Abstract
Delayed reward discounting (DRD) is a behavioral economic measure of impulsivity, reflecting how rapidly a reward loses value based on its temporal distance. In humans, more impulsive DRD is associated with susceptibility to a number of psychiatric diseases (e.g., addiction, ADHD), health outcomes (e.g., obesity), and lifetime outcomes (e.g., educational attainment). Although the determinants of DRD are both genetic and environmental, this review focuses on its genetic basis. Both rodent studies using inbred strains and human twin studies indicate that DRD is moderately heritable, a conclusion that was further supported by a recent human genome-wide association study (GWAS) that used single nucleotide polymorphisms (SNP) to estimate heritability. The GWAS of DRD also identified genetic correlations with psychiatric diagnoses, health outcomes, and measures of cognitive performance. Future research priorities include rodent studies probing putative genetic mechanisms of DRD and human GWASs using larger samples and non-European cohorts. Continuing to characterize genomic influences on DRD has the potential to yield important biological insights with implications for a variety of medically and socially important outcomes.
Keywords: delayed reward discounting, impulsivity, genetics, genomics
1. Introduction
The preference for smaller immediate rewards relative to larger delayed rewards is a behavioral economic concept that reflects the capacity to delay gratification (Green et al., 1994). Delayed reward discounting (DRD) is used to measure how rapidly a reward loses its value based on its temporal distance. Thus, greater DRD reflects a preference for smaller immediate rewards rather than larger, delayed rewards and is one form of impulsivity. Meta-analyses show consistent associations between greater DRD and adverse psychiatric outcomes including substance use disorders, gambling disorder, and attention-deficit/hyperactivity disorder (ADHD) (Amlung et al., 2016a; Jackson & MacKillop, 2016; MacKillop et al., 2011). In terms of nonpsychiatric health outcomes, greater DRD is positively associated with obesity (Amlung et al., 2016b), and negatively associated with glycemic adherence in type 2 diabetes (Lebeau et al., 2016; Reach et al., 2011), obtaining preventative medical care (e.g., flu shots, breast and prostate exams; (Bradford, 2010)), and seatbelt use (Bradford et al., 2014). Finally, even after attempting to control for parental income and cognitive ability, DRD is negatively associated with lifetime outcomes including educational attainment, income, and employment (Golsteyn et al., 2014). Individuals with high DRD appear to be less thoughtful of their future selves, which leads to increased risks for a multitude of deleterious mental, physical, and social outcomes. As such, DRD has been proposed as a target for treatment (Gray & MacKillop, 2015; Lowe et al., 2018; Sheffer et al., 2018) and is one component of the Research Domain Criteria (RDoC) (Lempert et al., 2018), a National Institute of Mental Health (NIMH) initiative that emphasizes basic dimensions of functioning that span the full range of human behavior from normal to abnormal.
This mini-review will highlight current research relating to the genetic basis of DRD, including data from animal models. We begin with a summary of DRD measurement in humans and nonhuman animals, followed by a review of findings from heritability and genome-wide association studies (GWASs). We conclude our review by identifying promising future research directions. We will not review the many candidate gene studies that have been conducted on this topic, in part because of the consistent difficulty in replicating candidate loci for complex traits (Chabris et al., 2012; Farrell et al., 2015; Hart et al., 2013), and because candidate genes for DRD have been summarized in two of our recent publications (MacKillop et al., 2019; Sanchez-Roige et al., 2018).
Like all psychological traits, DRD is influenced by environmental and genetic factors and presumably also their many interactions. With regard to environmental influences, research indicates that child maltreatment (Oshri et al., 2018a, 2018b), trauma (van den Berk-Clark et al., 2018), and substance use (Mendez et al., 2010; Mitchell et al., 2014; Setlow et al., 2009; Simon et al., 2007) appear to increase levels of DRD. While no-well powered studies have investigated gene-by-environment interactions relevant to DRD, it is likely that certain environmental exposures modulate DRD in a genotype-specific manner. Thus, while this mini-review is focused on the genetic basis of DRD, research seeking to understand environmental and gene-by-environment interactions also represent important lines of inquiry.
2. Delayed Reward Discounting Measurement
DRD is typically assessed by providing organisms with a choice between smaller immediate and larger delayed rewards. In humans, these rewards are usually choices between smaller amounts of money today versus larger amounts of money after a delay, though food and drugs have been in place of money (Green and Lawyer, 2014; Odum and Rainaud, 2003; Robertson and Rasmussen, 2018). For example, one of the most widely-used measures, the Monetary Choice Questionnaire (MCQ), consists of 27 questions such as “Would you rather have $24 today or $35 in 29 days?” (Gray et al., 2016; Kirby et al., 1999). Although the rewards are typically hypothetical rather than real, this does not appear to impact responding (Madden et al., 2003; Matusiewicz et al., 2013; Robertson & Rasmussen, 2018). In animals such as pigeons and rodents, DRD is typically assessed using delayed food or water rewards and the animals always receive the rewards associated with their choice (Isles et al., 2004; Mazur, 1987; Mitchell, 2014; Richards et al., 2013).
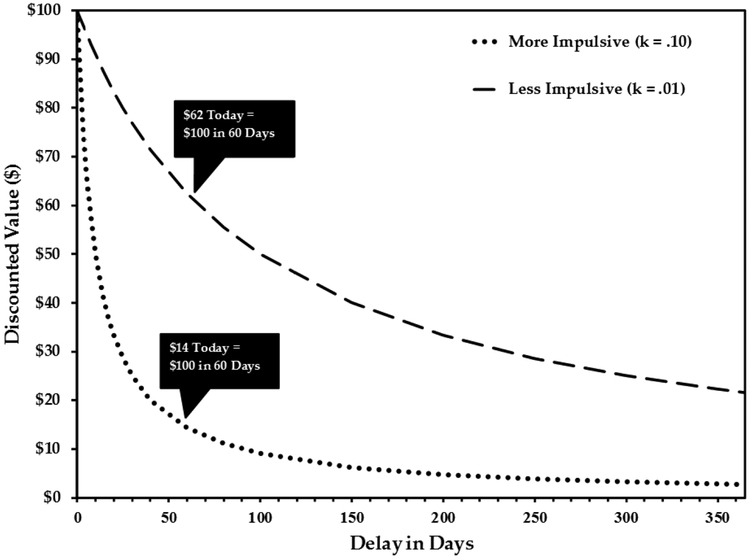
In both humans and non-human species, organisms typically devalue delayed rewards in a nonlinear fashion, modeled as a hyperbolic function (Vanderveldt et al., 2016). The extent of DRD can be quantified in several ways (Myerson et al., 2014), such as calculating the slope of the hyperbolic discounting function (k) or model-free methods such as area under the curve and immediate choice ratio (Green & Myerson, 2004; Myerson et al., 2001). Figure 1 shows two prototypic hyperbolic demand curves in humans with differing slopes (more impulsive k = .1, less impulsive k = .01) that exhibit the discounted subjective value of $100 delayed from 1 day to 1 year. For example, at 60 days, $100 is equal in subjective value to $62 today for the less impulsive profile and $14 today for the more impulsive profile.
Figure 1.
Prototypic hyperbolic delayed reward discounting curves. The curves reflect the discounted subjective value of $100 delayed from 1 day to 1 year. The k values refer to the slopes of the two discounting curves. The k values are derived from the Mazur (1987) equation: V = A/(1 + kD), where V is the present value of the delayed reward A at delay D, and k is a free parameter that determines the discount rate. The higher one’s discount rate (k) is, the more they discount larger future rewards.
Although there are many parallels between the DRD models used with humans and laboratory animals, there are also several notable differences that may affect generalizability across species (for an in depth discussion see Vanderveldt et al., 2016). First, in humans there is a well-documented magnitude effect, whereby humans discount small, delayed rewards more steeply than larger delayed rewards. This effect has been shown across reward types including money (Johnson and Bickel, 2002; Madden et al., 2003), food (Odum et al., 2006), and liquid rewards (Jimura et al., 2009). However, the magnitude effect has not been consistently observed in nonhuman animals (e.g., Green et al., 2004; Richards et al., 1997). Second, the time frame of the procedures, and presumably the time frame for self-control, differs in humans and laboratory animals. In the animal procedures the delays are in seconds or minutes whereas in most human procedures the delays are days to months. Moreover, in the animal procedures, the delays are experienced directly and relate to their immediate thirst or hunger, whereas in humans the delays are communicated by instructions and typically involve a secondary reinforcer (money) (de Wit et al., 2018). Nonetheless, both humans and laboratory animals discount delayed rewards in an orderly manner, suggesting a fundamental behavioral homology.
3. Heritability
The heritability of DRD has been examined in both humans and rodents. In humans, studies with monozygotic and dizygotic adolescent twins provide evidence of robust heritability, which tends to increase through development (i.e. 12 years old (yo) [30%] and 14 yo [51%], (Anokhin et al., 2011); 16 yo [35–46%], 17 yo [47–51%], and 18 yo [55–62%] (Anokhin et al., 2015; Isen et al., 2014; Sparks et al., 2014)). The increase in genetic influence on DRD throughout development may reflect the changing importance of competing environmental factors and the maturation of the prefrontal cortex in adolescence (Argyriou et al., 2018), a critical region for DRD (Wesley & Bickel, 2014).
In mice and rats, a significant proportion of the variance in DRD can be attributed to between-strain versus within-strain differences (16–50%), which is analogous to the twin model design (Anderson & Woolverton, 2005; Isles et al., 2004; Madden et al., 2008; Richards et al., 1997; Stein et al., 2012; Wilhelm and Mitchell, 2009). The lowest estimate (16%) came from the only study with mice conducted to date (Isles et al., 2004), whereas estimates of heritability in rats were much higher (40–50%) (Richards et al., 2013; Wilhelm and Mitchell, 2009). However, comparisons across strains of rodents have some limitations. First, strains were sometimes obtained from different vendors and thus genotype and the different environment of each vendor facility are confounded. Second, studies vary with regard to training procedure, type of reinforcer (e.g., condensed milk, water), delay range (e.g., 8 vs. 16 seconds maximum delay), number of sessions, and dependent variable (e.g., ratio of delayed choices, AUC, k). On balance, findings from both humans and rodents suggest that DRD is a moderately heritable trait, although the variability in estimates suggests significant moderators of its heritability.
4. Genome-wide Association Studies
A GWAS is a study of a set of genetic variants sampled across the whole genome to identify polymorphisms associated with a trait (Visscher et al., 2017). The primary goal of GWAS is to better understand the biology of the trait. Because millions of variants are tested, a stringent significance testing threshold must be employed. It is generally accepted that the significance threshold for any single polymorphism is p < 5 × 10−8. This threshold accounts for an estimated 1 million independent tests, and variants beyond this threshold tend to replicate (McCarthy et al., 2008; Visscher et al., 2017). Over the past decade, it has become clear that for virtually all common traits, associations tend to be numerous small-effect variants spread across most of the genome, in or near genes that have no obvious biological connection to the trait (e.g., Boyle et al., 2017). Nonetheless, GWASs are thought to yield new insights into the biology of complex traits (Visscher et al., 2017) and ultimately facilitate the discovery of novel treatments (Cook et al., 2014; Nelson et al., 2015).
To date, two GWASs have been conducted on DRD. The first was conducted in collaboration with the genetics company 23andMe, Inc., and included 23,217 adults of European ancestry (Sanchez-Roige et al., 2018). This study found single nucleotide polymorphism (SNP)-based heritability of DRD of 12.2%. This SNP-based heritability is lower than heritability estimates obtained using human twins and rodent inbred strains for a number of reasons, including that the SNP-based heritability is an underestimation due to the absence of rare variants (Marouli et al., 2017; Yang et al., 2015), and that pedigree estimates are inflated due to shared environmental and non-additive genetic effects (Polderman et al., 2015). In Sanchez-Roige et al., (2018), one SNP, rs6528024, which is located in an intron of the gene GPM6B (Neuronal Membrane Glycoprotein M6B), reached genome-wide significance (p = 2.40 × 10−8). This association was supported by an independent cohort of 928 participants (meta-analysis p = 1.44 × 10−8).
GPM6B encodes a protein that is involved in the internalization of the serotonin transporter and has been implicated in prepulse inhibition and altered response to the 5-HT2A/C agonist DOI in mice (Dere et al., 2015; Fjorback et al., 2009). A large body of research has explored the relationship between serotonergic functioning and DRD; the findings are inconsistent and have primarily relied on rodent models. For example, there is some evidence that serotonin may be more related to increased confidence in reward delivery than to increased capacity to wait for a delayed reward (Dalley and Ersche, 2019; Miyazaki et al., 2018). In humans, GPM6B expression is downregulated in the brains of depressed suicide victims (Fuchsova et al., 2015) and DRD has been linked to suicide attempts with a pooled odds ratio = 3.14 (95% confidence interval: 1.48–6.67) (Liu et al., 2017). The link between DRD and suicidality is further supported by genetic correlations identified in the study by Sanchez-Roige et al (2018), which found positive genetic correlations between DRD and major depression and neuroticism as well as smoking behaviors, ADHD, BMI, and negative associations with years of education and childhood IQ.
The second DRD GWAS used a sample of 986 healthy young adults of European ancestry (MacKillop et al., 2019). That study identified a genome-wide significant variant (p = 2.8 × 10−8), rs13395777, on chromosome 2, an association that was not observed in the 23andMe cohort (p = .45). There are two most likely explanations for this failure to replicate. The finding may have been a false positive, which would explain why it was not detected in a cohort that was ~25x larger. Alternatively, the smaller study was comprised of young adults and required low levels of substance use, whereas the larger study included a wider age range, resulting in substantially higher mean age and income, and allowed for psychopathology.
5. Future Directions
DRD is a moderately heritable phenotype that is both phenotypically and genetically associated with an array of negative psychological, cognitive, and health outcomes. The largest GWAS to date identified a single locus that was associated with DRD and showed that genetic predisposition to high DRD is positively genetically correlated with many of the negative outcomes that have been previously associated with higher DRD. Future studies will be required to further define the genetic basis of DRD. We are currently using rodents with mutations in GPM6B to examine DRD and related behavioral traits. We are also continuing to increase the sample size for future GWASs of DRD, which may allow us to identify additional loci (Marouli et al., 2017; Visscher et al., 2017). Another future direction may be to study diverse ancestral groups, expanding current data from individuals of European ancestry (Duncan et al., 2018; Locke et al., 2015). Additionally, it will be important to further parse causality between DRD and associated outcomes (e.g., addiction, years of education) using methods such as longitudinal designs and Mendelian randomization (Burgess et al., 2015; Grant and Chamberlain, 2014). Finally, DRD is only one element of impulsivity, which is a broader construct that appears to comprise three broad and generally independent domains (MacKillop et al., 2016). Thus, understanding the genetics of impulsivity will also require exploration of other measures of impulsivity (e.g., response inhibition and impulsive personality traits; Gray et al., 2018; Sanchez-Roige et al., 2019; Weafer et al., 2017).
6. Conclusion
DRD is a heritable trait that can be assessed quickly and reliably both in person and over the internet (Koffarnus & Bickel, 2014; Sanchez-Roige et al., 2018; MacKillop et al., 2018), and influences a variety of health-related outcomes. Although at an early stage, GWASs have begun to identify loci and genes that influence variability in DRD, setting the stage for a deeper understanding of its molecular, cellular and circuit-level bases, and perhaps ultimately informing the treatment of psychiatric disorders and other conditions to which it confers risk.
Highlights.
Delayed reward discounting (DRD) is a measure of capacity to delay gratification.
DRD is moderately heritable and associated with mental, physical, and social outcomes.
DRD is a component of Research Domain Criteria and a putative target for treatment.
The largest GWAS to date yielded a SNP heritability of 12% and one significant SNP.
Future priorities include GWAS with larger samples and non-European cohorts.
Funding:
HDW was supported by the National Institutes of Health [NIH; DA032015, DA02812, DA037011]; JM was supported by the NIH [R01AA025911, R01AA025849, R01AA024930] and the Peter Boris Chair in Addictions Research; SSR was supported by the Frontiers of Innovation Scholars Program [FISP; 3-P3029], the Interdisciplinary Research Fellowship in NeuroAIDS [IRFN; MH081482], and a pilot award from the NIH [DA037844]; SSR and AAP were supported by the California Tobacco-Related Disease Research Program [TRDRP; 28IR-0070] and NIH [P50DA037844].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
References
- 1.Amlung MT, Vedelago L, Acker J, Balodis I, MacKillop J, 2016a. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction 112, 51–62. 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amlung MT, Petker T, Jackson J, Balodis I, MacKillop J, 2016b. Steep discounting of delayed monetary and food rewards in obesity: A meta-analysis. Psychol. Med 46, 2423–2434. 10.1017/s0033291716000866 [DOI] [PubMed] [Google Scholar]
- 3.Anderson KG, Woolverton WL, 2005. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol. Biochem. Behav 80, 387–393. https://doi.org/10.10167j.pbb.2004.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anokhin AP, Golosheykin S, Grant JD, Heath AC, 2011. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav. Genet 41, 175–183. 10.1007/s10519-010-9384-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anokhin AP, Grant JD, Mulligan RC, Heath AC, 2015. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol. Psychiatry 77, 887–894. 10.1016/j.biopsych.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argyriou E, Um M, Carron C, Cyders MA, 2018. Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacol. Biochem. Behav 164, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnau-Soler A, Adams MJ, Clarke TK, MacIntyre DJ, Milburn K, Navrady L, Hayward C, McIntosh A, Thomson PA, 2019. A validation of the diathesis-stress model for depression in Generation Scotland. Transl. Psychiatry 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle EA, Li YI, Pritchard JK, 2017. An expanded view of complex traits: From polygenic to omnigenic. Cell 169, 1177–1186. 10.1016/J.CELL.2017.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford WD, 2010. The association between individual time preferences and health maintenance habits. Med. Decis. Mak 30, 99–112. 10.1177/0272989X09342276 [DOI] [PubMed] [Google Scholar]
- 10.Bradford WD, Courtemanche C, Heutel G, McAlvanah P, 2014. Time preferences and consumer behavior. Natl. Bur. Econ. Res. Work. Pap. Ser [Google Scholar]
- 11.Burgess S, Scott RA, Timpson NJ, Smith GD, Thompson SG, Consortium., E.-I., 2015. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol 30, 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabris CF, Hebert BM, Benjamin DJ, Beauchamp J, Cesarini D, van der Loos M, Johannesson M, Magnusson PKE, Lichtenstein P, Atwood CS, Freese J, Hauser TS, Hauser RM, Christakis N, Laibson D, 2012. Most reported genetic associations with general intelligence are probably false positives. Psychol. Sci 23, 1314–1323. 10.1177/0956797611435528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colodro-Conde L, Couvy-Duchesne B, Zhu G, Coventry WL, Byrne EM, Gordon S, Wright MJ, Montgomery GW, Madden PA, Ripke S, Eaves LJ, 2018. A direct test of the diathesis–stress model for depression. Mol. Psychiatry 23, 1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook D, Brown D, Alexander R, March R, Morgan P, Satterthwaite G, Pangalos MN, 2014. Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov. 13, 419–431. 10.1038/nrd4309 [DOI] [PubMed] [Google Scholar]
- 15.Dalley JW, Ersche KD, 2019. Neural circuitry and mechanisms of waiting impulsivity: relevance to addiction. Philos. Trans. R. Soc. B Biol. Sci 374, 20180145 10.1098/rstb.2018.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wit H, Epstein DH, Preston KL, 2018. Does human language limit translatability of clinical and preclinical addiction research? Neuropsychopharmacology 43, 1985–1988. 10.1038/s41386-018-0095-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dere E, Winkler D, Ritter C, Ronnenberg A, 2015. Gpm6b deficiency impairs sensorimotor gating and modulates the behavioral response to a 5-HT2A/C receptor agonist. Behav. Brain Res. 277, 254–263. [DOI] [PubMed] [Google Scholar]
- 18.Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, Beckham JC, Bierut LJ, Bisson J, Bradley B, 2018. Largest GWAS of PTSD (N= 20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol. Psychiatry 23, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell MS, Werge T, Sklar P, Owen MJ, Ophoff RA, O’Donovan MC, Corvin A, Cichon S, Sullivan PF, 2015. Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry 20, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fjorback AW, Müller HK, Wiborg O, 2009. Membrane Glycoprotein M6B interacts with the human serotonin transporter. J. Mol. Neurosci 37, 191–200. 10.1007/s12031-008-9092-4 [DOI] [PubMed] [Google Scholar]
- 21.Fuchsova B, Alvarez Juliá A, Rizavi HS, Frasch AC, Pandey GN, 2015. Altered expression of neuroplasticity-related genes in the brain of depressed suicides. Neuroscience 299, 1–17. 10.1016/j.neuroscience.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golsteyn BHH, Grönqvist H, Lindahl L, 2014. Adolescent Time Preferences Predict Lifetime Outcomes. Econ. J 124, F739–F761. 10.1111/ecoj.12095 [DOI] [Google Scholar]
- 23.Grant JE, Chamberlain SR, 2014. Impulsive action and impulsive choice across substance and behavioral addictions: cause or consequence? Addict. Behav 39, 1632–1639. [DOI] [PubMed] [Google Scholar]
- 24.Gray JC, Amlung MT, Palmer AA, MacKillop J, 2016. Syntax for calculation of discounting indices from the monetary choice questionnaire and probability discounting questionnaire. J. Exp. Anal. Behav 106 10.1002/jeab.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray JC, MacKillop J, 2015. Impulsive delayed reward discounting as a genetically-influenced target for drug abuse prevention: a critical evaluation. Front. Psychol 6, 1104 10.3389/fpsyg.2015.01104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray JC, MacKillop J, Weafer J, Hernandez KM, Gao J, Palmer AA, de Wit H, 2018. Genetic analysis of impulsive personality traits: Examination of a priori candidates and genome-wide variation. Psychiatry Res. 259 10.1016/j.psychres.2017.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green L, Fry AF, Myerson J, 1994. Discounting of delayed rewards: A life-span comparison. Psychol. Sci 5, 33–36. 10.1111/j.1467-9280.1994.tb00610.x [DOI] [Google Scholar]
- 28.Green L, Myerson J, 2004. A discounting framework for choice with delayed and probabilistic rewards. Psychol. Bull 130, 769–792. 10.1037/0033-2909.130.5.769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green L, Myerson J, Holt DD, Slevin JR, Estle SJ, 2004. Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? J. Exp. Anal. Behav 81, 39–50. 10.1901/jeab.2004.81-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green RM, Lawyer SR, 2014. Steeper delay and probability discounting of potentially real versus hypothetical cigarettes (but not money) among smokers. Behav. Processes 108, 50–56. [DOI] [PubMed] [Google Scholar]
- 31.Hart AB, de Wit H, Palmer AA, 2013. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology 38, 802–816. 10.1038/npp.2012.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isen JD, Sparks JC, Iacono WG, 2014. Predictive validity of delay discounting behavior in adolescence: A longitudinal twin study. Exp. Clin. Psychopharmacol 22, 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isles AR, Humby T, Walters E, Wilkinson LS, 2004. Common genetic effects on variation in impulsivity and activity in mice. J. Neurosci 24, 6733–6740. 10.1523/JNEUROSCI.1650-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson JNS, MacKillop J, 2016. Attention deficit hyperactivity disorder and monetary delay discounting: A meta-analysis of case-control studies. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 316–325. 10.1016/j.bpsc.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimura K, Myerson J, Hilgard J, Braver TS, Green L, 2009. Are people really more patient than other animals? Evidence from human discounting of real liquid rewards. Psychon. Bull. Rev 16, 1071–1075. 10.3758/PBR.16.6.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson MW, Bickel WK, 2002. Within-subject comparison of real and hypothetical money rewards in delay discounting. J. Exp. Anal. Behav 77, 129–146. 10.1901/jeab.2002.77-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirby KN, Petry NM, Bickel WK, 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol 128, 78–87. 10.1037/0096-3445.128.L78 [DOI] [PubMed] [Google Scholar]
- 38.Koffarnus MN, Bickel W, 2014. A 5-trial adjusting delay discounting task: Accurate discount rates in less than one minute. Exp. Clin. Psychopharmacol 22, 222–228. 10.1037/a0035973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lebeau G, Consoli SM, Bouc RL, Sola-Gazagnes A, Hartemann A, Simon D, Reach G, Altman J-J, Pessiglione M, Limosin F, Lemogne C, 2016. Delay discounting of gains and losses, glycemic control and therapeutic adherence in type 2 diabetes. Behav. Processes 132, 42–48. 10.1016/J.BEPR0C.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB, 2018. Can delay discounting deliver on the promise of RDoC? Psychol. Med 1–10. 10.1017/S0033291718001770 [DOI] [PubMed] [Google Scholar]
- 41.Liu RT, Trout ZM, Hernandez EM, Cheek SM, Gerlus N, 2017. A behavioral and cognitive neuroscience perspective on impulsivity, suicide, and non-suicidal self-injury: Meta-analysis and recommendations for future research. Neurosci. Biobehav. Rev 83, 440–450. 10.1016/J.NEUBI0REV.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Croteau-Chonka DC, 2015. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe CJ, Manocchio F, Safati AB, Hall PA, 2018. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: A systematic review and meta-analysis. Neuropsychologia 111, 344–359. 10.1016/J.NEUR0PSYCH0L0GIA.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 44.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR, 2011. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl). 216, 305–21. 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacKillop J, Gray JC, Weafer J, Sanchez-Roige S, Palmer AA, de Wit H, 2018. Genetic influences on delayed reward discounting: A genome-wide prioritized subset approach. Exp. Clin. Psychopharmacol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKillop J, Weafer J, Gray J, Oshri A, Palmer AA, de Wit H, 2016. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacol. 233, 3361–3370. 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madden GJ, Begotka AM, Raiff BR, Kastern LL, 2003. Delay discounting of real and hypothetical rewards. Exp. Clin. Psychopharmacol 11, 139–145. 10.1901/jeab.2008.90-333 [DOI] [PubMed] [Google Scholar]
- 48.Madden GJ, Smith NG, Brewer AT, Pinkston JW, Johnson PS, 2008. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J. Exp. Anal. Behav 90, 333–344. 10.1901/jeab.2008.90-333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marouli E, Graff M, Medina-Gomez C, Lo KS, Wood AR, Kjaer TR, Fine RS, Lu Y, Schurmann C, Highland HM, Rüeger S, Thorleifsson G, Justice AE, Lamparter D, Stirrups KE, Turcot V, Young KL, Winkler TW, Esko T, Karaderi T, Locke AE, Masca NGD, Ng MCY, Mudgal P, Rivas MA, Vedantam S, Mahajan A, Guo X, Abecasis G, Aben KK, Adair LS, Alam DS, Albrecht E, Allin KH, Allison M, Amouyel P, Appel EV, Arveiler D, Asselbergs FW, Auer PL, Balkau B, Banas B, Bang LE, Benn M, Bergmann S, Bielak LF, Blüher M, Boeing H, Boerwinkle E, Böger CA, Bonnycastle LL, Bork-Jensen J, Bots ML, Bottinger EP, Bowden DW, Brandslund I, Breen G, Brilliant MH, Broer L, Burt AA, Butterworth AS, Carey DJ, Caulfield MJ, Chambers JC, Chasman DI, Chen Y-DI, Chowdhury R, Christensen C, Chu AY, Cocca M, Collins FS, Cook JP, Corley J, Galbany JC, Cox AJ, Cuellar-Partida G, Danesh J, Davies G, de Bakker PIW, de Borst GJ, de Denus S, de Groot MCH, de Mutsert R, Deary IJ, Dedoussis G, Demerath EW, den Hollander AI, Dennis JG, Di Angelantonio E, Drenos F, Du M, Dunning AM, Easton DF, Ebeling T, Edwards TL, Ellinor PT, Elliott P, Evangelou E, Farmaki A-E, Faul JD, Feitosa MF, Feng S, Ferrannini E, Ferrario MM, Ferrieres J, Florez JC, Ford I, Fornage M, Franks PW, Frikke-Schmidt R, Galesloot TE, Gan W, Gandin I, Gasparini P, Giedraitis V, Giri A, Girotto G, Gordon SD, Gordon-Larsen P, Gorski M, Grarup N, Grove ML, Gudnason V, Gustafsson S, Hansen T, Harris KM, Harris TB, Hattersley AT, Hayward C, He L, Heid IM, Heikkilä K, Helgeland Ø, Hernesniemi J, Hewitt AW, Hocking LJ, Hollensted M, Holmen OL, Hovingh GK, Howson JMM, Hoyng CB, Huang PL, Hveem K, Ikram MA, Ingelsson E, Jackson AU, Jansson J-H, Jarvik GP, Jensen GB, Jhun MA, Jia Y, Jiang X, Johansson S, Jørgensen ME, Jørgensen T, Jousilahti P, Jukema JW, Kahali B, Kahn RS, Kähönen M, Kamstrup PR, Kanoni S, Kaprio J, Karaleftheri M, Kardia SLR, Karpe F, Kee F, Keeman R, Kiemeney LA, Kitajima H, Kluivers KB, Kocher T, Komulainen P, Kontto J, Kooner JS, Kooperberg C, Kovacs P, Kriebel J, Kuivaniemi H, Küry S, Kuusisto J, La Bianca M, Laakso M, Lakka TA, Lange EM, Lange LA, Langefeld CD, Langenberg C, Larson EB, Lee I-T, Lehtimäki T, Lewis CE, Li H, Li J, Li-Gao R, Lin H, Lin L-A, Lin X, Lind L, Lindström J, Linneberg A, Liu Y, Liu Y, Lophatananon A, Luan J, Lubitz SA, Lyytikäinen L-P, Mackey DA, Madden PAF, Manning AK, Männistö S, Marenne G, Marten J, Martin NG, Mazul AL, Meidtner K, Metspalu A, Mitchell P, Mohlke KL, Mook-Kanamori DO, Morgan A, Morris AD, Morris AP, Müller-Nurasyid M, Munroe PB, Nalls MA, Nauck M, Nelson CP, Neville M, Nielsen SF, Nikus K, Njølstad PR, Nordestgaard BG, Ntalla I, O’Connel JR, Oksa H, Loohuis LMO, Ophoff RA, Owen KR, Packard CJ, Padmanabhan S, Palmer CNA, Pasterkamp G, Patel AP, Pattie A, Pedersen O, Peissig PL, Peloso GM, Pennell CE, Perola M, Perry JA, Perry JRB, Person TN, Pirie A, Polasek O, Posthuma D, Raitakari OT, Rasheed A, Rauramaa R, Reilly DF, Reiner AP, Renström F, Ridker PM, Rioux JD, Robertson N, Robino A, Rolandsson O, Rudan I, Ruth KS, Saleheen D, Salomaa V, Samani NJ, Sandow K, Sapkota Y, Sattar N, Schmidt MK, Schreiner PJ, Schulze MB, Scott RA, Segura-Lepe MP, Shah S, Sim X, Sivapalaratnam S, Small KS, Smith AV, Smith JA, Southam L, Spector TD, Speliotes EK, Starr JM, Steinthorsdottir V, Stringham HM, Stumvoll M, Surendran P, ‘t Hart LM, Tansey KE, Tardif J-C, Taylor KD, Teumer A, Thompson, Thorsteinsdottir U, Thuesen BH, Tönjes A, Tromp G, Trompet S, Tsafantakis E, Tuomilehto J, Tybjaerg-Hansen A, Tyrer JP, Uher R, Uitterlinden AG, Ulivi S, van der Laan SW, Van Der Leij AR, van Duijn CM, van Schoor NM, van Setten J, Varbo A, Varga TV, Varma R, Edwards DRV, Vermeulen SH, Vestergaard H, Vitart V, Vogt TF, Vozzi D, Walker M, Wang F, Wang CA, Wang S, Wang Y, Wareham NJ, Warren HR, Wessel J, Willems SM, Wilson JG, Witte DR, Woods MO, Wu Y, Yaghootkar H, Yao J, Yao P, Yerges-Armstrong LM, Young R, Zeggini E, Zhan X, Zhang W, Zhao JH, Zhao W, Zhao W, Zheng H, Zhou W, EPIC-InterAct Consortium, E.-C., CHD Exome+ Consortium, T.E.-I., ExomeBP Consortium, C.E., T2D-Genes Consortium, E., GoT2D Genes Consortium, T.-G., Global Lipids Genetics Consortium, G.G., ReproGen Consortium, G.L.G., MAGIC Investigators, R., Rotter JI, Boehnke M, Kathiresan S, McCarthy MI, Willer CJ, Stefansson K, Borecki IB, Liu DJ, North KE, Heard-Costa NL, Pers TH, Lindgren CM, Oxvig C, Kutalik Z, Rivadeneira F, Loos RJF, Frayling TM, Hirschhorn JN, Deloukas P, Lettre G, Lettre G, 2017. Rare and low-frequency coding variants alter human adult height. Nature 542, 186–190. 10.1038/nature21039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matusiewicz AK, Carter AE, Landes RD, Yi R, 2013. Statistical equivalence and test–retest reliability of delay and probability discounting using real and hypothetical rewards. Behav. Processes 100, 116–122. 10.1016/J.BEPROC.2013.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazur JE, 1987. An adjusting procedure for studying delayed reinforcement, in: Mazur JE, Nevin JA, Rachlin H (Eds.), Quantitative Analysis of Behavior: Vol 5. The Effects of Delay and Intervening Events on Reinforcement Value. Erlbaum, Hillsdale, pp. 55–73. [Google Scholar]
- 52.McCarthy M, Abecasis G, … L. C-N reviews, 2008, U., 2008. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat. Rev. Genet 9, 356369. [DOI] [PubMed] [Google Scholar]
- 53.Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B, 2010. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav. Neurosci 124, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, Setlow B, 2014. Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav. Neurosci 128, 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell SH, 2014. Assessing delay discounting in mice. Curr. Protoc. Neurosci 66, Unit 8.30. 10.1002/0471142301.ns0830s66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazaki K, Miyazaki KW, Yamanaka A, Tokuda T, Tanaka KF, Doya K, 2018. Reward probability and timing uncertainty alter the effect of dorsal raphe serotonin neurons on patience. Nat. Commun 9, 2048 10.1038/s41467-018-04496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myerson J, Baumann AA, Green L, 2014. Discounting of delayed rewards:(A) theoretical interpretation of the Kirby questionnaire. Behav. Processes 107, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Myerson J, Green L, Warusawitharana M, 2001. Area under the curve as a measure of discounting. J. Exp. Anal. Behav 76, 235–243. 10.1901/jeab.2001.76-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson M, Tipney H, Painter J, Shen J, 2015. The support of human genetic evidence for approved drug indications. Nature. [DOI] [PubMed] [Google Scholar]
- 60.Odum AL, Baumann AAL, Rimington DD, 2006. Discounting of delayed hypothetical money and food: Effects of amount. Behav. Processes 73, 278–284. 10.1016/J.BEPROC.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 61.Odum AL, Rainaud CP, 2003. Discounting of delayed hypothetical money, alcohol, and food. Behav. Processes 64, 305–313. [DOI] [PubMed] [Google Scholar]
- 62.Oshri A, Kogan SM, Kwon JA, Wickrama KAS, Vanderbroek L, Palmer AA, Mackillop J, 2018a. Impulsivity as a mechanism linking child abuse and neglect with substance use in adolescence and adulthood. Dev. Psychopathol 30, 417–435. [DOI] [PubMed] [Google Scholar]
- 63.Oshri A, Liu S, Duprey EB, MacKillop J, 2018b. Child maltreatment, delayed reward discounting, and alcohol and other drug use problems: The moderating role of heart rate variability. ACER 42, 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polderman TJ, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM Posthuma D, 2015. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat. Genet 47, 702–709. [DOI] [PubMed] [Google Scholar]
- 65.Reach G, Michault A, Bihan H, Paulino C, Cohen R, Clésiaub H Le, 2011. Patients’ impatience is an independent determinant of poor diabetes control. Diabetes Metab. 37, 497504 10.1016/J.DIABET.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 66.Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, Palmer AA, 2013. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes. Brain. Behav 12, 490–502. 10.1111/gbb.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards JB, Mitchell SH, de Wit H, Seiden LS, 1997. Determination of discount functions in rats with an adjusting-amount procedure. J. Exp. Anal. Behav 67, 353–66. 10.1901/jeab.1997.67-353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson SH, Rasmussen EB, 2018. Comparison of potentially real versus hypothetical food outcomes in delay and probability discounting tasks. Behav. Processes 149, 8–15. 10.1016/J.BEPROC.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Roige S, Fontanillas P, Elson SL, 23andMe Research Team, Pandit A, Schmidt EM, Foerster JR, Abecasis GR, Gray JC, de Wit H, Davis LK, MacKillop J, Palmer AA, 2018. Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat. Neurosci 21, 16–18. 10.1038/s41593-017-0032-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanchez-Roige S, Fontanillas P, Elson SL, 23andMe Research Team, Gray JC, de Wit H, MacKillop J, Palmer AA, 2019. Genome-wide association studies of impulsive personality traits (BIS-11 and UPPSP) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J. Neurosci 10.1523/JNEUROSCI.2662-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Setlow B, Mendez IA, Mitchell MR, Simon NW, 2009. Effects of chronic administration of drugs of abuse on impulsive choice (delay discounting) in animal models. Behav. Pharmacol 20, 380–9. 10.1097/FBP.0b013e3283305eb4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheffer CE, Bickel WK, Brandon TH, Franck CT, Deen D, Panissidi L, Abdali SA, Pittman JC, Lunden SE, Prashad N, Malhotra R, Mantovani A, 2018. Preventing relapse to smoking with transcranial magnetic stimulation: Feasibility and potential efficacy. Drug Alcohol Depend. 182, 8–18. 10.1016/j.drugalcdep.2017.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simon NW, Mendez IA, Setlow B, 2007. Cocaine exposure causes long-term increases in impulsive choice. Behav. Neurosci 121, 543–9. 10.1037/0735-7044.121.3.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sparks JC, Isen JD, Iacono WG, 2014. Preference on cash-choice task predicts externalizing outcomes in 17-year-olds. Behav. Genet 44, 102–112. 10.1007/s10519-013-9638-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, Toro R, Appel K, Bartecek R, Bergmann Ø, Bernard M, Brown AA, Cannon DM, Chakravarty MM, Christoforou A, Domin M, Grimm O, Hollinshead M, Holmes AJ, Homuth G, Hottenga J-J, Langan C, Lopez LM, Hansell NK, Hwang KS, Kim S, Laje G, Lee PH, Liu X, Loth E, Lourdusamy A, Mattingsdal M, Mohnke S, Maniega SM, Nho K, Nugent AC, O’Brien C, Papmeyer M, Pütz B, Ramasamy A, Rasmussen J, Rijpkema M, Risacher SL, Roddey JC, Rose EJ, Ryten M, Shen L, Sprooten E, Strengman E, Teumer A, Trabzuni D, Turner J, van Eijk K, van Erp TGM, van Tol M-J, Wittfeld K, Wolf C, Woudstra S, Aleman A, Alhusaini S, Almasy L, Binder EB, Brohawn DG, Cantor RM, Carless MA, Corvin A, Czisch M, Curran JE, Davies G, de Almeida MAA, Delanty N, Depondt C, Duggirala R, Dyer TD, Erk S, Fagerness J, Fox PT, Freimer NB, Gill M, Göring HHH, Hagler DJ, Hoehn D, Holsboer F, Hoogman M, Hosten N, Jahanshad N, Johnson MP, Kasperaviciute D, Kent JW, Kochunov P, Lancaster JL, Lawrie SM, Liewald DC, Mandl R, Matarin M, Mattheisen M, Meisenzahl E, Melle I, Moses EK, Muhleisen TW, Nauck M, Nöthen MM, Olvera RL, Pandolfo M, Pike GB, Puls R, Reinvang I, Rentería ME, Rietschel M, Roffman JL, Royle NA, Rujescu D, Savitz J, Schnack HG, Schnell K, Seiferth N, Smith C, Steen VM, Valdés Hernández MC, Van den Heuvel M, van der Wee NJ, Van Haren NEM, Veltman JA, Völzke H, Walker R, Westlye LT, Whelan CD, Agartz I, Boomsma DI, Cavalleri GL, Dale AM, Djurovic S, Drevets WC, Hagoort P, Hall J, Heinz A, Jack CR, Foroud TM, Le Hellard S, Macciardi F, Montgomery GW, Poline JB, Porteous DJ, Sisodiya SM, Starr JM, Sussmann J, Toga AW, Veltman DJ, Walter H, Weiner MW, Bis JC, Ikram MA, Smith AV, Gudnason V, Tzourio C, Vernooij MW, Launer LJ, DeCarli C, Seshadri S, Andreassen OA, Apostolova LG, Bastin ME, Blangero J, Brunner HG, Buckner RL, Cichon S, Coppola G, de Zubicaray GI, Deary IJ, Donohoe G, de Geus EJC, Espeseth T, Fernández G, Glahn DC, Grabe HJ, Hardy J, Hulshoff Pol HE, Jenkinson M, Kahn RS, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meyer-Lindenberg A, Morris DW, Müller-Myhsok B, Nichols TE, Ophoff RA, Paus T, Pausova Z, Penninx BW, Potkin SG, Sämann PG, Saykin AJ, Schumann G, Smoller JW, Wardlaw JM, Weale ME, Martin NG, Franke B, Wright MJ, Thompson PM, 2012. Identification of common variants associated with human hippocampal and intracranial volumes. Nat. Genet 44, 552–61. 10.1038/ng.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tucker-Drob EM, Bates TC, 2016. Large cross-national differences in gene× socioeconomic status interaction on intelligence. Psychol. Sci 27, 138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van den Berk-Clark C, Myerson J, Green L, Grucza RA, 2018. Past trauma and future choices: differences in discounting in low-income, urban African Americans. Psychol. Med 48, 2702–2709. [DOI] [PubMed] [Google Scholar]
- 78.Vanderveldt A, Oliveira L, Green L, 2016. Delay discounting: Pigeon, rat, human--does it matter? J. Exp. Psychol. Anim. Learn. Cogn 42, 141–62. 10.1037/xan0000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Visscher PM, Wray NR, Zhang Q, Sklar P, McCarthy MI, Brown MA, Yang J, 2017. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet 101, 5–22. 10.1016/J.AJHG.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weafer J, Gray JC, Hernandez K, Palmer AA, MacKillop J, de Wit H, 2017. Hierarchical investigation of genetic influences on response inhibition in healthy young adults. Exp. Clin. Psychopharmacol 25 10.1037/pha0000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wesley MJ, Bickel WK, 2014. Remember the future II: meta-analyses and functional overlap of working memory and delay discounting. Biol. Psychiatry 75, 435–448. 10.1016/j.biopsych.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilhelm CJ, Mitchell SH, 2009. Strain differences in delay discounting using inbred rats. Genes, Brain Behav. 8, 426–434. 10.1111/j.1601-183X.2009.00484.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AAE, Lee SH, Robinson MR, Perry JRB, Nolte IM, van Vliet-Ostaptchouk JV, Snieder H, LifeLines Cohort Study TLC, Esko T, Milani L, Mägi R, Metspalu A, Hamsten A, Magnusson PKE, Pedersen NL, Ingelsson E, Soranzo N, Keller MC, Wray NR, Goddard ME, Visscher PM, 2015. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat. Genet 47, 1114–20. 10.1038/ng.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]