Abstract
Objectives:
Bevacizumab with chemotherapy improved overall survival (OS) in the E4599 trial in metastatic non-squamous Non-Small Cell Lung Cancer (NS-NSCLC). A meta-analysis (Soria et al. Ann. Oncol 2012) demonstrated an OS benefit with bevacizumab in a subset of non-white patients. We explored the efficacy of anti-VEGF antibodies (AVA) in a diverse cohort.
Methods:
Patients with advanced (stage IIIB/IV, AJCC 7th edition) recurrent or metastatic NS-NSCLC diagnosed 1/2006–12/2017 at a single medical center were included. Survival analysis was performed with log-rank testing of Kaplan-Meier estimator. Univariate models were constructed, and significant variables, age sex, race were incorporated into a multi-variate Cox proportional hazard model. Data analysis was performed on SAS.
Results:
171 patients, 80 were treated with AVA and 91 were untreated. Median age: 63 years, 55% females, 19% Non-Hispanic Whites, 44% Blacks and 32% Hispanic Whites; median 40 pack-years of smoking; 11.7% had sensitizing EGFR mutations. Patients who received AVA had a survival benefit (26.6 vs. 19 months, p=0.025). Adjusting for age, sex, race/ethnicity, EGFR mutations, ECOG performance status and number of metastases; AVA therapy was associated with improved OS (aHazard Ratio = 0.62; p=0.049). In a sub-group analysis, females had survival benefit with AVA (median survival 29.1 months vs. 14.2 months; log-rank p=0.02) which was significant in the adjusted model (aHR=0.52; p=0.049).
Conclusions:
In a diverse cohort of patients with advanced NS-NSCLC, a survival benefit was confirmed with AVA. The greatest magnitude of benefit was in Blacks and Non-Hispanic Whites. A significant survival benefit was limited to female patients.
Keywords: Bevacizumab, Ramucirumab, Anti-VEGF, Non-small cell lung cancer, Minorities
Micro-Abstract
There is evidence of the differential efficacy of Anti-VEGF antibodies (AVA) based on race in colorectal cancer but it is unknown whether this extends to non-squamous non-small cell lung cancer (Nsq-NSCLC). A review of 171 patients with advanced Nsq-NSCLC from a single institution was performed which revealed a survival benefit by multivariate cox regression that was restricted to Non-Hispanic Whites and Blacks but not Hispanic Whites. In a sub-group analysis, this survival benefit was noted only in female patients indicating race and sex specific differences in the efficacy of AVA.
Introduction
Tumor-associated angiogenesis is critical to the growth, survival and metastasis of many solid malignancies.[1] Angiogenesis in the malignant context is predominantly mediated by the VEGF-A ligand through its interaction with the VEGFR-2 receptor.[2] Numerous strategies to disrupt this axis have been devised including monoclonal antibodies, VEGF traps and tyrosine kinase inhibitors. In non-small cell lung cancer (NSCLC), combinations of these agents with chemotherapy have shown survival benefit in the front-line and beyond.[3–5] Bevacizumab in combination with carboplatin and paclitaxel chemotherapy improved overall survival (OS) in the treatment-naive setting for the management of advanced non-squamous NSCLC in the ECOG 4599 trial. However, subsequent trials failed to show a significant OS benefit.[6] Ramucirumab, a human IgG1 that binds to the extracellular domain of the VEGF receptor-2 improved OS in a mixed cohort of patients with advanced squamous and non-squamous NSCLC in the second-line setting. [3] However, the efficacy of anti-VEGF antibodies in racial/ethnic minorities with non-squamous NSCLC in the real-world setting is currently lacking. A meta-analysis by Soria et al. demonstrated a statistically significant OS benefit with bevacizumab restricted to a subset (20%) of non-white patients mostly driven by the ECOG 4599 trial.[7] Similarly, within the context of colorectal cancer, there is evidence that bevacizumab, when combined with chemotherapy, provides additional benefit, predominantly among the Non-Hispanic Whites, but not so in Blacks.[8, 9] We explored the efficacy of anti-VEGF antibodies from real-world data in a diverse cohort of patients treated at a single academic institution.
Material and Methods
Clinical Data Collection
The initial cohort was obtained from the Albert Einstein Cancer Center cancer registry database. The included patients had a diagnosis of advanced (stage IIIB/IV, American Joint Committee Cancer 7th edition) recurrent or metastatic non-squamous NSCLC diagnosed between 1/2006–12/2017. Local IRB approval from Albert Einstein College of Medicine was received. Inclusion was restricted to patients who had received treatment and had complete records. Patients who were lost to follow-up within 30 days or lacked complete treatment history were excluded.. Demographic and clinical data including age, sex, race, progression-free survival (PFS), overall survival (OS), TNM stage, number and sites of metastases, diabetes, hypertension, smoking, EGFR/ALK/ROS mutations and ECOG performance status were collected from the database and a retrospective medical record review using MMC’s clinical information system (Centricity Enterprise, version 6.6.3 and EPIC). Ethnicity/Race data were recorded from patient self-report. All patient information was de-identified and stored as a Microsoft Excel spreadsheet (Version 2016) that was password protected. Progression-free survival was defined as the interval from the start of therapy to documented progression or death. OS was defined as the interval from the start of therapy to the date of death. For patients with unknown vital status, the survival time was censored at the date of the last follow-up visit.
Statistical Analysis
Patients were grouped into two groups a) those who were treated with anti-VEGF antibodies (bevacizumab and ramucirumab) and b) those who were not treated with anti-VEGF antibodies. Kaplan-Meier survival curves were generated by treatment groups and a log-rank test was performed to test the difference of the treatment groups in survival experience. Log-rank test was also done in specific gender and treatment sub-groups to test the difference of the age groups in survival experience. Bi-variate Cox proportional hazard regression analysis was performed to check the presence of cofounding effects. If adding a potential confounder to the model made the hazard ratio (HR) of treatment groups change by 10% or more, we considered it a confounder and included it in the next stage multi-variate Cox proportional hazard regression analysis. We also included age, sex and race in the multi-variate cox regression analysis. Adjusted HR and 95% confidence interval (CI) of each factor of the final multi-variate cox model was assessed. A two-sided p-value of 0.05 was deemed to be statistically significant. All data analysis was performed on SAS 9.4 (SAS Institute, Cary NC).
Results
We initially identified more than 2500 patients from the cancer registry with a diagnosis of advanced NS-NSCLC from 1/2006 to 12/2017. We narrowed our analysis to 171 patients who had been treated within our medical center with complete treatment records. This included 80 patients treated with anti-VEGF antibodies and 91 who did not receive anti-angiogenic therapy. The median age of the cohort was 63 years. The majority (87%) of patients had advanced disease at the time of diagnosis. Females constituted 55% of the cohort. While Non-Hispanic Whites comprised 19%, Blacks and Hispanic Whites were 44% and 32% respectively. Greater than 80% of the cohort comprised of patients with adenocarcinoma. Close to 80% had an ECOG PS of 0 or 1 at diagnosis. Sensitizing EGFR mutations (Exon 19 deletion or L858R substitution) were found in 11.7% of patients. The cohort was well-balanced in terms of age, sex, recurrent versus de-novo advanced disease, smoking history, number of extra-thoracic metastases, total number of metastases, hypertension, weight loss, and LDH at diagnosis. More patients not treated with anti-VEGF antibodies had ECOG PS >2 (19% versus 3%, p=0.008) and sensitizing EGFR mutations (18% versus 5%, p=0.02). The treated arm had a larger proportion of Non-Hispanic Whites compared to the non-treated arm (30% versus 11%, p=0.008). Complete demographic characteristics of patients treated with Anti-VEGF antibodies and those not treated is summarized in Table 1.
Table 1:
Demographics
| Attribute | Description | Treated with AVA (n=80) | Not Treated with AVA (n=91) | p-value* |
|---|---|---|---|---|
| Age | Median (IQR) | 63 (56–72) | 63 (58–71) | 0.44 |
| Sex | Male | 33 (41%) | 44 (48%) | 0.35 |
| Female | 47 (59%) | 47 (52%) | ||
| Status | Recurred | 10 (13%) | 13 (14%) | |
| Initial Stage IIIb or higher | 70 (88%) | 78 (86%) | ||
| Smoking | Never Smoker | 19 (24%) | 23 (25%) | |
| Ever Smoker | 37 (46%) | 44 (48%) | ||
| Not recorded | 24 (30%) | 24 (26%) | ||
| Number of pack-years | Median (IQR) | 23 (0–40) | 30 (0–50) | 0.47 |
| Pathology | Adenocarcinoma | 60 (75%) | 78 (86%) | |
| Other variants | 20 (25%) | 13 (14%) | ||
| ECOG PS | 0 | 18 (23%) | 21 (23%) | 0.008 |
| 1 | 48 (60%) | 50 (55%) | ||
| 2 | 2 (3%) | 15 (16%) | ||
| 3 | 0 (0%) | 3 (3%) | ||
| Not recorded | 12 (15%) | 2 (2%) | ||
| EGFR mutation | Deletion 19/Exon 21 L858R | 4 (5%) | 16 (18%) | 0.02 |
| Anti-VEGF antibody | Bevacizumab | 75 (94%) | N/A | |
| Ramucirumab | 6 (8%) | N/A | ||
| Immune Checkpoint blockade | Number (%) | 4 (5%) | 3 (3%) | |
| Extra-thoracic Metastases | Number (%) | 63 (80%) | 77 (85%) | 0.47 |
| Total Number of Metastases | 0 | 11 (14%) | 13 (14%) | 0.09 |
| 1 | 18 (23%) | 35 (38%) | ||
| 2 | 15 (19%) | 21 (23%) | ||
| 3 | 11 (14%) | 6 (7%) | ||
| 4 | 2 (3%) | 2 (2%) | ||
| ≥5 | 22 (28%) | 14 (15%) | ||
| Race/Ethnicity | Non-Hispanic Whites | 23 (30%) | 9 (11%) | 0.008 |
| Blacks | 30 (38%) | 46 (54%) | ||
| Hispanic Whites | 25 (32%) | 30 (35%) | ||
| Diabetes | Number (%) | 13 (23%) | 30 (33%) | 0.01 |
| Hypertension | Number (%) | 48 (60%) | 64 (70%) | 0.16 |
| Weight Loss | Number (%) | 24 (31%) | 34 (37%) | 0.40 |
| LDH at diagnosis | Median(IQR) | 213(179–289) | 216(183–289) | 0.57 |
P-value for Wilcoxon Rank-sum test, Chi-square test or fisher’s exact test, when appropriate. AVA = anti-VEGF antibody
A statistically significant improvement in median OS was noted in patients who received anti-VEGF antibodies when compared to un-exposed patients (26.6 months vs. 19 months, log-rank p=0.025). This improvement in survival was seen among all racial/ethnic sub-groups but differed in magnitude. A survival benefit approaching statistical significance was found in Non-Hispanic Whites (36.4 months vs. 15.4 months, log-rank p= 0.19) and Blacks (26.6 months vs 13 months log-rank p=0.13) but not in Hispanic Whites (23.2 months vs 22.9 months, log-rank p= 0.34). (Figure 1)
Figure 1:
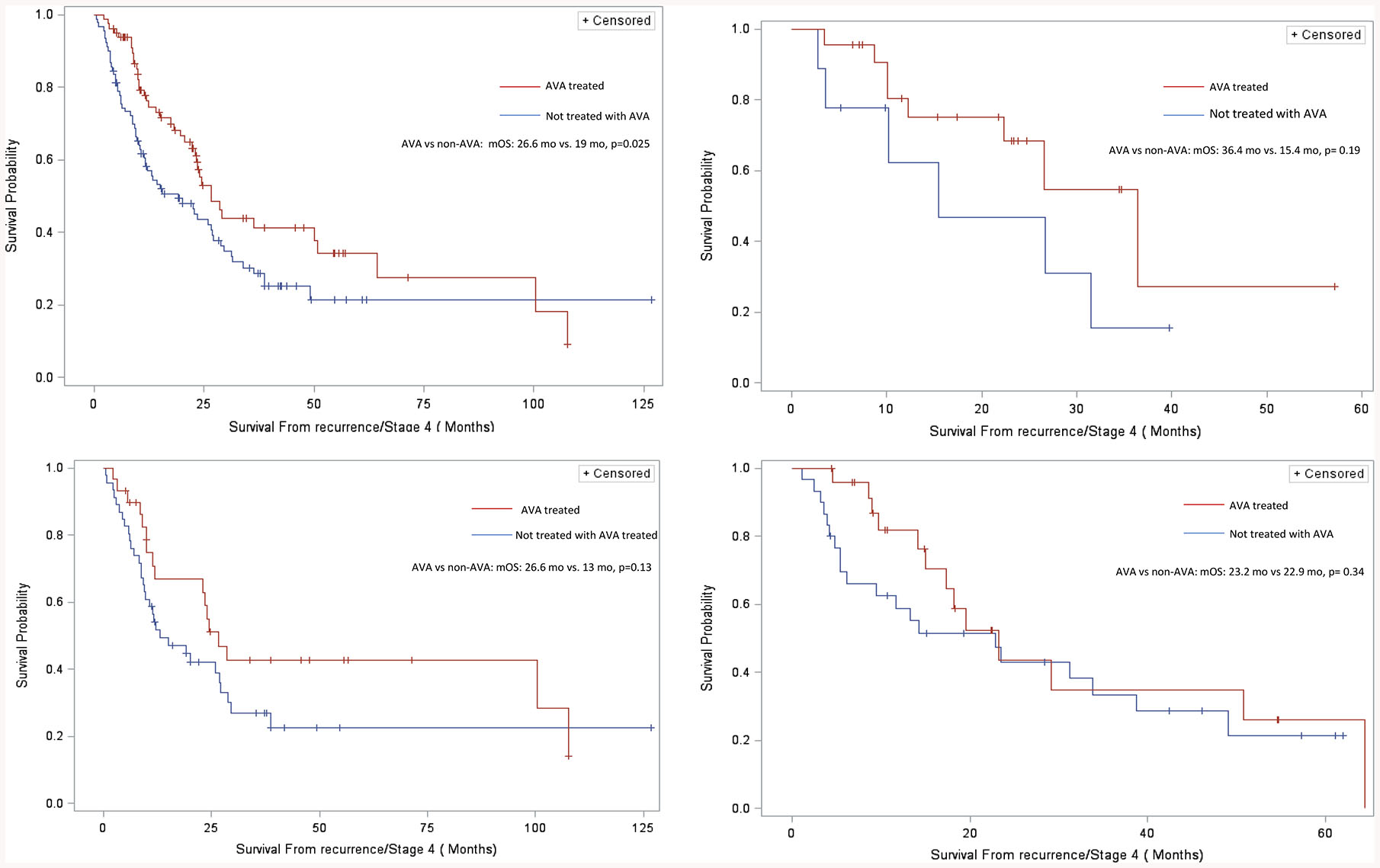
Survival Curves for Patients Receiving Chemotherapy Alone (Controls) Versus Those Receiving Chemotherapy Plus Anti-VEGF antibodies (AVA). (A) All Patients (B) Non-Hispanic Whites (C) Blacks (D) Hispanic Whites
A multi-variate cox regression model that included age, sex, race/ethnicity, sensitizing EGFR mutations, ECOG performance status, total number of sites of metastases and anti-VEGF antibody therapy was constructed. No statistically significant association was found between overall survival and age, sex or race/ethnicity in this model. ECOG performance status was associated with worsened survival which trended towards significance. (HR=1.34; 95% CI 0.97–1.85; p=0.07) While total number of metastases was significantly associated with worse overall survival (HR=1.19; 95% CI 1.05–1.35; p=0.005); the presence of sensitizing mutations in EGFR were associated with improved survival (HR=0.33; 95% CI 0.14–0.79; p=0.01). Treatment with anti-VEGF antibodies was independently associated with improved OS with an adjusted Hazard Ratio of 0.62 in this model (95% CI 0.39–0.99, p=0.049). (Table 2)
Table 2:
Multivariate Cox Proportional Hazard Regression Model for Overall Survival
| Variable | Adjusted Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Treatment with anti-VEGF antibody | 0.62 (0.28–0.99) | 0.049 |
| Age | 0.92 (0.82–1.03) | 0.16 |
| Sex | 0.86 (0.55–1.34) | 0.51 |
| Race/Ethnicity | ||
| 1) Blacks vs. Non-Hispanic Whites | 1.25 (0.65–2.39) | 0.48 |
| 2) Hispanic Whites vs. Non-Hispanic Whites | 1.28 (0.65–2.53) | 0.47 |
| 3) Blacks vs. Hispanic Whites | 0.97 (0.60–1.57) | 0.92 |
| ECOG Performance Status | 1.34 (0.97–1.85) | 0.07 |
| Total Number of Metastases | 1.19 (1.05–1.35) | 0.005 |
| EGFR mutation status (ex19del/ex21 L858R) | 0.33 (0.14–0.79) | 0.01 |
In a sub-group analysis, females treated with anti-VEGF antibodies had prolonged survival compared to non-treated females (median survival 29.1 months vs. 14.2 months; log-rank p=0.02). Female patients also had improved overall survival that was independently significant in the multi-variate cox model previously described. (aHR=0.52; 95% CI 0.27–0.99; p=0.049) In contrast, no survival benefit was forthcoming in male patients by Kaplan-Meier estimator. (23.5 months vs. 19 months, log-rank p=0.61) Also, there was no statistically significant survival benefit in males treated with anti-VEGF antibodies in the multivariate cox regression model. (aHR=0.76; 95% CI 0.33–1.80; p=0.54). (Figure 2) These observations confirm that the survival benefit with anti-VEGF antibody therapy in the cohort was limited to female patients alone.
Figure 2:
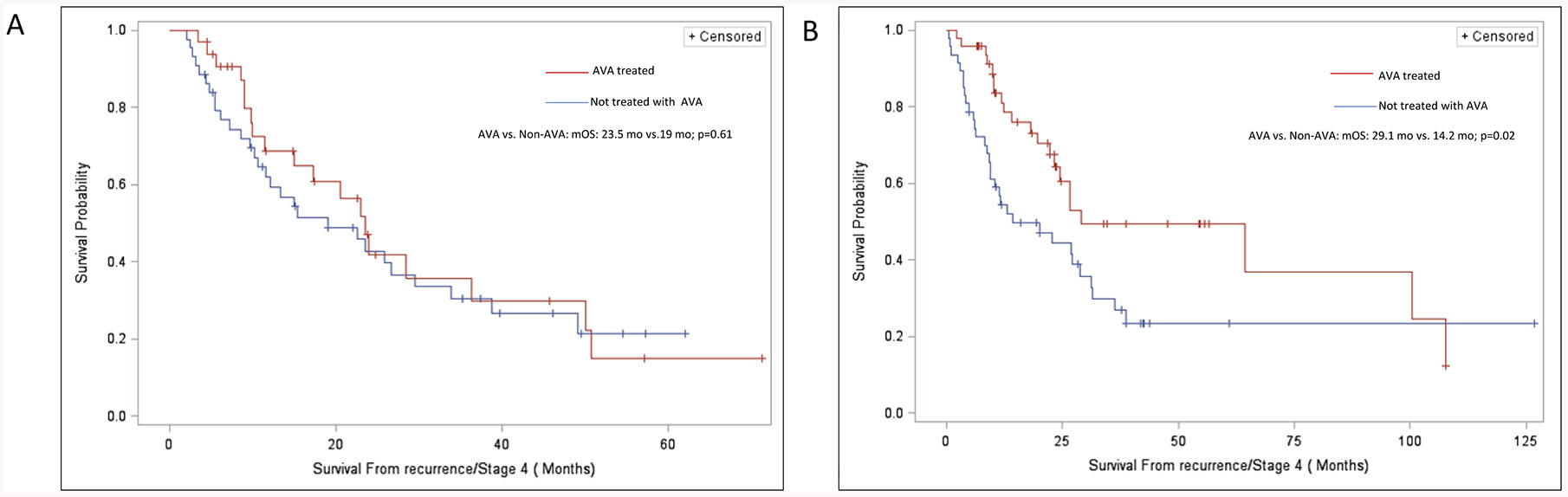
Survival Curves for Patients Receiving Chemotherapy Alone (Controls) Versus Those Receiving Chemotherapy Plus Anti-VEGF antibodies (AVA) in subsets (A) Males (B) Females
Discussion
In a diverse cohort of patients with advanced or recurrent non-squamous non-small cell lung cancer, anti-VEGF antibody therapy was associated with an absolute survival benefit of 7.6 months with a 38% reduction in risk of mortality after adjusting for age, sex, race/ethnicity, ECOG performance status, total number of metastatic sites and EGFR mutational status. In the patients who were not treated with Anti-VEGF antibodies, Non-Hispanic Whites and Blacks had worse survival when compared to Hispanic Whites but these groups derived the greatest absolute improvement in survival from anti-VEGF therapy. Female patients had a statistically significant prolongation of survival with anti-VEGF biologics.
In a sub-group analysis of the E4599 trial, though women <60 and <45 had a 4.5 month and 11 month improvement in OS, respectively with combination bevacizumab that approached statistical significance, no survival benefit was seen in women >60 [10]. While these findings seem contradictory, the discrepancy is likely explained by the important confounding effect of treatment with EGFR Tyrosine Kinase Inhibitors (TKI). There is now emerging evidence that EGFR TKI therapy has differential efficacy based on sex and age. An individual patient meta-analysis of randomized trials of erlotinib or gefitinib against chemotherapy showed statistically significant improved OS in female patients only (aHR=0.83, p=0.05) and prolonged progression free survival in patients ≥65 (aHR=0.77, p=0.001).[11] Recent evidence indicates a hitherto un-appreciated sensitivity for anti-VEGF treatments in female patients receiving EGFR TKI therapy. NEJ-026, a randomized phase III trial of bevacizumab in combination with erlotinib in the frontline treatment of EGFR mutation driven NSCLC showed a 55% reduction in risk of disease progression limited to female subjects.[12] Our study lacked statistical power to study the differential efficacy of anti-VEGF therapy among subsets of EGFR mutated and non-mutated patients to derive definitive conclusions.
Anti-VEGF strategies in combination with platinum-based chemotherapy improve OS in a number of gynecological malignancies including ovarian cancer, cervical cancer and possibly endometrial carcinoma confirming the importance of angiogenesis in the biology of these tumors.[13–15] Tumor-derived VEGF inhibits immune cell activity while anti-VEGF augments intra-tumoral chemokine expression, T-cell trafficking, MHC-I, Th1 and T-effector marker expression.[16] The combination of anti-VEGF antibodies and checkpoint blockade improves OS in non-squamous NSCLC, prolongs progression free-survival in renal cell carcinoma and is associated with emerging signals of robust durable responses in hepatocellular carcinoma.[17–19]. There are differences in the efficacy of checkpoint inhibitors based on sex; a meta-analysis of randomized trials comparing checkpoint blockade alone to chemotherapy in advanced NSCLC found limited benefit in women.[20]
Taken together, anti-angiogenic strategies may be especially effective in augmenting the efficacy of chemotherapy, EGFR TKI therapy and checkpoint blockade in female patients in whom overactive VEGF signaling augments tumor growth and immune evasion. Limitations of the study include single center, retrospective nature of the findings and limited sample sizes that restrict the ability to detect small inter-group differences. Nevertheless, evidence of efficacy of anti-VEGF antibody therapy in a diverse cohort of patients with NSCLC derived from real-world practice was a lacuna in knowledge that this study fills.
Conclusion
In a diverse cohort of patients with advanced non-squamous NSCLC derived from real-world practice, a significant survival benefit was confirmed with anti-VEGF antibody therapy after adjusting for age, sex, race/ethnicity, ECOG PS, total number of metastases and EGFR mutation status. The greatest magnitude of benefit was seen in Blacks and Non-Hispanic Whites. A subgroup analysis revealed a significant survival benefit limited to female patients with the addition of anti-VEGF therapies.
Clinical practice points.
The addition of anti-VEGF antibodies (AVA) to chemotherapy for the treatment of advanced non-squamous NSCLC was associated with improved survival in the predominantly Caucasian population enrolled in the ECOG 4599 trial. There is some evidence of differential efficacy of AVA in colorectal cancer based on race; with the most benefit being seen in Non-Hispanic Whites and the least in Blacks. In a review of 80 patients with advanced non-squamous NSCLC treated with AVA compared to 91 patients treated with conventional chemotherapy alone; a statistically significant improvement in median survival was noted in patients who received AVA (26.6 months vs. 19 months, log-rank p=0.025) which remained significant after adjusting for age, sex, race/ethnicity, sensitizing EGFR mutations, ECOG performance status and total number of sites of metastases (HR 0.62, p=0.049). This improvement in survival was most prominent in Non-Hispanic Whites (36.4 months vs. 15.4 months, log-rank p= 0.19) and Blacks (26.6 months vs. 13 months log-rank p=0.13) but not in Hispanic Whites (23.2 months vs. 22.9 months, log-rank p= 0.34). In a sub-group analysis, females treated with AVA had prolonged survival compared to non-treated females (median survival 29.1 months vs. 14.2 months; log-rank p=0.02). These findings may be indicative of sex-specific differences in VEGF signaling which is supported by evidence of benefit of AVA in advanced cervical and ovarian carcinomas. These findings indicate that race and sex may be important factors to consider when deciding optimal therapy for patients with advanced non-squamous NSCLC in the clinic.
Acknowledgements
The work was supported by LCFA/IASLC Foundation Lori Monroe Scholarship Award (to H. Cheng).
Abbreviations:
- VEGF
Vascular Endothelial Growth Factor
References
- [1].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144(5) (2011) 646–74. [DOI] [PubMed] [Google Scholar]
- [2].Ferrara N, Pathways mediating VEGF-independent tumor angiogenesis, Cytokine Growth Factor Rev 21(1) (2010) 21–6. [DOI] [PubMed] [Google Scholar]
- [3].Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel J, Czyzewicz G, Orlov SV, Lewanski CR, Thomas M, Bidoli P, Dakhil S, Gans S, Kim JH, Grigorescu A, Karaseva N, Reck M, Cappuzzo F, Alexandris E, Sashegyi A, Yurasov S, Perol M, Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial, Lancet 384(9944) (2014) 665–73. [DOI] [PubMed] [Google Scholar]
- [4].Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, von Pawel J, Gottfried M, Bondarenko I, Liao M, Gann CN, Barrueco J, Gaschler-Markefski B, Novello S, L.U.-L.S. Group, Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial, Lancet Oncol 15(2) (2014) 143–55. [DOI] [PubMed] [Google Scholar]
- [5].Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH, Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer, N Engl J Med 355(24) (2006) 2542–50. [DOI] [PubMed] [Google Scholar]
- [6].Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C, Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil, J Clin Oncol 27(8) (2009) 1227–34. [DOI] [PubMed] [Google Scholar]
- [7].Soria JC, Mauguen A, Reck M, Sandler AB, Saijo N, Johnson DH, Burcoveanu D, Fukuoka M, Besse B, Pignon JP, N.c.g. meta-analysis of bevacizumab in advanced, Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer, Ann Oncol 24(1) (2013) 20–30. [DOI] [PubMed] [Google Scholar]
- [8].Goel S, Negassa A, Khot A, Goyal D, Guo S, Nandikolla A, Bakirhan K, Polineni R, Shah U, Chaudhary I, Ghalib MH, Rajdev L, Kaubisch A, Chuy J, Aparo S, Comparative Effectiveness Research: The Impact of Biologic Agents in Ethnic Minorities With Metastatic Colorectal Cancer, Clin Colorectal Cancer 16(4) (2017) 286–292. [DOI] [PubMed] [Google Scholar]
- [9].Catalano PJ, Mitchell EP, Giantonio BJ, Meropol NJ, B. AB III, Outcomes differences for African Americans and Caucasians treated with bevacizumab, FOLFOX4 or the combination in patients with metastatic colorectal cancer (MCRC): Results from the Eastern Cooperative Oncology Group Study E3200, Journal of Clinical Oncology 25(18_suppl) (2007) 4100–4100. [Google Scholar]
- [10].Wakelee HA, Dahlberg SE, Brahmer JR, Schiller JH, Perry MC, Langer CJ, Sandler AB, Belani CP, Johnson DH, G. Eastern Cooperative Oncology, Differential effect of age on survival in advanced NSCLC in women versus men: analysis of recent Eastern Cooperative Oncology Group (ECOG) studies, with and without bevacizumab, Lung Cancer 76(3) (2012) 410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee CK, Davies L, Wu YL, Mitsudomi T, Inoue A, Rosell R, Zhou C, Nakagawa K, Thongprasert S, Fukuoka M, Lord S, Marschner I, Tu YK, Gralla RJ, Gebski V, Mok T, Yang JC, Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: Individual Patient Data Meta-Analysis of Overall Survival, J Natl Cancer Inst 109(6) (2017). [DOI] [PubMed] [Google Scholar]
- [12].Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, Nakachi I, Gemma A, Azuma K, Kurimoto F, Tsubata Y, Fujita Y, Nagashima H, Asai G, Watanabe S, Miyazaki M, Hagiwara K, Nukiwa T, Morita S, Kobayashi K, Maemondo M, Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial, Lancet Oncol 20(5) (2019) 625–635. [DOI] [PubMed] [Google Scholar]
- [13].Wu YS, Shui L, Shen D, Chen X, Bevacizumab combined with chemotherapy for ovarian cancer: an updated systematic review and meta-analysis of randomized controlled trials, Oncotarget 8(6) (2017) 10703–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, DiSaia PJ, Copeland LJ, Creasman WT, Stehman FB, Brady MF, Burger RA, Thigpen JT, Birrer MJ, Waggoner SE, Moore DH, Look KY, Koh WJ, Monk BJ, Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240), Lancet 390(10103) (2017) 1654–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aghajanian C, Filiaci V, Dizon DS, Carlson JW, Powell MA, Secord AA, Tewari KS, Bender DP, O’Malley DM, Stuckey A, Gao J, Dao F, Soslow RA, Lankes HA, Moore K, Levine DA, A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer, Gynecol Oncol 150(2) (2018) 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, Denker M, Leveque V, Canamero M, Babitski G, Koeppen H, Ziai J, Sharma N, Gaire F, Chen DS, Waterkamp D, Hegde PS, McDermott DF, Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma, Nat Commun 7 (2016) 12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA, I.M.S. Group, Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial, Lancet Respir Med 7(5) (2019) 387–401. [DOI] [PubMed] [Google Scholar]
- [18].Rini BI, Powles T, Atkins MB, Escudier B, McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL, Hawkins R, Ravaud A, Alekseev B, Staehler M, Uemura M, De Giorgi U, Mellado B, Porta C, Melichar B, Gurney H, Bedke J, Choueiri TK, Parnis F, Khaznadar T, Thobhani A, Li S, Piault-Louis E, Frantz G, Huseni M, Schiff C, Green MC, Motzer RJ, I.M.S. Group, Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial, Lancet (2019). [DOI] [PubMed] [Google Scholar]
- [19].Pishvaian MJ, Lee MS, Ryoo B-Y, Stein S, Lee K-H, Verret W, Spahn J, Shao H, Liu B, Iizuka K, Hsu C-H, LBA26Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC), Annals of Oncology 29(suppl_8) (2018). [Google Scholar]
- [20].Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, Gelber RD, Goldhirsch A, Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis, Lancet Oncol 19(6) (2018) 737–746. [DOI] [PubMed] [Google Scholar]


