% increments. Regions showing a tendency toward increased activation from pre- to post-treatment are presented first, followed by those showing a tendency toward decreasing activation.
Abbreviations: PSC: percent signal change (pictures > fixation); LAG: left angular gyrus; LMFG: left middle frontal gyrus; LIFGorb: left inferior frontal gyrus pars orbitalis; LSMG: left supramarginal gyrus; LMTG: left middle temporal gyrus; LPCG: left precentral gyrus; LIFGop: left inferior frontal gyrus pars opercularis; LIFGtri: left inferior frontal gyrus pars triangularis.
3.2.5. The relationship between treatment response and changes in activation
As described above in the behavioral results (section 3.1), TxPWA were classified as responders or nonresponders based on whether or not they achieved a small effect size in at least one trained category. The regression model predicting PSC from time, ROI, and treatment response (i.e., responders or nonresponder) indicated that the effects of time (F1, 802 = 8.155, p = .004), ROI (F15, 802 = 2.269, p = .004), and the time-byresponse interaction (F1, 802 = 8.978, p = .003) were significant. Because our interest was in understanding if and how activation changed over time as a function of treatment response, a post hoc EM means test was performed to explore the interaction term. The test showed that responders had a significant increase in activation from pre- to post-treatment, averaged across all ROIs (t869 = −4.765, p < .001), whereas nonresponders showed virtually no change in activation (t869 = .084, p = .933) (see Figure 6, which also depicts average PSC in healthy controls as a point of reference).
Figure 6.
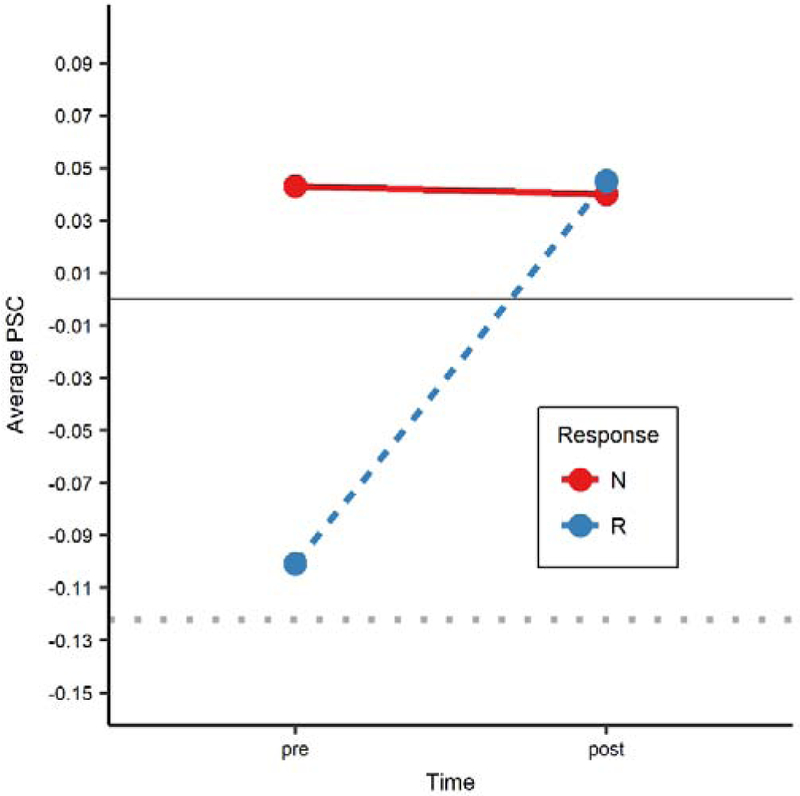
Average activation across all ROIs at pre- and post-treatment in responders (blue dots/dashed line) and non-responders (red dots/solid line). Responders had a significant increase in activation (p < .001), while nonresponders showed no significant change. For reference, average activation for healthy controls (HC) is plotted as a dotted gray line.
N: nonresponders; R: responders
3.2.6. Longitudinal activation in unPWA
Finally, to further determine if treatment was responsible for the changes observed in TxPWA (i.e., aim 3), the effect of repeated scanning in the absence of treatment was investigated via an LMEM predicting PSC in unPWA from time (i.e., scan 1 vs. scan 2, which were separated by approximately 12 weeks without intervention), ROI, and their interaction, with a random effect for participants. The effect of ROI (F15, 310 = 2.539, p = .001) was significant, but time and the time-by-ROI interaction were not (p = .200 and p = .860, respectively); thus, in contrast to treated patients, there were no significant longitudinal changes in activation in untreated patients.
4. Discussion
We examined neural activation during picture naming in PWA who received semantic naming treatment, PWA who did not receive treatment, and healthy controls. There were several notable findings. First, treatment-related naming improvement in patients with chronic aphasia coincided with an overall increase in activation, particularly in those patients who responded most favorably to treatment. In contrast, activation did not increase in nonresponders or untreated patients. Second, results showed that the proportion of spared tissue in left hemisphere ROIs had a differential effect on activation changes depending on the region, such that changes in most ROIs were larger when there was less spared tissue and smaller when there was more spared tissue. The direction of this association varied across the ROIs, with activation increasing in some regions as a function of treatment and decreasing in others. Finally, region-specific differences in activation between patients and healthy controls provided further insights into how the functional architecture for naming was altered in patients and changed in response to treatment. All of these results are discussed in further detail below.
4.1. Semantic treatment improves naming accuracy
Similar to previous treatment studies that targeted underlying lexical-semantic representations (Boyle, 2004, 2010; Boyle & Coelho, 1995; Kiran, 2008; Kiran & Johnson, 2008; Kiran et al., 2015; Kiran & Thompson, 2003), the majority of TxPWA in our study benefitted from therapy, as indicated by treatment effect sizes and accuracy on the naming battery and scan task5. Furthermore, 12-week follow-up assessments of naming indicated that, despite a decline in accuracy relative to their immediate post-treatment performance, treated patients experienced some maintenance of treatment effects. Importantly, these findings were observed in participants who were all in the chronic stage of aphasia recovery, demonstrating that treatment-related language recovery can be achieved many months, or even years, after onset.
As described in detail in Gilmore et al. (2018), the theoretical basis for the treatment used in this study is that the semantic representations of trained items can be strengthened through repeated analysis of their features. These strengthened representations can then be accessed more easily than their semantic competitors, which, in turn, improves selection of the correct lexical representation and, subsequently, its phonological form, during oral naming. This is presumed to be the mechanism underlying improvement in those patients who responded to treatment. However, nine patients did not improve with treatment, indicating that our lexical-semantic training was not uniformly beneficial to all PWA. While more research is needed to fully elucidate the circumstances that explain such variability in treatment response, we found critical differences in functional activation between responders and nonresponders that are likely related to their divergent behavioral outcomes, as discussed in section 4.2 below.
4.2. Change in activation differentiates responders from nonresponders
With respect to neuroimaging outcomes, a key finding of this study was that of a differential effect of treatment on activation depending on treatment response, such that those who benefited from treatment had a significant increase in average activation, while those who did not respond had virtually no change in activation. This result demonstrates a meaningful relationship between behavioral improvement due to treatment and changes in neural function in our sample of treated patients. Additionally, as shown in Figure 6, responders’ average pre-treatment activation was more comparable to that of healthy controls than nonresponders, even though post-treatment activation was similar in both patient subgroups. One possible explanation for this observation is that lower activation reflects more efficient neural processing, which would be consistent with interpretations of reduced activation following treatment in prior studies of PWA (e.g., Abel et al., 2015; Breier, Maher, Schmadeke, Hasan, & Papanicolaou, 2007; Nardo et al., 2017; Richter, Miltner, & Straube, 2008). The pre-treatment difference between responders and nonresponders may indicate that responders-to-be had an advantage over nonresponders-to-be in terms of their capacity to benefit from treatment. Thus, patients whose naming architecture was operating at a level that approximated “normal” (i.e., control-like) levels of engagement with the task from the outset may have been better equipped to encode trained items during treatment, learn compensatory strategies, and effectively allocate and utilize their spared neural resources (i.e., cortical regions) when attempting to name items after treatment. This does not necessarily mean that nonresponders are incapable of benefiting from treatment, but rather that they might be at a disadvantage a priori due to the relative “overactivation” of their naming resources. It is possible that nonresponders might have benefited from additional time in treatment or a different treatment, or that they may be good candidates for noninvasive brain stimulation. In summary, while it is premature to suggest that elevated pre-treatment activation in the regions we have examined is a biomarker for recovery with semantic naming therapy, we would argue that our results provide an important foundation for further investigations along these lines.
4.3. Treatment resulted in upregulation of most ROIs
As described in the results, the main effect of treatment in this study was a significant increase in activation across the regions of interest in the full group of treated patients. Since nonresponders showed no change in activation, we can conclude that this result was driven by the responders. Furthermore, we identified a trending time-by-hemisphere interaction, which was driven by more consistent increases in activation in right hemisphere ROIs than left hemisphere ROIs. The right hemisphere, including some of the ROIs examined in the present study, has previously been implicated in treatment-related language recovery, though most of the evidence comes from case reports or studies with fairly small samples (Crosson et al., 2005, 2009; Fridriksson et al., 2006; Kiran et al., 2015; Meinzer et al., 2006; Menke et al., 2009; Nardo et al., 2017; Raboyeau et al., 2008; Vitali et al., 2007). The largest of these studies, by Nardo et al. (2017), described 18 PWA who engaged right IFGop, anterior insula, dorsal anterior cingulate cortex, and left premotor cortex during post-treatment naming, leading the authors to emphasize the important contributions of language homologues to post-stroke language processing and rehabilitation. We concur with this perspective and suggest that our results offer further evidence of the right hemisphere’s potential to support recovery. However, increased activation was not exclusive to right hemisphere ROIs in the present study and was, in fact, found in LIFGorb, LMTG, LSMG, and LAG. Upregulation of these regions has been reported in prior studies that employed a variety of treatments (see Table II), including semantic (Davis et al., 2006; Kiran et al., 2015; Menke et al., 2009), phonological/articulatory (Fridriksson et al., 2006; Léger et al., 2002; Rochon et al., 2010; Vitali et al., 2007), or intention/attention (Crosson et al., 2005) training, and is consistent with the view that rehabilitation engages preserved tissue in traditional language areas. Collectively, the results of the present study suggest that treatment elicits functional changes bilaterally, particularly given that the patient control group (unPWA), like nonresponders, showed no longitudinal changes across a 12-week span without treatment. Although there were fewer unPWA than TxPWA, their inclusion is a key strength of this study, given that few imaging treatment studies have employed patient control groups and, thus, the extent to which activation in PWA changes over time without intervening treatment has not been clearly established.
4.4. Regional activation differs between healthy controls and patients before and after treatment
While longitudinal analyses of patients indicated that treatment resulted in bilateral functional changes, comparisons between healthy controls and patients provide insight into the effect of stroke and treatment on specific regions involved in naming in PWA. That regional differences between groups were identified in both hemispheres before (i.e., bilateral IFGtri and AG) and after (i.e., bilateral IFGtri, RIFGop, and RMFG) treatment provides further evidence that rehabilitation-related recovery may be underpinned by modulation of a bilateral network, rather than one hemisphere or the other. Each of the ROIs found to show a significant group difference and the potential implications of these differences are addressed below.
4.4.1. Bilateral AG
Relative to TxPWA before treatment, controls had significantly higher activation (or nearly so) in the bilateral AG. In fact, these were the only regions in which controls showed positive activation for the contrast of interest. AG has frequently been implicated in semantic tasks, though its specific contribution to semantic processing has been the topic of much debate and investigation (Binder et al., 2009; Davey et al., 2015; Humphreys & Lambon Ralph, 2015; Jackson, Hoffman, Pobric, & Lambon Ralph, 2016; Noonan et al., 2013; Price, 2010; Seghier, 2013; Seghier et al., 2010; Vigneau et al., 2006). There is evidence that AG is part of the default mode network (DMN) and plays a role in episodic and self-referential semantic processing irrespective of the presence of a task or stimulus (Humphreys & Lambon Ralph, 2015; Seghier et al., 2010); that it is involved in searching for semantic content or representations in visual stimuli (Seghier et al., 2010); that it supports later stages of visually driven semantic processing (Seghier et al., 2010) and/or more complex semantic functions as part of a frontoparietal executive semantic control network (Noonan et al., 2013; Seghier et al., 2010); and that it contributes to automatic/bottom-up conceptual processing and retrieval (Davey et al., 2015; Humphreys & Lambon Ralph, 2015). All of these functions relate to conceptual semantics, which may explain why the AG were recruited by healthy controls during picture naming, a semantically driven task.
In patients in the present study, bilateral AG exhibited relative deactivation (i.e., less activation for the trained condition than the fixation) both before and after treatment; this sort of task-based deactivation is consistent with AG’s role in the DMN. Notably, however, there was less deactivation in the AG after treatment than before, to such an extent that patients no longer had significantly lower AG activation than controls. As noted above, there is evidence that AG is involved in semantic processing and, furthermore, that it may be particularly sensitive to concrete, relative to abstract, representations (Binder, Westbury, McKiernan, Possing, & Medler, 2005; Wang, Conder, Blitzer, & Shinkareva, 2010). Binder and Desai (2011) posited that AG is part of a convergence zone that stores and processes supramodal representations (i.e., representations formed by the integration of multiple sources of modality-specific information. In accordance with this view, the reduction in deactivation in AG from pre- to post-treatment in the present study may reflect a corresponding change in the strength of supramodal representations of trained items as they became more concrete through repeated analysis of their features during treatment.
Alternatively, recent work by Humphreys and Lambon Ralph (2015, 2017) suggests that AG is sensitive to task or item difficulty and that this sensitivity extends across cognitive domains and is not exclusive to semantic processing. These authors found no difference in activation in AG between a semantic task and a visuospatial task and instead found that activation varied for both tasks depending on the difficulty of the items presented, with greater deactivation in response to hard trials and less deactivation in response to easy trials (Humphreys & Lambon Ralph, 2017). They argue that AG is involved in domain-general, automatic, bottom-up processing of all sorts of incoming information, and that when tasks are more difficult, these automatic functions are suppressed to allow for more effective utilization of top-down executive control functions. Under this account, patients in the present study may have had more deactivation in AG when the task was more difficult for them (i.e., before treatment) and less deactivation when the task was easier (i.e., after treatment).
Given the nature of the picture-naming task utilized in this study, it is not feasible to determine if changes in AG deactivation in the present study reflect an increase in the concreteness of trained stimuli or a reduction in task difficulty through training; however, both interpretations would be indicative of a desirable treatment effect.
4.4.2. Bilateral IFGtri
Another pair of regions showing group differences in activation were the bilateral IFGtri, which were more active in patients than healthy controls before and after treatment. This finding is particularly interesting given that, as depicted in Figure 3, left and right IFGtri responded differently to treatment, with RIFGtri showing a substantial increase in activation and LIFGtri showing a small decrease. Post-treatment recruitment of RIFGtri is notable as activation in the right hemisphere (including in RIFGtri) was reported in conjunction with correct naming in a non-treatment study of PWA (Fridriksson et al., 2009). It should be noted, however, that although we assume RIFGtri made a beneficial contribution to naming after treatment, there is evidence that this region can actually interfere with accurate naming, at least in some PWA (Crosson et al., 2007; Naeser et al., 2005, 2011; Winhuisen et al., 2005, 2007).
Prior studies of healthy subjects (Price, 2012; Thompson-Schill et al., 1997; Vigneau et al., 2006, 2011) and PWA (Sebastian & Kiran, 2011; Sims et al., 2016) have variously linked LIFGtri to semantic and phonological processing, lexico-semantic control, selection, and/or retrieval, so it is possible that patients’ recruitment of LIFGtri at pre- and post-treatment in the present study reflects the preservation of relatively normal language functions for that region. Additionally, a few recent studies have also identified decreased activation in LIFGtri in conjunction with positive treatment outcomes (Abel et al., 2015; van Hees et al., 2014). Such reductions have been interpreted as a product of greater efficiency during neural processing (Abel et al., 2015; Breier et al., 2007; Nardo et al., 2017; Richter et al., 2008; van Hees et al., 2014), which could explain why activation decreased in LIFGtri, and to an even greater extent LPCG, LIFGop, and LMFG, even as naming accuracy improved in treated patients.
4.4.3. RIFGop
Unlike IFGtri, RIFGop activation did not significantly differ between patients and controls at pre-treatment. However, after treatment, patients recruited RIFGop to the extent that it was significantly more active than the level observed in healthy controls. Like its left-hemisphere counterpart, RIFGop has been implicated in aspects of lexical-semantic and phonological processing in studies of healthy individuals (Vigneau et al., 2006, 2011). In PWA, Naeser and colleagues proposed that RIFGop supports language processing and recovery on the basis that inhibitory transcranial magnetic stimulation (TMS) applied to RIFGop increased response latencies during naming (Naeser et al., 2011). This effect was most prominent in a patient with very severe aphasia, which may suggest that RIFGop is especially critical when language functions are highly impaired. More recently, Skipper-Kallal et al. found an association between lesion volume and RIFGop (as well as other right hemisphere regions), such that PWA with larger lesions had greater activation in RIFGop during a covert naming task (Skipper-Kallal et al., 2017). In our study, RIFGop was recruited for the contrast of interest only after treatment, which may indicate that, in addition to potentially playing a role in post-stroke language functions in patients with large lesions and/or severe impairment, it may become more engaged in naming as a function of treatment in chronic aphasia.
4.4.4. RMFG
Similar to RIFGop, RMFG was not recruited by patients or controls before treatment but was sufficiently activated by patients after treatment to result in a group difference that trended toward significance. We provide a brief explanation of the potential role of RMFG even though the p-value was greater than .05 because of the potential importance of this finding. In a meta-analysis by Vigneau and colleagues (2011), the authors suggested that although RMFG has been shown to activate in the context of phonological tasks, it likely supports attention processing and is not specifically engaged for language. Consistent with this view, Fedorenko and colleagues subsequently found that right MFG was among a number of regions recruited for tasks covering a variety of domains (e.g., math, spatial and verbal working memory, Stroop, etc.) and thus proposed that it is a domain general processing region (Fedorenko, Duncan, & Kanwisher, 2013). Therefore, the increase in RMFG after treatment may be indicative of engagement of domain general regions due to changes in language and/or domain general functions (e.g., attention, executive control).
Interestingly, RMFG’s homologue in the left hemisphere was positively activated both before and after treatment. As noted in section 4.4.2, above, LMFG was also one of the few regions in which there was a decrease in activation from pre- to post-treatment. Thus, while treatment appears to have encouraged the recruitment of a domain general region that was not especially engaged in the task before treatment (RMFG), it may have improved the functional efficiency of a different domain general region that was previously engaged in the task (LMFG). Future research should investigate the role of domain general regions in language recovery and the differences between treatment-related recruitment of regions that were not associated with a given task at baseline and modulation of those regions that were.
4.5. Spared tissue affects the magnitude of activation change in some left hemisphere regions
The follow-up analysis of the influence of spared tissue on activation in left hemisphere ROIs indicated that, for most regions, less spared tissue (i.e., more damage), was associated with a larger change in activation from pre- to post-treatment, while more spared tissue (i.e., less damage) was associated with a smaller change. This was the case in all ROIs except for LMTG, where the pre-/post- difference in activation was virtually the same regardless of the extent to which it was spared. We also found that the direction of activation change (i.e., increase or decrease) associated with various quantities of spared tissue was different for different regions. Specifically, activation increased after treatment irrespective of how much damage there was in IFGorb, SMG, and MTG. It also increased in AG and MFG, except when they were completely intact, and decreased in PCG, IFGtri, and IFGop, except when they were completely intact.
Prior studies indicate that perfusion and functional activation are abnormally reduced in the ipsilesional hemisphere, especially in tissue that is proximal to the lesion (de Haan, Rorden, & Karnath, 2013; Krainik, Hund-Georgiadis, Zysset, & von Cramon, 2005; Richardson et al., 2011; Robson et al., 2017). Therefore, a tentative explanation for the present results is that when regions are more highly damaged, they are more dysfunctional during task performance; this may necessitate a larger change in activation (i.e., a larger “correction” of abnormal function) in order for the region to successfully contribute to task performance after treatment. Conversely, regions that are wholly or mostly intact may function more optimally than those that are highly damaged, and therefore treatment may have a less dramatic effect on intact regions than highly damaged regions. This study is among the first to examine how the proportion of spared tissue in specific regions relates to treatment-related changes in activation in the same regions, so this interpretation is preliminary. Crucially, however, our results indicate that even highly damaged regions are amenable to functional changes in response to treatment.
Further, this is the first examination of treatment effects in a large sample of PWA (including an untreated patient control group) to detail activation changes in both hemispheres at the level of task-related ROIs while also using a novel method to address the effects of lesion variability. The results of the present study help explain the variability observed in previous treatment studies, including those outlined in Table II, by providing a nuanced but comprehensive explanation of factors that affect functional activation, and may serve as a promising foundation for updated models of treatment-induced neuroplasticity in chronic stroke aphasia.
5. Conclusion
This study demonstrated that semantic-based naming treatment resulted in improved naming accuracy and bilateral changes in neural function in a group of patients with chronic post-stroke aphasia. Those who responded best to treatment had a significant increase in cortical activation, while those who did not respond or did not undergo treatment showed neither behavioral improvement nor functional changes. Furthermore, responders and nonresponders had different levels of pre-treatment activation, but not post-treatment activation, which suggests that their divergent behavioral outcomes may have been associated with pre-existing differences in cortical function prior to the initiation of treatment. We also found that the magnitude of changes in activation from pre- to post-treatment in most left hemisphere ROIs varied as a function of the proportion of residual tissue therein. Finally, patients exhibited regional differences in activation relative to healthy controls, some of which were present before and after treatment and others that were present at just one point in time. This nuanced set of results provides insights into the effect of treatment on neural function and the mechanisms underlying naming improvement.
Acknowledgments
The authors extend their gratitude to all of the individuals who participated in this study. We also thank Stefano Cardullo, Yansang Geng, and Yorghos Tripodis for support and consultation on statistical and functional imaging analyses, and Jennifer Michaud, Natalie Gilmore, Kushal Kapse, Maria Dekhtyar, Kelly Martin, Brett McCardel, and Talia Raney for their assistance in data collection. This work was supported by NIH/NIDCD grants 1P50DC012283 and 1F31DC015940. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transparency and Openness
Informed consent documents for the present study did not include permissions for public archiving of data; thus, the data used in this study have not been made publicly available at the time of submission. However, anonymized data can be obtained via written request submitted to Swathi Kiran (kirans@bu.edu). Requests should include the requestor’s name, affiliation, and contact information, a list of desired data, and a description of the purpose for the request. R code for the primary statistical analyses reported in this study and experimental E-Prime scripts for the imaging and treatment tasks are available on Open Science Framework (https://osf.io/j23s5/). Associated picture stimuli were obtained via Internet image searches conducted in 2012-2013, prior to study initiation. Due to copyright restrictions, these pictures have not been publicly archived, but interested readers may contact Swathi Kiran (kirans@bu.edu) to request access to the stimuli or for guidance in generating a similar set of stimuli. As with data requests, stimuli requests should include the requestor’s name, affiliation, and contact information, as well as a description of the rationale for the request. This study was registered on ClinicalTrials.gov in August 2013 (ClincialTrials.gov identifier: ; https://clinicaltrials.gov/ct2/show/NCT01927302?cond=Aphasia&cntry=US&state=US%3AMA&rank=7). Some modifications to the originally-stated inclusion/exclusion criteria were subsequently made, allowing for the inclusion of patient participants who were at least six months removed from their stroke at enrollment and/or those who were pre-morbidly left handed (as long as they met the other criteria, including confirmation of aphasia following left-hemisphere stroke). The analyses utilized in this study were not pre-registered.
Conflicts of Interest
None of the authors have a financial conflict of interest with respect to the work reported here.
Inclusion/exclusion criteria were established prior to data collection and analysis and registered on ClinicalTrials.gov (identifier number ; https://clinicaltrials.gov/ct2/show/NCT01927302?cond=Aphasia&cntry=US&state=US%3AMA&rank=7) on August 22, 2013. However, some modifications of these criteria were subsequently allowed, as described in the Transparency and Openness section at the end of this manuscript.
Note that 36 pictures of items from participants’ experimental categories that were not trained during treatment were also presented in order to monitor for generalization effects from treatment; however, generalization is beyond the scope of the present study; thus, while these untrained items were modeled in first-level analyses, they were not analyzed further in the present study.
Extensive calibration of the scanners at each site was performed to equate their performance in data acquisition as part of the larger study by the Center for the Neurobiology of Language Recovery.
One TxPWA, BU23, was lost to follow-up after completing the immediate post-treatment testing and neuroimaging protocols.
Readers interested in the efficacy of this treatment and its capacity for generalization (i.e., improvements in untrained items, tasks, or contexts) are directed to Gilmore et al. (2018), which provides comprehensive behavioral outcomes for most participants in the present study, as well as group analyses indicating that treated patients experienced improvements on naming trained items, with generalization to untrained but semantically related items, measures of semantic and phonological processing, and standardized assessments of cognitive-linguistic functions.
References
- Abel S, Weiller C, Huber W, & Willmes K (2014). Neural underpinnings for model-oriented therapy of aphasic word production. Neuropsychologia, 57, 154–165. 10.1016/j.neuropsychologia.2014.03.010 [DOI] [PubMed] [Google Scholar]
- Abel S, Weiller C, Huber W, Willmes K, & Specht K (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138(4), 1097–1112. 10.1093/brain/awv022 [DOI] [PubMed] [Google Scholar]
- Anglade C, Thiel A, & Ansaldo AI (2014). The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: A critical review of literature. Brain Injury, 28(2), 138–145. 10.3109/02699052.2013.859734 [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Beeson PM, & Robey RR (2006). Evaluating single-subject treatment research: lessons learned from the aphasia literature. Neuropsychology Review, 16(4), 161–169. 10.1007/s11065-006-9013-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, & Desai RH (2011). The neurobiology of semantic memory. Trends in Cognitive Sciences, 15(11), 527–536. 10.1016/j.tics.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, & Medler DA (2005). Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience, 17(6), 905–917. 10.1162/0898929054021102 [DOI] [PubMed] [Google Scholar]
- Birn RM, Cox RW, & Bandettini PA (2004). Experimental designs and processing strategies for fMRI studies involving overt verbal responses. NeuroImage, 23(3), 1046–1058. 10.1016/j.neuroimage.2004.07.039 [DOI] [PubMed] [Google Scholar]
- Boyle M (2004). Semantic feature analysis treatment for anomia in two fluent aphasia syndromes. American Journal of Speech-Language Pathology, 13(3), 236 10.1044/1058-0360(2004/025) [DOI] [PubMed] [Google Scholar]
- Boyle M (2010). Semantic feature analysis treatment for aphasic word retrieval impairments: what’s in a name? Topics in Stroke Rehabilitation, 17(6), 411–422. 10.1310/tsr1706-411 [DOI] [PubMed] [Google Scholar]
- Boyle M, & Coelho CA (1995). Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology, 4(4), 94 10.1044/1058-0360.0404.94 [DOI] [Google Scholar]
- Breier JI, Maher LM, Schmadeke S, Hasan KM, & Papanicolaou AC (2007). Changes in language-specific brain activation after therapy for aphasia using magnetoencephalography: a case study. Neurocase, 13(3), 169–177. 10.1080/13554790701448200 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16(2), S497. [Google Scholar]
- Brett M, Leff AP, Rorden C, & Ashburner J (2001). Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage, 14(2), 486–500. 10.1006/nimg.2001.0845 [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, & Welch KMA (1999). Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke, 30(11), 2331–2340. [DOI] [PubMed] [Google Scholar]
- Cappa SF (2011). The neural basis of aphasia rehabilitation: Evidence from neuroimaging and neurostimulation. Neuropsychological Rehabilitation, 21(5), 742–754. 10.1080/09602011.2011.614724 [DOI] [PubMed] [Google Scholar]
- Coltheart M (1981). The MRC psycholinguistic database. The Quarterly Journal of Experimental Psychology Section A, 33(4), 497–505. 10.1080/14640748108400805 [DOI] [Google Scholar]
- Cornelissen K, Laine M, Tarkiainen A, Järvensivu T, Martin N, & Salmelin R (2003). Adult brain plasticity elicited by anomia treatment. Journal of Cognitive Neuroscience, 15(3), 444–461. 10.1162/089892903321593153 [DOI] [PubMed] [Google Scholar]
- Crinion JT, & Leff AP (2007). Recovery and treatment of aphasia after stroke: functional imaging studies: Current Opinion in Neurology, 20(6), 667–673. 10.1097/WCO.0b013e3282f1c6fa [DOI] [PubMed] [Google Scholar]
- Crosson B, McGregor K, Gopinath KS, Conway TW, Benjamin M, Chang Y-L, … White KD (2007). Functional mri of language in aphasia: a review of the literature and the methodological challenges. Neuropsychology Review, 17(2), 157–177. 10.1007/s11065-007-9024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson B, Moore AB, Gopinath K, White KD, Wierenga CE, Gaiefsky ME, … Rothi LJG (2005). Role of the right and left hemispheres in recovery of function during treatment of intention in aphasia. Journal of Cognitive Neuroscience, 17(3), 392–406. 10.1162/0898929053279487 [DOI] [PubMed] [Google Scholar]
- Crosson B, Moore AB, McGregor KM, Chang Y-L, Benjamin M, Gopinath K, … White KD (2009). Regional changes in word-production laterality after a naming treatment designed to produce a rightward shift in frontal activity. Brain and Language, 111(2), 73–85. 10.1016/j.bandl.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, & Jefferies E (2015). Automatic and controlled semantic retrieval: tms reveals distinct contributions of posterior middle temporal gyrus and angular gyrus. Journal of Neuroscience, 35(46), 15230–15239. 10.1523/JNEUROSCI.4705-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CH, Harrington G, & Baynes K (2006). Intensive semantic intervention in fluent aphasia: A pilot study with fMRI. Aphasiology, 20(1), 59–83. 10.1080/02687030500331841 [DOI] [Google Scholar]
- de Haan B, Rorden C, & Karnath H-O (2013). Abnormal perilesional BOLD signal is not correlated with stroke patients’ behavior. Frontiers in Human Neuroscience, 7 10.3389/fnhum.2013.00669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E, Duncan J, & Kanwisher N (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J (2003). Effect Displays in R for Generalised Linear Models. Journal of Statistical Software, 5(15). 10.18637/jss.v008.i15 [DOI] [Google Scholar]
- Fridriksson J (2010). Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30(35), 11558–11564. 10.1523/JNEUROSCI.2227-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Baker JM, & Moser D (2009). Cortical mapping of naming errors in aphasia. Human Brain Mapping, 30(8), 2487–2498. 10.1002/hbm.20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Morrow-Odom L, Moser D, Fridriksson A, & Baylis G (2006). Neural recruitment associated with anomia treatment in aphasia. Neuroimage, 32(3), 1403–1412. 10.1016/j.neuroimage.2006.04.194 [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Fillmore P, & Cai B (2012). Left hemisphere plasticity and aphasia recovery. Neuroimage, 60(2), 854–863. 10.1016/j.neuroimage.2011.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, & Kiran S (2018). Typicality-based semantic treatment for anomia results in multiple levels of generalisation. Neuropsychological Rehabilitation, 1–27. 10.1080/09602011.2018.1499533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, & Kertesz A (2000). Right hemisphere semantic processing of visual words in an aphasic patient: an fmri study. Brain and Language, 73(3), 456–465. 10.1006/brln.2000.2317 [DOI] [PubMed] [Google Scholar]
- Heiss W-D, & Thiel A (2006). A proposed regional hierarchy in recovery of post-stroke aphasia. Brain and Language, 98(1), 118–123. 10.1016/j.bandl.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Howard D, & Patterson KE (1992). The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Thames Valley Test Company. [Google Scholar]
- Humphreys GF, & Lambon Ralph MA (2015). Fusion and fission of cognitive functions in the human parietal cortex. Cerebral Cortex, 25(10), 3547–3560. 10.1093/cercor/bhu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GF, & Lambon Ralph MA (2017). Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: Evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cerebral Cortex, 27(8), 4199–4212. 10.1093/cercor/bhx107 [DOI] [PubMed] [Google Scholar]
- Indefrey P (2011). The spatial and temporal signatures of word production components: a critical update. Frontiers in Psychology, 2 10.3389/fpsyg.2011.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, & Levelt WJ (2004). The spatial and temporal signatures of word production components. Cognition, 92(1–2), 101–144. 10.1016/j.cognition.2002.06.001 [DOI] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, & Lambon Ralph MA (2016). The semantic network at work and rest: differential connectivity of anterior temporal lobe subregions. Journal of Neuroscience, 36(5), 1490–1501. 10.1523/JNEUROSCI.2999-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (2001). Boston Naming Test (2nd ed.). Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Kertesz A (2007). Western Aphasia Battery - Revised. San Antonio, TX: Psych Corp. [Google Scholar]
- Kiran S (2008). Typicality of inanimate category exemplars in aphasia treatment: further evidence for semantic complexity. Journal of Speech Language and Hearing Research, 51(6), 1550 10.1044/1092-4388(2008/07-0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Ansaldo A, Bastiaanse R, Cherney LR, Howard D, Faroqi-Shah Y, … Thompson CK (2013). Neuroimaging in aphasia treatment research: Standards for establishing the effects of treatment. NeuroImage, 76, 428–435. 10.1016/j.neuroimage.2012.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, & Bassetto G (2008). Evaluating the effectiveness of semantic-based treatment for naming deficits in aphasia: what works? Seminars in Speech and Language, 29(1), 071–082. 10.1055/s-2008-1061626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, & Johnson L (2008). Semantic complexity in treatment of naming deficits in aphasia: evidence from well-defined categories. American Journal of Speech-Language Pathology, 17(4), 389 10.1044/1058-0360(2008/06-0085) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, Meier EL, Kapse KJ, & Glynn PA (2015). Changes in task-based effective connectivity in language networks following rehabilitation in post-stroke patients with aphasia. Frontiers in Human Neuroscience, 9 10.3389/fnhum.2015.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiran S, & Thompson CK (2003). The role of semantic complexity in treatment of naming deficits: training semantic categories in fluent aphasia by controlling exemplar typicality. Journal of Speech Language and Hearing Research, 46(4), 773 10.1044/1092-4388(2003/061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krainik A, Hund-Georgiadis M, Zysset S, & von Cramon DY (2005). Regional impairment of cerebrovascular reactivity and bold signal in adults after stroke. Stroke, 36(6), 1146–1152. 10.1161/01.STR.0000166178.40973.a7 [DOI] [PubMed] [Google Scholar]
- Krieger-Redwood K, & Jefferies E (2014). TMS interferes with lexical-semantic retrieval in left inferior frontal gyrus and posterior middle temporal gyrus: Evidence from cyclical picture naming. Neuropsychologia, 64, 24–32. 10.1016/j.neuropsychologia.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, & Christensen RHB (2016). Lmertest: tests in linear mixed effects models. (Version R package version 2.0-32). Retrieved from https://CRAN.R-project.org/package=lmerTest
- Léger A, Démonet J-F, Ruff S, Aithamon B, Touyeras B, Puel M, … Cardebat D (2002). Neural substrates of spoken language rehabilitation in an aphasic patient: an fmri study. NeuroImage, 17(1), 174–183. 10.1006/nimg.2002.1238 [DOI] [PubMed] [Google Scholar]
- Lenth R (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. Retrieved from https://CRAN.R-project.org/package=emmeans
- Leonard C, Rochon E, & Laird L (2008). Treating naming impairments in aphasia: Findings from a phonological components analysis treatment. Aphasiology, 22(9), 923–947. 10.1080/02687030701831474 [DOI] [Google Scholar]
- Lüdecke D (2018). sjPlot: Data Visualization for Statistics in Social Science. Retrieved from https://CRAN.R-project.org/package=sjPlot
- Marcotte K, Adrover-Roig D, Damien B, de Préaumont M, Généreux S, Hubert M, & Ansaldo AI (2012). Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia, 50(8), 1776–1786. 10.1016/j.neuropsychologia.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Marcotte K, & Ansaldo A (2010). The neural correlates of semantic feature analysis in chronic aphasia: discordant patterns according to the etiology. Seminars in Speech and Language, 37(01), 052–063. 10.1055/s-0029-1244953 [DOI] [PubMed] [Google Scholar]
- Meinzer M, Beeson PM, Cappa S, Crinion J, Kiran S, Saur D, … Thompson CK (2013). Neuroimaging in aphasia treatment research: Consensus and practical guidelines for data analysis. Neuroimage, 73, 215–224. 10.1016/j.neuroimage.2012.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, & Rockstroh B (2008). Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. Neuroimage, 39(4), 2038–2046. 10.1016/j.neuroimage.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Meinzer M, Flaisch T, Obleser J, Assadollahi R, Djundja D, Barthel G, & Rockstroh B (2006). Brain regions essential for improved lexical access in an aged aphasic patient: a case report. BMC Neurology, 6(1). 10.1186/1471-2377-6-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Postman-Caucheteux WA, McArdle JJ, & Braun AR (2009). Strategies for longitudinal neuroimaging studies of overt language production. Neuroimage, 47(2), 745–755. 10.1016/j.neuroimage.2009.04.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke R, Meinzer M, Kugel H, Deppe M, Baumgärtner A, Schiffbauer H, … Breitenstein C (2009). Imaging short- and long-term training success in chronic aphasia. BMC Neuroscience, 10(1), 118 10.1186/1471-2202-10-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Theoret H, Kobayashi M, Fregni F, Nicholas M, … Pascual-Leone A (2011). TMS suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain and Language, 119(3), 206–213. 10.1016/j.bandl.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin P, Nicholas M, Baker E, Seekins H, Kobayashi M, … Kurland J (2005). Improved picture naming in chronic aphasia after TMS to part of right Broca?s area: An open-protocol study. Brain and Language, 93(1), 95–105. 10.1016/j.bandl.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Nardo D, Holland R, Leff AP, Price CJ, & Crinion JT (2017). Less is more: neural mechanisms underlying anomia treatment in chronic aphasic patients. Brain, 140(11), 3039–3054. 10.1093/brain/awx234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels L (2002). Therapy for naming disorders: Revisiting, revising, and reviewing. Aphasiology, 16(10–11), 935–979. 10.1080/02687030244000563 [DOI] [Google Scholar]
- Noonan KA, Jefferies E, Visser M, & Lambon Ralph MA (2013). Going beyond inferior prefrontal involvement in semantic control: evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. Journal of Cognitive Neuroscience, 25(11), 1824–1850. 10.1162/jocn_a_00442 [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, & Gabrieli JDE (1999). Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage, 10(1), 15–35. 10.1006/nimg.1999.0441 [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, … Braun AR (2010). Single-trial fmri shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience, 22(6), 1299–1318. 10.1162/jocn.2009.21261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191(1), 62–88. 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, & Crinion J (2005). The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology, 18(4), 429–434. [DOI] [PubMed] [Google Scholar]
- Psychology Software Tools, Inc. [E-Prime 2.0]. (2012). Retrieved from http://www.pstnet.com.
- Raboyeau G, De Boissezon X, Marie N, Balduyck S, Puel M, Bezy C, … Cardebat D (2008). Right hemisphere activation in recovery from aphasia Lesion effect or function recruitment? Neurology, 70(4), 290–298. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, & Fridriksson J (2011). Cerebral perfusion in chronic stroke: Implications for lesion-symptom mapping and functional MRI. Behavioural Neurology, (2), 117–122. 10.3233/BEN-2011-0283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Miltner WHR, & Straube T (2008). Association between therapy outcome and right-hemispheric activation in chronic aphasia. Brain, 131(5), 1391–1401. 10.1093/brain/awn043 [DOI] [PubMed] [Google Scholar]
- Robson H, Specht K, Beaumont H, Parkes LM, Sage K, Lambon Ralph MA, & Zahn R (2017). Arterial spin labelling shows functional depression of non-lesion tissue in chronic Wernicke’s aphasia. Cortex, 92, 249–260. 10.1016/j.cortex.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochon E, Leonard C, Burianova H, Laird L, Soros P, Graham S, & Grady C (2010). Neural changes after phonological treatment for anomia: An fMRI study. Brain and Language, 114(3), 164–179. 10.1016/j.bandl.2010.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, & Brett M (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12(4), 191–200. 10.1155/2000/421719 [DOI] [PubMed] [Google Scholar]
- RStudio Team. (2015). RStudio: Integrated Development for R. Boston, MA: RStudio, Inc. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Sebastian R, & Kiran S (2011). Task-modulated neural activation patterns in chronic stroke patients with aphasia. Aphasiology, 25(8), 927–951. 10.1080/02687038.2011.557436 [DOI] [Google Scholar]
- Seghier ML (2013). The angular gyrus multiple functions and multiple subdivisions. The Neuroscientist, 19(1), 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, & Price CJ (2010). Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. Journal of Neuroscience, 30(50), 16809–16817. 10.1523/JNEUROSCI.3377-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JA, Kapse K, Glynn P, Sandberg C, Tripodis Y, & Kiran S (2016). The relationships between the amount of spared tissue, percent signal change, and accuracy in semantic processing in aphasia. Neuropsychologia, 84, 113–126. 10.1016/j.neuropsychologia.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper-Kallal LM, Lacey EH, Xing S, & Turkeltaub PE (2017). Right hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Neural Plasticity, 2017, 1–17. 10.1155/2017/8740353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, & den Ouden D-B (2008). Neuroimaging and recovery of language in aphasia. Current Neurology and Neuroscience Reports, 8(6), 475–483. 10.1007/s11910-008-0076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, & Farah MJ (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America, 94(26), 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Messing S, Norise C, & Hamilton RH (2011). Are networks for residual language function and recovery consistent across aphasic patients? Neurology, 76(20), 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wouden T (1990). Celex: Building a multifunctional polytheoretical lexical data base. In T. Magay & J. Zigány (Eds.), BudaLEX ‘88 proceedings: papers from the 3rd International EURALEX Congress, Budapest, 4-9 September 1988 (pp. 363–373). Budapest: Akadémiai Kiadó. [Google Scholar]
- van Hees S, Angwin A, McMahon K, & Copland D (2013). A comparison of semantic feature analysis and phonological components analysis for the treatment of naming impairments in aphasia. Neuropsychological Rehabilitation, 23(1), 102–132. 10.1080/09602011.2012.726201 [DOI] [PubMed] [Google Scholar]
- van Hees S, McMahon K, Angwin A, de Zubicaray G, & Copland DA (2014). Neural activity associated with semantic versus phonological anomia treatments in aphasia. Brain and Language, 129, 47–57. 10.1016/j.bandl.2013.12.004 [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, … Tzourio-Mazoyer N (2006). Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage, 30(4), 1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé P-Y, Jobard G, Petit L, Crivello F, … Tzourio-Mazoyer N (2011). What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? NeuroImage, 54(1), 577–593. 10.1016/j.neuroimage.2010.07.036 [DOI] [PubMed] [Google Scholar]
- Vitali P, Abutalebi J, Tettamanti M, Danna M, Ansaldo A-I, Perani D, … Cappa SF (2007). Training-induced brain remapping in chronic aphasia: a pilot study. Neurorehabilitation and Neural Repair, 21(2), 152–160. 10.1177/1545968306294735 [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, & Shinkareva SV (2010). Neural representation of abstract and concrete concepts: A meta-analysis of neuroimaging studies. Human Brain Mapping, 31(10), 1459–1468. 10.1002/hbm.20950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, & Jefferies E (2011). The neural organization of semantic control: tms evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex, 21(5), 1066–1075. 10.1093/cercor/bhq180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph MA, & Jefferies E (2012). Executive semantic processing is underpinned by a large-scale neural network: revealing the contribution of left prefrontal, posterior temporal, and parietal cortex to controlled retrieval and selection using tms. Journal of Cognitive Neuroscience, 24(1), 133–147. 10.1162/jocn_a_00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; Retrieved from http://ggplot2.org [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, & Heiss WD (2005). Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke, 36(8), 1759–1763. 10.1161/01.STR.0000174487.81126.ef [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, & Heiss WD (2007). The right inferior frontal gyrus and poststroke aphasia: a follow-up investigation. Stroke, 38(4), 1286–1292. 10.1161/01.STR.0000259632.04324.6c [DOI] [PubMed] [Google Scholar]
- Wisenburn B, & Mahoney K (2009). A meta-analysis of word-finding treatments for aphasia. Aphasiology, 23(11), 1338–1352. 10.1080/02687030902732745 [DOI] [Google Scholar]