Abstract
Structural remodeling is central to the initiation and progression of many chronic lung diseases, representing an important unmet need. We examine the evidence supporting bromodomain-containing protein 4 (BRD4) as a validated biological target for treatment of airway remodeling. In epithelial cells and fibroblasts, BRD4 serves as a scaffold for chromatin remodeling complexes in active super-enhancers. In response to inflammatory stimuli, BRD4 is repositioned to innate and mesenchymal genes activating their production. Proof-of-concept studies show promising benefit of selective BRD4 inhibitors in disrupting epithelial mesenchymal transition and myofibroblast transition in diverse models of lung injury. Recent identification of biomarkers of BRD4 provides a basis for further drug development for application in viral-induced airway inflammation, COPD and interstitial lung diseases.
Keywords: Bromodomain-containing protein 4, BRD4, airway remodeling, mesenchymal transition, myofibroblast, inflammatory diseases, epigenetics
Introduction
Chronic environmental injury–repair triggers pathological organ fibrosis, accounting for substantial morbidity and end-organ dysfunction. In the pulmonary system, the two most common obstructive airway diseases: asthma and chronic obstructive pulmonary disease (COPD), are characterized by periodic acute exacerbations (AEs). AEs are clinical episodes of acute inflammation provoked by infections, allergens or environmental exposures that result in impairment of air exchange and account for substantial morbidity and healthcare costs. For example in allergic asthma, AEs account for 15 million outpatient visits, 2 million emergency room visits and 500 000 hospitalizations annually in the USA alone [1]. Conventional treatments for AEs involve antibiotics and increased anti-inflammatory corticosteroids and/or bronchodilators; there are no FDA-approved therapeutics for reducing the frequency of AEs.
In addition to acute inflammation precipitating medical interventions, AEs produce epithelial injury and repair processes. Epithelial barrier injuries from environmental pollutants, allergens and viruses activate a coordinated, multicellular reparative response termed ‘airway remodeling’. Airway remodeling involves cell state changes of structural components of the airway including epithelial mesenchymal transition (EMT), mucous metaplasia, enhanced collage deposition in the lamina reticularis and transdifferentiation of stromal myofibroblasts. This remodeling process is the result of myofibroblast growth factor secretion, including transforming growth factor (TGF)β, epidermal growth factor (EGF), connective tissue growth factor (CTGF) and fibrogenic cytokines [e.g., interleukin (IL)-6, thymic stromal lymphopoietin (TSLP), IL-33, IL-25, among others], initiating the injury–repair processes. Unabated, pathogenic remodeling and fibrosis ensues. Functionally, airway remodeling enhances mucin secretion, producing small-airway obstruction, airway hyperreactivity to nonspecific triggers and reduced lung compliance [2]. The common therapeutics used in chronic lung diseases do not affect structural remodeling of the airway.
Improved treatments for airway remodeling represent an important unmet need in the management of chronic lung diseases. In this concise review, using work from the academic arena demonstrating the mechanisms of epithelial injury response in cellular and in vivo models of asthma, we will examine the case for the epigenetic reader bromodomain-containing protein 4 (BRD4) as a validated biological target for drug development for the treatment of airway inflammation and relevant lung diseases. We focus on biological target validation informed by the paradigm AstraZeneca’s 5Rs model (the right target, patient, tissue, safety and commercial potential) which has been extensively applied to the drug development pipeline [3]. The intended goal of this framework is to reduce the substantial fraction of failures of lead candidates in human efficacy trials [3]. A robust understanding of a biological target in a pathway (i.e., target validation) is crucial for selection of druggable therapeutic targets. We will review the deep mechanistic understanding of BRD4 as a drug target for airway inflammation and structural remodeling (cell state change and myofibroblast transdifferentiation) in preclinical models of AEs relevant to allergic asthma and COPD.
The right target: BRD4 plays a central part in lower airway epithelial-driven inflammation
Epithelial cells are the primary target of respiratory infections and environmental exposures [4]. Innate signaling of the epithelium plays a central part in triggering AEs by producing mucin, stimulating leukocytic infiltration and EMT, which further promotes fibrosis and remodeling through epithelial production of extracellular matrix (ECM) and TGFβ [5]. The epithelial innate response shapes the evolution of downstream T helper lymphocyte (Th)2- and Th17-type adaptive immunity characteristic of asthma. In AA, aeroallergens induce a robust small airway epithelial TGFβ response, important in activation of IL-13-producing innate lymphoid type 2 cells and atopy [6].
Upper airway epithelial injury produces distinct immunological signals from those produced by lower airway epithelium. Compared to proximal (tracheal) epithelial cells, we demonstrated that virus infection triggered bronchiolar-derived epithelial cells to produce >106 proteins; these included a core of nuclear factor (NF)-κB-dependent Th2-polarizing chemokines, including CC-motif chemokine ligand (CCL)-20, TSLP and CCL3-like 1 [7]. To provide greater insight into the functional role of the small airway epithelial cells in the viral-induced inflammation, we examined the response of a conditional knockout of the NF-κB transcriptional subunit RelA selectively in small-airway bronchiolar cells. Interestingly, these animals are protected from Toll-like receptor (TLR)3-induced neutrophilia [8] and respiratory syncytial virus (RSV)-induced inflammation [9], indicating that the bronchiolar epithelium is a major sentinel cell responsible for viral-induced inflammation.
Mechanistic studies have shown that virus infection results in signaling via NF-κB and interferon regulatory factor (IRF)3 transcription actors. When activated, these factors form a complex with positive transcriptional elongation factor (PTEFb), a complex composed of cyclin-dependent kinase (CDK)9·BRD4. Activated NF-κB•PTEFb complex recruits to immediate early cytokine genes maintained in an open chromatin conformation with inactive and/or hypophosphorylated RNA polymerase II (RNA Pol II) bound to its promoter. The presence of BRD4 facilitates phosphorylation of RNA Pol II [10], regulating its enzymatic processivity and RNA splicing functions, resulting in the rapid expression of inflammatory genes [11,12]. Consequently, inhibition of BRD4 prevents acute viral-induced cytokine production, neutrophilia, leukocytic infiltration and clinical manifestations of disease [8,13–15]. Collectively, these data indicate that BRD4 actions are essential for the production of acute inflammation by the activated lower airway epithelial cell, satisfying the ‘right target’ in the ‘right tissue’ criteria of the 5R paradigm for treatment of acute airway inflammation.
The right target: BRD4 functions at the interface of epithelial-injury–cell-state transition responses
Disruption of the epithelial barrier releases fibrogenic cytokines, including latent TGF, sequestered within the ECM that coordinate expression of gene regulatory networks to promote repair. Phenotypically, TGFβ orchestrates a dedifferentiation program known as type II EMT. Type II EMT involves extensive cytosolic restructuring, resulting in the loss of apical–basal polarity, dissolution of adherens junctions, enhanced motility and expression of fibrotic genes (see [16] for an in-depth review). As a consequence of this process, epithelial cells acquire stem-cell-like properties, permitting the transitioned mesenchymal cell to repopulate regions of denuded epithelium, promoting tissue repair and ECM remodeling [16]. The EMT program is a BRD4-dependent event that involves coordinating epigenetic reprogramming of ~3000 genes mediated by a core group of mesenchymal transcription factors, including Snail family transcriptional repressor (SNAI)1 and RelA [17–19] (Figure 1). This program inhibits expression of differentiated epithelial cadherin (CDH1) and upregulates core EMT transcription factors, mesenchymal intermediate filaments and ECM-modifying genes. Transition to the mesenchymal state is the product of sequential cell-state changes beginning from the differentiated epithelial state transitioning into the uncommitted ‘partial EMT’ (pEMT) state(s) [20]. Transition into pEMT is initiated by TGFβ binding to the TGFβ receptor type II (TGFβRII), signaling intracellularly via the Smad-dependent canonical and Smad-independent noncanonical pathways [21]. These two arms function coordinately to activate a cascade of transcription factors that result in ordered patterns of gene expression and inhibition, resulting in a metastable pEMT state [22]. The transition to stable EMT requires ongoing growth factor stimulation, activating autoregulatory loops of master transcription factors and formation of acetylated histone-rich super-enhancers on mesenchymal genes promoting chromatin remodeling [18,23].
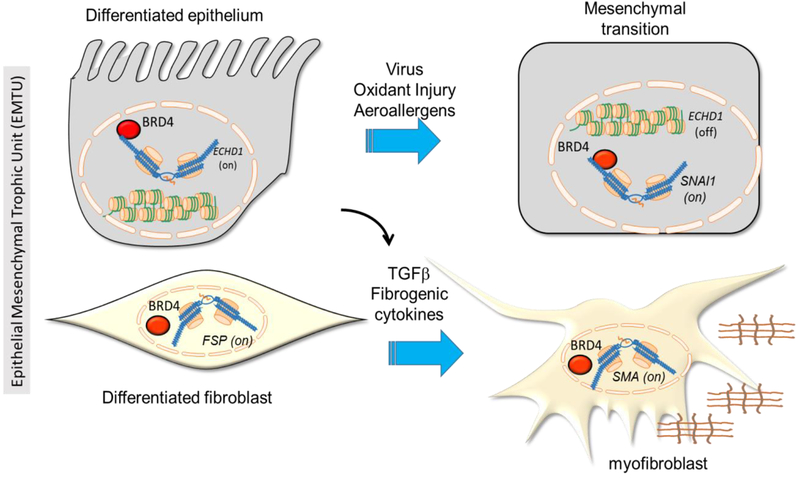
Figure 1.
Central role of bromodomain-containing protein 4 (BRD4) in activating remodeling through the epithelial mesenchymal trophic unit (EMTU). Schematic view of a differentiated airway epithelial cell in normal (left) and after mesenchymal (right) transition. At top left, BRD4 plays a part in maintaining normal airway phenotype by expression of epithelial cadherin (ECDH1), maintained in an open 30 nm chromatin fiber formation. The mesenchymal fibrotic program, Snail family transcriptional repressor 1 (SNAI1), vimentin (VIM) and fibronectin (FN) genes are in inactive heterochromatin states. Right, in response to viral infection or allergen exposure, injury–repair mechanisms trigger epigenetic reprogramming, silencing ECDH1 and activating SNAI1, VM and FN genes. Similarly, BRD4 is involved in maintenance of the normal fibroblast phenotype maintaining differentiated fibroblast markers such as fibroblast-specific protein (FSP)/S100A4. In response to epithelial injury, secretion of transforming growth factor (TGF)β and fibrogenic cytokines produces myofibroblast transdifferentiation (bottom right) with activation of extracellular matrix production including alpha smooth muscle actin (SMA). In these cell-type selective manners, BRD4 controls the activation state of the EMTU.
SNAI1 and zinc finger E-box binding homeobox 1 (ZEB1) are cooperating master transcriptional regulators of the mesenchymal transition [24]. In differentiated epithelial cells, SNAI1 and ZEB1 expression is silenced by a double-negative feedback loop mediated by the miR34 and miR200 microRNAs [25,26]. miR34 post- transcriptionally inhibits SNAI1 expression, whereas miR200 blocks ZEB1 expression. Equilibrium of the double-negative feedback loops is perturbed by TGFβ-induced transcriptional upregulation of SNAI1, which inhibits miR200 expression, releasing ZEB1. Using genome-wide RNA sequencing of airway cells after RelA silencing, we discovered that RelA plays a direct part in triggering this SNAI1—ZEB1 negative feedback loop by directly activating SNAI1 expression by binding to its proximal promoter. SNAI1 upregulation is mediated through coordinate transcription factors, initially by pSmad3 [22,27] but later requires RelA activation for sustained expression. RelA activation of SNAI1 is mediated by a RelA-dependent coactivator recruitment of BRD4 [28]. BRD4 activates SNAI1 by phosphorylation of the carboxy terminal domain (CTD) of RNA polymerase II, promoting its transcriptional elongation.
BRD4 is a dynamically responsive chromatin-modifying and -organizing factor
Through its acetyl lysine-binding bromodomains, BRD4 is essential for the maintenance of higher order chromatin configuration [29]. In particular, BRD4 is enriched with enhancer regions (super-enhancers) with other chromatin-modifying factors controlling the expression of tissue-specific genes. These super-enhancers result in high-level, constitutive gene expression and coordinate with expression of distant genes through looping interactions controlling cell identity [30]. In response to inflammatory signaling through the conserved pattern recognition receptors, BRD4 super-enhancers are repositioned to inflammatory and fibrotic gene expression networks.
Activated NF-κB drives BRD4-dependent cell-state transition
Our recent unbiased RNA sequencing studies discovered that NF-κB is upstream of the ‘core’ mesenchymal transcription factors SNAI1, ZEB1 and the Jun proto-oncogene (JUN), qualifying its designation as a ‘master transcriptional regulator’ of EMT [18]. Master regulators are engaged in the coordinate regulation of cliques of downstream transcription factors by maintaining their own expression by the formation of super-enhancers [18,30]. Indeed, RelA expression itself is induced by virus and TGFβ and requires activity of the RelA•BRD4 complex. Systems-level studies have shown the essential role of BRD4 in mediating the coordinated gene expression changes underlying EMT [18]. Our earlier work demonstrated the mechanism underlying this expression to be medicated through transcriptional elongation following de-repression of their chromatin environment [28].
BRD4 is central for NF-κB-dependent EMT at the level of transcriptional elongation
RelA association with BRD4 is required for the process of transcriptional elongation, promoting the TGFβ-induced mesenchymal transition program [28]. Here, Ser276 phosphorylated RelA is acetylated by p300/CBP and bound by BRD4 through bromodomain (BD) interactions [11,31]. Through site-specific DNA binding, RelA repositions BRD4 to the promoters of mesenchymal genes, where its kinase activity for the CTD of RNA Pol II phosphorylates Ser 2 on heptad repeat motifs. Phospho Ser2 is a post-translational modification licensing RNA Pol II to become processive, producing full length mRNA transcripts [32]. In addition, we recently found that the association of RelA also induced the atypical HAT activity of BRD4, acetylating histone H3 on Lys122, a modification that destabilizes nucleosomes, enhancing transcription through gene bodies [15,33]. We have recently discovered that NF-κB triggers TGFβ-induced EMT by recruiting BRD4, inducing phosphorylation of RNA Pol II and enhancing mesenchymal gene and growth factor expression via the process of transcriptional elongation in vivo and in vitro [34]. Collectively, these data provide detailed mechanistic understanding for how BRD4 actions mediate mesenchymal transition addressing the ‘right target’ and ‘differentiated efficacy’ criteria of the 5R paradigm for treatment of mucosal remodeling in chronic airway diseases.
The right target: BRD4 plays a direct part in myofibroblast transdifferentiation
In addition to its role in modifying epithelial-driven mesenchymal transition, BRD4 is a key factor in transition of quiescent fibroblasts into ECM-producing myofibroblasts. Myofibroblasts are central effector cells responsible for excessive ECM deposition of collagen (COL) I, COL III and fibronectin (FN) in the lamina reticularis. Myofibroblast transdifferentiation plays a crucial part in tissue response to injury in the skin, kidney, liver and lung. Like EMT, myofibroblast transition is a TGFβ-induced epigenomic reprogramming event for which substantial evidence indicates that BRD4 plays a central part [35]. The myofibroblast population is dynamic, increasing in patients with refractory asthma [36] actively progressing COPD or recurrent asthma [37]. Myofibroblasts originate from a variety of sources, including resident mesenchymal cells, epithelial and endothelial cells (EMT/EndMT) undergoing mesenchymal transition, as well as circulating bone marrow stem cells (fibrocytes) [38]. Through the epithelial mesenchymal trophic unit (EMTU) [39] there is a close functional interrelationship between the epithelium and myofibroblasts. BRD4 inhibitors reduce the subepithelial myofibroblast population in response to allergen [5] and viral pattern-induced AEs [5]. These data indicate that BRD4 action in the quiescent fibroblast is required for production of ECM in the diseased airway addressing the ‘right tissue’ of the 5R paradigm.
Advances in discovery of potent and selective BRD4 inhibitors
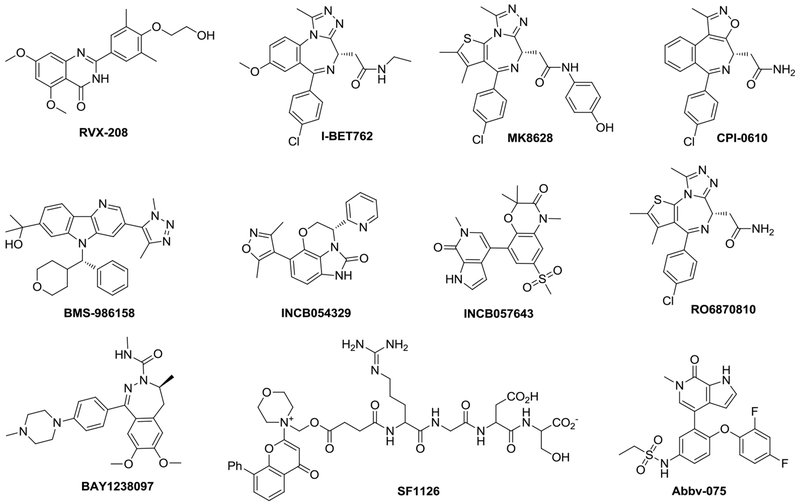
The advancement of small-molecule inhibitors of BRD4 has been the subject of intense medicinal chemistry work, particularly in cancer drug discovery, and has been the topic of many recent reviews [13,40–43]. BRD4 belongs to the bromodomain and extraterminal (BET) family of proteins, which consists of four members (BRD2, BRD3, BRD4 and BRDT), each with two distinct bromodomains: BD1 and BD2 [44]. BET family proteins, especially BRD4, are emerging as promising therapeutic targets for a variety of human diseases and conditions including cancer [45,46], inflammation [47], HIV infection [48,49], heart failure [50] and central nervous system (CNS) disorders [51]. Several BET/BRD4 inhibitors have been advanced into human clinical trials (Table 1) with the chemical structures of selected representative inhibitors shown in Figure 2, whereas most pan-BET inhibitors are not very selective toward BRD4 or its bromodomains. Many of them are (+)-JQ1 analogs with the diazepine or azepine core scaffolds.
Table 1.
Representative small-molecule bromodomain and extra-terminal (BET) inhibitors advanced into human clinical trialsa
| BET inhibitors | Pharmaceuticals | Stage | Indications |
|---|---|---|---|
| RVX-208 | Resverlogix | Phase III | Stable coronary artery disease; type II diabetes |
| I-BET762 | GSK | Phase II | Nut midline carcinoma; other cancer |
| MK8628 | OncoEthix | Phase II | Acute leukemia; other hematological malignancies |
| CPI-0610 | Constellation | Phase II | Multiple myeloma; acute myelogenous leukemia |
| BMS-986158 | BMS | Phase II | Advanced tumors |
| INCB054329 | Incyte | Phase II | Advanced malignancies |
| INCB057643 | Incyte | Phase II | Solid tumors |
| ZEN003964 | Zenith Epigenetics | Phase II | Metastatic castration-resistant prostate cancer |
| ODM-207 | Orion | Phase I/II | Solid tumors |
| RO6870810 | Tensha | Phase I | Acute myelogenous leukemia; myelodysplastic syndrome; cancer |
| SF1126 | SignalRX | Phase I | Advanced hepatocellular carcinoma |
| Abbv-075 | AbbVie | Phase I | Advanced cancer; breast cancer; non-small-cell lung cancer; acute myelogenous leukemia; multiple myeloma |
| Abbv-744 | AbbVie | Phase I | Prostate cancer; acute myelogenous leukemia |
| BAY1238097 | Bayer | Phase I | Neoplasms |
| INCB059872 | Incyte | Phase I | Relapsed Ewing sarcoma |
| CC90010 | Celgene | Phase I | Lymphoma, non-Hodgkin; neoplasms |
| AZD5153 | AstraZeneca | Phase I | Lymphoma; ovarian cancer and solid tumors |
| FT-1101 | Forma Therapeutics | Phase I | Acute myelogenous leukemia; myelodysplastic syndromes; non-Hodgkin lymphoma |
| GSK2820151 | GSK | Phase I | Advanced/recurrent solid tumors |
Information taken from www.clinicaltrial.gov
Figure 2.
Chemical structures of selected representative small-molecule bromodomain and extra-terminal (BET) inhibitors in human clinical trials.
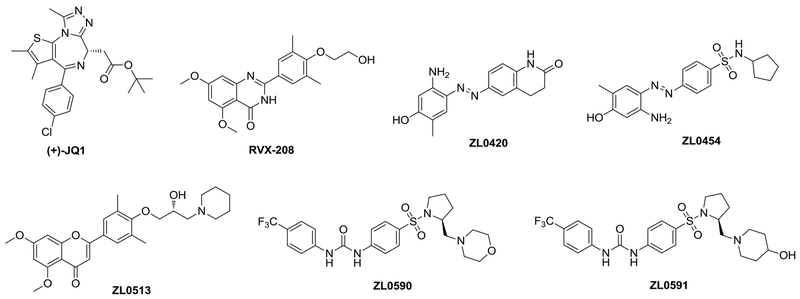
The lack of highly selective inhibitors among BRD4 and close family members as well as the challenges in selectively targeting either of the two bromodomains (BD1 and BD2) of BRD4, because of >90% conserved identity for crucial residues around the acetylated lysine (KAc) binding site, have stalled the collective understanding of this important system and the progress toward clinical development. Our recent discovery and validation of highly specific BRD4 inhibitors with nanomolar binding affinity and >30-fold specificity over the closely related BRD2 isoform have advanced the field by providing useful pharmacological probes such as ZL0420, ZL0454, ZL0513, ZL0590 and ZL0591 (Figure 3) with three diversified core scaffolds for the testing of the role of BRD4 in pathophysiological conditions in vivo with an efficacy even greater than that of RVX-208 - a Phase III clinical trial drug candidate - and the widely used pharmacological tool inhibitor (+)-JQ1 in blocking poly(I:C)-induced airway inflammation [8,14,52]. Our recent X-ray co-crystal structural analysis of one BRD4 BD1 domain-selective inhibitor (ZL0590) in complex with human BRD4 BD1 illustrates a first-in-class non-KAc binding site located at the helix αB and αC surface (unpublished data), whereas the classic KAc recognition site is at the end of four helix bundles. The selective targeting of BRD4 bromodomains might facilitate further elucidation of the complex biology underpinning bromodomain specificity among BRD4 protein partners and exploration of distinct new biological functions. Notably, we recently discovered that ZL0580, a structurally close analog of ZL0590, can epigenetically suppress HIV [53], whereas (+)-JQ1 was reported to activate latent HIV [48,54].
Figure 3.
Chemical structures of bromodomain and extra-terminal (BET) inhibitors (+)-JQ1, RVX-208 and representative bromodomain-containing protein bromodomain-containing protein 4 (BRD4)-selective inhibitors with reported in vivo efficacy in blocking poly(I:C)-induced airway inflammation.
The right target: mechanisms of inhibition and BRD4 biomarkers
The 5R framework specifies that preclinical biomarkers are available for monitoring the relationship between drug exposure and consequent downstream biological effects. Understanding the mechanisms of inhibition has advanced the identification of biomarkers of BRD4 inhibition. At the cellular level, BD-targeted inhibitors disrupt BRD4 activity in multiple ways, including inhibition of the BRD4 protein–protein interaction complex, disruption of BRD4-rich super-enhancers, inhibition of its atypical histone acetyl transferase (HAT) activity and degradation of acetylated transcription factors. Chromatin immunoprecipitation experiments have shown that RelA recruitment and Pol II phosphorylation are disrupted by BD-directed small-molecule inhibitors disrupting the formation of a stable pre-initiation complex [9]. Similarly, BRD4 BD inhibitors displace BRD4 from high-affinity chromatin binding sites.
Unbiased protein–protein interaction studies have found that BRD4 forms complexes with ~1000 interacting proteins including the AP-2 adapter complex, DNA-dependent RNA transcription, ATP chromatin remodeling factors, RNA spliceosome factors and others [34]. BRD4 inhibitors disrupt BRD4 binding with Pol II and RelA [9,55]. In one study, BD inhibitor-induced disruption of BRD4 binding to acetylated RelA resulted in the degradation of nuclear RelA complex – a mechanism that further potentiates inhibition of the NF-κB signaling pathway [56]. More-recent identification of the atypical HAT activity of BRD4 has shown that BD binding inhibitors result in reduction of the unique HAT H3K122 acetylation marks [5,57]. Consequently, BRD4 inhibition can be observed in tissues through disruption of RNA Pol II binding, displacement from super-enhancers and/or reduction in H3K122 acetylation.
The effects of highly selective BRD4 inhibitors have been observed in preclinical studies where virus-induced inducible epithelial chemokine production, neutrophilia [8,14] and allergen-induced remodeling [5] were reduced. A recent systems-level pharmacoproteomics study of TLR3-NF-κB/RelA innate inflammation coupled remodeling [58] extends the understanding of the effect of BRD4 inhibition on epithelial signals associated with remodeling. We identified upregulation of orosomucoid (ORM2), pentraxin member amyloid P (APCS), fibrinogen (FgA/B), fibronectin (FN1) and SPARC like 1 (SPARCL1) proteins in the bronchoalveolar fluid (BALF). The abundance of all of these proteins was increased in BALF undergoing remodeling and substantially reduced with selective BRD4 inhibitor treatment, making these proteins a biomarker panel indicative of BRD4 inhibitor effect on airway remodeling.
The right patient
Preclinical advancement is dependent on identifying patients who have active airway remodeling. To address this unmet need, we developed highly specific selected reaction monitoring (SRM) assays for each of the BRD4-inhibition-sensitive BALF markers identified in our mouse pharmacoproteomics study. SRMs are antibody-independent quantitative proteomics assays. Using this method, we were able to observe increases in these proteins in human BALF from patients with severe asthma, relative to controls [58]. Identification of biomarkers of predictive response to BRD4 inhibition will be invaluable in advancing selective BRD4 inhibitors to treating airway remodeling.
Broader applications of BRD4 inhibitors in treatment of airway remodeling
Although the focus of this review has been on the biological validation of BRD4 in AA-induced remodeling, it is important to emphasize that BRD4 antagonists can have broad application in disrupting inflammation remodeling in several lung diseases. This includes virus-induced acute inflammation, such as RSV bronchiolitis. RSV remains a significant human pathogen worldwide, responsible for >64 million cases of acute infections globally [59]. BRD4 inhibitors substantially prevent disease manifestations in preclinical models [8,9]. BRD4 has been validated as a promising therapeutic target in idiopathic pulmonary fibrosis using a preclinical bleomycin model, with functional demonstration in explanted myofibroblasts [60].
Concluding remarks and future perspectives
In this review, we have examined the case for the epigenetic reader BRD4 as a validated biological target in epithelial-driven myofibroblast remodeling and myofibroblast transition. The mechanism for BRD4 as the nexus in inflammation-mediated remodeling is compelling. Bromodomain-targeted small-molecule inhibitors disrupt BRD4 activity by disrupting formation of a protein–protein interaction complex with other coactivators and chromatin super-enhancers, as well as inhibiting its atypical HAT activity and degradation of BRD4-associated acetylated transcription factors. As more-selective, -potent and tissue-targeted BRD4 inhibitors are developed, this class of epigenomic inhibitors will find numerous clinical applications in airway remodeling and relevant lung diseases.
Highlights:
BRD4 plays a central part in epithelial-driven innate inflammation
BRD4 is a dynamically responsive chromatin-modifying and organizing factor
BRD4 is central for NF-κB-dependent EMT at the level of transcriptional elongation
BRD4 has a direct role in myofibroblast transdifferentiation
BRD4 is a validated epigenetic therapeutic target for airway remodeling
Acknowledgments
This work was supported by grants NIAID 2P01AI062885 and NCATS UL1TR00237 from the National Institutes of Health, UTMB Technology Commercialization Program, Sanofi Innovation Awards (iAwards), Crohn’s & Colitis Foundation Entrepreneurial Investing Initiative (EI) award from the Crohn’s & Colitis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no competing financial interest. A.R.B. and J.Z. are co-founders of QuadraGenics.
Teaser: Targeting BRD4 offers a novel and effective epigenetic therapy for treatment of airway remodeling in lung diseases.
References
- 1.Dougherty RH and Fahy JV (2009) Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin. Exp. Allergy 39, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takizawa H (2008) Remodeling in small airways of asthma. Respiratory Medicine CME 1, 69–74 [Google Scholar]
- 3.Cook D et al. (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat. Rev. Drug Discov 13, 419–431 [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN and Hammad H (2012) The airway epithelium in asthma. Nat. Med 18, 684–692 [DOI] [PubMed] [Google Scholar]
- 5.Tian B et al. (2019) Mucosal bromodomain-containing protein 4 mediates aeroallergen-induced inflammation and remodeling. J. Allergy Clin. Immunol 143, 1380–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denney L et al. (2015) Pulmonary epithelial cell-derived cytokine TGF-betal is a critical cofactor for enhanced innate lymphoid cell function. Immunity 43, 945–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y et al. (2017) Systematic analysis of cell-type differences in the epithelial secretome reveals insights into the pathogenesis of respiratory syncytial virus-induced lower respiratory tract infections. J. Immunol 198, 3345–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian B et al. (2018) Selective antagonists of the bronchiolar epithelial NF-kappaB-bromodomain-containing protein 4 pathway in viral-induced airway inflammation. Cell Rep. 23, 1138–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian B et al. (2018) Central role of the NF-kappaB pathway in the Scgb1a1-expressing epithelium in mediating respiratory syncytial virus-induced airway inflammation. J. Virol 92, doi: 00410.01128/JVI.00441-00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaiah BN et al. (2012) BRD4 is an atypical kinase that phosphorylates serine2 of the RNA polymerase II carboxy-terminal domain. Proc. Natl. Acad. Sci. U. S. A 109, 6927–6932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brasier AR et al. (2011) RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J. Virol 85, 11752–11769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J et al. (2015) Systematic determination of human cyclin dependent kinase (CDK)-9 interactome identifies novel functions in RNA splicing mediated by the DEAD box (DDX)-5/17 RNA helicases. Mol. Cell Proteomics 14, 2701–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z et al. (2017) Drug discovery targeting bromodomain-containing protein 4. J. Med. Chem 60, 4533–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z et al. (2018) Discovery of potent and selective BRD4 inhibitors capable of blocking TLR3-induced acute airway inflammation. Enr. J. Med. Chem 151, 450–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian B et al. (2017) BRD4 couples NF-kappaB/RelA with airway inflammation and the IRF-RIG-I amplification loop in respiratory syncytial virus infection. J. Virol 91, doi: 00010.01128/JVI.00007-00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalluri R and Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J. Clin. Invest 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian B et al. (2015) Analysis of the TGFbeta-induced program in primary airway epithelial cells shows essential role of NF-kappaB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genomics 16, 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang H et al. (2016) Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res. 44, 2514–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ijaz T et al. (2014) Systems biology approaches to understanding epithelial mesenchymal transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J 7, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan NV et al. (2011) Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell Cycle 10, 2865–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuno Y et al. (2013) TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr. Opin. Oncol 25, 76–84 [DOI] [PubMed] [Google Scholar]
- 22.Yang YC et al. (2003) Hierarchical model of gene regulation by transforming growth factor beta. Proc. Natl. Acad. Sci. U. S. A. 100, 10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullen AC et al. (2011) Master transcription factors determine cell-type-specific responses to TGF-beta signaling. Cell 147, 565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamouille S et al. (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol 15, 178–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bracken CP et al. (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 68, 7846–7854 [DOI] [PubMed] [Google Scholar]
- 26.Lu M et al. (2013) MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc. Natl. Acad. Sci. U. S. A 110, 18144–18149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoot KE et al. (2010) HGF upregulation contributes to angiogenesis in mice with keratinocyte-specific Smad2 deletion. J. Clin. Invest 120, 3606–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian B et al. (2016) BRD4 mediates NF-kappaB-dependent epithelial-mesenchymal transition and pulmonary fibrosis via transcriptional elongation. Am. J. Physiol. Lung Cell Mol. Physiol 311, L1183–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R et al. (2012) Bromodomain protein Brd4 associated with acetylated chromatin is important for maintenance of higher-order chromatin structure. J. Biol. Chem 287, 10738–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whyte WA et al. (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B et al. (2009) Brd4 coactivates transcriptional activation of NF-kappaB via specific binding to acetylated RelA. Mol. Cell Biol 29, 1375–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian B et al. (2013) CDK9-dependent transcriptional elongation in the innate interferon-stimulated gene response to respiratory syncytial virus infection in airway epithelial cells. J. Virol 87, 7075–7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devaiah BN et al. (2016) BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat. Struct. Mol. Biol 23, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y et al. (2017) Quantitative assessment of the effects of trypsin digestion methods on affinity purification-mass spectrometry-based protein–protein interaction analysis. J. Proteome Res 16, 3068–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ijaz T et al. (2017) Coordinate activities of BRD4 and CDK9 in the transcriptional elongation complex are required for TGFb-induced Nox4 expression and myofibroblast transdifferentiation. Cell Death Differ. 8, e2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll NG et al. (2000) The airway longitudinal elastic fiber network and mucosal folding in patients with asthma. Am. J. Respir. Crit. Care Med 161, 244–248 [DOI] [PubMed] [Google Scholar]
- 37.Karvonen HM et al. (2013) Myofibroblast expression in airways and alveoli is affected by smoking and COPD. Respiratory Res. 14, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KK et al. (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. U. S. A 103, 13180–13185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans MJ et al. (1999) The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am. J. Respir. Cell Mol. Biol 21, 655–657 [DOI] [PubMed] [Google Scholar]
- 40.Duan Y et al. (2018) Targeting Brd4 for cancer therapy: inhibitors and degraders. MedChemComm 9, 1779–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang CY et al. (2019) Small-molecule PROTAC degraders of the bromodomain and extra terminal (BET) proteins – a review. Drug Discov. Today Technol. 31, 43–51 [DOI] [PubMed] [Google Scholar]
- 42.Kharenko OA and Hansen HC (2017) Novel approaches to targeting BRD4. Drug Discov. Today Technol. 24, 19–24 [DOI] [PubMed] [Google Scholar]
- 43.Cochran AG et al. (2019) Bromodomains: a new target class for drug development. Nat. Rev. Drug Discov 18, 609–628 [DOI] [PubMed] [Google Scholar]
- 44.Dhalluin C et al. (1999) Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 [DOI] [PubMed] [Google Scholar]
- 45.Sun C et al. (2018) BRD4 inhibition is synthetic lethal with PARP inhibitors through the induction of homologous recombination deficiency. Cancer Cell 33, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkina AC and Denis GV (2012) BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer 12, 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao Y et al. (2017) Brd4 modulates the innate immune response through Mnk2-eIF4E pathway-dependent translational control of IkappaBalpha. Proc. Natl. Acad. Sci. U. S. A 114, E3993–4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu J et al. (2012) Reactivation of latent HIV-1 by inhibition of BRD4. Cell Rep. 2, 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niu Q et al. (2019) Structure-guided drug design identifies a BRD4-selective small molecule that suppresses HIV. J. Clin. Invest 129, 3361–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duan Q et al. (2017) BET bromodomain inhibition suppresses innate inflammatory and profibrotic transcriptional networks in heart failure. Sci. Transl. Med 9, eaah5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korb E et al. (2015) BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat. Neurosci 18, 1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou J et al. (2018) Inhibitors of Bromodomain-Containing Protein 4 (BRD4). WO 2018/112037 A1 [Google Scholar]
- 53.Niu Q et al. (2019) Structure-guided drug design identifies a BRD4-selective small molecule that suppresses HIV. J. Clin. Invest 130, 3361–3373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z et al. (2013) The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res. 41, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brewster CE et al. (1990) Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol 3, 507–511 [DOI] [PubMed] [Google Scholar]
- 56.Zou Z et al. (2014) Brd4 maintains constitutively active NF-kappaB in cancer cells by binding to acetylated RelA. Oncogene 33, 2395–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tian B et al. (2019) Efficacy of novel highly specific bromodomain-containing protein 4 inhibitors in innate inflammation-driven airway remodeling. Am. J. Respir. Cell Mol. Biol 60, 68–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y et al. (2019) Pharmacoproteomics reveal novel protective activity of bromodomain containing 4 inhibitors on vascular homeostasis in TLR3-mediated airway remodeling. J. Proteomics 205, 103415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair H et al. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang X et al. (2013) Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am. J. Pathol 183, 470–479 [DOI] [PubMed] [Google Scholar]